Abstract
Environmental 222radon exposure is a human health concern, and many studies demonstrate that very low doses of high LET α-particle irradiation initiate deleterious genetic consequences in both irradiated and non-irradiated bystander cells. One consequence, radiation-induced genomic instability (RIGI), is a hallmark of tumorigenesis and is often assessed by measuring delayed chromosomal aberrations. We utilised a technique that facilitates transient immobilization of primary lymphocytes for targeted microbeam irradiation and have reported that environmentally relevant doses, e.g. a single 3He2+ particle traversal to a single cell, are sufficient to induce RIGI. Herein we sought to determine differences in radiation response in lymphocytes isolated from five healthy male donors. Primary lymphocytes were irradiated with a single particle per cell nucleus. We found evidence for inter-individual variation in radiation response (RIGI, measured as delayed chromosome aberrations). Although this was not highly significant, it was possibly masked by high levels of intra-individual variation. While there are many studies showing a link between genetic predisposition and RIGI, there are few studies linking genetic background with bystander effects in normal human lymphocytes. In an attempt to investigate inter-individual variation in the induction of bystander effects, primary lymphocytes were irradiated with a single particle under conditions where fractions of the population were traversed. We showed a marked genotype-dependent bystander response in one donor after exposure to 15% of the population. The findings may also be regarded as a radiation-induced genotype-dependent bystander effect triggering an instability phenotype.
Keywords: Genomic instability, Bystander effects, Individual variation, Radiosensitivity, Human lymphocytes, Microbeam
1. Introduction
Radiation exposure can lead to a number of cellular changes that resemble those observed during tumorigenesis. A hallmark of tumorigenesis, genomic instability (GI), describes an increased rate in the accumulation of new genetic alterations and is frequently assessed using cytogenetic analysis of chromosomes both in clinical and research settings. In addition to being a characteristic of tumorigenesis and being observed in a large fraction of the progeny of irradiated cells, radiation-induced genomic instability (RIGI) has also been reported in unirradiated cells (bystander cells) that are in the vicinity of, and able to communicate with, irradiated cells [1,2]. Understanding radiation-induced bystander effects (RIBE) is crucial for a complete elucidation of radiation exposures, including those used for therapy [3] and several possible mechanisms and candidate genes responsible have been identified [4]. In particular, significant evidence for abscopal effects [2] in patients treated with radiation could be manifestations of bystander responses. Radon and its degradation progeny are ubiquitous in the environment and exposure may be linked to lung cancer in miners [5] and lung fibrosis in plutonium workers [6]; furthermore, indoor radon exposure has been associated with lung cancer risk [7] and leukaemia [8]. At relevant exposure levels, cells at risk in the human body will predominantly only see a single particle traversal and therefore bystander responses may be important in overall risk.
Measurement of cytogenetic aberrations in peripheral lymphocytes is commonly used for biological dosimetry following occupational or accidental exposure to individuals [9] or for retrospective dosimetry [10,11], and can also help to predict the risk of side effects after radiation therapy [12,13], and may even be helpful in predicting cancer risk [14,15].
Inter-individual differences in chromosome aberration frequencies after low linear energy transfer (LET) γ-irradiation have been reported [16]. This study also found substantial intra-individual variation with time, which might confound interpretation of inter-individual variation. However, over extended periods (12 years and 200 samplings), it has been reported that mean spontaneous chromosome aberration frequency for a single subject is relatively constant [17]. Inter-individual variation in the induction of chromosome damage has been associated with for instance, DNA repair gene polymorphisms [18] and even seasonal variations in ambient temperature [19].
In our previous studies with several normal human bone marrow samples, we demonstrated inter-individual variation in the level of RIGI after high LET α-particle irradiation, such as would occur with radon exposure, where only 2/4 individuals were sensitive to the induction of GI [20,21]. However, these studies were performed using a broad-beam 238Pu source, where the number of particle traversals per cell follows a Poisson distribution. Exposure to radon to the vast majority of the population rarely occurs and is generally such that very few cells in the lung will ever be traversed and those that do will only ever see a single charged particle traversal [22], suggesting the need for more precisely targeted high LET sources to faithfully replicate the in vivo or environmental exposure condition. Charged particle microbeams have emerged as powerful tools to target exact numbers of cells for irradiation by precise numbers of particle, and have been used in recent years to study a wide range of biological endpoints [23]. Additionally, as precise fractions of the cellular population may be targeted, microbeams are ideal to study effects in unirradiated bystander cells [24,25].We have developed techniques that allow the transient immobilization of primary human lymphocytes [26], which are otherwise non-adherent and thus not amenable to microbeam studies; in doing so, we combine irradiation of a common cell type used in biological dosimetry and exposure assessments with precise irradiation conditions that mimic the environmental exposure situation under controlled laboratory conditions. Using lymphocytes from a single donor, we have previously reported that nuclear traversal of all cells in a population with a single or two 3He2+ particles results in a significant induction of RIGI [27]. Also, nuclear traversal of fractions of the cellular population with a single 3He2+ particle results in a marked induction of RIGI, suggesting a significant contribution of bystander effects.
In this study we couple the measurement of delayed chromosome aberrations (RIGI) in primary lymphocytes from five donors following targeted microbeam irradiation to address the hypothesis that inter-individual differences in radiation response in terms of induction of RIGI will be evident between healthy donors, and that intra-individual variations will also exist under bystander experimental conditions.
2. Materials and methods
Primary human T-lymphocytes were isolated from five healthy donors as described [28] and in accordance with the guidelines of Responsibility in Investigations on Human Participants and Material and on Personal Information (MRC Ethics Series, November 1992). Ampoules were stored in liquid nitrogen. Donors were numerically coded as 12, 21, 31, 51 and 61. Mean donor age (years) was 42.4 (±5.13 SD), ranging between 35 and 48. All donors were healthy non-smoking males. Data for ambient temperature at time of blood collection are from the UK Met Office, http://www.metoffice.com/climate/uk/.
At least 6 hr before the experiment, 4 μm thick based polypropylene tissue culture plates (microbeam dishes) were treated with the biological adherent Cell Tak (Fahrenheit Scientific) as described [26]. On the day of the experiment, a single ampoule of cells was thawed and washed. The cells were resuspended in fresh complete media; RPMI 1640 (Gibco, UK) supplemented with 10% heat-inactivated foetal bovine serum (PAA, UK) 2 mM sodium pyruvate, 100 μg/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 100 IU/ml interleukin 2 (Sigma, UK) with 2× normal concentration Phytohaemagglutinin (100 μl/ml, Invitrogen, UK) to facilitate attachment and stained with a 0.8 μM of Hoechst 33258 to highlight the cell nucleus for targeting [26]. Microbeam dishes were seed with approximately 1 × 104 cells in 30 μl complete medium. Unattached cells were removed by washing in supplemented RPMI with 20 mM HEPES. Cells were plated at low density to minimize the potential for overlap and approximately 500-1000 cells remained attached after washing. Exact cell number per plate was determined when plates were scanned on the microbeam. The cells were irradiated in 2 ml of supplemented RPMI with 20 mM HEPES. All irradiations were performed at room temperature.
The Gray Cancer Institute charged particle microbeam was used for all irradiations, described in [26]. The efficiency of delivering a single particle to a specified target (±2 μm) is >99%, in comparison to the size of human lymphocytes (6–7 μm diameter). Fractions of the population were irradiated at G0/G1 of the cell cycle, with precisely one 3He2+ particle (105 KeV/μm) delivered through the centre of each nucleus. Irradiation of a single cell with a single particle took less than a second and total exposure time per plate took up to 15 min. For bystander studies, 15% of cells were automatically selected at random from the total population of cells for irradiation. The groups have been abbreviated throughout the text as described [27]. Briefly: 1/n (100%)–1 traversal per nucleus, with 100% of the population targeted, or 1/n (15%)–1 traversal per nucleus, 15% of cells in the population targeted. Sham-irradiated controls were included. Parallel control sets were included to assess the effects of plating conditions on doubling times and unpublished data from our group demonstrate that microbeam plating conditions do not alter doubling times or GI (data not shown).
After irradiation, cells were processed for long term culture and delayed chromosome instability analysis as described previously [23]. Briefly, cells and irradiation media (2 ml) were removed immediately from the microbeam dish with gentle aspiration and placed into a flask with 4 ml tissue culture media for expansion. Twenty-one days after irradiation (12–13 population doublings), exponentially growing cells were harvested for chromosome instability analysis as described in [26]. At least fifty well spread metaphases were analysed per sample (i.e. per microbeam dish) using a light microscope.
RIGI is characterised in this study by de novo aberrations as described [26,27]. With solid staining, chromatid-type aberrations are readily visible and provide a useful marker of de novo chromosomal alterations [29] and they presumably arise during the cell cycle in which they are observed. Acentric fragments and double minute chromosomes were included in the present study as delayed aberrations associated with ongoing RIGI.
Comparisons between treatments within individual donors and between donors within a treatment group were made using a two-sided unpaired t-test. Because we had some preliminary unpublished data from a previous study [27] to suggest that cell number/dish at time of irradiation may be important to the induction of RIGI, data were sorted by number of cells/microbeam dish after the aberration values from each treatment [1/n (100%) and 1/n (15%) across all donors was grouped when non-significant by Kruskal–Wallis ANOVA. In all statistical tests, p = 0.05 was used as the significance cut-off. Statistical comparisons were made using GraphPad InStat software (version 3.06, GraphPad Software, Inc.).
3. Results and discussion
Despite showing that genetic factors significantly influence RIGI under conditions where all cells/tissue are exposed to ionising radiation, there are no sufficient studies to relate genetic factors and RIGI under bystander conditions in a normal population, although some studies have carried out using established cell lines [30]. To address this issue, this study was designed to investigate the influence of genetic background in the induction of RIGI in bystander cell populations and test the hypothesis that similar inter-individual and genetic variations are operable under bystander conditions. In the current study of primary T-lymphocytes isolated from five healthy donors.
3.1. Inter-individual variation in RIGI in primary human lymphocytes: variation in the induction of cytogenetic damage in fully irradiated lymphocytes
Results for the five donors are shown in Fig. 1. In donors 12, 21 and 51, chromosome aberration yields in cells following one particle hit to each nucleus (1/n (100%) were similar to or less than those in corresponding controls, partly due to intra-individual (inter-microbeam dish) variability. In contrast, in donor 31 and 61, there was a trend of an increase in aberration yield in the fully irradiated groups compared to controls, i.e. 1.8 (p = 0.28) and 2.5-fold (p = 0.22) increase in donor 31 and 61, respectively, however they were not statistically significant. Genomic instability was not induced significantly in any of the fully irradiated groups (1/n (100%). The increase over controls in donor 61 (2.5-fold increase) in the fully irradiated group was similar in magnitude to previous reports from our group (2.5–3-fold increase) [27], suggesting that the lack of response observed in the other donors in this study (Fig. 1) is not an artefact as all experiments were performed under identical conditions (i.e. same batch of medium, serum, growth factors and the same chromosome analysis criteria).
Fig. 1.
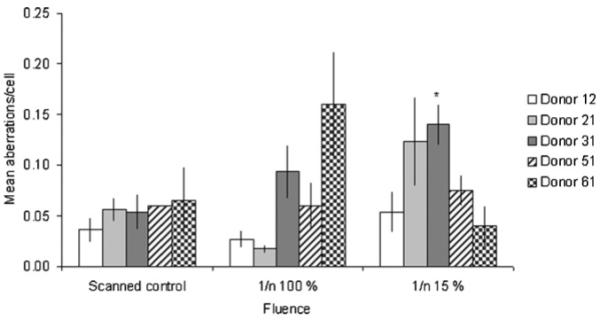
Inter-individual differences in radiosensitivity. Primary lymphocytes were collected from five different donors and irradiated. Mean aberrations per cell (±SEM) from 2 to 6 experiments are plotted. 1/n (100%) indicates a single 3He2+ track to all of the cells (fully irradiated), and 1/n (15%) indicates a single 3He2+ track to 15% of the cells on the microbeam dish. Increases in aberrations are taken to indicate radiation-induced genomic instability (RIGI) when significantly elevated compared to control values (indicated by * when p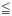 0.05).
0.05).
3.2. Inter-individual variation in bystander response as measured by delayed chromosomal aberration
Similar to RIGI induction, RIBE have been demonstrated in both in vitro and in vivo systems [31,32] and like GI response it has been reported that not all cell types produce bystander signals and not all cell types respond [33-35]. This variable expression of RIBE could be due to the presence of multiple pathways involved in the various bystander phenomena. Recent studies have shown that the extent of bystander effect varied according to the genetic background of cells [36,37].
In the current study we investigated the inter-individual variation in the induction of RIBE under controlled conditions in order to investigate the hypothesis that inter-individual differences in radiation-induced chromosomal instability will be evident between healthy lymphocyte donors, in situations where fractions of the cellular population were irradiated (Fig. 1).
In the majority (4/5) of groups where 15% of the cells were irradiated with 3He2+ particles, chromosomal aberrations were not significantly elevated over controls. However, in several donors (12 and 21) there were slight increases in aberrations above controls and another donor (51) there was a marked (although not significant elevation in damage above control levels and more so than in the 100% irradiated group. In one donor (31) with 15% cell population exposure, there was a significant increase in aberration yield over the respective controls (p = 0.05). It is of note, that this donor (31) expresses more chromosome instability response when all cells were irradiated compared to other donors (Fig. 1) although this increase was not statistically significant. In stark contrast, the final donor (61) exhibited decreased aberration yield above control levels. These results suggest that there is genotype dependence in radiation-induced bystander effects.
3.3. Variation is not a function of donor age or ambient temperature at time of collection
Finally we examined some common factors that might underlie the inter-individual variations. Within the available statistics there was no obvious relationship between donor age or ambient temperature and background aberrations (Table 1).
Table 1.
Potential sources of variability in chromosomal instability: donor age, ambient temperature and background levels of chromosomal damage. Temperature data is from http://www.metoffice.com/climate/uk/.
| Donor | Age (years) | Mean temp (°C) | Mean aberrations/cell background levels (±SEM) |
|---|---|---|---|
| 12 | 35 | 14.7 | 0.036 ± 0.0117 |
| 21 | 41 | 6.6 | 0.056 ± 0.0117 |
| 31 | 43 | 6.6 | 0.053 ± 0.0176 |
| 51 | 46 | 12.5 | 0.060 ± 0.0000 |
| 61 | 48 | 15.4 | 0.065 ± 0.0333 |
Despite the small number of donors (n = 5), and the usual variation in age (mean = 42.4±5.13 years; low = 35, high = 48), we expected a correlation between donor age and mean aberrations per cell, as reviewed in [38]. However, there was no obvious relationship between donor age and mean aberrations per cell (Table 1). We also determined ambient temperature at time of lymphocyte collection as a recent report showed a significant correlation between ambient temperature and chromosome damage in a large group of donors [19]. Within the available statistics there was no obvious relationship between ambient temperature at time of collection and mean aberrations per cell (Table 1). These results support the assertion that the current finding of very little RIGI (Fig. 1) was not due to an experimental artefact or indication of some underlying differences in the particulars of the current studies, but rather is biologically relevant.
4. Conclusions
Following targeted microbeam irradiation with a single helium ion, whilst we did not find significant evidence for inter-individual variation in the level of RIGI in the fully irradiated normal human lymphocytes, we do report for the first time, intra-individual variation in the level of RIGI under bystander condition with only one of five donors showing a significant increase in aberrations in the bystander group. These results suggest that, in the bystander groups, inter-cellular communication within the bystander cell population may be critical for the increases in chromosome aberrations associated with RIGI. This relationship may have important consequences for the role of the bystander response in vivo, where cell context at the time of exposure may be important, and thus may have implications for environmental dosimetry and radiotherapy.
Acknowledgements
We are grateful to Professor Melvyn Folkard for helpful discussion and advice regarding the microbeam irradiation system, to Mr. Stuart Gilchrist for assistance with the microbeam irradiation and also to Mrs. Debbie Bowler and Dr. Sarah Irons for valuable technical assistance and guidance. This work was supported by the UK Department of Health grant (RRX-100) to MAK and KMP, Medical Research Council, The European Union NOTE project (FI6R 036465), US Department of Energy Grant (DE-FG02-05ER64079) and Cancer Research UK ([CR-UK] C1513/A7047).
Abbreviations
- GI
genomic instability
- BE
bystander effects
- LET
linear energy transfer
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- [1].Chapman KL, Kelly JW, Lee R, Goodwin EH, Kadhim MA. Tracking genomic instability within irradiated and bystander populations. J. Pharm. Pharmacol. 2008;60:959–968. doi: 10.1211/jpp.60.8.0003. [DOI] [PubMed] [Google Scholar]
- [2].Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat. Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [3].Mothersill C, Rea D, Wright EG, Lorimore SA, Murphy D, Seymour CB, O’Malley K. Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis. 2001;22:1465–1471. doi: 10.1093/carcin/22.9.1465. [DOI] [PubMed] [Google Scholar]
- [4].Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J. Pharm. Pharmacol. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gilliland FD, Hunt WC, Archer VE, Saccomanno G. Radon progeny exposure and lung cancer risk among non-smoking uranium miners. Health Phys. 2000;79:365–372. doi: 10.1097/00004032-200010000-00004. [DOI] [PubMed] [Google Scholar]
- [6].Newman LS, Mroz MM, Ruttenber AJ. Lung fibrosis in plutonium workers. Radiat. Res. 2005;164:123–131. doi: 10.1667/rr3407. [DOI] [PubMed] [Google Scholar]
- [7].Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Makelainen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Rosario AS, Tirmarche M, Tomasek L, Whitley E, Wichmann HE, Doll R. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case–control studies. BMJ. 2005;330:223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Henshaw DL. Radon and childhood cancer. Br. J. Cancer. 2002;87:1336–1337. doi: 10.1038/sj.bjc.6600671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wojcik A, Gregoire E, Hayata I, Roy L, Sommer S, Stephan G, Voisin P. Cytogenetic damage in lymphocytes for the purpose of dose reconstruction: a review of three recent radiation accidents. Cytogenet. Genome Res. 2004;104:200–205. doi: 10.1159/000077489. [DOI] [PubMed] [Google Scholar]
- [10].Hande MP, Azizova TV, Burak LE, Khokhryakov VF, Geard CR, Brenner DJ. Complex chromosome aberrations persist in individuals many years after occupational exposure to densely ionizing radiation: an mFISH study. Genes Chromosomes Cancer. 2005;44:1–9. doi: 10.1002/gcc.20217. [DOI] [PubMed] [Google Scholar]
- [11].Mitchell CR, Azizova TV, Hande MP, Burak LE, Tsakok JM, Khokhryakov VF, Geard CR, Brenner DJ. Stable intrachromosomal biomarkers of past exposure to densely ionizing radiation in several chromosomes of exposed individuals. Radiat. Res. 2004;162:257–263. doi: 10.1667/rr3231. [DOI] [PubMed] [Google Scholar]
- [12].Wang WD, Chen ZT, Li DZ, Cao ZH, Pu P, Fu SZ, Chen J, Sun SL, Chen XP. Detecting normal cell radiosensitivity via assay of DNA damage in lymphocytes for individualizing radiotherapy in head and neck cancer patients. Oncology. 2005;69:208–213. doi: 10.1159/000088332. [DOI] [PubMed] [Google Scholar]
- [13].Borgmann K, Hoeller U, Nowack S, Bernhard M, Roper B, Brackrock S, Petersen C, Szymczak S, Ziegler A, Feyer P, Alberti W, Dikomey E. Individual radiosensitivity measured with lymphocytes may predict the risk of acute reaction after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:256–264. doi: 10.1016/j.ijrobp.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [14].Kolusayin Ozar MO, Orta T. The use of chromosome aberrations in predicting breast cancer risk. J. Exp. Clin. Cancer Res. 2005;24:217–222. [PubMed] [Google Scholar]
- [15].Bonassi S, Norppa H, Ceppi M, Stromberg U, Vermeulen R, Znaor A, Cebulska-Wasilewska A, Fabianova E, Fucic A, Gundy S, Hansteen IL, Knudsen LE, Lazutka J, Rossner P, Sram RJ, Boffetta P. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis. 2008;29:1178–1183. doi: 10.1093/carcin/bgn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vral A, Thierens H, Baeyens A, De, Ridder L. Chromosomal aberrations and in vitro radiosensitivity: intra-individual versus inter-individual variability. Toxicol. Lett. 2004;149:345–352. doi: 10.1016/j.toxlet.2003.12.044. [DOI] [PubMed] [Google Scholar]
- [17].Andersson HC. The spontaneous frequency of chromosomal aberrations and sister-chromatid exchanges in cultured peripheral lymphocytes of a single blood donor sampled more than 200 times. Mutat. Res. 1993;286:281–292. doi: 10.1016/0027-5107(93)90193-j. [DOI] [PubMed] [Google Scholar]
- [18].Kiuru A, Lindholm C, Heilimo I, Ceppi M, Koivistoinen A, Ilus T, Hirvonen A, Norppa H, Salomaa S. Influence of DNA repair gene polymorphisms on the yield of chromosomal aberrations. Environ. Mol. Mutagen. 2005;46:198–205. doi: 10.1002/em.20155. [DOI] [PubMed] [Google Scholar]
- [19].Giovannelli L, Pitozzi V, Moretti S, Boddi V, Dolara P. Seasonal variations of DNA damage in human lymphocytes: correlation with different environmental variables. Mutat. Res. 2006;593:143–152. doi: 10.1016/j.mrfmmm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- [20].Kadhim MA, Lorimore SA, Hepburn MD, Goodhead DT, Buckle VJ, Wright EG. Alpha-particle-induced chromosomal instability in human bone marrow cells. Lancet. 1994;344:987–988. doi: 10.1016/s0140-6736(94)91643-8. [DOI] [PubMed] [Google Scholar]
- [21].Kadhim MA, Lorimore SA, Townsend KM, Goodhead DT, Buckle VJ, Wright EG. Radiation-induced genomic instability: delayed cytogenetic aberrations and apoptosis in primary human bone marrow cells. Int. J. Radiat. Biol. 1995;67:287–293. doi: 10.1080/09553009514550341. [DOI] [PubMed] [Google Scholar]
- [22].Health Effects of Exposure to Radon: BEIR VI. National Research Council; Washington DC: 1999. [PubMed] [Google Scholar]
- [23].Bigelow A, Brenner D, Garty G, Randers-Pehrson G. Single particle/single-cell ion microbeams as probes of biological mechanisms. IEEE Trans. Plasma Sci. 2008;36:1424–1431. [Google Scholar]
- [24].Prise KM, Schettino G, Vojnovic B, Belyakov O, Shao C. Microbeam studies of the bystander response. J. Radiat. Res. (Tokyo) 2009;50(Suppl. A):A1–6. doi: 10.1269/jrr.09012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- [26].Kadhim MA, Marsden SJ, Goodhead DT, Malcolmson AM, Folkard M, Prise KM, Michael BD. Long-term genomic instability in human lymphocytes induced by single-particle irradiation. Radiat. Res. 2001;155:122–126. doi: 10.1667/0033-7587(2001)155[0122:ltgiih]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [27].Moore SR, Marsden S, Macdonald D, Mitchell S, Folkard M, Michael B, Goodhead DT, Prise KM, Kadhim MA. Genomic instability in human lymphocytes irradiated with individual charged particles: involvement of tumor necrosis factor alpha in irradiated cells but not bystander cells. Radiat. Res. 2005;163:183–190. doi: 10.1667/rr3298. [DOI] [PubMed] [Google Scholar]
- [28].Anderson RM, Marsden SJ, Wright EG, Kadhim MA, Goodhead DT, Griffin CS. Complex chromosome aberrations in peripheral blood lymphocytes as a potential biomarker of exposure to high-LET alpha-particles. Int. J. Radiat. Biol. 2000;76:31–42. doi: 10.1080/095530000138989. [DOI] [PubMed] [Google Scholar]
- [29].Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature. 1992;355:738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- [30].Mothersill C, Seymour CB, Joiner MC. Relationship between radiation-induced low-dose hypersensitivity and the bystander effect. Radiat. Res. 2002;157:526–532. doi: 10.1667/0033-7587(2002)157[0526:rbrild]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [31].Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat. Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [32].Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat. Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [33].Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nagar S, Smith LE, Morgan WF. Characterization of a novel epigenetic effect of ionizing radiation: the death-inducing effect. Cancer Res. 2003;63:324–328. [PubMed] [Google Scholar]
- [35].Hagelstrom RT, Askin KF, Williams AJ, Ramaiah L, Desaintes C, Goodwin EH, Ullrich RL, Bailey SM. DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene. 2008;27:6761–6769. doi: 10.1038/onc.2008.276. [DOI] [PubMed] [Google Scholar]
- [36].Frankenberg D, Greif KD, Beverung W, Langner F, Giesen U. The role of nonhomologous end joining and homologous recombination in the clonogenic bystander effects of mammalian cells after exposure to counted 10 MeV protons and 4.5 MeV alpha-particles of the PTB microbeam. Radiat. Environ. Biophys. 2008;47:431–438. doi: 10.1007/s00411-008-0187-7. [DOI] [PubMed] [Google Scholar]
- [37].Nagasawa H, Wilson PF, Chen DJ, Thompson LH, Bedford JS, Little JB. Low doses of alpha particles do not induce sister chromatid exchanges in bystander Chinese hamster cells defective in homologous recombination. DNA Repair (Amst.) 2008;7:515–522. doi: 10.1016/j.dnarep.2007.11.014. [DOI] [PubMed] [Google Scholar]
- [38].Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]


