Abstract
Evidence is accumulating that irradiated cells produce signals, which interact with non-exposed cells in the same population. Here, we analysed the mechanism for bystander signal arising in wild-type CHO cells and repair deficient varients, focussing on the relationship between DNA repair capacity and bystander signal arising in irradiated cells. In order to investigate the bystander effect, we carried out medium transfer experiments after X-irradiation where micronuclei were scored in non-targeted DSB repair deficient xrs5 cells. When conditioned medium from irradiated cells was transferred to unirradiated xrs5 cells, the level of induction was independent of whether the medium came from irradiated wild-type, ssb or dsb repair deficient cells. This result suggests that the activation of a bystander signal is independent of the DNA repair capacity of the irradiated cells. Also, pre-treatment of the irradiated cells with 0.5% DMSO, which suppresses micronuclei induction in CHO but not in xrs5 cells, suppressed bystander effects completely in both conditioned media, suggesting that DMSO is effective for suppression of bystander signal arising independently of DNA damage in irradiated cells. Overall the work presented here adds to the understanding that it is the repair phenotype of the cells receiving bystander signals, which determines overall response rather than that of the cell producing the bystander signal.
Keywords: Bystander effect, DNA repair, DMSO, Signal transduction
1. Introduction
Significant evidence has accumulated for radiation-induced bystander effects, where irradiated cells produce signals that interact with non-exposed cells in the same population. It has been reported that the bystander effect can be mediated via gap junction intercellular communication (GJIC) [1-3] and also factors secreted from irradiated cells via the culture medium in vitro [4-6]. Recent quantitative analysis by microbeam irradiation showed little relationship between the radiation dose delivered to the targeted cells and responses in non-targeted bystander cells [6-9]. In microbeam studies it has also been shown that irradiation of only a single cell within a population causes a significant bystander effect [8-10].
We have shown previously that DNA repair deficient cells show higher induction of bystander effects [10]. Specifically, DNA double-strand break repair deficient cells, xrs5, are more sensitive [10]. However, it is unknown whether the differences in bystander effects in DNA repair deficient cells result from higher bystander signal production, or from higher susceptibility to a normal bystander signal. It has not been clear whether the bystander signal is affected by DNA repair capacity that is related to remaining unrepaired DNA damage.
Free radical scavengers have been used to identify the radical species involved in the bystander response, however, most previous studies have shown that radical scavengers affected both targeted and non-targeted cells. For example, it is difficult in microbeam experiments to treat only targeted or non-targeted cells with radical scavengers because these cells are seeded on the same culture dish. To know whether radical species are involved in bystander signaling in irradiated cells we should treat only the irradiated cells with radical scavengers. Medium transfer is a useful approach to distinguish irradiated cells from non-irradiated bystander cells, and it can be used to determine whether radical species in irradiated cells are involved in bystander effects. Our results showed clearly that unrepaired DNA damage and DNA repair capacity are independent of bystander signal production in irradiated cells.
2. Materials and methods
2.1. Cell culture
Chinese hamster ovary (CHO) cells and xrs5 cells were kindly supplied by Dr. Tom K. Hei, Columbia University, New York, and EM9 cells were purchased from ATCC (American Type Culture Collection, VA, USA). Cells were cultured in MEM alpha medium (Invitrogen Ltd., Paisley, UK) supplemented with 10% FBS (Gibco, UK), 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen Ltd., Paisley, UK). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
2.2. Micronucleus assay
To investigate the induction of micronuclei by direct X-irradiation, the cells were irradiated with 0.2 and 1 Gy of conventional X-rays. Exponentially growing cells in T25 flasks were irradiated with X-rays operating at 240 kVp and 13 mA with a filter system composed of 0.25 mm Cu plus 1 mm Al filter and 4.3 mm Al flattening filter, at a dose rate of 0.5 Gy/min. Immediately after irradiation, cells were treated with 2 μg/ml cytochalasin B for 24 h in a T25 flask. They were then harvested and treated with 3 ml of hypotonic (0.1 M) KCl for 20 min, and fixed with 3 ml of methanol–acetic acid (5:1). The cell suspensions were centrifuged at 1200 rpm for 5 min, the super-natant removed and cells resuspended in 4 ml methanol–acetic acid solution and incubated on ice for 5 min. After further centrifugation, the supernatant was removed and 0.5–1 ml methanol–acetic acid solution was added. Cells were resus-pended and a sample was dropped onto slides and stained with 7.5% Giemsa for 40 min. Micronuclei per 2000 binucleated cells were counted.
2.3. Medium transfer experiment
Cells (5 × 104) were seeded onto six well plates one day prior to X-irradiation. Fifty minutes before irradiation the medium was changed to DMSO containing medium and incubated. As it took about 10 min for X-irradiation and DMSO was kept in the medium during irradiation, cells were treated with DMSO for 1 h. Cells were irradiated with 1 Gy of conventional X-rays. Immediately after irradiation, medium was changed to normal medium and cells were incubated for 24 h following irradiation in order to prepare the conditioned medium. After the incubation, the conditioned medium was filtered through a 0.22 μm filter and transferred to unirradiated cultured cells on six well plates that had been seeded 2 days earlier. Cytochalasin B was added at the same time as the medium transfer, and cells were incubated for 24 h. Micronucleus samples were prepared as described above.
2.4. Statistical analysis
The statistical analysis in the present study was performed using Student's t-test.
3. Results
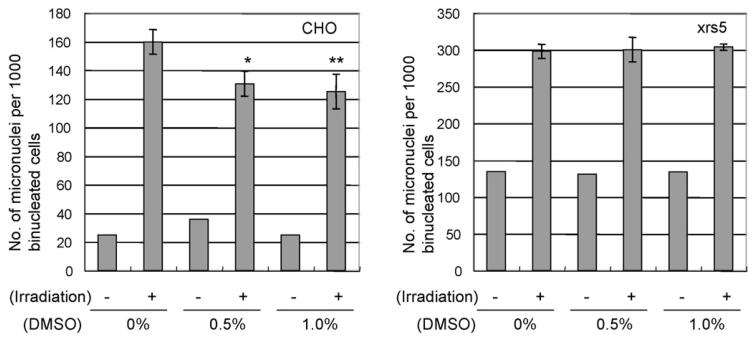
Firstly we examined the effects of DMSO on irradiated cells. Low concentrations of DMSO, that are non-toxic, were used. CHO and xrs5 cells were treated with 0.5% or 1.0% DMSO 1 h before irradiation and DMSO was removed immediately after X-irradiation. In the case of CHO cells, induction of micronuclei were significantly suppressed by treatment of 0.5% or 1.0% DMSO (Fig. 1). The levels of suppression observed between 0.5% and 1.0% were similar. However, significant suppressing effects of DMSO on micronuclei induction were not observed in 0.2 Gy—irradiated xrs5 cells (Fig. 1). The suppressing effects of DMSO were not observed when xrs5 cells were irradiated with 0.5 or 1.0 Gy.
Fig. 1.
Effect of DMSO treatment on induced micronuclei in 1 Gy-irradiated CHO and 0.2 Gy-irradiated xrs5 cells. Cells were treated with 0.5% or 1.0% DMSO for 1 h and DMSO was present during X-irradiation. Results show mean numbers of micronuclei±S.E.M., per 1000 binucleated cells from three independent experiments. A significant difference was observed between non-treated CHO and DMSO treated CHO after X-irradiation (Student's t-test *p < 0.05, **p < 0.01).
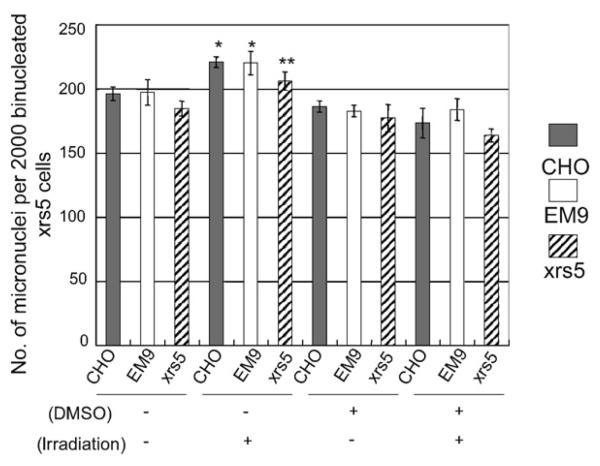
In medium transfer experiments, we examined two unknown mechanisms. Firstly, we examined whether micronuclei induction by conditioned medium from irradiated cells is dependent on the DNA repair capacity of the irradiated cells from which conditioned medium was prepared. Secondly, we examined whether micronuclei induced in unirradiated cells could be suppressed by medium transferred from DMSO treated irradiated cells. The results are summarized in Fig. 2. We used conditioned media from three cell lines that show different repair capacities for DNA damage. Cells treated with conditioned medium from irradiated cells produced significantly higher numbers of micronuclei than cells treated with medium from unirradiated cells (Fig. 2). Medium transferred from DMSO treated irradiated cells completely suppressed the induction of micronuclei in all three cell lines. These results suggest that secretion of bystander factors into the medium is inhibited by DMSO treatment in the irradiated cells. As shown in Fig. 2, micronuclei induction by conditioned medium from irradiated cells was not dependent on the DNA damage repair capacity of the irradiated cells, suggesting the signal for secretion of bystander factors is not associated with DNA damage and signal transduction following DNA damage.
Fig. 2.
Micronuclei induction in xrs5 cells exposed to conditioned medium from irradiated CHO, EM9 and xrs5 cells, and the effect of DMSO. Cells were treated with 0.5% DMSO for 1 h and DMSO was present during X-irradiation. Results show mean numbers of micronuclei ± S.E.M., per 2000 binucleated cells from three independent experiments. Significant differences were observed between non-irradiated and irradiated conditions in all three cell lines (Student's t-test *p < 0.05, **p < 0.01).
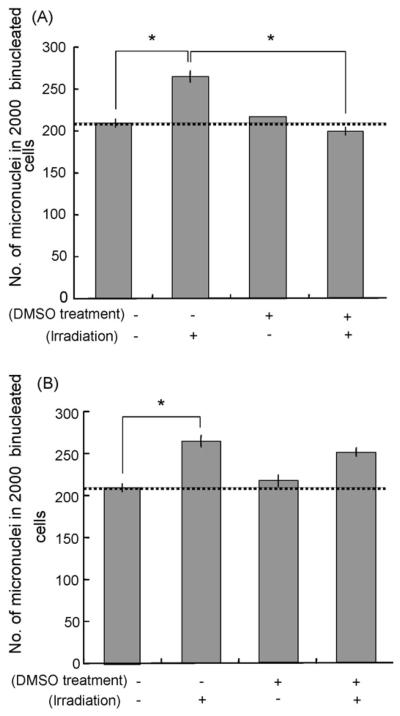
In Fig. 3A and B, irradiated cells or bystander cells treated with conditioned medium were treated with 0.5% DMSO separately. When irradiated xrs5 cells were pre-treated with 0.5% DMSO, induction of micronuclei was suppressed in medium transferred to irradiated xrs5 cells (Fig. 3A). On the other hand, little inhibition was observed in DMSO treated bystander cells (Fig. 3B). These results suggest that the signal for the bystander response was suppressed by pre-treating irradiated cells with DMSO.
Fig. 3.
(A) Suppression of bystander micronuclei induction in xrs5 cells by DMSO treatment. xrs5 cells were treated with 0.5% DMSO 1 h before X-irradiation. Significant differences were observed between irradiated cells and non-irradiated cells without DMSO treatment, and between with and without DMSO treatment in irradiated conditions (Student's t-test *p < 0.05). (B) Lack of suppression of micronuclei induction by DMSO treatment as bystander response by DMSO treatment of medium transferred bystander cells. xrs5 cells were treated with 0.5% DMSO at the same time as receiving medium transferred from irradiated cells. A significant difference was observed between irradiated cells and non-irradiated cells without DMSO treatment (Student's t-test *p < 0.05), but no significant difference was observed between with and without DMSO treatment under irradiated conditions.
4. Discussion
In the present study, we demonstrated that conditioned medium from irradiated CHO cell lines can induce micronuclei in unirradiated xrs5 cells. It has been reported that conditioned medium from irradiated cells induces some DNA damage [6,11,15,17] and signal activation [12,13,17,18], and these signals are related to radical species such as reactive oxygen species and nitric oxide [14,15,18]. Our results suggest that signals involved in bystander factor secretion result from the formation of radical species which are suppressed by the treatment of 0.5% DMSO, and these radicals are not involved in DNA damage because 0.5% DMSO was not effective at reducing micronuclei formation in irradiated xrs5 cells although the bystander factor secretion is thought to be suppressed in this cell line. Therefore, there is a possibility that radical formation in bystander signaling occurs outside of the nucleus by pathways independent of DNA damage. This idea is supported by the result that the level of bystander effect is independent of the DNA repair capacity of the irradiated cells (Fig. 2), that is, the yield of DNA damage in the directly irradiated cell is independent of signal activation for bystander effects.
Our previous work revealed that repair deficient xrs5 cells have a higher susceptibility to bystander signals compared to normal CHO cells [10]. In addition to this, we have preliminary data showing that a xrs5 population is more sensitive to the bystander signal from a co-cultured single CHO cell which was irradiated with a soft-X-ray microbeam. In contrast, a co-cultured CHO population was not sensitive to a bystander signal from irradiated CHO cells in the same dish measured using the colony formation assay (data not shown). Therefore, we conclude that the repair phenotype of the cells receiving bystander signals determines overall response rather than that of the cell producing the bystander signal.
Using microbeam irradiation it is possible to irradiate targets within a single cell. Shao et al. [7] reported that cytoplasmic irradiation causes similar levels of bystander effects compared to nuclear irradiation, suggesting that the bystander signal in targeted cells comes from outside the nucleus. Also it has been reported that the level of bystander effects in the population is independent of the number of cells targeted and dose absorbed [4,7,8,16]. It seems likely that bystander signals arising in targeted cells are triggered by relatively small energy depositions from ionizing radiation. In the case of soft-X-ray microbeam irradiation, cell kill effects resulting from a single cell within a population being irradiated were not dependent on the X-ray dose above 0.3 Gy [8]. Further studies suggested that the bystander signal arises from a biphasic reaction [9], with up to 0.3 Gy of X-ray, triggering of bystander signals depending on the probability of reaction between energy deposition and signal activation. The fact that cytoplasmic irradiation caused similar bystander effects shows that this bystander signal trigger is happening outside the nucleus. Our present study strongly confirms this possibility.
It is interesting that DMSO was not effective for the suppression of micronuclei induction after irradiation in the repair deficient xrs5 cells, although this treatment can suppress micronuclei induction after X-irradiation in normal CHO cells. DMSO has been used as a radical scavenger in irradiation experiments. However, if the action of DMSO is scavenging OH radicals, it is difficult to explain why suppressing effects were not observed in xrs5 cells. CHO and xrs5 differ in their ability to repair DNA double strand breaks because of differences in Ku 80 status. Therefore, one possibility is that this different radioprotective reaction causes variations in repair ability for the Ku 80 dependent non-homologous end joining pathway. Alternatively, DMSO may only be quenching less complex damage formation rather than clustered damage, which may be responsible for important biological effects. In contrast, bystander effects were suppressed completely by treatment of irradiated xrs5 cells with 0.5% DMSO. Overall this suggests that bystander signals arise independently of DNA damage directly induced by ionizing radiation in targeted cells and it's processing the repair pathways that are present. It supports the hypothesis that it is the repair phenotype of the cell receiving a bystander signal that is a critical determinant of overall response.
Acknowledgements
The authors are grateful to the Center of Excellence Program of Nagasaki University, Cancer Research UK and the Gray Cancer Institute for supporting this work. We also acknowledge the assistance of Gaurang Patel and Stuart Gilchrist during these studies.
References
- 1.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 2.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat. Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 3.Shao C, Furusawa Y, Aoki M, Ando K. Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat. Res. 2003;160:318–323. doi: 10.1667/rr3044. [DOI] [PubMed] [Google Scholar]
- 4.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 5.Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 6.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 7.Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schettino G, Folkard M, Prise KM, Vojnovic B, Held KD, Michael BD. Low-dose studies of bystander cell killing with targeted soft X rays. Radiat. Res. 2003;160:505–511. doi: 10.1667/rr3060. [DOI] [PubMed] [Google Scholar]
- 9.Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killng after microbeam irradiation of a single cell with focused c(k) X-rays. Radiat. Res. 2005;163:332–336. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- 10.Kashino G, Prise KM, Schettino G, Folkard M, Vojnovic B, Michael BD, Suzuki K, Kodama S, Watanabe M. Evidence for induction of DNA double strand breaks in the bystander response to targeted soft X-rays in CHO cells. Mutat. Res. 2004;556:209–215. doi: 10.1016/j.mrfmmm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 12.Iyer R, Lehnert BE. Alpha-particle induced increases in the radioresistance of normal human bystander cells. Radiat. Res. 2002;157:3–7. doi: 10.1667/0033-7587(2002)157[0003:apiiit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat. Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T, Kano E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Azzam EI, de Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 16.Liu Z, Mothersill CE, McNeill FE, Lyng FM, Byun SH, Seymour CB, Prestwich WV. A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiat. Res. 2006;166:19–23. doi: 10.1667/RR3580.1. [DOI] [PubMed] [Google Scholar]
- 17.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2006 Aug 7; doi: 10.1038/sj.onc.1209863. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat. Res. 2006;165:400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]