Abstract
Caspase activation is a hallmark of apoptosis. However, the molecular mechanisms underlying the regulation of caspase-8 activation within the extrinsic death pathway are not well understood. In this study, we demonstrate that procaspase-8 is phosphorylated in mitotic cells by Cdk1/cyclin B1 on Ser-387, which is located at the N terminus of the catalytic subunit p10. This phosphorylation of procaspase-8 on Ser-387 occurs in cancer cell lines, as well as in primary breast tissues and lymphocytes. Furthermore, RNA interference-mediated silencing of cyclin B1 or treatment with the Cdk1 inhibitor RO-3306 enhances the Fas-mediated activation and processing of procaspase-8 in mitotic cells. A nonphosphorylatable procaspase-8 (S387A) facilitates Fas-induced apoptosis during mitosis. Our findings suggest that Cdk1/cyclin B1 activity shields human cells against extrinsic death stimuli and unravel the molecular details of the cross talk between cell cycle and extrinsic apoptotic pathways. Finally, this new mechanism may also contribute to tumorigenesis.
Intact apoptotic machinery is essential to maintain the integrity and homeostasis of multicellular organisms (8). The evasion of apoptosis is a hallmark of cancer, as demonstrated by the inability of cancer cells to respond appropriately to signals that normally control unrestricted growth (11). In mammalian cells, the apoptotic response is mediated by either the intrinsic or the extrinsic pathway, depending on the origin of the death stimulus. After stimulation of the death receptor Fas (APO-1/CD95) the death-inducing signaling complex (DISC) assembles, which contains the oligomerized receptor, the adaptor molecule FADD, two isoforms of procaspase-8 (procaspase-8a and -8b), procaspase-10, and c-FlipL/S/R (28, 37). In accordance with the induced proximity model, immediately after DISC formation, procaspase-8, which consists of two death effector domains (DED) and a protease domain containing the p18 and p10 subunits, is proteolytically processed (28). This autoprocessing follows a sequential order of events: while the first cleavage step occurs at Asp-374 and results in the formation of the subunits p43/p41 and p12, the second cleavage at Asp-216 and Asp-384 produces the enzymatically active subunits p18, p10, and the prodomain p26/p24 (5, 14, 27, 40). The mature caspase-8 heterotetramer p182-p102 then translocates from the DISC to the cytosol, where it cleaves several substrates, such as Bid, and effector caspases to initiate the apoptotic cascade (22).
Increasing evidence highlights the functional importance of procaspase-8 for carcinogenesis; several findings suggest that the impairment of procaspase-8 function by genetic and epigenetic mechanisms correlates with the malignant potential of different types of cancer (6, 12, 43, 44). In the present study, we investigated the functional correlation of the cell cycle with the extrinsic death pathway. The cyclin-dependent kinase 1 (Cdk1) in complex with cyclin B1 (Cdk1/cyclin B1) is one of the key mitotic kinases. The kinase activity of Cdk1/cyclin B1 governs the entry into mitosis from G2 phase of the cell cycle (29, 30). Through mediating phosphorylation of a variety of substrates, Cdk1/cyclin B1 also plays an important role in multiple processes during mitosis, including chromosome condensation, nuclear envelope breakdown, centrosome separation, regulation of spindle microtubule dynamics, and metaphase-to-anaphase transition (4, 21, 31, 36). In the present investigation, our molecular analyses of the roles that cell cycle kinases play in the apoptosis signaling pathway demonstrated that Cdk1/cyclin B1 and procaspase-8 interact in vitro and in vivo. The phosphorylation of procaspase-8 by Cdk1/cyclin B1 at Ser-387 inhibits processing of procaspase-8. In a physiological situation, such as breast cancer and proliferating T cells, where Cdk1/cyclin B1 activity is upregulated, we observed elevated procaspase-8 phosphorylation on Ser-387. We suggest a model in which Cdk1/cyclin B1 helps to protect mitotic cells against extrinsic death stimuli.
MATERIALS AND METHODS
Antibodies.
Mouse monoclonal antibodies against Fas (CD95/Apo-1, clone 2R2 [Calbiochem]), β-actin (clone AC-15), Flag tag (FlagM2 F1804 [Sigma]), Plk1 (F-8, sc-17783), Cdk1 (17, sc-54), cyclin B1 (GNS1, sc-245), glutathione S-transferase (GST; B-15, sc138 [Santa Cruz]), phospho-histone H3 Ser10 (clone 3H10 [Millipore]), V5 (Invitrogen), and caspase-8 (1C12 [Cell Signaling Technology] and C15 [Alexis]) and a rabbit polyclonal antibody against poly(ADP-ribose) polymerase (PARP) (46D11 [Cell Signaling Technology]) were used according to the manufacturers' recommendations. To generate a polyclonal antibody against caspase-8 phosphorylated on Ser-387, rabbits were immunized by Eurogentec using the peptide YLEMDLSpSPQTRY. According to the immunization procedure, antibodies were affinity purified. Secondary antibodies conjugated to CY2 and CY3 were obtained from Jackson Immunoresearch Labs.
Cell culture, transfection, and synchronization.
Cell lines were purchased from the American Type Culture Collection and grown in the recommended medium supplemented with 10% heat-inactivated fetal calf serum at 37°C in a humidified environment with 5% CO2. Human lymphocytes from peripheral blood were cultured as described previously (13).
To generate knockdown clones for cyclin B1 and caspase-8, cells were transfected with plasmids in which the H1 promoter drives the expression of an shRNA targeting the open reading frame of caspase-8 (5′-CCTGGTACATCCAGTCACT-3′) or cyclin B1 (5′-AAGAAATGTACCCTCCAGA-3′). Stably transfected cells were selected for 4 weeks using 1.5 mg of G418/ml (PAA Laboratories). Transfection and synchronization were performed as described previously (48). For the induction of apoptosis, cells were stimulated with FasL (100 ng/ml), TRAIL (10 ng/ml), or tumor necrosis factor alpha (TNF-α; 10 ng/ml) (Enzo Life Sciences).
Generation of plasmids and mutagenesis.
Sequences of the primers used in the present study will be provided upon request. Caspase-8 was inserted into the pGEX (GE Healthcare) and the 3×Flag-tagged pcDNA3.1-Hygro+ (Invitrogen) vectors. The N-terminal domain was cloned into the BamHI/EcoRI sites of the pGEX vector. The 3×Flag-tagged caspase-8 sequence was inserted into the pcDNA3.1-V5 vector (Invitrogen). Deletion clones were generated by standard techniques. Mutants were generated by site-directed mutagenesis using the QuikChange protocol and Pfu Ultra II Fusion HS DNA polymerase (Stratagene). RNA interference (RNAi) vectors were constructed as described previously (18). Sequences for efficient silencing of caspase-8 and cyclin B1 were obtained using our web-based shRNA design tool (www.molgyn.kgu.de/genesilencer). All constructs were confirmed by sequencing. Information on cloning procedures is available from the authors.
Immunoprecipitation and phospho amino acid analysis.
Both methods were performed as described previously (34).
GST pulldown.
The expression of recombinant glutathione S-transferase (GST) proteins was induced in Escherichia coli BL21 cells at 37°C for 2 h by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (33). GST-fused proteins were purified first and then incubated with lysates of HeLa cells transfected with the Flag-Cdk1 expression vector in TBSN buffer (20 mM Tris [pH 8.0], 150 mM NaCl, 0.5% Nonidet P-40, 5 mM EGTA, 0.5 mM Na3VO4, 20 mM p-nitrophenyl phosphate) supplemented with protease inhibitors (Complete Mini EDTA-free Protease Inhibitor Cocktail; Roche Diagnostics) at 4°C for 2 h. GST, GST-caspase-8, and GST-fused subdomains of caspase-8 were adsorbed to glutathione-Sepharose 4B (GE Healthcare) for an additional 1 h. The bound proteins were resolved by SDS-PAGE and transferred to an Immobilon-P membrane (Millipore). The blots were then probed with anti-Flag antibodies to detect the Flag-Cdk1 protein.
Cell cycle analysis.
Cell cycle analysis was carried out as described previously (49). Briefly, cells were washed with phosphate-buffered saline (PBS), treated with RNase A, and stained with propidium iodide. Flow cytometry was performed by using a FACScan machine and CellQuest software (Becton Dickinson). The determination of the mitotic indices and mitotic phase distribution was performed as previously described (35). All experiments were performed in triplicate.
Immunofluorescence assays.
For immunofluorescence analysis, HeLa cells were grown on a glass coverslip, fixed with 4% paraformaldehyde, washed with PBS, and permeabilized with 0.2% Triton X-100 before addition of the appropriate primary and secondary antibodies (33). Microscopy was performed with a Zeiss Axio Imager Z1 microscope equipped with a 40× objective lens, and the images were captured and processed by using AxioVision Software (Zeiss).
Analysis of apoptosis.
Cells were processed by using a Vybrant apoptosis assay kit 2 (Alexa Fluor 488-annexin V/propidium iodide staining) according to the manufacturer's protocol (Invitrogen) and analyzed by flow cytometry using a FACScan (Becton Dickinson).
Cdk1/cyclin B1 and caspase-8 assays.
Immunoprecipitations with a cyclin B1-specifc antibody to measure the kinase activity of Cdk1/cyclin B1 were performed as described previously (48). In vitro kinase assays were performed using 10× Cdk1 buffer (New England Biolabs) supplemented with 0.05 mM ATP and 1 μCi of [γ-32P]ATP (3,000 Ci/mmol; Amersham Pharmacia) for 30 min at 30°C in the presence of bacterially expressed purified GST-caspase-8 fusion proteins. For the inhibition of Cdk1 activity in kinase assays, RO-3306 was diluted 3-fold in assay buffer in a final volume of 20 μl, and the assay was initiated by the addition of 40 μl of assay buffer containing the caspase-8 substrate. Samples were resolved by SDS-PAGE and subjected to autoradiography. Phosphate groups were removed by incubation with λ phosphatase (New England Biolabs). Caspase-8 activity was determined by using a Caspase Glo 8 assay kit (Promega) according to the manufacturer's instructions.
Patients and tumor samples.
The study group consisted of 13 patients who were treated at the Department of Obstetrics and Gynecology of J. W. Goethe University School of Medicine (Frankfurt, Germany). All breast cancer patients met the following major inclusion criteria: unilateral primary carcinoma of the breast confirmed histologically by core-cut needle or incisional biopsy (fine-needle aspiration was not considered sufficient); a tumor measurable two dimensionally by mammography, breast ultrasound, or breast magnetic resonance imaging; a primary tumor ≥3 cm at its largest diameter (in patients with multifocal or multicentric breast cancer, the largest lesion was measured); and a patient age between 18 and 70 years. In all cases, vital tumor biopsy specimens from the primary site were removed during surgery, immediately frozen in liquid nitrogen, and stored at −80°C. All histological evaluations were carried out by two independent investigators.
Statistical methods.
Experimental in vitro data are presented as mean ± the standard deviations from three or more independent experiments. Two-way analysis of variance (ANOVA; GraphPad Prism; GraphPad Software, Inc., San Diego, CA) was done to consider random effects of individual gels and different treatments. For two-way ANOVAs, all treatment groups were compared to control cells.
RESULTS
Signaling via the extrinsic death pathway is attenuated in mitotic cells.
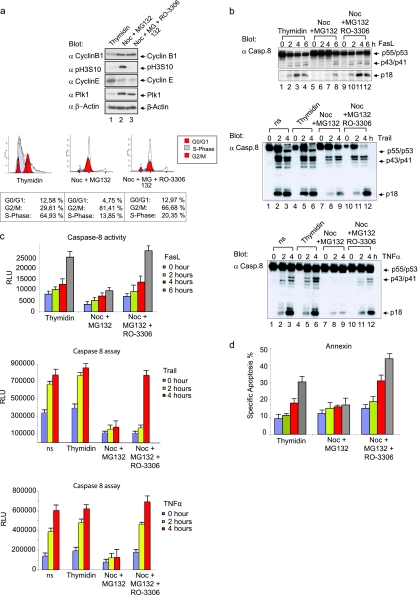
The apoptosis signaling pathway was evaluated in mitotic and nonmitotic B lymphoblastoid SKW 6.4 cells to determine whether the Fas-induced cellular response is regulated during the cell cycle. Cells were treated once with excess thymidine to accumulate the majority of them at G1/S (Fig. 1a, lane 1). In addition, we performed the analyses in the presence of the microtubule depolymerizing drug nocodazole combined with MG132 to prevent mitotic exit, as indicated by the phosphorylation of histone H3 at Ser10 (pH3S10), by the high abundance of polo-like kinase 1 (Plk1) and cyclin B1 (Fig. 1a, lane 2), and by fluorescence-activated cell sorting analysis (Fig. 1a, lower panel). When we compared the regulation of the extrinsic death pathway status in mitotic and nonmitotic cells, we noted that Fas-mediated procaspase-8 processing is impaired in mitotic cells: procaspase-8a/b was barely processed, and reduced levels of the previously described procaspase-8a/b cleavage products p43/p41 and p18 were observed (Fig. 1b) (37). To investigate this mechanism in other caspase-8-dependent pathways, we used the death ligands TRAIL or TNF-α (Fig. 1b). Again, upon stimulation with TRAIL or TNF-α procaspase-8a/b processing was also impaired in mitotic cells compared to nonmitotic control cells. Caspase-8 activity was also significantly blocked following stimulation with different death ligands (FasL, TRAIL, or TNF-α) in a mitotic cell-enriched population (Fig. 1c). These results prompted us to investigate the regulation of procaspase-8 in mitotic cells during Fas-induced apoptosis.
FIG. 1.
Inhibition of the extrinsic death pathway in Fas-induced mitotic cells. (a) B lymphoblastoid SKW6.4 cells were enriched in G1/S phase by treatment with thymidine (lane 1), in pro-/metaphase with nocodazole for 16 h, followed by MG132 for 2 h (lane 2) or in a G1-like state with nocodazole for 16 h, followed by MG132 for 2 h and RO-3306 for the inhibition of Cdk1/cyclin B1 (lane 3) (upper panel). Cell lysates were immunoblotted for cyclin B1, histone H3 phosphorylated at Ser10 (H3 pS10), cyclin E, Plk1, and β-actin. The cell cycle status was monitored by flow cytometry (lower panel). (b) Cells treated as described in panel a were stimulated with 100 ng of FasL/ml, 10 ng of TRIAL/ml, or 10 ng of TNF-α/ml for the times indicated, and total cellular lysates were analyzed by Western blotting with anti-caspase-8 MAb C15. (c) Caspase-8 activity was determined by measuring the cleavage of the luminogenic substrate containing the IETD peptide. All experiments were performed in triplicate. Error bars represent the standard deviation (SD). (d) Apoptosis analyses were performed by using an annexin V kit. All experiments were performed in triplicate. Error bars represent the SD.
Although it has been reported that overexpressed kinases can protect cells against apoptosis (11), the underlying mechanisms, particularly those regulating the extrinsic death pathway, remain elusive. Interestingly, the inhibition of certain cell cycle kinases, including Cdks, polo-like kinase 1 (Plk1), and Aurora kinases, which often exhibit elevated activity in human cancers, triggers apoptosis in many experimental contexts (13, 20, 24, 39, 41, 42). To shed more light on the role cell cycle kinases play for the regulation of the extrinsic death pathway, we used at first the selective ATP-competitive inhibitor RO-3306. This small molecule inhibits Cdk1/cyclin B1 activity with a 10-fold selectivity compared to Cdk2/cyclin E and >50-fold relative to Cdk4/cyclin D (45). The treatment of mitotic SKW 6.4 cells with RO-3306 restored the sensitivity to Fas stimulation as indicated by enhanced enzymatic activity and processing of procaspase-8 with elevated levels of p43/p41 and p18 (Fig. 1a to c). Most interestingly, RO-3306 enhanced the susceptibility of mitotic cells to Fas-induced cell death (Fig. 1d). These findings pointed out the possible role of Cdk1/cyclin B1 in the regulation of Fas-mediated procaspase-8 activation.
Cdk1/cyclin B1 phosphorylates procaspase-8 on Ser-387 in vitro.
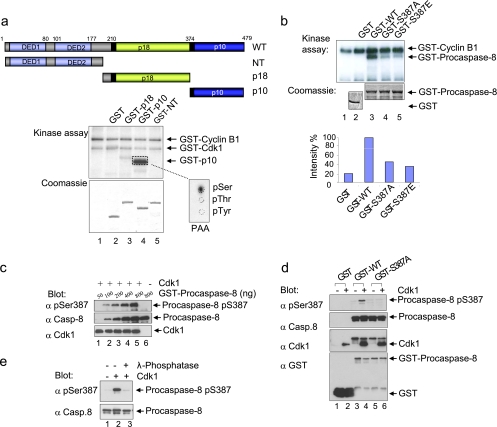
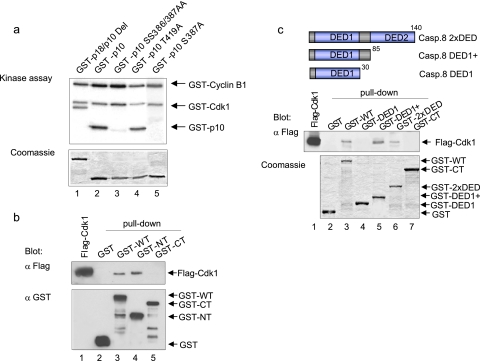
To explore the role of Cdk1/cyclin B1 in the modulation of the extrinsic death pathway in more detail, we started to directly investigate the possible interactions between procaspase-8 and Cdk1/cyclin B1. Recombinant subdomains of human procaspase-8 expressed as GST fusion proteins were analyzed in kinase assays to determine whether caspase-8 is an in vitro substrate of Cdk1/cyclin B1 (Fig. 2a, upper and middle panels). Under these conditions, only the p10 subdomain was labeled (Fig. 2a, middle panel). To investigate whether this phosphorylation occurs on a common residue, the labeled band was excised and analyzed. The amino acid analysis demonstrated that the site(s) phosphorylated by Cdk1/cyclin B1 was mainly serine residues (Fig. 2a, lower right panel). A putative Cdk1 phosphorylation site within p10, Ser-387, is followed immediately by a proline residue; this is characteristic of sites phosphorylated by kinases such as Cdk1. Point mutants of p10 expressed as GST fusion proteins (S387A, SS386/387AA, and T419A) were analyzed in kinase assays, and the substitution of Ser-387 led to a strong inhibition of Cdk1/cyclin B1-mediated phosphorylation of p10 (Fig. 3a). To further determine whether procaspase-8 is a bona fide substrate of Cdk1/cyclin B1, we examined the phosphorylation of the full-length protein. Site-directed mutagenesis of Ser-387 followed by in vitro kinase assays, did not block the phosphorylation of full-length procaspase-8 completely but led to a massive downregulation of the phosphorylation signal, suggesting that Ser-387 is the major phosphorylation site for Cdk1/cyclin B1 in full-length procaspase-8 (Fig. 2b). We generated a phospho-S387-specific antibody to explore the significance of Ser-387 phosphorylation in vitro and in vivo. Phosphorylation by Cdk1/cyclin B1 was required for the pS387-specific antibody to recognize procaspase-8 (Fig. 2c). Furthermore, the mutation of Ser-387 to Ala or the treatment of phosphorylated wild-type procaspase-8 with λ-phosphatase abolished this recognition completely, validating the specificity of our affinity-purified antibody for the Ser387-phosphorylated form of procaspase-8 (Fig. 2d and e).
FIG. 2.
Cdk1/cyclin B1 phosphorylates procaspase-8 in vitro at Ser-387. (a) Recombinant GST-fused subdomains of procaspase-8 were analyzed by SDS-PAGE (lower left panel). Subdomains were incubated with active Cdk1/cyclin B1 and [γ-32P]ATP, analyzed by SDS-PAGE, and visualized by autoradiography (upper left panel). This was followed by phospho amino acid analysis (lower right panel). (b) Wild-type (WT) and mutated (S387A, S387E) recombinant full-length GST-caspase-8 proteins were subjected to kinase assays using Cdk1/cyclin B1, analyzed by SDS-PAGE, and autoradiography. (c) Recombinant caspase-8 (WT) was incubated with active Cdk1/cyclin B1. Samples were immunoblotted with antibodies specific for caspase-8, for a caspase-8 peptide [ABcasp.-8(pSer387)] containing phospho-Ser387 (YLEMDLSpSPQTRY) and for Cdk1. (d) GST, recombinant caspase-8 (GST-WT), and a mutated form (GST-S387A) were incubated with or without active Cdk1/cyclin B1 and immunoblotted with ABcasp.-8(pSer387) (e) Recombinant caspase-8 was incubated with or without active Cdk1/cyclin B1 in kinase buffer, followed by treatment with 100 U of λ-phosphatase and immunoblotted with ABcasp.-8(pSer387).
FIG. 3.
Cdk1/cyclin B1 associates with and phosphorylates procaspase-8 in vitro at Ser-387. (a) The p10 subunit was mutated at different sites (S387A, SS386/387AA, and T419A), and the corresponding GST fusion proteins were incubated with active Cdk1/cyclin B1 and [γ-32P]ATP, analyzed by SDS-PAGE and visualized by autoradiography. (b) Bacterially expressed, purified GST-fused full-length caspase-8 (GST-WT), and the N-terminal (GST-NT) and C-terminal (GST-CT) subdomains of caspase-8 were incubated with lysates of HeLa cells that had been transfected with a Flag epitope-tagged Cdk1 (Flag-Cdk1) expression construct for pulldown assays. The Flag-Cdk1 protein that associated with GST-caspase-8 or its subdomains was detected by immunoblot with an anti-Flag antibody. (c) The binding of the GST-fused N-terminal subdomains (GST-2xDED, -DED1+, and -DED1) was also analyzed in pulldown assays. The Flag-Cdk1 protein that associated with GST-fused subdomains of the N-terminal portion of caspase-8 was detected by immunoblotting with an anti-Flag antibody. A 5% total input lane was also run.
Next, we analyzed whether procaspase-8 and Cdk1/cyclin B1 interact in vitro. Using lysates of cells transfected with Flag-Cdk1 expression constructs, we performed pulldown assays with procaspase-8 or its subdomains fused to GST to determine the regions involved in the association of both proteins in vitro. Flag-Cdk1 was associated with procaspase-8, its N-terminal portion (GST-NT), and all fragments of GST-NT comprising the linker region between DED1 and DED2 (Fig. 3b and c). We concluded that the region connecting both DED domains is involved in binding to Cdk1/cyclin B1.
Inhibition of Cdk1/cyclin B1 activity impairs the phosphorylation of procaspase-8 at Ser-387 in vivo.
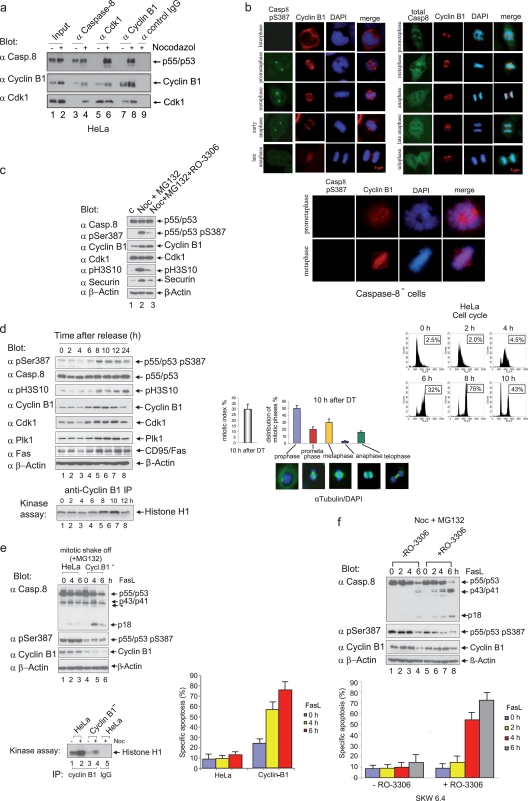
We evaluated whether Cdk1/cyclin B1 and procaspase-8 also interact in vivo. In contrast to extracts of asynchronous HeLa cells, specific complexes containing procaspase-8, Cdk1, and cyclin B1 were detected following the immunoprecipitation of mitotic cell extracts using caspase-8-, Cdk1-, or cyclin B1-specific antibodies (Fig. 4a). Next, we used indirect immunofluorescence to visualize the subcellular localization of caspase-8, caspase-8 (pS387), and cyclin B1. Whereas caspase-8 was found predominantly in the cytoplasm, the Ser-387 phosphorylated form was also found in the cytoplasm associated with intense staining of centrosomes from prometaphase to anaphase (Fig. 4b, upper panels). Cyclin B1 also localized to the centrosomes and microtubules, confirming previous findings (3, 16). The merged image demonstrates a colocalization of caspase-8 (pS387) and cyclin B1 at the centrosomes. Depletion of caspase-8 by RNAi abolished the staining of cytoplasm and centrosomes using our phospho-caspase-8-specific antibodies completely (Fig. 4b, lower panels).
FIG. 4.
The phosphorylation of procaspase-8 correlates with Cdk1/cyclin B1 activity in vivo. (a) Lysates from asynchronous (−) or mitotic (+) HeLa cells were immunoprecipitated with caspase-8-, Cdk1-, or cyclin B1-specific antibodies. Anti-mouse IgG was used as a control. The immunoprecipitated proteins were resolved by SDS-PAGE and immunoblotted for caspase-8, cyclin B1, and Cdk1. Lanes 1 and 2 show 2.5% total input. (b) HeLa cells were arrested in G1/S using a double-thymidine block and released into fresh medium for 10 h. Semisynchronized cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) and subsequently categorized according to their staining patterns. Cells were labeled using ABcasp.-8 (pSer387), caspase-8, or cyclin B1 antibodies for analysis by microscopy. Caspase-8-deficient HeLa cells were arrested in G1/S using a double-thymidine block and released into fresh medium for 10 h. Semisynchronized cells were stained with DAPI or labeled using ABcasp.-8 (pSer387) or cyclin B1 antibodies for analysis by microscopy. (c) To enrich for mitotic HeLa cells, an incubation with nocodazole (Noc) for 16 h was carried out; cells were captured by shake-off and then treated with MG132 for 2 h (lane 2). Shake-off cells were treated as described for lane 2, except that, 1 h after MG132 addition, the Cdk1 inhibitor RO-3306 was added (lane 3). The cell lysates were immunoblotted for caspase-8, caspase-8 (pS387), cyclin B1, Cdk1, pH3S10, securin, and β-actin. (d) HeLa cells were arrested in G1/S using a double-thymidine block (time zero) and released for the times indicated. The cellular lysates were immunoblotted for caspase-8 (pS387), caspase-8, pH3S10, cyclin B1, Cdk1, Plk1, CD95/Fas, and β-actin (upper left panels). To measure the Cdk1/cyclin B1 activity in cell lysates of synchronized cells, immunoprecipitation assays were performed with cyclin B1-specific antibodies, and immunoprecipitated proteins were then subjected to kinase assays with histone H1 as the substrate (lower left panel). The cell cycle status was monitored using flow cytometry (upper right panel). Boxed numbers exhibit the percentage of cells with 4 N DNA content. The mitotic index and distribution of mitotic phases of HeLa cells 10 h after release from the G1/S block are shown: the leftmost bar graph indicates 30% mitotic index 10 h after G1/S (of these, 46% were in prophase and 25% were in metaphase). The mitotic index was determined by counting 6 times 250 to 300 cells (lower right panel). All experiments were performed in triplicate. The error bars represent the SD. (e) Characterization of mock-transfected HeLa cells and of a cyclin B1-depleted HeLa clone using a stably integrated RNAi cassette (Cycl.B1−-HeLa). Mitotic shake-off cells (mock-transfected and Cycl.B1−) were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the caspase-8 MAb C15 and antibodies for caspase-8 (pS387), cyclin B1, and β-actin (*, unspecific band). To measure Cdk1/cyclin B1 activity, immunoprecipitation assays were performed using cyclin B1-specific antibodies, and the immunoprecipitated proteins were subjected to kinase assays with histone H1 as the substrate (middle panel). Apoptosis analyses were performed by using an annexin V kit (lower panel). All experiments were performed in triplicate. Error bars represent the SD. (f) To enrich for mitotic SKW 6.4 cells, an incubation with nocodazole (Noc) for 16 h was carried out; cells were captured by shake-off and then treated with MG132 for 2 h. For the inhibition of Cdk1 activity, RO-3306 was added 1 h after MG132 treatment. Cells were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the anti-caspase-8 MAb C15. Apoptosis analyses were performed by using an annexin V kit (lower panel). All experiments were performed in triplicate. Error bars represent the SD.
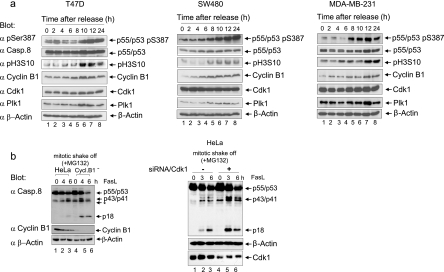
To further address the significance of S387 phosphorylation during mitosis, cells treated with nocodazole and MG132 were collected by mitotic shake-off. Although procaspase-8 showed strong S387 phosphorylation only in mitotic cells, the treatment of shake-off cells with the Cdk1 inhibitor RO-3306 led to an almost complete loss of the phosphorylation signal (Fig. 4c). We also determined the kinetics of procaspase-8 phosphorylation in vivo. The quality of the synchronization of the double thymidine-blocked HeLa cells was monitored by analyzing the expression of cyclin B1 and Plk1, the phosphorylation of histone H3 (Fig. 4d, upper left panel), and the mitotic index (Fig. 4d, lower right panel), as well as by flow cytometry (Fig. 4d, upper right panel). In these cells, the peak levels of procaspase-8 phosphorylation at Ser-387 at 8 to 10 h after release from the double-thymidine block (Fig. 4d, upper left panel) coincided with maximum activity of the Cdk1/cyclin B1 complex (Fig. 4d, lower left panel). In semisynchronized colon (SW480) and breast cancer (T47D, MDA-MB-231) cells, the phosphorylation of procaspase-8 also peaked in mitosis, supporting a correlation between Cdk1/cyclin B1 activity and procaspase-8 Ser-387 phosphorylation in cell lines of different origins (Fig. 5a).
FIG. 5.
The phosphorylation of procaspase-8 correlates with Cdk1/cyclin B1 activity in cell lines of different origin. (a) T47D, SW480, and MDA-MB-231 cells were arrested in G1/S using a double-thymidine block (time zero) and released for the times indicated. The cellular lysates were immunoblotted for caspase-8 (pS387), caspase-8, pH3S10, cyclin B1, Cdk1, Plk1, and β-actin. (b) Characterization of a cyclin B1-depleted clone using a stably integrated RNAi cassette (Cycl.B1−-HeLa). Mitotic shake-off cells (wild-type and Cycl.B1−) were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the caspase-8 MAb C15 and antibodies for caspase-8 (pS387), cyclin B1, and β-actin (*, unspecific band) (left panel). Characterization of Cdk1-depleted cells by RNAi. Mitotic shake-off cells (wild-type and Cdk1-depleted cells) were stimulated with 100 ng of FasL/ml for the times indicated. Lysates were immunoblotted to analyze procaspase-8 processing using the caspase-8 MAb C15 and antibodies for caspase-8 (pS387), Cdk1, and β-actin (*, unspecific band) (right panel).
To investigate this correlation in more detail, we depleted cyclin B1 using our previously developed RNAi vectors (18, 19, 26, 49). Because the complete small interfering RNA (siRNA)-mediated knockdown of cyclin B1 in our earlier studies induced apoptosis in a number of cancer cell types (49), we selected stable HeLa clones that exhibited only a partial knockdown of cyclin B1 (up to 80%) (Fig. 4e and 5b). Furthermore, consistent with the downregulation of the cyclin B1 protein, the kinase activity of Cdk1/cyclin B1 was decreased to ca. 20% in the cellular extract of the analyzed Cycl.B1− cell clones compared to mock-transfected cells (Fig. 4e and Fig. 5b), which confirms our previous results (48). Similar Cdk1/cyclin B1-kinase activities were also measured in different Cycl.B1− clones (data not shown). Upon Fas stimulation of mitotic Cycl.B1− cells procaspase-8a/b processing products p43/p41 and p18 were significantly upregulated compared to mitotic mock-transfected cells (Fig. 4e, upper panel, and Fig. 5b, left panel). Upon Fas stimulation, cyclin B1-depleted mitotic cells also showed an elevated level of apoptosis compared to their mock-transfected mitotic counterparts (Fig. 4e, lower right panel). By knocking down Cdk1 by RNAi, we could also sensitize mitotic cells to Fas-mediated apoptosis (Fig. 5b, right panel).
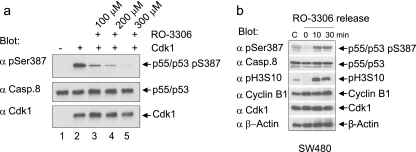
To further evaluate the role of Cdk1/cyclin B1 activity for the phosphorylation of procaspase-8 at Ser-387 and its processing, we used the Cdk1 inhibitor RO-3306 in vitro and in vivo (45). The level of procaspase-8 (pS387) correlated inversely with the concentration of RO-3306 in our in vitro kinase assays (Fig. 6a). By treating proliferating cells for 18 h with RO-3306, we confirmed the previous finding that RO-3306 arrests cells at the G2/M border. Phospho-histone H3 staining demonstrated that mitotic cells appeared as early as 10 min after drug removal, coinciding with the appearance of phosphorylated procaspase-8 (pS387) in SW480 cells (Fig. 6b). This suggested that the enzymatic activity of Cdk1/cyclin B1 is important for procaspase-8 phosphorylation at Ser-387. To further study the correlation of Cdk1 activity and processing of procaspase-8 in living cells, we analyzed protein extracts of mitotic B lymphoblastoid SKW 6.4 cells treated with FasL in the absence or presence of the Cdk inhibitor RO-3306. Fas stimulation led to increased levels of p43/p41 and p18 in mitotic cells with downregulated Cdk1/cyclin B1 activity compared to mitotic control cells (Fig. 4f, upper panel). Treatment of mitotic SKW 6.4 cells with RO-3306 also induced enhanced apoptosis upon Fas stimulation compared to untreated mitotic SKW 6.4 cells (Fig. 4f, lower panel). Interestingly, we observed that an increase in caspase-8 activation upon the addition of RO-3306 occurred in type I, e.g., B lymphoblastoid SKW 6.4 cells. Caspase-8 activation, which triggers the apoptotic cascade in type I cells, takes place directly at the DISC, independently of mitochondria and caspase-9. Therefore, experiments in SKW6.4 cells demonstrate that RO-3306 influences caspase-8 activation directly at the DISC independently of caspase-9 activation.
FIG. 6.
Inhibition of Cdk1 activity using the small molecule RO-3306 suppresses the phosphorylation of procaspase-8 at Ser-387 in vitro and in vivo. (a) Procaspase-8 purified from bacteria was subjected to kinase assays with Cdk1/cyclin B1 and increasing concentrations of RO-3306. Samples were immunoblotted for Cdk1, caspase-8, and caspase-8 (pSer387). (b) Treatment of proliferating human SW480 cancer cells with RO-3306 for 14 h led to a complete block of the cell cycle at the G2/M border. After releasing SW480 cells from RO-3306 a Western blot analysis was performed for caspase-8 (pS387), caspase-8, pH3S10, cyclin B1, Cdk1, and β-actin.
Mimicking the phosphorylation at Ser-387 modulates processing of procaspase-8 and Fas-mediated apoptosis in mammalian cells.
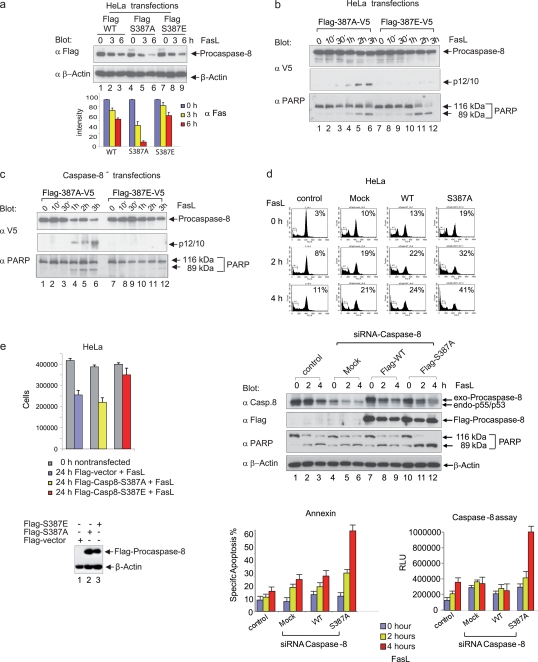
Interestingly, Ser-387 is located at the N-terminal end of p10 close to the cleavage residue Asp-384, which, in conjunction with Asp-374, facilitates the separation of the two catalytic subunits. To evaluate whether the phosphorylation of Ser-387 by Cdk1 can affect the processing and concomitantly the stability of procaspase-8 upon Fas stimulation in nonsynchronized cells, HeLa cells were transfected with Flag-tagged procaspase-8 wild type (WT) or the procaspase-8 mutants S387A and S387E. Extracts from Fas-stimulated transfected HeLa cells were analyzed by immunoblotting with a Flag-specific antibody. Whereas the S387A mutant, which mimics the nonphosphorylated form, disappeared rapidly after Fas stimulation, the S387E mutant, which mimics the phosphorylated form, was more stable (Fig. 7a).
FIG. 7.
A nonphosphorylatable S387 mutant of procaspase-8 sensitizes mitotic cells to Fas-mediated apoptosis. (a) HeLa cells were transiently transfected with vectors encoding Flag-tagged caspase-8 (Flag-WT, Flag-S387A, and Flag-S387E). At 18 h after transfection, cells were incubated with 100 ng of FasL/ml plus 1 μg of cycloheximide/ml for 3 and 6 h. Lysates were immunoblotted for exogenous caspase-8 with a Flag-specific antibody and for β-actin (upper panels). The signal intensity of immunoblotted, Flag-tagged caspase-8 was standardized to the level of β-actin expression (lower panel). All experiments were performed in triplicate. The error bars represent the SD. (b) Analysis of procaspase-8 processing in HeLa cells expressing different double-tagged (N-terminal Flag, C-terminal V5) forms of procaspase-8 (S387A, S387E). Lysates were immunoblotted for V5 and PARP. (c) Analysis of procaspase-8 processing in caspase-8 knockdown HeLa cell clones (caspase-8−) expressing different double-tagged (N-terminal Flag, C-terminal V5) forms of procaspase-8 (S387A, S387E). Lysates were immunoblotted for V5 and caspase-8. (d) On day 1, HeLa cells were transfected with siRNA targeting the untranslated region of caspase-8, followed by the transfection of Flag-tagged caspase-8 (mock, WT, or S387A) on day 2. On day 3, the cells were treated overnight with nocodazole, and then a mitotic shake-off was performed on day 4. Subsequently, cells were reseeded in nocodazole-containing medium and stimulated with 100 ng of FasL/ml. The cell cycle status was monitored by flow cytometry (upper panels). Lysates were immunoblotted for exogenous caspase-8 using a Flag-specific antibody, caspase-8, PARP, and β-actin (middle panels). Caspase-8 activity and apoptosis (annexin staining) were determined (lower panels). All experiments were performed in triplicate. Error bars represent the SD. (e) On day 1 HeLa cells were transfected with siRNA targeting the untranslated region of caspase-8, followed by the transfection of Flag-tagged caspase-8 (mock, S387A, or S387E) on day 2. On day 3 cells were stimulated with 100 ng of FasL/ml. After 24 h, the cell numbers were determined, and lysates were immunoblotted for exogenous caspase-8 using a Flag-specific antibody and β-actin. The experiment was performed in triplicate. Error bars represent the SD.
In our previous experiments we demonstrated that Cdk1 phosphorylates caspase-8 at Ser-387 in mitotic cells. Since caspase-8 is the main initiator of the extrinsic death pathway, it would be interesting to monitor first the sensitivity of nonmitotic cells expressing either the nonphosphorylatable form S387A or the phosphomimic form S387E to Fas-induced apoptosis. We generated double-tagged (N-terminal Flag, C-terminal V5) mutated versions of caspase-8 and monitored the kinetics of p12/p10 production as an early step of procaspase-8 processing. The p12/p10 subunit was detectable in HeLa cells transfected with caspase-8 (S387A) expression constructs within 1 h of Fas stimulation, and its level increased after 2 to 3 h (Fig. 7b, upper panel). In contrast, V5-tagged p12/p10 was at the limit of detection in HeLa cells transfected with the caspase-8 (S387E) expression construct and became visible only after prolonged exposure. PARP cleavage in these cells indicated efficient Fas-induced signaling via endogenous caspase-8 (Fig. 7b, lower panel). To evaluate the correlation between procaspase-8 processing as measured by p12/p10 cleavage and apoptosis in a rescue experiment of nonsynchronized cells, caspase-8-depleted cells (caspase-8−) were transfected with the constructs expressing double-tagged caspase-8 (S387A or S387E) mutants (Fig. 7c). The S387A mutant expressed in caspase-8-depleted cells was processed with the passing of time upon Fas stimulation, as indicated by decreasing amounts of procaspase-8 and increasing levels of p12/p10 (Fig. 7c, upper and middle panels). First signs of procaspase-8 processing correlated with the onset of apoptosis as measured by PARP cleavage (Fig. 7c, lower panel). In contrast, the S387E mutant was not processed and blocked the apoptotic response in caspase-8-depleted cells upon Fas stimulation (Fig. 7c). These data indicate that both mutants displayed a different apoptotic response even in nonmitotic cells where the activity of Cdk1 is nearly inexistent, showing that the replacement of endogenous procaspase-8 by a procaspase-8 mutant mimicking phosphorylation at S387 inhibits the extrinsic death pathway even in an unsynchronized cell population. This corroborates our previous findings that the mitotic phosphorylation of caspase-8 by Cdk1 at Ser-387 blocks the Fas-induced apoptotic response.
Still, since our data demonstrate that the phosphorylation of procaspase-8 at Ser-387 occurs exclusively during mitosis and is mediated by Cdk1/cyclin B1, we focused in subsequent experiments on the role of procaspase-8 phosphorylation in mitotic cells. First, we sought to determine whether phosphorylation of Ser-387 controls Fas-induced apoptosis in mitotic cells. We used a strategy based on the depletion of endogenous caspase-8 by siRNA targeting the untranslated region and its replacement in mitotic cells by similar amounts of transfected caspase-8, either with or without mutation of Ser387 to a nonphosphorylatable Ala residue (Fig. 7d, upper and middle panel). Upon Fas stimulation of mitotic cell cultures, only a small increase in caspase-8 activity and a small increase in apoptosis were determined (Fig. 7d, lower left and right panels). In contrast to the replacement of endogenous caspase-8 with wild-type caspase-8, the replacement with the S387A mutant led to elevated caspase-8 activity and to an increased incidence of apoptosis, indicating that prevention of Ser-387 phosphorylation increases sensitivity to Fas-induced apoptosis in mitotic cells (Fig. 7d, lower left and right panels). Moreover, the expression of the S387E mutant in an exponentially growing, caspase-8-depleted HeLa cell population, which encompasses ≥80% nonmitotic cells, protected against Fas-mediated apoptosis in a prolonged observation period of 24 h compared to S387A-expressing cells (Fig. 7e).
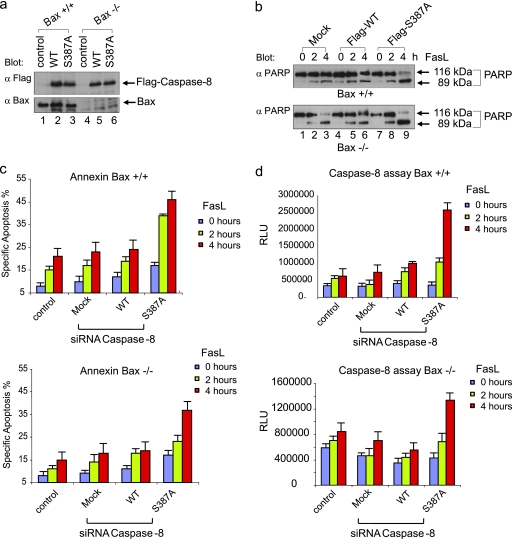
Furthermore, to evaluate a putative contribution of the intrinsic death pathway to the apoptotic response that we observed upon Fas stimulation, we used HCT116 cells with a disrupted mitochondrial death pathway due to a lack of a functional BAX gene (50). We replaced endogenous caspase-8 with wild-type caspase-8 or the S387A mutant in mitotic BAX-negative HCT116 cells (Fig. 8a). Expression of the nonphosphorylatable caspase-8/S387A-form in caspase-8-depleted, BAX-negative HCT116 cells led to elevated caspase-8 activity and to an increased incidence of apoptosis upon Fas stimulation during mitosis. The replacement experiment in BAX-positive cells revealed an elevated level of apoptosis compared to the corresponding experiment using BAX-negative cells, indicating a weak contribution of the intrinsic pathway to overall apoptosis (Fig. 8c). Our data suggest that prevention of Ser-387 phosphorylation of procaspase-8 increases sensitivity to Fas-induced apoptosis also in mitotic BAX-negative HCT116 cells with a disrupted mitochondrial pathway (Fig. 8b to d).
FIG. 8.
A nonphosphorylatable S387 mutant of procaspase-8 sensitizes mitotic BAX-deficient HCT116 cells to Fas-mediated apoptosis. (a) On day 1, wild-type (BAX-positive) and BAX-deficient HCT116 cells were transfected with siRNA targeting the untranslated region of caspase-8, followed by the transfection of Flag-tagged caspase-8 (mock, WT, or S387A) on day 2. On day 3, cells were treated overnight with nocodazole, and then a mitotic shake-off was performed on day 4. Subsequently, the cells were reseeded in nocodazole-containing medium and stimulated with 100 ng of FasL/ml. Lysates were immunoblotted for exogenous caspase-8 using a Flag-specific antibody and BAX. (b) Lysates of BAX-positive and -negative cells that were treated as described in panel a were immunoblotted for PARP. (c and d) Apoptosis (annexin staining) and caspase-8 activity were determined. All experiments were performed in triplicate. Error bars represent the SD.
Phosphorylation of procaspase-8 in primary cells and tissues.
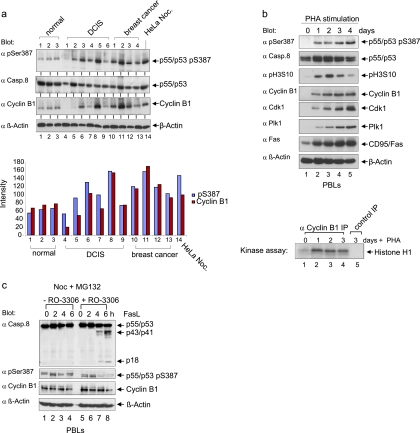
Because alterations in cyclin B1 and Cdk1 are a widespread feature of tumorigenesis (46), we assessed whether procaspase-8 is phosphorylated at Ser-387 in breast cancer cells. We analyzed primary normal breast epithelial cells, premalignant ductal carcinomas in situ (DCIS) and invasive breast cancers (Fig. 9a, upper panel). The level of cyclin B1 was low in normal epithelial cells and higher in the more proliferative DCIS and invasive breast cancer. Interestingly, elevated cyclin B1 expression in the breast tissue often correlated with an increased level of procaspase-8 phosphorylation at Ser-387 (Fig. 9a, lower panel). We detected phosphorylated procaspase-8 (S387) in 12 of 13 breast cancer samples, suggesting a pathophysiological role for this event in primary breast cancer.
FIG. 9.
Phosphorylation of procaspase-8 at Ser-387 in primary cells and tissues. (a) Lysates were prepared from human breast cancer tissues, premalignant ductal carcinoma in situ (DCIS) samples and normal breast tissues. Total protein was resolved by SDS-PAGE and immunoblotted for caspase-8 (pSer387), caspase-8, cyclin B1, and β-actin (top panel). Blue bars represent the level of caspase-8 (pSer387) normalized to the level of β-actin expression (lower panel). Red bars represent the level of cyclin B1 expression standardized to the level of β-actin expression. (b) Peripheral blood lymphocytes (PBLs) were treated with PHA for the times indicated. Cell lysates were immunoblotted for caspase-8 (pS387), caspase-8, histone H3 phosphorylated at Ser10 (H3 pS10), cyclin B1, Cdk1, Plk1, CD95/Fas, and β-actin (upper panels). To measure Cdk1/cyclin B1 activity in cell lysates of synchronized cells, immunoprecipitation assays were performed using a cyclin B1-specific antibody, and the purified proteins were subjected to kinase assays with histone H1 as the substrate (lower panel). (c) PBLs were cultured with PHA for 16 h. Cells were then washed three times and cultured for an additional 5 days in the presence of interleukin-2, followed by nocodazole treatment for 16 h and MG132 for 2 h. For the inhibition of Cdk1/cyclin B1, RO-3306 was added.
To investigate the physiological role of procaspase-8 phosphorylation in primary cells of different origin, we analyzed the extrinsic pathway in T cells. Resting peripheral blood lymphocytes (PBLs) stimulated with phytohemagglutinin (PHA) started to proliferate within 1 to 2 days depending on the donor. The onset of proliferation was associated with increased Plk1 and cyclin B1 expression, as well as Cdk1/cyclin B1 activity (Fig. 9b, upper panels). The detection of phosphorylated procaspase-8 (S387) correlated with the pronounced increase of Cdk1/cyclin B1 activity (Fig. 9b, lower panel). Cycling PBLs, trapped in mitosis with nocodazole and MG132, were treated with or without RO-3306 and FasL. Incubation with RO-3306 decreased the level of procaspase-8 phosphorylation at Ser-387 within 2 to 3 h and led to elevated processing (Fig. 9c). Therefore, the inhibition of procaspase-8 phosphorylation at Ser-387 by downregulating Cdk1/cyclin B1 activity correlates with a more robust Fas-induced response also in primary mitotic lymphocytes.
DISCUSSION
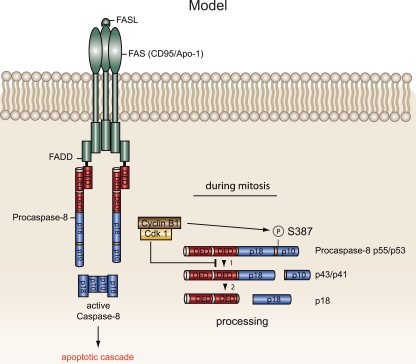
Because many types of cancer are characterized by accelerated cell proliferation driven by the increased activity of cell cycle kinases and by resistance to death stimuli, in this study we examined the impact of these protein kinases on the apoptotic cascade. Although various genetic and epigenetic mechanisms, including mutations, homo- or heterozygous genomic deletions, or allelic imbalance on chromosome 2q that inactivate caspase-8, have been described previously (9), our understanding of the posttranslational regulation of procaspase-8 is limited. Here, we describe for the first time the phosphorylation of procaspase-8 on a serine residue. We propose a model in which death receptor signaling is inhibited by the Cdk1/cyclin B1-mediated phosphorylation of procaspase-8 on Ser-387 in mitotic cells (Fig. 10).
FIG. 10.
Proposed model for the impact of the active Cdk1/cyclin B1 complex on the extrinsic death pathway. During the cell cycle, Cdk1/cyclin B1 associates with procaspase-8. As cyclin B1 becomes abundant in G2, the active Cdk1/cyclin B1 complex phosphorylates Ser-387 on procaspase-8 at the centrosomes. This phosphorylation event inhibits the processing of procaspase-8, thus blocking its enzymatic activation and the downstream initiation of apoptotic signaling. This cellular barrier seems to protect mitotic cells against Fas-mediated apoptosis. The identification of Ser-387 phosphorylation of procaspase-8 in primary lymphocytes and breast tissues suggests both a physiological and a pathophysiological role for this process in vivo.
Various investigations established a two-step cleavage model for the processing of procaspase-8, and it was proposed that its activation occurs in a strict sequence of events: Although the cleavage between the small and the large catalytic subunit represents the first step of autoprocessing, the second step occurs between the prodomain and the large catalytic subunit (5, 27). A phosphorylation of procaspase-8 at Ser-387 close to Asp-384 and Asp-374, which are used to generate p43/p41 and the p12/p10 subunits, inhibits also the generation of p18. From these data, we conclude that a phosphorylation at the N-terminal end of p10 inhibits the liberation of the p12/p10 subunits by blocking the Asp-374/Asp-384 cleavage sites and thereby also influences the two-step cleavage of procaspase-8. Our observations suggest that both steps of caspase-8 processing occur on a mutual basis. By controlling the autoprocessing of procaspase-8, Cdk1/cyclin B1 seems to elevate the threshold of cells against extrinsic death signals during mitosis. This mechanism is not specific for Fas-induced cell death, it can be generalized to other caspase-8-dependent pathways including the stimulation of cells with TRAIL or TNF-α.
Whereas the activation of effector caspases by Fas-induced stimulation of caspase-8 suffices for cell killing in type I cells, caspase cascade amplification through caspase-8 mediated activation of the proapoptotic BCL-2 family member BID leading to BAX/BAK-dependent activation of caspases-9 is essential in type 2 cells. Using type I cells such as the B lymphoblastoid cell line SKW 6.4 or HCT116 cells with a disrupted mitochondrial death pathway due to a lack of a functional BAX gene, we could demonstrate that the posttranslational modification of procaspase-8 regulates Fas-mediated apoptosis independent of caspases cascade amplification.
A previous study has described the src-mediated phosphorylation of caspase-8 on Tyr-380, which is located in the linker region between the p18 and p11 subunits. The overexpression of a hyperactive mutant of c-src resulted in a delayed accumulation of the large catalytic subunit p18 after Fas stimulation. Therefore, src activity triggers endogenous caspase-8 tyrosine phosphorylation and also protects cells from Fas-induced apoptosis. Although elevated Cdk1/cyclin B1 activity is a more general feature of mammalian tumors, the protective effect of elevated src activity under physiological conditions is limited to certain types of cancers such as human colon cancer (7). The present study also highlights the functional importance of posttranslational modifications within the region linking the small and large catalytic subunits in the regulation of the activity of caspase-8. Phosphorylation of Tyr-380 and/or Ser-387 represses the activation of procaspase-8, which requires two cleavage events to remove the linker region between the p18 and the p10 subunits to liberate p10 (7). In addition, Cdk1/cyclin B1 was shown to phosphorylate caspase-9 (Thr-125) and most recently also caspase-2 (Ser-340) and thereby blocks apoptosis in mitotic cells (1, 2). Most interestingly, these posttranslational events, including the phosphorylation of caspase-8 (Tyr-380) by src (7), all modulate the processing of caspases by targeting different linker regions connecting functional domains of caspases. Taken together, the description of caspases-2, -8, and -9 as substrates of Cdk1/cyclin B1 suggests that Cdk1/cyclin B1 targets predominantly initiator caspases that act upstream in the apoptotic cascade thereby blocking apoptotic signaling in an early stage.
Furthermore, pTyr-380 of caspase-8 can function as a docking site for the p85 subunit of phosphatidylinositol 3-kinase (PI3K), which affects Rac activation, thereby promoting cell adhesion and motility (38). Hence, caspase-8 can be considered as a molecular switch involved in the cellular decision to initiate apoptosis or migration. Whether phosphorylation at Ser-387 by Cdk1/cyclin B1 regulates this balance between the apoptotic and nonapoptotic functions of caspase-8 by influencing its interaction with PI3K will require further study.
In many cancers, the normal apoptotic program is dysregulated, which can lead to uncontrolled proliferation and the development of resistance to conventional chemotherapy and radiotherapy. Many tumors become particularly resistant to death receptor-induced apoptosis with increasing treatment modalities (15). Thus, it is important to identify and develop therapies that restore the sensitivity of cancer cells to Fas-mediated apoptosis. Interestingly, the potentiation of Cdk1 via overexpression of its cognate partner cyclin B1 and the inactivation of Cdk inhibitors is often linked to tumor development (23, 25, 32, 39). Our observations suggest that procaspase-8 phosphorylation on Ser-387 raises the threshold for Fas-mediated apoptosis in mitotic cells of different origin. In addition, antimitotic drugs that inhibit microtubule dynamics cause cells to arrest in mitosis with elevated levels of cyclin B1 and activated Cdk1/cyclin B1 kinase activity. Although this treatment elevates the threshold toward extrinsic and intrinsic apoptotic stimuli considering the Cdk1/cyclin B1-mediated phosphorylation of capases-8, -9, and -2, the activation of the spindle assembly checkpoint by antimitotic drugs is of central importance for the fate of cancer cells.
Because increased proliferation is one trait of cancer, neoplastic cells could have hijacked the protective effect of elevated Cdk1/cyclin B1 activity to evade apoptosis by raising the threshold for extrinsic death signals originating from the infiltration of immune cells into tumors. The selective inhibition of Cdk1/cyclin B1 leading to reduced procaspase-8 phosphorylation may constitute a novel approach to fighting the resistance of cancer to therapeutic strategies targeting the extrinsic death pathway. Moreover, several strategies have been developed to elevate caspase-8 expression to restore its functions in human cancer and to resensitize cancer cells to extrinsic death stimuli: the treatment of cancer cells with demethylating agents, interferons, and retinoic acid are promising strategies to raise the levels of the initiator caspase-8 in tumors lacking this critical apoptosis regulator (10, 17, 47). Taken together, combinations of these agents with Cdk1/cyclin B1 inhibitors might further increase the sensitivity of cancer cells to extrinsic cell death.
Acknowledgments
We thank Kaufmann for providing the tissue samples. We thank Vogelstein for the generous gift of BAX-negative HCT116 cells.
This study was supported by grants from the Else Kröner-Fresenius/Carls-Stiftung, the Deutsche Krebshilfe, and the LOEWE Centre (Frankfurt, Germany).
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Allan, L. A., and P. R. Clarke. 2007. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol. Cell 26:301-310. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. L., C. E. Johnson, C. D. Freel, A. B. Parrish, J. L. Day, M. R. Buchakjian, L. K. Nutt, J. W. Thompson, M. A. Moseley, and S. Kornbluth. 2009. Restraint of apoptosis during mitosis through interdomain phosphorylation of caspase-2. EMBO J. 28:3216-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley, A. M., G. Normand, J. Hoyt, and R. W. King. 2007. Distinct sequence elements of cyclin B1 promote localization to chromatin, centrosomes, and kinetochores during mitosis. Mol. Biol. Cell 18:4847-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blangy, A., H. A. Lane, P. d'Herin, M. Harper, M. Kress, and E. A. Nigg. 1995. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83:1159-1169. [DOI] [PubMed] [Google Scholar]
- 5.Chang, D. W., Z. Xing, V. L. Capacio, M. E. Peter, and X. Yang. 2003. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 22:4132-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, A., A. M. Dunning, M. Garcia-Closas, S. Balasubramanian, M. W. Reed, K. A. Pooley, S. Scollen, C. Baynes, B. A. Ponder, S. Chanock, J. Lissowska, L. Brinton, B. Peplonska, M. C. Southey, J. L. Hopper, M. R. McCredie, G. G. Giles, O. Fletcher, N. Johnson, S. S. dos, I. L. Gibson, S. E. Bojesen, B. G. Nordestgaard, C. K. Axelsson, D. Torres, U. Hamann, C. Justenhoven, H. Brauch, J. Chang-Claude, S. Kropp, A. Risch, S. Wang-Gohrke, P. Schurmann, N. Bogdanova, T. Dork, R. Fagerholm, K. Aaltonen, C. Blomqvist, H. Nevanlinna, S. Seal, A. Renwick, M. R. Stratton, N. Rahman, S. Sangrajrang, D. Hughes, F. Odefrey, P. Brennan, A. B. Spurdle, G. Chenevix-Trench, J. Beesley, A. Mannermaa, J. Hartikainen, V. Kataja, V. M. Kosma, F. J. Couch, J. E. Olson, E. L. Goode, A. Broeks, M. K. Schmidt, F. B. Hogervorst, L. J. Van't Veer, D. Kang, K. Y. Yoo, D. Y. Noh, S. H. Ahn, S. Wedren, P. Hall, Y. L. Low, J. Liu, R. L. Milne, G. Ribas, A. Gonzalez-Neira, J. Benitez, A. J. Sigurdson, D. L. Stredrick, B. H. Alexander, J. P. Struewing, P. D. Pharoah, and D. F. Easton. 2007. A common coding variant in CASP8 is associated with breast cancer risk. Nat. Genet. 39:352-358. [DOI] [PubMed] [Google Scholar]
- 7.Cursi, S., A. Rufini, V. Stagni, I. Condo, V. Matafora, A. Bachi, A. P. Bonifazi, L. Coppola, G. Superti-Furga, R. Testi, and D. Barila. 2006. Src kinase phosphorylates caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J. 25:1895-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degterev, A., and J. Yuan. 2008. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell. Biol. 9:378-390. [DOI] [PubMed] [Google Scholar]
- 9.Fulda, S. 2009. Caspase-8 in cancer biology and therapy. Cancer Lett. 281:128-133. [DOI] [PubMed] [Google Scholar]
- 10.Fulda, S., M. U. Kufer, E. Meyer, V. F. van, B. Dockhorn-Dworniczak, and K. M. Debatin. 2001. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 20:5865-5877. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 12.Harada, K., S. Toyooka, N. Shivapurkar, A. Maitra, J. L. Reddy, H. Matta, K. Miyajima, C. F. Timmons, G. E. Tomlinson, D. Mastrangelo, R. J. Hay, P. M. Chaudhary, and A. F. Gazdar. 2002. Deregulation of caspase 8 and 10 expression in pediatric tumors and cell lines. Cancer Res. 62:5897-5901. [PubMed] [Google Scholar]
- 13.Holtrich, U., G. Wolf, A. Brauninger, T. Karn, B. Bohme, H. Rubsamen-Waigmann, and K. Strebhardt. 1994. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. U. S. A. 91:1736-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes, M. A., N. Harper, M. Butterworth, K. Cain, G. M. Cohen, and M. MacFarlane. 2009. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol. Cell 35:265-279. [DOI] [PubMed] [Google Scholar]
- 15.Igney, F. H., and P. H. Krammer. 2002. Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 16.Jackman, M., C. Lindon, E. A. Nigg, and J. Pines. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143-148. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, M., K. Zhu, J. Grenet, and J. M. Lahti. 2008. Retinoic acid induces caspase-8 transcription via phospho-CREB and increases apoptotic responses to death stimuli in neuroblastoma cells. Biochim. Biophys. Acta 1783:1055-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappel, S., Y. Matthess, M. Kaufmann, and K. Strebhardt. 2007. Silencing of mammalian genes by tetracycline-inducible shRNA expression. Nat. Protoc. 2:3257-3269. [DOI] [PubMed] [Google Scholar]
- 19.Kappel, S., Y. Matthess, B. Zimmer, M. Kaufmann, and K. Strebhardt. 2006. Tumor inhibition by genomically integrated inducible RNAi-cassettes. Nucleic Acids Res. 34:4527-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keen, N., and S. Taylor. 2004. Aurora-kinase inhibitors as anticancer agents. Nat. Rev. Cancer 4:927-936. [DOI] [PubMed] [Google Scholar]
- 21.Kimura, K., O. Cuvier, and T. Hirano. 2001. Chromosome condensation by a human condensin complex in Xenopus egg extracts. J. Biol. Chem. 276:5417-5420. [DOI] [PubMed] [Google Scholar]
- 22.Lavrik, I., A. Krueger, I. Schmitz, S. Baumann, H. Weyd, P. H. Krammer, and S. Kirchhoff. 2003. The active caspase-8 heterotetramer is formed at the CD95 DISC. Cell Death. Differ. 10:144-145. [DOI] [PubMed] [Google Scholar]
- 23.Malumbres, M., and M. Barbacid. 2001. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer 1:222-231. [DOI] [PubMed] [Google Scholar]
- 24.Malumbres, M., and M. Barbacid. 2009. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9:153-166. [DOI] [PubMed] [Google Scholar]
- 25.Mashal, R. D., S. Lester, C. Corless, J. P. Richie, R. Chandra, K. J. Propert, and A. Dutta. 1996. Expression of cell cycle-regulated proteins in prostate cancer. Cancer Res. 56:4159-4163. [PubMed] [Google Scholar]
- 26.Matthess, Y., S. Kappel, B. Spankuch, B. Zimmer, M. Kaufmann, and K. Strebhardt. 2005. Conditional inhibition of cancer cell proliferation by tetracycline-responsive, H1 promoter-driven silencing of PLK1. Oncogene 24:2973-2980. [DOI] [PubMed] [Google Scholar]
- 27.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 29.Nigg, E. A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell. Biol. 2:21-32. [DOI] [PubMed] [Google Scholar]
- 30.Nurse, P. 1990. Universal control mechanism regulating onset of M-phase. Nature 344:503-508. [DOI] [PubMed] [Google Scholar]
- 31.Peter, M., J. Nakagawa, M. Doree, J. C. Labbe, and E. A. Nigg. 1990. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61:591-602. [DOI] [PubMed] [Google Scholar]
- 32.Pines, J. 2006. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16:55-63. [DOI] [PubMed] [Google Scholar]
- 33.Raab, M., S. Pfister, and C. E. Rudd. 2001. CD28 signaling via VAV/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity 15:921-933. [DOI] [PubMed] [Google Scholar]
- 34.Raab, M., M. Yamamoto, and C. E. Rudd. 1994. The T-cell antigen CD5 acts as a receptor and substrate for the protein-tyrosine kinase p56lck. Mol. Cell. Biol. 14:2862-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reindl, W., J. Yuan, A. Kramer, K. Strebhardt, and T. Berg. 2008. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem. Biol. 15:459-466. [DOI] [PubMed] [Google Scholar]
- 36.Rudner, A. D., and A. W. Murray. 2000. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149:1377-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 38.Senft, J., B. Helfer, and S. M. Frisch. 2007. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 67:11505-11509. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro, G. I. 2006. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 24:1770-1783. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, G. Litwack, and E. S. Alnemri. 1996. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc. Natl. Acad. Sci. U. S. A. 93:14486-14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strebhardt, K. 2010. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat. Rev. Drug Discov. 9:643-660. [DOI] [PubMed] [Google Scholar]
- 42.Strebhardt, K., and A. Ullrich. 2006. Targeting polo-like kinase 1 for cancer therapy. Nat. Rev. Cancer 6:321-330. [DOI] [PubMed] [Google Scholar]
- 43.Stupack, D. G., T. Teitz, M. D. Potter, D. Mikolon, P. J. Houghton, V. J. Kidd, J. M. Lahti, and D. A. Cheresh. 2006. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 439:95-99. [DOI] [PubMed] [Google Scholar]
- 44.Teitz, T., T. Wei, M. B. Valentine, E. F. Vanin, J. Grenet, V. A. Valentine, F. G. Behm, A. T. Look, J. M. Lahti, and V. J. Kidd. 2000. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med. 6:529-535. [DOI] [PubMed] [Google Scholar]
- 45.Vassilev, L. T., C. Tovar, S. Chen, D. Knezevic, X. Zhao, H. Sun, D. C. Heimbrook, and L. Chen. 2006. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. U. S. A. 103:10660-10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winters, Z. E., N. C. Hunt, M. J. Bradburn, J. A. Royds, H. Turley, A. L. Harris, and C. J. Norbury. 2001. Subcellular localization of cyclin B, Cdc2, and p21WAF1/CIP1 in breast cancer. association with prognosis. Eur. J. Cancer 37:2405-2412. [DOI] [PubMed] [Google Scholar]
- 47.Yang, X., M. S. Merchant, M. E. Romero, M. Tsokos, L. H. Wexler, U. Kontny, C. L. Mackall, and C. J. Thiele. 2003. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 63:1122-1129. [PubMed] [Google Scholar]
- 48.Yuan, J., A. Kramer, Y. Matthess, R. Yan, B. Spankuch, R. Gatje, R. Knecht, M. Kaufmann, and K. Strebhardt. 2006. Stable gene silencing of cyclin B1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene 25:1753-1762. [DOI] [PubMed] [Google Scholar]
- 49.Yuan, J., R. Yan, A. Kramer, F. Eckerdt, M. Roller, M. Kaufmann, and K. Strebhardt. 2004. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 23:5843-5852. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]