Abstract
Ataxia telangiectasia (A-T) patients can develop multiple clinical pathologies, including neuronal degeneration, an elevated risk of cancer, telangiectasias, and growth retardation. Patients with A-T can also exhibit an increased risk of insulin resistance and type 2 diabetes. The ATM protein kinase, the product of the gene mutated in A-T patients (Atm), has been implicated in metabolic disease, which is characterized by insulin resistance and increased cholesterol and lipid levels, blood pressure, and atherosclerosis. ATM phosphorylates the p53 tumor suppressor on a site (Ser15) that regulates transcription activity. To test whether the ATM pathway that regulates insulin resistance is mediated by p53 phosphorylation, we examined insulin sensitivity in mice with a germ line mutation that replaces the p53 phosphorylation site with alanine. The loss of p53 Ser18 (murine Ser15) led to increased metabolic stress, including severe defects in glucose homeostasis. The mice developed glucose intolerance and insulin resistance. The insulin resistance correlated with the loss of antioxidant gene expression and decreased insulin signaling. N-Acetyl cysteine (NAC) treatment restored insulin signaling in late-passage primary fibroblasts. The addition of an antioxidant in the diet rendered the p53 Ser18-deficient mice glucose tolerant. This analysis demonstrates that p53 phosphorylation on an ATM site is an important mechanism in the physiological regulation of glucose homeostasis.
Ataxia telangiectasia (A-T) is a recessive progeroid disease of early childhood, with poor prognosis. Patients can develop a constellation of clinical pathologies, including neuronal degeneration, an elevated risk of cancer, telangiectasias, accelerated aging, growth retardation, and pulmonary disease (25). In addition, A-T patients exhibit an increased risk of insulin resistance and type 2 diabetes (3). The gene mutated in A-T patients (Atm) encodes a protein kinase that phosphorylates the p53 tumor suppressor on a site (Ser15) that regulates transcription activity (10, 21, 43). The loss of Atm has been implicated in metabolic disease, which is characterized by insulin resistance and increased cholesterol and lipid levels, blood pressure, and atherosclerosis (42).
Studies have attributed most of the pathology in A-T patients and Atm-deficient cells to increased levels of reactive oxygen species (ROS) and oxidative stress (4, 15, 20). Thus, the loss of ATM function has been hypothesized to lead to a state of chronic oxidative stress, which can contribute to insulin resistance. However, how ATM senses ROS levels is not clear. p53 has been shown to have a dual role in the regulation of reactive oxygen species, a major source of cellular stress (8, 27, 35). On one hand, very high levels of p53 (such as in overexpression studies) or high levels of stress will induce p53-dependent prooxidant genes (36), which can facilitate apoptosis. On the other hand, endogenous levels of p53 have been shown to regulate antioxidant gene expression (9, 38). Perhaps more physiologically relevant is the observation that p53 induces a transcriptional program in response to mild stresses, which may operate during physiological aging (46). Studies demonstrate a role of endogenous p53 levels in the regulation of genes involved in antioxidant defense, such as those encoding the sestrins (Sesn) (9) and glutathione peroxidase 1 (GPX-1) (38). Therefore, ATM and p53 both regulate the levels of ROS in vivo.
The development of insulin resistance in patients with A-T and in Atm mutant mice is poorly understood. ATM is activated by a number of stresses that have a direct impact on metabolism. A number of these cellular stresses, including atherosclerosis, trauma, hypoxia, and infections, are associated with p53 activation (12, 30, 32, 39, 44, 50, 51). Many of these stresses also cause phosphorylation of p53 on the ATM site (Ser15 in humans, Ser18 in mice). ATM-mediated p53 phosphorylation therefore represents a possible mechanism of physiological regulation of insulin sensitivity.
Additional support for p53 in regulating the metabolic effects of ATM has come from studies investigating the role of p53 in energy metabolism (7). p53 has been shown in vitro and in vivo to regulate the expression of genes involved in metabolism, such as the cytochrome c oxidase 2 gene (Sco2), which is required for mitochondrial respiration (29), glucose transporter genes, including Glut1 and Glut4, in cancer cell lines (41, 48), the phosphoglycerate mutase gene (PGM) (24), Tigar (7), and the gene encoding which is zinc finger protein 385a (Zfp385a, also known as hzf), involved in adipocyte function and glucose homeostasis (22). p53 has been implicated in the development of insulin resistance through the regulation of senescence in adipose tissue (31). p53 has also been implicated in autoimmune disease and the macrophage response in a streptozotocin-induced type 1 diabetes model (52). Mice deficient for p53 were found to be more susceptible to streptozotocin-induced diabetes than wild-type mice. Thus, p53 had a protective role in this system. Although the p53 tumor suppressor has been implicated in the streptozotocin model of type 1 diabetes, the role of p53 in type 2 diabetes and insulin resistance has not been established.
The connection of p53 activity and p53 Ser18 phosphorylation to metabolic regulation supports a model whereby the mechanism of ATM-mediated physiological regulation of insulin sensitivity may involve p53 activity and ATM-mediated p53 phosphorylation. To test this hypothesis, we compared metabolic regulation in mice without and with a germ line mutation in the p53 phosphorylation site Ser18. These p53S18A mice exhibit defects in p53-mediated apoptosis and gene expression (10, 43), but unlike p53-null mice, p53S18A mice develop tumors only at advanced age (1). Analysis of various metabolic parameters indicated that p53S18A mice have increased metabolic stress. This metabolic deregulation is likely mediated through increased levels of ROS, as antioxidant treatment restores insulin sensitivity. Indeed, we observed impaired glucose homeostasis and insulin resistance in these animals, indicating that p53 Ser18 participates in a metabolic checkpoint.
MATERIALS AND METHODS
Mouse strains and diet information.
The methods employed for the generation and genotyping of p53S18A mice (43), p53−/− mice (11), and Atm+/− mice (5) have been reported previously. p53S18A/+ mice were intercrossed to obtain p53S18A/S18A, p53S18A/+, and p53+/+ (wild-type) mice. Cohorts of male mice on a mixed 129/Sv × C57BL/6 genetic background were established for analysis. The mice were maintained on a standard chow diet. For antioxidant treatment, mice were given a diet containing 1.5% butylated hydroxyanisole (BHA; Bioserv; custom diet). All mice were housed in specific-pathogen-free facilities accredited by the American Association for Laboratory Animal Care. The Institutional Animal Care and Use Committees of the University of Massachusetts Medical School and Pennsylvania State University approved all studies using animals.
Glucose and insulin tolerance tests.
Mice were examined using a glucose tolerance test (GTT) and an insulin tolerance test (ITT) with methods described previously (33). Mice were fasted overnight and challenged by the intraperitoneal administration of glucose (1 g/kg body weight) or insulin (0.75 U/kg body weight). Blood glucose was measured with an Ascenzia Breeze 2 glucometer (Bayer), and blood insulin was measured by enzyme-linked immunosorbent assay (ELISA) (Luminex 200, Millipore).
Hyperinsulinemic-euglycemic clamp studies.
The clamp studies were performed at the Pennsylvania State Diabetes and Obesity Mouse Phenotyping Center. Whole-body fat and lean mass were noninvasively measured using 1H-MRS (Echo Medical Systems). Following an overnight fast, a 2-h hyperinsulinemic-euglycemic clamp study was conducted in conscious mice with a primed and continuous infusion of human insulin (150 mU/kg body weight priming followed by 2.5 mU/kg/min; Humulin, Eli Lilly), and 20% glucose was infused at variable rates to maintain euglycemia (23). Whole-body glucose turnover was assessed with a continuous infusion of [3-3H]glucose (PerkinElmer), and 2-deoxy-d-[1-14C]glucose (PerkinElmer) was administered as a bolus (10 μCi) at 75 min after the start of the clamp study to measure insulin-stimulated glucose uptake in individual organs. At the end of the clamp study, mice were anesthetized and tissues were taken for biochemical analysis (23).
Analysis of tissue sections.
Upon necropsy, gross organ analysis was performed. In addition, samples of liver, spleen, thymus, lymph nodes, kidney, heart, pancreas, fat, and muscle were removed. Histology was performed using tissue fixed in 10% formalin for 24 h, dehydrated, and embedded in paraffin. Sections (7 μm) were cut, stained using hematoxylin and eosin (American Master Tech Scientific), and examined by a board-certified veterinary pathologist.
Insulin treatment and immunoblot analysis.
For insulin response in vivo, mice were fasted overnight. Mice were injected with 1.5 U of insulin, and tissues were harvested 30 min later. Tissues were homogenized in Triton lysis buffer (20 mM Tris [pH 7.4], 1% Triton X-100, 10% glycerol, 137 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml of aprotinin and leupeptin). Tissue extracts (50 μg) were examined by immunoblot analysis using antibodies to Akt, phospho-Ser473 Akt, and phospho-Thr172 AMPKα (Cell Signaling). Flag-sestrin 2 was detected with anti-Flag antibody (Sigma).
Cytokine analysis.
The amount of cytokines in plasma was measured by multiplexed ELISA by using a Luminex 200 machine (Millipore) and serum mouse adipokine, adiponectin, and cytokine kits (Millipore).
Blood lipid analysis.
The amount of triglyceride was measured using the CardioCheck meter and triglyceride strips (Polymer Technology Systems, Indianapolis, IN).
RNA preparation and analysis.
RNA was prepared from tissues collected in RNAlater (Ambion) and snap-frozen in liquid nitrogen. Total RNA was prepared with RNeasy kits (Qiagen) by following the manufacturer's instructions. The purified RNA was subjected to an additional DNase treatment (Ambion) to ensure the removal of contaminating genomic DNA prior to final column purification. The relative expression of mRNA was examined by quantitative PCR analysis. cDNA was prepared using Superscript III (Invitrogen) with random hexamers and 0.5 μg to 1 μg of RNA per tissue. Quantitative real-time PCR was performed on a Bio-Rad iCycler using Sybr green master mix (Bio-Rad). The primer sequences for the murine genes were as follows: Gapdh (5′-CTTCACCACCATGGAGAAGGC-3′, 5′-GGCATGGACTGTGGTCAT-3′), p53 (5′-TGAAACGCCGACCTATCCTTA-3′, 5′-GGCACAAACACGAACCTCAAA-3′), Mdm2 (5′-TGACACCAGAGCTTAGTCCTG-3′, 5′-GCGTCTCGTAACGAATAAGGC-3′), Zfp385a (5′-ACATTGAGCACCGCTATGTCT-3′, 5′-CTCTCTTGGATGAGGGTCTGATA-3′), Sesn1 (5′-GTGGACCCAGAACGAGATGACGTGGC-3′, 5′-GACACTGTGGAAGGCAGCTATGTGC-3′), Sesn2 (5′-TCCGAGTGCCATTCCGAGAT-3′, 5′-TCCGGGTGTAGACCCATCAC-3′), Sesn3 (5′-GCGAGGAGAAGAACATTTGCC-3′, 5′-CCAAACATACAGTGAACATAGT-3′), Sco2 (5′-GTGGACCCAGAACGAGATGACGTGGC-3′, 5′-GACACTGTGGAAGGCAGCTATGTGC-3′), and Tigar (5′-CAAGCAGCGGCCGCCGGGCAGTTTCTG-3′, 5′-CTCCTGGCAACGAGCATCTGAGGTCAC-3′). All samples were examined in triplicate, and values were normalized for baseline expression and for expression of Gapdh. Calculations of values were made using the ΔΔCT method. Statistical significance was calculated using threshold cycle (CT) values.
Tissue culture.
Primary murine embryo fibroblasts (MEFs) were prepared from E14 wild-type and p53S18A embryos (11). The MEFs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen). Early-passage MEFs were at passage 2 and later-passage MEFs were passage 4 (p53S18A MEFs lose viability at this time). For insulin response analysis, cells were starved 15 to 18 h in Dulbecco's phosphate-buffered saline (D-PBS) with calcium, magnesium, and 5 mM glucose. Cells were treated with insulin (10 nM) and harvested at the indicated time points. For N-acetyl cysteine (NAC) treatment, cells were thawed and fed with medium containing 0.5 mM NAC (Sigma) for four passages and examined for insulin response. For hydrogen peroxide treatment, cells were plated (12,000 onto a 6-well plate) and incubated with hydrogen peroxide (0, 44, 88, and 176 μM) and harvested at 24 h. Relative viability was determined by the trypan blue staining method.
Reactive oxygen species.
For the detection of intracellular ROS levels, MEFs were incubated with 10 μg/ml 2′,7′-dichlorofluorescein diacetate (DCF; Sigma) in PBS supplemented with 5 mM glucose (20 min, 37°C), washed twice with PBS-glucose, suspended in Dulbecco's modified Eagle's medium (5 mM glucose, no phenol red), incubated at 37°C (20 min), and examined by flow cytometry (28). For sestrin expression, lentivirus expressing Sesn2 and green fluorescent protein (GFP) (pVL-Sesn2F and pVL-GFP [9]) were produced by the University of Massachusetts Medical School vector core. Cells (0.5 × 106) were infected with 10 μl concentrated virus. Transduction was confirmed by GFP and Flag-sesn 2 expression.
Data analysis.
To calculate statistical changes in metabolic parameters, statistically significant differences (P < 0.05) between groups were examined using the two-tailed Student t test. Microsoft Excel was used for statistical calculations.
RESULTS
Expression of antioxidant sestrins and metabolic genes mediated by p53 Ser18.
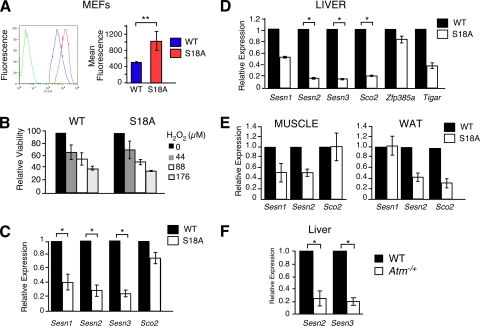
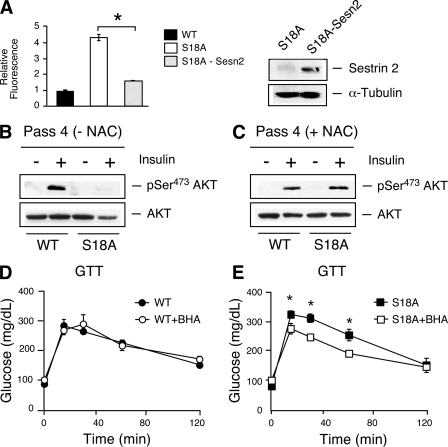
Increased levels of reactive oxygen species (ROS) have been proposed as the major cause of pathologies in A-T patients and Atm mutant mice (47). A-T patients present with increased lipid peroxidation and oxidative DNA damage (37), and tissues from Atm−/− mice exhibit ROS-induced damage (20). Increased ROS production (e.g., as a by-product of mitochondrial oxidative phosphorylation) can contribute to inflammation and insulin resistance (14, 34, 40). p53 has been implicated in the regulation of oxidative stress. We therefore examined whether a mutation of the ATM phosphorylation site on p53 caused increased ROS accumulation. We found that primary fibroblasts prepared from p53S18A mice generated increased amounts of ROS compared with ROS levels in wild-type fibroblasts (Fig. 1A). In contrast, treatment of wild-type and p53S18A fibroblasts with H2O2 (a physiological form of ROS) caused a similar decrease in viability (Fig. 1B). The increased ROS production may therefore account for previous reports that p53S18A fibroblasts, like Atm−/− fibroblasts (49), exhibit decreased growth and accelerated senescence compared to wild-type fibroblasts (1). It has been reported that p53 can regulate the expression of the synthesis of the cytochrome c oxidase 2 gene (Sco2), which is required for mitochondrial respiration (29). We examined Sco2 expression and found no difference between p53S18A and wild-type fibroblasts (Fig. 1C). There was no significant difference in p53 expression between p53S18A and wild-type fibroblasts (data not shown). Thus, increased ROS levels in p53S18A fibroblasts may not be due to defective mitochondrial function.
FIG. 1.
Regulation of antioxidant and metabolic genes by p53 Ser18. (A) The amount of reactive oxygen species (ROS) in MEFs was examined by DCF staining and analysis by flow cytometry. Left, representative relative fluorescence of non-DCF-stained cells (green line) and DCF-stained wild-type (WT) (blue line) and p53 S18A (red line) cells; right, quantification of fluorescence. Data are presented as means ± standard errors of the means (SEM; n = 5). (B) Viability of MEFs treated with hydrogen peroxide at the indicated doses and harvested 18 h posttreatment. Data are presented as means ± SEM (n = 5). (C to F) The expression of Sesn1, -2, and -3, Sco2, or Zfp385a, and Gapdh mRNA was measured by quantitative real-time PCR analysis of the MEFs, liver, white adipose tissue (WAT), and skeletal muscle of 6- to 7-month-old wild-type mice and compared to that of p53S18A mice (C to E) and Atm−/+ mice (F). The amount of Gapdh mRNA in each sample was used to calculate relative mRNA expression (means ± SEM; n = 3). Statistically significant differences between WT and p53S18A mice are indicated (*, P < 0.05).
Cellular antioxidants represent an important mechanism of ROS regulation. Indeed, p53 can regulate the expression of antioxidants (8, 38). Thus, the increased ROS levels in p53S18A fibroblasts may be caused by decreased antioxidant gene expression (38). To test this hypothesis, we examined the expression of Sestrins, p53 target genes that modulate the function of peroxiredoxin (9). The expression of Sestrin 1, 2, and 3 was significantly reduced in p53S18A fibroblasts compared to that in wild-type fibroblasts (Fig. 1C). These defects in Sestrin gene expression may contribute to the increased ROS levels detected in p53S18A fibroblasts (Fig. 1A). Similarly, Sestrin gene expression was significantly reduced in the livers of p53S18A mice compared to that in wild-type mice (Fig. 1D). In contrast, Tigar expression was not significantly reduced in the livers of p53S18A mice (Fig. 1D). This further supports that defects in homeostasis are not due to mitochondrial respiration abnormalities. The decrease in Sestrin expression in the adipose tissue and muscle of p53S18A mice was not significant (Fig. 1E). We examined Atm−/+ mice that were insulin resistant (data not shown) and observed a significant decrease in Sestrin 2 and Sestrin 3 expression (Fig. 1F), suggesting that this activation of p53 is mediated by ATM phosphorylation in the liver.
Increased metabolic stress in p53 Ser18 phosphorylation-deficient animals.
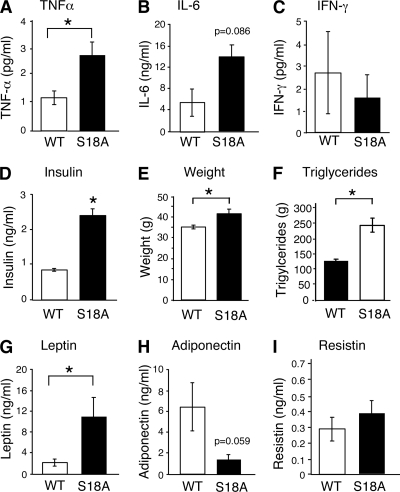
The mutation of ATM leads to increased inflammation, including the expression of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and C-reactive peptide (47). These cytokines are implicated in insulin resistance (6). We examined cytokine expression in young wild-type and p53S18A animals and observed no difference (data not shown). We also examined cytokine expression in 6-month-old p53S18A animals. We found a significant increase in the circulating levels of TNF-α in p53S18A mice compared with levels in wild-type mice (Fig. 2A). Although not significant, IL-6 levels in the blood were also elevated (Fig. 2B), and gamma interferon (IFN-γ) levels were reduced (Fig. 2C).
FIG. 2.
Analysis of cytokines and metabolic parameters. Wild-type (WT) and p53S18A (S18A) mice were maintained on a standard chow diet. Experiments were performed on 6- to 7-month-old animals. (A to C) WT and p53S18A mice were fasted overnight, and the concentrations of TNF-α, IL-6, and IFN-γ in blood were measured (means ± SEM; n = 6 animals). (D) Insulin measurement in mice fasted overnight (means ± SEM; n = 9). (E) Body weight was measured at 6 months (means ± SEM; n = 15). (F) Concentration of triglyceride in the blood of WT and p53S18A mice was measured (means ± standard deviation [SD]; n = 3). (G to I) The concentrations of the adipokines leptin, adiponectin, and resistin in the blood of mice fasted overnight were measured (means ± SEM; n = 7). Statistically significant differences between WT and p53S18A mice are indicated (*, P < 0.05).
The finding that p53S18A mice have reduced antioxidant gene expression (e.g., Sestrins) and increased expression of inflammatory cytokines (e.g., TNF-α) suggests that these mice may exhibit metabolic stress. To test this hypothesis, we compared various metabolic parameters in wild-type and p53S18A mice. Insulin levels were significantly increased in p53S18A animals (Fig. 2D). There was a modest but significant (P < 0.05) increase in body weight in the 24-week-old p53S18A mice compared to that in wild-type mice (Fig. 2E), with the difference attributed mainly to an increased weight of fat pads (data not shown). The fasting blood triglyceride was increased in p53S18A mice compared with that in wild-type mice (Fig. 2F). Studies of adipokines demonstrated a significant increase in blood leptin levels (Fig. 2G). These data are consistent with the observation that the levels of circulating leptin are proportional to the total amount of body fat, as leptin is primarily made in adipocytes (13). There was also a decrease in adiponectin levels; however, it was of borderline significance (P = 0.059) (Fig. 2H). Resistin levels were not different (Fig. 2I). Furthermore, the pancreatic islets of p53S18A mice exhibited hypertrophy compared with those of wild-type mice (unpublished observation).
Defective glucose homeostasis and insulin resistance in p53S18A mice.
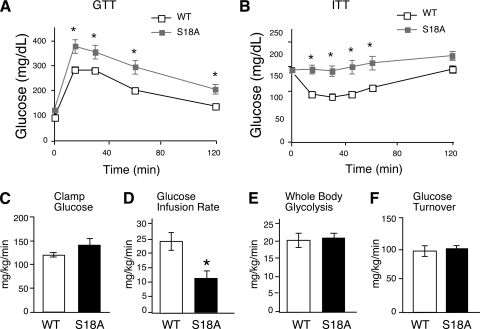
The observation of decreased antioxidant defense, increased markers of inflammation and metabolic stress, and deregulation of genes important for metabolic control suggested that p53 Ser18 could be a good candidate for mediating glucose homeostasis mediated by ATM. We analyzed glucose metabolism of p53S18A animals by performing a glucose tolerance test and insulin tolerance test on tumor-free animals (Fig. 3). Wild-type and p53S18A mice were maintained on a chow diet. Baseline glucose tolerance and insulin tolerance tests were performed at 3 months, and no significant difference was observed (data not shown). However, we hypothesized that the effects of antioxidant loss could exhibit a time-dependent effect. Thus, we examined glucose homeostasis in animals at 6 months of age. p53S18A mice exhibited glucose intolerance (Fig. 3A) and insulin resistance (Fig. 3B) at 6 months of age. Together, these data indicate that adult p53S18A mice are insulin resistant. To test this hypothesis, we performed a hyperinsulinemic-euglycemic clamp study to examine insulin sensitivity in conscious mice.
FIG. 3.
Insulin resistance in p53S18A mice. Wild-type (WT) and p53S18A (S18A) mice were maintained on a standard chow diet. Experiments were performed on 6- to 7-month-old animals. (A) Glucose tolerance test (GTT). Mice fasted overnight were treated with glucose (1 g/kg) by intraperitoneal injection. Blood glucose concentration was measured at the indicated times (means ± SEM; n = 15 to 20). (B) Insulin tolerance test (ITT). Mice fed ad libitum were treated with insulin (0.75 U/kg) by intraperitoneal injection. Blood glucose levels were measured at the indicated times (means ± SEM; n = 15 to 20). (C to F) Hyperinsulinemia-euglycemic clamp analysis (means ± SEM; n = 7 or 8). (C) Blood glucose concentration during the hyperinsulinemia-euglycemic clamp analysis; (D) steady-state glucose infusion rates to maintain euglycemia during the clamp; (E) whole-body glycolysis; (F) insulin-stimulated whole-body glucose turnover. Statistically significant differences are indicated (*, P < 0.05).
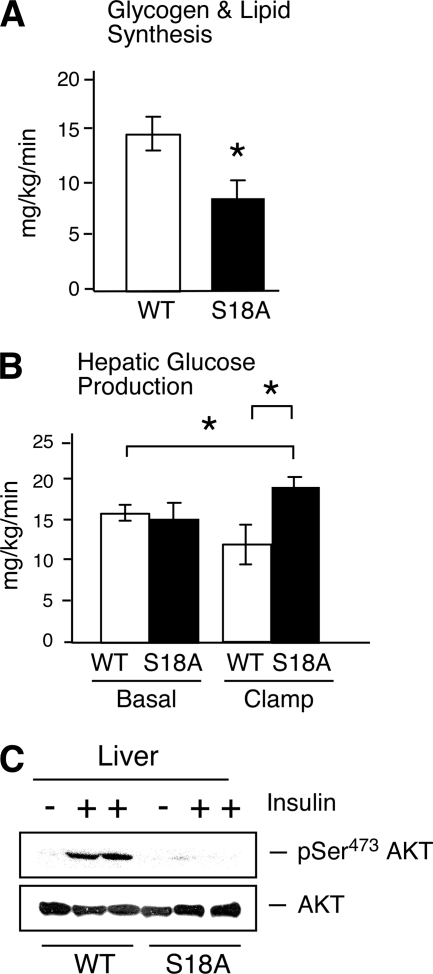
During the euglycemic clamp study, plasma glucose levels were similarly maintained at ∼130 mg/dl in both groups of mice (Fig. 3C). The steady-state glucose infusion rate during the clamp study was significantly reduced in p53S18A mice compared to that in wild-type mice (Fig. 3D). Insulin-stimulated whole-body glucose turnover and glycolysis were not altered in p53S18A mice (Fig. 3E and F). In contrast, insulin-stimulated whole-body glycogen synthesis was reduced in p53S18A mice compared to that in wild-type mice (Fig. 4A). Levels of basal hepatic glucose production (HGP) by wild-type and p53S18A mice were similar (Fig. 4B). However, insulin failed to suppress HGP during the clamp study in p53S18A mice, indicating severe hepatic insulin resistance in these mice (Fig. 4B). To obtain biochemical evidence for selective insulin resistance in p53S18A mice, we examined insulin-stimulated activation of Akt in liver (Fig. 4C). We found that insulin caused Akt activation in the muscles of both wild-type and p53S18A mice. In contrast, insulin caused Akt activation in the livers of wild-type mice but not in p53S18A mice. Importantly, the animals used in these experiments were determined to be tumor free. Together, these data demonstrate that p53S18A mice exhibit hepatic insulin resistance.
FIG. 4.
p53S18A mice exhibit hepatic insulin resistance. (A and B) Wild-type (WT) and p53S18A (S18A) mice were maintained on a standard chow diet. The data presented are the means ± SEM (n = 7 or 8). Statistically significant differences are indicated (*, P < 0.05). (A) Whole-body glycogen synthesis; (B) basal hepatic glucose production (HGP) (left) and insulin-stimulated HGP during the hyperinsulinemia-euglycemic clamp analysis (right). (C) Extracts prepared from the liver of WT and p53S18A mice were examined by immunoblot analysis using antibodies to Akt and pSer473 Akt. The mice were fasted overnight and treated without and with insulin (1.5 U/kg body mass) by intraperitoneal injection (30 min).
The increased production of ROS and loss of antioxidant gene expression suggested that reactive oxygen species could be mediating the metabolic effects observed in the p53S18A animals. In order to first examine this, we tested whether sestrin expression could reduce the levels of ROS observed in p53S18A MEFs (Fig. 1A). We generated p53S18A MEFs expressing sestrin 2 and determined that increased levels of sestrin 2 decreased the levels of ROS in p53S18A MEFs (Fig. 5A). We observed decreased Akt phosphorylation in p53S18A MEFs at passage 4 (Fig. 5B). However, the examination of the phosphorylation of Thr172 of AMPKα showed no difference (data not shown). The p53S18A cells exhibit morphological characteristics of senescence and exhibit greatly reduced viability at increasing passages (1). The treatment of the cells with the antioxidant NAC restored insulin signaling and improved the morphology of the cells (Fig. 5C). We next tested whether increased ROS levels are affecting the glucose intolerance observed in the p53S18A animals. We placed wild-type and p53S18A animals on a diet supplemented with 1.5% BHA and compared results to those for animals whose diet did not contain BHA. BHA treatment of the wild-type animals had no effect on glucose homeostasis (Fig. 5D). However, dietary supplementation with BHA significantly reduced the insulin sensitivity in p53S18A animals (Fig. 5E) to a level where the GTT results exhibited no difference compared to results for wild-type animals. These data indicate that deregulated ROS contributes to the imbalance in glucose homeostasis in p53S18A animals.
FIG. 5.
Role of increased ROS levels in metabolic defects in p53S18A mice. (A) Reduction of ROS levels by the expression of sestrin 2. Left, the amount of reactive oxygen species (ROS) in p53S18A MEFs was examined by DCF staining and analysis by flow cytometry and compared to that in wild-type cells. Data are presented as relative fluorescence (means ± SEM; n = 5). Right, Western analysis of the cells used in the experiment shown in the left panel, indicating expression of sestrin 2. (B and C) Insulin signaling is improved with NAC treatment. Passage 4 cells that were untreated (B) or treated with 0.5 mM NAC (C) were starved for 18 h and treated with 10 nM insulin. Akt and pSer473 Akt were examined by immunoblot. (D and E) Wild-type (WT) and p53S18A (S18A) mice were maintained on a standard chow diet or a diet including 1.5% BHA for approximately 4 weeks (at 6 months of age). Glucose tolerance test (GTT) on wild-type mice (D) and p53S18A mice (E). Mice fasted overnight were treated with glucose (1 g/kg) by intraperitoneal injection. The blood glucose concentration was measured at the indicated times (means ± SEM; n = 15 to 20). Statistically significant differences are indicated (*, P < 0.05).
DISCUSSION
ATM is activated by a number of stresses that have a direct impact on metabolism. A number of these cellular stresses, including atherosclerosis, trauma, hypoxia, and infections, are associated with p53 activation and p53 Ser18 phosphorylation (12, 30, 32, 39, 44, 50, 51). A-T patients exhibit an increased risk of insulin resistance and type 2 diabetes (3). In addition, loss of Atm has been implicated in metabolic disease (42). Atm mutant animals have been shown to be glucose intolerant and insulin resistant. Our analysis demonstrates that the ATM phosphorylation site on murine p53 (Ser18) exerts a protective role in maintaining glucose homeostasis. p53 Ser18-deficient animals exhibited glucose intolerance and insulin resistance. Although multiple factors can contribute to the development of insulin resistance, including oxidative stress, inflammation, and obesity (6), oxidative stress has been proposed to be the major contributor to pathology in A-T patients (4, 15, 20). One important substrate for ATM is the p53 tumor suppressor. We observed that cells from p53 Ser18-defective mice (p53S18A mice) exhibited increased levels of reactive oxygen species (ROS). In addition, we determined that the p53 Ser18 phosphorylation site is required for p53-dependent antioxidant gene expression (e.g., Sestrin) in MEFs and in the liver (Fig. 1). Although we did not observe statistically significant changes in other mediators of mitochondrial respiration, further examination needs to be made to test whether the changes we observed in TIGAR expression have an effect on homeostasis.
Another clinical observation in A-T patients and Atm mutant mice is increased metabolic stress, such as increased proinflammatory cytokine expression. We observed significantly increased plasma levels of the proinflammatory cytokine TNF-α in p53S18A animals. Analysis of other metabolic parameters indicated deregulated metabolism in these animals. For instance, the p53S18A animals had increased body weight and elevated basal serum insulin levels compared to those in wild-type animals. We propose that these effects are indirect effects of the changes to the expression of the metabolic genes regulated by p53 Ser18. The role of sestrins in oxidative stress provides data that they may have a substantial role in the defects observed in the p53S18A animals. Furthermore, we observed insulin resistance in older animals, suggesting that the pathologies that lead to defects in glucose homeostasis accumulate with time. The role of oxidative stress in aging is well established (2, 17). Importantly, hydrogen peroxide, whose levels are regulated indirectly by the sestrin gene family, is also implicated in aging (16). Our data are in agreement with the model that accumulated oxidative damage affects glucose homeostasis.
Through clamp analysis, we have identified the liver as the primary organ of insulin resistance. We also demonstrated reduced Akt activation in the liver, following insulin stimulation. Interestingly, sestrin expression was also significantly reduced in the liver. This suggests that a loss of sestrin expression leads to increased oxidative stress in the liver, which disrupts insulin sensitivity. In support of this model, treatment of p53S18A mice leads to restored glucose tolerance, whereas wild-type mice remain unchanged. This demonstrates a specific improvement in the p53S18A mice due to decreased levels of ROS in vivo. In addition, studies in MEFs indicate that NAC treatment restores insulin sensitivity. This has been observed in Atm-deficient MEFs as well (18). AMPKα has been shown to phosphorylate p53 Ser18 in vitro (19); however, a role in AMPK-mediated response to glucose is not clear (26). In addition, AMPKα-deficient mice develop muscle insulin resistance (45), which is not observed in p53S18A mice. Thus, although there may be additional kinases that regulate p53 Ser18 in vivo, there is a clear connection with Atm deficiency, increased oxidative stress, and insulin resistance. Together, these data indicate that the ATM-p53 pathway causes tissue-specific changes in gene expression that function collectively to maintain insulin sensitivity and glucose homeostasis. Thus, mice with a targeted mutation in the ATM phosphorylation site on p53 (Ser18) exhibit insulin resistance. Moreover, disruption of this pathway in A-T patients may contribute to deregulation of metabolism. These data establish that the modulation of the ATM-p53 pathway is an important mechanism that contributes to normal glucose homeostasis.
Acknowledgments
We thank Roger Davis for critical reading of the manuscript; Guadalupe Sabio, Anja Jaeschke, and other members of the Davis laboratory for helpful discussions and invaluable technical assistance; David Garlick for histology analysis; Ceren Acer, Chrisitine Delaney, and Charissa Cottonham for assistance with real-time PCR analysis; Punita Shroff for help with hydrogen peroxide experiments; and Andrei Budanov for kindly providing plasmids.
These studies were supported by a Pilot & Feasibility grant from the UMass Diabetes Endocrinology Research Center (NIH P30 DK32520 to H.K.S.), Worcester Foundation for Biomedical Research (to H.S.K.), and by grants from the NIH (DK80756 to J.K.K.) and the American Diabetes Association (7-07-RA-80 to J.K.K.). Core resources supported by the Diabetes Endocrinology Research Center grant (DK32520) were also used. Hayla K. Sluss and Jason K. Kim are members of the UMass DERC (DK32520). Part of this study was performed at the Penn State Diabetes & Obesity Mouse Phenotyping Center (supported by the Pennsylvania Department of Health and Tobacco Settlement Funds).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Armata, H. L., D. S. Garlick, and H. K. Sluss. 2007. The ataxia telangiectasia-mutated target site Ser18 is required for p53-mediated tumor suppression. Cancer Res. 67:11696-11703. [DOI] [PubMed] [Google Scholar]
- 2.Balaban, R. S., S. Nemoto, and T. Finkel. 2005. Mitochondria, oxidants, and aging. Cell 120:483-495. [DOI] [PubMed] [Google Scholar]
- 3.Bar, R. S., W. R. Levis, M. M. Rechler, L. C. Harrison, C. Siebert, J. Podskalny, J. Roth, and M. Muggeo. 1978. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N. Engl. J. Med. 298:1164-1171. [DOI] [PubMed] [Google Scholar]
- 4.Barlow, C., P. A. Dennery, M. K. Shigenaga, M. A. Smith, J. D. Morrow, L. J. Roberts III, A. Wynshaw-Boris, and R. L. Levine. 1999. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc. Natl. Acad. Sci. U. S. A. 96:9915-9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow, C., S. Hirotsune, R. Paylor, M. Liyanage, M. Eckhaus, F. Collins, Y. Shiloh, J. N. Crawley, T. Ried, D. Tagle, and A. Wynshaw-Boris. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. [DOI] [PubMed] [Google Scholar]
- 6.Bastard, J. P., M. Maachi, C. Lagathu, M. J. Kim, M. Caron, H. Vidal, J. Capeau, and B. Feve. 2006. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 17:4-12. [PubMed] [Google Scholar]
- 7.Bensaad, K., and K. H. Vousden. 2007. p53: new roles in metabolism. Trends Cell Biol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 8.Bensaad, K., and K. H. Vousden. 2005. Savior and slayer: the two faces of p53. Nat. Med. 11:1278-1279. [DOI] [PubMed] [Google Scholar]
- 9.Budanov, A. V., A. A. Sablina, E. Feinstein, E. V. Koonin, and P. M. Chumakov. 2004. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304:596-600. [DOI] [PubMed] [Google Scholar]
- 10.Chao, C., S. Saito, C. W. Anderson, E. Appella, and Y. Xu. 2000. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc. Natl. Acad. Sci. U. S. A. 97:11936-11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 12.Eizenberg, O., A. Faber-Elman, E. Gottlieb, M. Oren, V. Rotter, and M. Schwartz. 1995. Direct involvement of p53 in programmed cell death of oligodendrocytes. EMBO J. 14:1136-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederich, R. C., A. Hamann, S. Anderson, B. Lollmann, B. B. Lowell, and J. S. Flier. 1995. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1:1311-1314. [DOI] [PubMed] [Google Scholar]
- 14.Fridlyand, L. E., and L. H. Philipson. 2006. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes. Metab. 8:136-145. [DOI] [PubMed] [Google Scholar]
- 15.Gatei, M., D. Shkedy, K. K. Khanna, T. Uziel, Y. Shiloh, T. K. Pandita, M. F. Lavin, and G. Rotman. 2001. Ataxia-telangiectasia: chronic activation of damage-responsive functions is reduced by alpha-lipoic acid. Oncogene 20:289-294. [DOI] [PubMed] [Google Scholar]
- 16.Giorgio, M., M. Trinei, E. Migliaccio, and P. G. Pelicci. 2007. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 8:722-728. [DOI] [PubMed] [Google Scholar]
- 17.Golden, T. R., D. A. Hinerfeld, and S. Melov. 2002. Oxidative stress and aging: beyond correlation. Aging Cell 1:117-123. [DOI] [PubMed] [Google Scholar]
- 18.Ito, K., K. Takubo, F. Arai, H. Satoh, S. Matsuoka, M. Ohmura, K. Naka, M. Azuma, K. Miyamoto, K. Hosokawa, Y. Ikeda, T. W. Mak, T. Suda, and A. Hirao. 2007. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J. Immunol. 178:103-110. [DOI] [PubMed] [Google Scholar]
- 19.Jones, R. G., D. R. Plas, S. Kubek, M. Buzzai, J. Mu, Y. Xu, M. J. Birnbaum, and C. B. Thompson. 2005. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18:283-293. [DOI] [PubMed] [Google Scholar]
- 20.Kamsler, A., D. Daily, A. Hochman, N. Stern, Y. Shiloh, G. Rotman, and A. Barzilai. 2001. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 61:1849-1854. [PubMed] [Google Scholar]
- 21.Kastan, M. B., and D. S. Lim. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 22.Kawagishi, H., T. Wakoh, H. Uno, M. Maruyama, A. Moriya, S. Morikawa, H. Okano, C. J. Sherr, M. Takagi, and M. Sugimoto. 2008. Hzf regulates adipogenesis through translational control of C/EBPalpha. EMBO J. 27:1481-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, H. J., T. Higashimori, S. Y. Park, H. Choi, J. Dong, Y. J. Kim, H. L. Noh, Y. R. Cho, G. Cline, Y. B. Kim, and J. K. Kim. 2004. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53:1060-1067. [DOI] [PubMed] [Google Scholar]
- 24.Kondoh, H., M. E. Lleonart, J. Gil, J. Wang, P. Degan, G. Peters, D. Martinez, A. Carnero, and D. Beach. 2005. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65:177-185. [PubMed] [Google Scholar]
- 25.Lavin, M. F. 2008. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signaling and cancer. Nat. Rev. Mol. Cell Biol. 9:759-769. [DOI] [PubMed] [Google Scholar]
- 26.Lee, C. H., K. Inoki, M. Karbowniczek, E. Petroulakis, N. Sonenberg, E. P. Henske, and K. L. Guan. 2007. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 26:4812-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, B., Y. Chen, and D. K. St. Clair. 2008. ROS and p53: a versatile partnership. Free Radic. Biol. Med. 44:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matheu, A., A. Maraver, P. Klatt, I. Flores, I. Garcia-Cao, C. Borras, J. M. Flores, J. Vina, M. A. Blasco, and M. Serrano. 2007. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448:375-379. [DOI] [PubMed] [Google Scholar]
- 29.Matoba, S., J. G. Kang, W. D. Patino, A. Wragg, M. Boehm, O. Gavrilova, P. J. Hurley, F. Bunz, and P. M. Hwang. 2006. p53 regulates mitochondrial respiration. Science 312:1650-1653. [DOI] [PubMed] [Google Scholar]
- 30.Mercer, J., N. Figg, V. Stoneman, D. Braganza, and M. R. Bennett. 2005. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ. Res. 96:667-674. [DOI] [PubMed] [Google Scholar]
- 31.Minamino, T., M. Orimo, I. Shimizu, T. Kunieda, M. Yokoyama, T. Ito, A. Nojima, A. Nabetani, Y. Oike, H. Matsubara, F. Ishikawa, and I. Komuro. 2009. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 15:1082-1087. [DOI] [PubMed] [Google Scholar]
- 32.Moon, C., S. Kim, M. Wie, H. Kim, J. Cheong, J. Park, Y. Jee, N. Tanuma, Y. Matsumoto, and T. Shin. 2000. Increased expression of p53 and Bax in the spinal cords of rats with experimental autoimmune encephalomyelitis. Neurosci. Lett. 289:41-44. [DOI] [PubMed] [Google Scholar]
- 33.Mora, A., K. Sakamoto, E. J. McManus, and D. R. Alessi. 2005. Role of the PDK1-PKB-GSK3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 579:3632-3638. [DOI] [PubMed] [Google Scholar]
- 34.Newsholme, P., E. P. Haber, S. M. Hirabara, E. L. Rebelato, J. Procopio, D. Morgan, H. C. Oliveira-Emilio, A. R. Carpinelli, and R. Curi. 2007. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 583:9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pani, G., O. R. Koch, and T. Galeotti. 2009. The p53-p66shc-manganese superoxide dismutase (MnSOD) network: a mitochondrial intrigue to generate reactive oxygen species. Int. J. Biochem. Cell Biol. 41:1002-1005. [DOI] [PubMed] [Google Scholar]
- 36.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 37.Reichenbach, J., R. Schubert, D. Schindler, K. Muller, H. Bohles, and S. Zielen. 2002. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxid. Redox Signal. 4:465-469. [DOI] [PubMed] [Google Scholar]
- 38.Sablina, A. A., A. V. Budanov, G. V. Ilyinskaya, L. S. Agapova, J. E. Kravchenko, and P. M. Chumakov. 2005. The antioxidant function of the p53 tumor suppressor. Nat. Med. 11:1306-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakhi, S., A. Bruce, N. Sun, G. Tocco, M. Baudry, and S. S. Schreiber. 1994. p53 induction is associated with neuronal damage in the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 91:7525-7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage, D. B., K. F. Petersen, and G. I. Shulman. 2005. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension 45:828-833. [DOI] [PubMed] [Google Scholar]
- 41.Schwartzenberg-Bar-Yoseph, F., M. Armoni, and E. Karnieli. 2004. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 64:2627-2633. [DOI] [PubMed] [Google Scholar]
- 42.Shoelson, S. E. 2006. Banking on ATM as a new target in metabolic syndrome. Cell Metab. 4:337-338. [DOI] [PubMed] [Google Scholar]
- 43.Sluss, H. K., H. Armata, J. Gallant, and S. N. Jones. 2004. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol. Cell. Biol. 24:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Vlijmen, B. J., G. Gerritsen, A. L. Franken, L. S. Boesten, M. M. Kockx, M. J. Gijbels, M. P. Vierboom, M. van Eck, B. van De Water, T. J. van Berkel, and L. M. Havekes. 2001. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ. Res. 88:780-786. [DOI] [PubMed] [Google Scholar]
- 45.Viollet, B., F. Andreelli, S. B. Jorgensen, C. Perrin, D. Flamez, J. Mu, J. F. Wojtaszewski, F. C. Schuit, M. Birnbaum, E. Richter, R. Burcelin, and S. Vaulont. 2003. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem. Soc. Trans. 31:216-219. [DOI] [PubMed] [Google Scholar]
- 46.Vousden, K. H., and D. P. Lane. 2007. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8:275-283. [DOI] [PubMed] [Google Scholar]
- 47.Watters, D. J. 2003. Oxidative stress in ataxia telangiectasia. Redox Rep. 8:23-29. [DOI] [PubMed] [Google Scholar]
- 48.Werner, H., E. Karnieli, F. J. Rauscher, and D. LeRoith. 1996. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. U. S. A. 93:8318-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu, Y., T. Ashley, E. E. Brainerd, R. T. Bronson, M. S. Meyn, and D. Baltimore. 1996. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10:2411-2422. [DOI] [PubMed] [Google Scholar]
- 50.Yamanishi, Y., D. L. Boyle, M. J. Pinkoski, A. Mahboubi, T. Lin, Z. Han, N. J. Zvaifler, D. R. Green, and G. S. Firestein. 2002. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am. J. Pathol. 160:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung, S. J., J. Pan, and M. H. Lee. 2008. Roles of p53, MYC and HIF-1 in regulating glycolysis—the seventh hallmark of cancer. Cell. Mol. Life Sci. 65:3981-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng, S. J., S. E. Lamhamedi-Cherradi, P. Wang, L. Xu, and Y. H. Chen. 2005. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes 54:1423-1428. [DOI] [PubMed] [Google Scholar]