Abstract
The RNA binding protein CPEB (cytoplasmic polyadenylation element binding) regulates cytoplasmic polyadenylation and translation in germ cells and the brain. In neurons, CPEB is detected at postsynaptic sites, as well as in the cell body. The related CPEB3 protein also regulates translation in neurons, albeit probably not through polyadenylation; it, as well as CPEB4, is present in dendrites and the cell body. Here, we show that treatment of neurons with ionotropic glutamate receptor agonists causes CPEB4 to accumulate in the nucleus. All CPEB proteins are nucleus-cytoplasm shuttling proteins that are retained in the nucleus in response to calcium-mediated signaling and alpha-calcium/calmodulin-dependent kinase protein II (CaMKII) activity. CPEB2, -3, and -4 have conserved nuclear export signals that are not present in CPEB. CPEB4 is necessary for cell survival and becomes nuclear in response to focal ischemia in vivo and when cultured neurons are deprived of oxygen and glucose. Further analysis indicates that nuclear accumulation of CPEB4 is controlled by the depletion of calcium from the ER, specifically, through the inositol-1,4,5-triphosphate (IP3) receptor, indicating a communication between these organelles in redistributing proteins between subcellular compartments.
The cytoplasmic polyadenylation element binding (CPEB) protein, a sequence-specific RNA binding protein, is found in the cell body and at synapses of hippocampal and other neurons; in response to synaptic experience, CPEB promotes polyadenylation and translation (37, 49, 50). CPEB knockout mice (44) have deficiencies in synaptic plasticity, particularly, theta burst-induced long-term potentiation (LTP) (1, 51), as well as in particular forms of hippocampal-dependent memories (3). Some of these CPEB-related functions may be related at least in part to its ability to direct CPE-containing RNA transport in dendrites (16), in addition to its regulation of translation (51).
In neurons, most of the CPEB-related proteins CPEB3 and CPEB4 are found in the cell soma. However, a relatively small amount of these proteins is localized to synaptic regions and cofractionates with postsynaptic density (PSD) proteins, suggesting possible roles in RNA translation and/or localization (17). While investigating the possible translocation of CPEB3 and -4 to dendritic spines in response to N-methyl-d-aspartate receptor (NMDAR) activation, as is the case with the fragile X mental retardation protein (FMRP) (11), we noticed a surprising relocalization of CPEB4 from the cell soma to the nucleus. While the treatment of neurons with ionotropic glutamate receptor-activating agents, such as glutamate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), as well as NMDA, induced nuclear localization of CPEB4, 3,5-dihydroxyphenylglycine (DHPG), an agonist of the metabotropic glutamate receptors, did not. CPEB4 remained cytoplasmic when calcium/calmodulin-dependent protein kinase II (CaMKII) activity was inhibited, suggesting a link between calcium levels and nuclear import and/or retention. When fused to epitope tags and expressed in neurons, CPEB, CPEB3, and CPEB4 also became concentrated in the nucleus in response to NMDAR activation. All three proteins, however, were also nuclear when neurons were treated with leptomycin B (LMB), an agent that inactivates the nuclear export factor Crm1, indicating that CPEB family proteins are continuously shuttling between nucleus and cytoplasm. Moreover, these data suggest that NMDAR activation does not necessarily induce nuclear translocation but, rather, induces nuclear retention of the CPEB proteins.
One physiological event that causes widespread glutamate overload is ischemia, which occurs when the brain's supply of glucose and oxygen is disrupted by stroke or cardiac arrest (35). ATP production is reduced when the blood supply is insufficient, causing neuron polarization and the accumulation of glutamate in the extracellular space due to reversed uptake (40). This excess glutamate is responsible for neuron death in transient ischemia (5), and its stimulation of massive calcium influx through the NMDA receptors plays a major role in excitotoxicity (45, 46). A mouse model for transient focal ischemia caused nuclear accumulation of CPEB4, as did an in vitro model for oxygen and glucose deprivation. Lentivirus-mediated small hairpin RNA (shRNA) knockdown of CPEB4 demonstrates that this protein is essential for neuron survival and might suggest that its nuclear accumulation is a physiological response to high levels of intracellular calcium. However, additional experiments with cultured neurons show that the depletion of calcium from the endoplasmic reticulum (ER) rather than elevated levels of cytosolic calcium per se is responsible for CPEB4 nuclear accumulation. We have also demonstrated the involvement of IP3 receptors in this ER calcium depletion-mediated nuclear accumulation of CPEB4. We propose that the dispatch of a signal that is dependent upon the expulsion of ER calcium signals CPEB4 to accumulate in the nucleus, where it may offer some protection against cell death.
MATERIALS AND METHODS
Hippocampal neuron culture.
The culture of primary rat hippocampal neurons was performed as described previously (2), with a typical plating density of 1.8 × 104 cells/cm2 cultured in Neurobasal medium (Invitrogen) containing B27 supplement (B27 medium) and glutamine (1 μg/ml). Cytosine arabinoside (Ara-C) (1 μM) was added at day 3 after plating in vitro (DIV3) to prevent glial cell proliferation.
Lentiviral vector construction and virus production.
Lentiviruses expressing CPEB3 and CPEB4 were constructed by inserting myc-CPEB3 and myc-CPEB4 into the BamHI and XhoI sites of pFugw vector (Addgene). For virus production, 10 μg of virus transfer vector that expressed various CPEBs, 7.5 μg of gag-pol-expressing vector psPAX2 (from Addgene), and 5 μg of vesicular stomatitis virus G (VSV-G)-expressing vector pMD2.G (from Addgene) were cotransfected into 1 × 107 293T cells plated in 10-cm culture dishes using Lipofectamine 2000 (Invitrogen). Three hours after transfection, the medium was replaced with Neurobasal medium containing B27 supplement (B27 medium). Forty-eight hours after transfection, the medium was collected and passed through a 0.45-mm filter; the virus titer in the filtrate was calculated by serial dilution to determine the minimum amount of virus that could infect 90% of neurons plated at 1.8 × 104 cells/cm as assayed by immunocytochemistry for the myc-tagged fusion proteins.
CPEB4 knockdown.
Lentiviruses expressing shRNAs against CPEB4 (pLL3.7-syn-KD2 and pLL3.7-syn-KD3) followed the procedure reported in reference 17. The primers for constructing pLL3.7-syn-KD2 were C4-KD2-F (TGGCTGCAGCATGGAGAGATAGATTTCAAGAGAATCTATCTCTCCATGCTGCAGCCTTTTTTC) and C4-KD2-R (TCGAGAAAAAAGGCTGCAGCATGGAGAGATAGATTCTCTTGAAATCTATCTCTCCATGCTGCAGCCA). The primers for constructing pLL3.7-syn-KD3 were C4-KD3-F (TGGCTGCCTCATTTGGCGAATAATTTCAAGAGAATTATTCGCCAAATGAGGCAGCCTTTTTTC) and C4-KD3-R (TCGAGAAAAAAGGCTGCCTCATTTGGCGAATAATTCTCTTGAAATTATTCGCCAAATGAGGCAGCCA). Lentivirus expressing shRNA was produced by transfecting 293T cells (1 × 106 cells/ml) with transfer vector together with packaging vectors pSPAX2 and pMD2.G using Lipofectamine 2000. Three hours after transfection, the cells were washed and then cultured for 48 h after transfection, when the culture medium was collected and filtered through a 0.2-mm syringe filter. The virus-containing medium was used directly without concentration; the titer was determined as the minimal amount needed to infect >90% of cultured neurons.
Image acquisition and processing.
A Nikon Eclipse E600 microscope was used to take fluorescence images; confocal images were acquired using a spinning-disk confocal microscope (CSU10B; Solamere Technology Group) controlled by Metamorph software. Most of the images were taken using a Plan Fluor objective lens with ×20 magnification and a numerical aperture (NA) of 0.5. The cooled charge-coupled device (CCD) RT (real time) color camera is made by Diagnostic Instruments, Inc. Images were taken and further processed using SPOT version 3.5.8 software. Where indicated, fluorescence intensity was quantified using Image J software (NIH).
Antibodies and immunohistochemistry.
CPEB4 antibody was produced as described previously (17); hemagglutinin (HA) (16B12) and myc (9E10) monoclonal antibodies were produced as ascites fluid (Covance); and C/EBP homology protein (CHOP) antibody was purchased from Santa Cruz Biotechnology. A terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay kit was purchased from MBL International. For CPEB4 immunostaining, cells were fixed in 2% paraformaldehyde-phosphate-buffered saline (PBS)-4% sucrose for 20 min and then blocked in 10% bovine serum albumin (BSA) for 20 min before overnight incubation with affinity-purified CPEB4 antibody at 4°C. Secondary antibody (Alexa 595-conjugated goat anti-rabbit and Alexa 488-conjugated goat anti-mouse antibodies) application and washing were performed as recommended by the manufacturer (Molecular Probes). For some experiments, neurons treated with tetrodotoxin (TTX) or TTX plus NMDA were collected and probed for CPEB4 and actin on Western blots.
MCAO and OGD.
Middle cerebral artery occlusion (MCAO) was performed as described previously (47), except that MCAO was extended to 90 min and reperfusion to 24 h before sacrifice. For oxygen and glucose deprivation (OGD), minimal essential medium (MEM) is purged with nitrogen for 20 min to remove oxygen from solution. The gas-purged medium was placed in an anaerobic chamber for 30 min to equilibrate pH. DIV14 hippocampal neurons were then cultured in oxygen-purged, glucose-deficient MEM in an anaerobic chamber with an air mixture of 0.5% oxygen, 10% carbon dioxide, and 89.5% nitrogen for 1 h. The cells were then moved to normal Neurobasal medium with B27 and cultured under standard conditions for various times before fixation.
In vitro nuclear import assay.
HeLa cells grown on Lab-Tek chamber slides were permeabilized with digitonin (40 mg/ml) in TB buffer [20 mM HEPES, pH 7.4, 110 mM potassium acetate (KOAc), 2 mM Mg(OAc)2, 2 mM dithiothreitol, 1 mM EGTA, and protease inhibitor] for 5 min on ice. The cells were then washed twice with TB buffer plus BSA (10 mg/ml). After the second wash, the import reaction mixture was added (2 μl 100 mg/ml BSA, 8 ml HeLa cytosol with ATP regeneration system, 2 ml glutathione S-transferase [GST]-CPEB4 RNA binding domain [RBD], and 8 ml TB buffer). The ATP regeneration system contained 1 mM ATP, 5 mM phosphocreatine, and 20 units/ml creatine phosphokinase. Permeabilized HeLa cells were incubated in nuclear import reaction mixture for 20 min at 25°C; the cells were then washed with cold TB buffer before fixation with 4% formaldehyde-PBS for 10 min. The fixed cells were stained with anti-GST antibody to detect nuclear import substrate and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI).
Pharmacological treatment of primary neuron culture.
Freshly prepared glutamate (100 μM), NMDA (100 μM), AMPA (300 μM), DHPG (100 μM), or ionomycin (5 μM) was applied to DIV16 hippocampal neurons for 1 h before fixation and immunostaining. In some experiments, APV (2-amino-5-phosphonovaleric acid, 20 μM), Ant-AIP-II (10 μM; Calbiochem), or EGTA (2 mM) was added to the cells 20 min before NMDA. BAPTA/AM [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester, 50 μM; Calbiochem] was added to cultures for 20 min and then replaced with culture medium for 40 min. Thapsigargin (TG, 2 mM stock; Calbiochem) and tunicamycin (TM, 5 mg/ml of 1,000× stock; Calbiochem) were suspended in dimethyl sulfoxide (DMSO) and added to cultures. 2-APB (2-aminoethoxydiphenyl borate, 20 μM) was added to neurons 45 min before NMDA.
Sucrose gradients.
Brain tissue from 2-month-old mice was washed once in 1× PBS, homogenized in 2 ml of 0.8 M sucrose, and centrifuged at 10,000 rpm for 10 min. An amount of 1.5 ml of the supernatant was subsequently removed and layered on 2.0 M sucrose in an SW41 centrifuge tube. Amounts of 2.25 ml of 1.3 M, 1.95 M, and 2.5 M sucrose were layered on top of the brain lysates, which was followed by centrifugation at 40,000 × g for 5 h. An 18-gauge needle was used to pierce the bottom of the tube, and 1-ml fractions were collected; 200 μl of each fraction was removed to a clean microcentrifuge, 2.5 volumes of 100% ethanol were added, and the tubes were stored overnight at −20°C. The precipitates were collected by centrifugation and with 70% ethanol. The pellets were suspended in 100 μl of 1× SDS sample buffer and analyzed by Western blotting for CPEB4 (antibody dilution of 1:1,000) or protein disulfide isomerase (PDI, antibody dilution of 1:750; Santa Cruz).
Immunoelectron microscopy.
CPEB4-enriched fractions were pooled and diluted 5-fold in homogenization buffer (20 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 50 mM KCl, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride). Pooled fractions were then centrifuged for 1 h at 36,000 rpm and the pellet washed once with 1× PBS. The pellets were then incubated with CPEB4 antibody (1:500) for 1 h at room temperature. The pellets were then washed twice with 1× PBS and blocked for 15 min with 5% BSA-PBS. Subsequently, the pellets were incubated with 10 nm goat anti-rabbit gold-conjugated antibody (1:25; Ted Pella, Inc.) for 1 h at room temperature. The pellets were then washed three times for 5 min each time with 1× PBS and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 8.0.
Measurement of intracellular Ca2+.
Neurons were plated at a density of 0.5 × 105 cells/well in a 96-well dish and maintained in Neurobasal medium containing B27. DIV16 hippocampal neurons were loaded with 1 μM Fluo-3/AM, a Ca2+-sensitive fluorescent probe (Invitrogen) containing 0.02% Pluronic F-127 (Molecular Probes) in Hanks' buffered salt solution for 30 min at 37°C. The plates were gently washed with buffer and incubated for another 30 min at 37°C to permit de-esterification of intracellular Fluo-3/AM. The fluorescence of Fluo-3 is a measure of the Ca2+ concentration and was determined using a POLARstar Optima fluorescence plate reader (BMG Labtech) equipped with an excitation filter set to 485 ± 10 nm and an emission filter set to 520 ± 10 nm. After dye loading, neurons were treated with various reagents (see Fig. 9 for a complete list), and the relative fluorescence intensity was determined.
Measurement of ER calcium.
ER calcium measurements were carried out using the ER-targeted cameleon construct D1ER (30). Primary hippocampal neurons (DIV12) were transfected with 1.6 mg of pD1ER using Lipofectamine 2000 and maintained in Neurobasal medium. Four days after transfection, the appropriate pharmacological agent (see Fig. 9) was added to the neurons and the fluorescence resonance energy transfer (FRET) from the donor fluorophore cyan fluorescent protein (CFP) (441/485 nm excitation/emission) to the acceptor fluorophore yellow fluorescent protein (YFP) (441/550 nm excitation/emission) was measured. The magnitude of the FRET signal is proportional to the amount of calcium in the ER. Cells were imaged on an Olympus IX 70 inverted light microscope. To calibrate the relative FRET signals, Rmin was obtained by treating the cells with 3 mM EGTA and 2 μM ionomycin and the FRET signal from untreated cells was set as Rmax. Fluorescence images were background corrected. The emission ratio (FRET/CFP) was quantified before and after treatment of the neurons, and the percent calcium change was calculated by setting Rmin as −100% change and Rmax as 0% change. See Fig. 9 for a schematic of the FRET assay.
Plasmid construction.
Plasmid construction is detailed in the supplemental material.
RESULTS
NMDA induces nuclear localization of CPEB4.
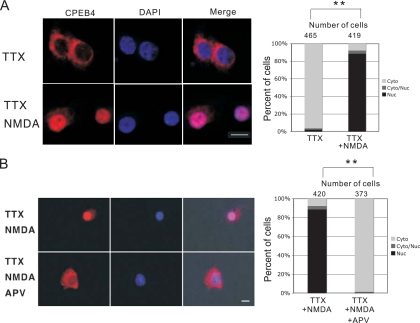
In cultured hippocampal neurons, CPEB4 is detected mainly in the cytoplasm and in dendrites; it is also enriched in postsynaptic density (PSD) fractions from adult rat brain and hippocampal neurons. To assess whether CPEB4 changes location in response to synaptic activity, neurons were treated with 0.1 mM NMDA for 40 min in the presence of tetrodotoxin (TTX). Compared to the results for a control, NMDA treatment unexpectedly caused strong and permanent nuclear CPEB4 immunostaining, which was prevented when APV, an NMDA antagonist, was added to the cells (Fig. 1A and B; also see Fig. S1 in the supplemental material for specificity of CPEB4 antibody). Although TTX was used here to silence spontaneous neural activity, we have observed similar nuclear localization of CPEB4 even in the absence of TTX (data not shown). The numbers of neurons in which CPEB4 was predominantly nuclear or cytoplasmic or was distributed in both compartments (see Fig. S2 in the supplemental material for representative images) as a function of activity show that NMDA caused a dramatic nuclear localization of CPEB4 that was nearly completely prevented by APV (Fig. 1A and B, right, comparison of TTX versus TTX-plus-NMDA treatment, P < 0.01, Student's t test). Also, upon treatment with lower concentrations of NMDA (0.01 mM and 0.001 mM), fewer neurons exhibited nuclear accumulation of CPEB4 (data not shown), indicating a dose-dependent response of CPEB4 localization to this neurotransmitter.
FIG. 1.
Stimulation of NMDA receptor causes CPEB4 nuclear localization. (A) DIV16 hippocampal neurons incubated in TTX for 24 h were treated with buffer alone (TTX) or NMDA (TTX NMDA) and then fixed and immunostained with affinity-purified CPEB4 antibody. DAPI shows nuclear DNA staining. The cells were examined by confocal microscopy. To quantify the localization of CPEB4 in the nucleus or cytoplasm, other cells were stained for CPEB4 where the protein was predominantly cytoplasmic (cyto), nuclear (nuc), or evenly distributed in cytoplasm and nucleus (cyto/nuc) (see Fig. S2 in the supplemental material for representative images). Using this protein distribution as a standard, the percentage of cells in which CPEB4 was in these compartments following TTX or NMDA treatment was determined (histogram). The asterisks refer to a statistically significant difference (P < 0.01, Student's t test) between the results for neuron samples treated with TTX versus those treated with TTX plus NMDA. (B) DIV16 hippocampal neurons incubated in TTX for 24 h were treated with APV (TTX NMDA APV) for 5 min prior to application of NMDA for 40 min and then fixed and immunostained with affinity-purified CPEB4 antibody. DAPI shows nuclear DNA staining. The cells were examined by fluorescence microscopy. The asterisks refer to statistical significance (P < 0.01, Student's t test). Size bar = 10 μm.
NMDA also caused a substantial loss of CPEB4 from dendrites. While NMDA did not significantly alter the overall amount of CPEB4 in neurons, it did lead to an increase in the fluorescence intensity of CPEB4 in the nucleus relative to that in the cell body without stimulation (see Fig. S3 in the supplemental material). These data suggest that dendritic (and cell body) CPEB4 was not destroyed in an activity-dependent manner but instead was probably transported retrogradely to the nucleus.
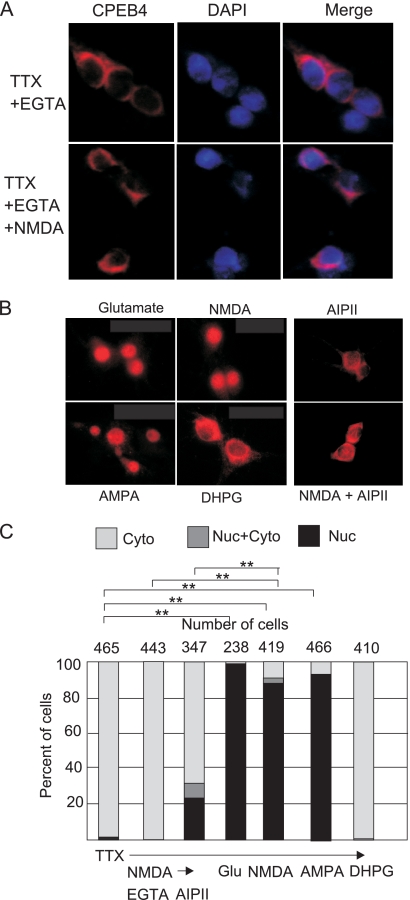
Ligand binding to ionotropic glutamate receptors causes calcium influx and induces downstream signaling events through CaMKII (18). To examine whether extracellular calcium is important for nuclear accumulation of CPEB4, EGTA was applied to neurons before NMDA; this caused CPEB4 to remain predominantly cytoplasmic (Fig. 2A). To assess whether NMDA/calcium-induced CPEB4 nuclear accumulation occurs via CaMKII, a membrane-permeable CaMKII inhibitory peptide, AIP-II (19, 48), was applied to neurons 20 min before NMDA treatment. This peptide reduced NMDA-induced CPEB4 nuclear translocation (Fig. 2B), indicating that extracellular calcium and CaMKII are part of an NMDA-induced signaling pathway that causes CPEB4 nuclear accumulation. In addition, treatment of neurons with glutamate, AMPA, or NMDA induced CPEB4 nuclear staining; DHPG, a metabotropic glutamate receptor agonist, had no effect, however (Fig. 2B), nor did acetylcholine (calcium opening neurotransmitter) or γ-aminobutyrate (GABA) (chloride channel neurotransmitter) (data not shown). These data, together with the determination of the number of cells that respond to these treatments (Fig. 2C), show that ionotropic glutamate receptor activation induces CPEB4 nuclear accumulation.
FIG. 2.
Effectors of CPEB4 nuclear localization. (A) DIV16 neurons were incubated with EGTA for 20 min prior to application of NMDA for 40 min; the cells were then fixed and stained for CPEB4. (B) TTX-treated DIV16 hippocampal neurons were treated with DHPG, AMPA, glutamate, or NMDA for 40 min before being immunostained with CPEB4 antibody. Other cells were treated with AIP-II for 20 min and then subjected to NMDA for 40 min before fixation. (C) Quantification of CPEB4 in the cytoplasm, nucleus, or both nucleus and cytoplasm was determined as described in the legend to Fig. 1. The asterisks refer to statistically significant differences (P < 0.01, Student's t test) between the results for the indicated samples.
CPEB family proteins are nucleus-cytoplasm shuttling proteins.
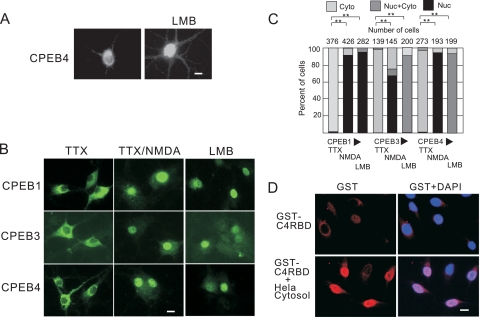
Nuclear accumulation of CPEB4 could be due to active cytoplasm-to-nucleus import or inhibited nuclear export if this protein continuously shuttles between the two compartments. To distinguish between these possibilities, neurons were treated with leptomycin B (LMB), an inhibitor of the nuclear export factor Crm1 (21, 29, 33). The application of LMB to cultured neurons resulted in nuclear accumulation of CPEB4, suggesting that CPEB4 is a nucleus-cytoplasm shuttling protein (Fig. 3A).
FIG. 3.
CPEB family proteins are nucleus-cytoplasm shuttling proteins. (A) DIV16 hippocampal neurons were treated with 0.1% ethanol alone or with 10 nM LMB for 40 min before fixation and then immunostained for endogenous CPEB4. (B) DIV16 neurons infected with lentivirus expressing HA-CPEB1, myc-CPEB3, or myc-CPEB4 for 2 days were treated with 10 nM LMB for 1 h and then immunostained with HA or myc antibodies. Other infected cells were stimulated with NMDA for 1 h prior to immunostaining. (C) Quantification of CPEB1, CPEB3, or CPEB4 in the nucleus or cytoplasm following the treatments described for panel B. The asterisks refer to statistically significant differences (P < 0.01, Student's t test) between the results for the indicated samples. C4RBD, CPEB4 RNA binding domain. (D) HeLa cells were treated with digitonin, and the permeabilized cells were incubated with an ATP-regenerating system, purified recombinant GST-CPEB4 RBD fusion protein, with or without HeLa cell cytosol. After 40 min, the cells were fixed and stained with GST antibody. Size bar = 10 μm.
To investigate whether other CPEB family proteins are nucleus-cytoplasm shuttling proteins and accumulate in the nucleus in response to NMDA, DIV14 hippocampal neurons were infected with lentiviruses expressing epitope-tagged CPEB1, CPEB3, and CPEB4, which were then subjected to either LMB or NMDA treatment. These proteins all accumulated in the nucleus when nuclear export was blocked by LMB, suggesting that they are all shuttling proteins (Fig. 3B). NMDA treatment also caused these proteins to accumulate in nuclei (Fig. 3B; quantification of responsive cells is presented in Fig. 3C), supporting the notion that nuclear accumulation is a common feature among CPEB proteins in neurons following synaptic stimulation. To determine whether nuclear accumulation of CPEB4 is neuron specific, we treated HeLa cells and 293T cells with LMB; in both cases, CPEB4 was retained in the nucleus (unpublished data). We also treated HeLa cells with digitonin, which causes cytoplasmic leakage through pores in the plasma membrane. When a fusion of the CPEB4 RNA binding domain (RBD, i.e., both RNA recognition motifs [RRMs] and both zinc fingers) to GST was added to the treated cells after they were washed to remove the leaked cytoplasm, there was no evidence of nuclear accumulation. However, when the permeabilized cells were supplemented with fresh HeLa cytosol, nuclear GST-CPEB4 was readily apparent (Fig. 3D). These data show that CPEB4 nuclear transport is not neuron specific and that the RBD contains the nuclear localization signal (NLS) of CPEB4.
Identification of CPEB4 nuclear import/export cis elements.
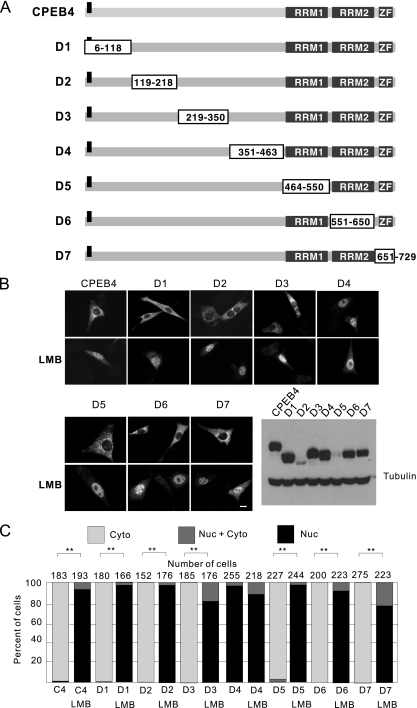
To identify the CPEB4 NLS and nuclear export signal (NES), plasmids encoding serial deletions of CPEB4 (mutants D1 to D7) were generated (Fig. 4A) and transfected into NIH 3T3 cells, which because of their flattened morphology, are particularly amenable for use in immunocytochemistry to distinguish between cytoplasmic and nuclear proteins. A CPEB4 truncation mutant that lacks the NLS would be expected to be constitutively cytoplasmic irrespective of LMB treatment; protein with an NES deletion should reside in the nucleus irrespective of LMB treatment.
FIG. 4.
Identification of the CPEB4 shuttling elements. (A) The CPEB4 internal deletion constructs are depicted. The boxes indicate the parts of the protein that were deleted, and the gray boxes indicate the known functional domains (RNA recognition motifs RRM1 and RRM2 and two zinc fingers [ZF]) of CPEB4. The bar in the N terminus is a myc epitope tag. (B) NIH 3T3 cells were transfected with plasmid DNA encoding the proteins shown in panel A; 12 h later, the cells were treated with LMB for 1 h prior to fixation. Antibody against the myc epitope was used for immunostaining. Exogenous proteins and α-tubulin were monitored by immunoblotting. Size bar = 10 μm. (C) Quantification of the CPEB4 proteins in the nucleus and cytoplasm as described in the legend to Fig. 1. The asterisks refer to statistically significant differences (P < 0.01, Student's t test) between the results for the indicated samples.
Although the entire CPEB4 protein was sequentially deleted (Fig. 4A), none of the mutants remained cytoplasmic when cells were treated with LMB. Thus, CPEB4 probably has two or more independently acting NLSs. However, CPEB4 truncation mutant D4, lacking residues 351 to 463, was nuclear in the absence or presence of LMB, indicating that this truncated region contains the NES (Fig. 4B; quantification of responsive cells is presented in Fig. 4C). The expression levels of the CPEB4 mutant proteins are shown on the lower right in Fig. 4B.
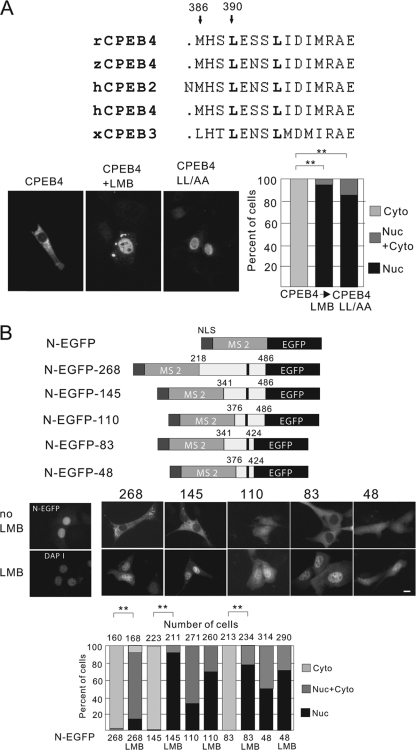
Using the Multalin program (6) that compares protein sequence similarity, a sequence from the peptide deleted in mutant D4 was aligned with corresponding regions from CPEB2, -3, and -4 from rat, human, Xenopus laevis, zebrafish, and Drosophila melanogaster (in this case, a single CPEB4-like protein called Orb2). As shown in Fig. 5A, CPEB4 residues 383 to 397 within the deleted peptide are highly conserved among all the protein sequences examined. Leucine residues are often found in NESs; mutation of two of them at positions 385 and 390 (arrows and denoted in boldface) to alanine (CPEB4 LL/AA) caused the accumulation of CPEB4 in the nucleus of transfected 3T3 cells irrespective of whether they were treated with LMB (Fig. 5A, bottom left; quantification of cells is at the bottom right). Thus, these leucine residues are essential for CPEB4 nuclear export. To assess sufficiency of nuclear export, various segments of CPEB4 containing the NES were fused to bacteriophage MS2 expressing enhanced green fluorescent protein (EGFP-MS2), which also contained a simian virus 40 (SV40) T NLS (Fig. 5B, N-EGFP). As expected, the control N-EGFP lacking a CPEB4 NES was nuclear in transfected 3T3 cells (Fig. 5B, middle, left). Upon fusion with the CPEB4 NES-containing fragment, three of the fusion proteins, N-EGFP-268, N-EGFP-145, and N-EGFP-83, became localized to the cytoplasm in the absence but to the nucleus in the presence of LMB. However, two proteins lacking CPEB4 residues 341 to 376 (N-EGFP-110 and N-EGFP-48) were evenly distributed in both the nuclear and cytoplasmic compartment, suggesting a lack of NES function (Fig. 5B, middle; quantification of cells is at the bottom). From these results, we conclude that CPEB4 residues 341 to 424 constitute a minimal NES.
FIG. 5.
Identification of CPEB4 nuclear export signal. (A) Alignment of the human CPEB4 protein sequence from residues 383 to 397 with homologous regions from rat and zebrafish CPEB4 and human and Xenopus CPEB2 and CPEB3. Two arrows point to conserved leucine residues that have been mutated to alanine in the LL-AA mutant. NIH 3T3 cells were transfected with DNA encoding myc-tagged wild-type or LL-AA mutant CPEB4 proteins for 12 h, treated with LMB for 1 h and then fixed and immunostained for the myc-tagged protein. Quantification of the CPEB4 proteins in the nucleus and cytoplasm are as described in the legend to Fig. 1. The asterisks refer to statistically significant differences (P < 0.01, Student's t test) between the results for the indicated samples. (B) Fusion proteins used to identify the minimal CPEB4 NES domain. Various regions of the CPEB4 coding region (the numbers refer to amino acid residues) that contain the NES were fused to the SV40 T NLS, the bacteriophage MS2 coat protein, and EGFP. The dark bar indicates the leucine residues indicated in panel A (top). Middle, NIH 3T3 cells were transfected with DNA encoding the fusion proteins noted above. The image in the left panel shows that the NLS-EGFP-MS2 protein, without any CPEB4 sequence, was nuclear. The other panels show the nuclear-cytoplasmic distribution of the fusion proteins in neurons, some of which were treated with LMB. Size bar = 10 μm. Bottom, quantification of the GFP-CPEB4 fusion proteins in the nucleus or cytoplasm as described in the legend to Fig. 1. The asterisks refer to statistically significant differences (P < 0.01, Student's t test) between the results for the indicated samples.
Brain ischemia causes nuclear accumulation of CPEB4.
Glutamate plays dual roles in the brain: at physiological levels, it induces excitatory synapse activation and plasticity, but under pathological conditions, such as ischemia and epilepsy, elevated levels of extracellular glutamate cause neuron degeneration through excitotoxicity. To determine whether CPEB4 nuclear accumulation is induced by glutamate release in vivo, brain sections from rats subjected to high-frequency stimulation (HFS) for 90 min were probed with CPEB4 antibody. While this HFS paradigm induced the expression of the immediate early gene that encodes activity-regulated cytoskeleton-association protein (Arc) in granular cells of the dentate gyrus (41), it did not cause CPEB4 nuclear accumulation (unpublished data).
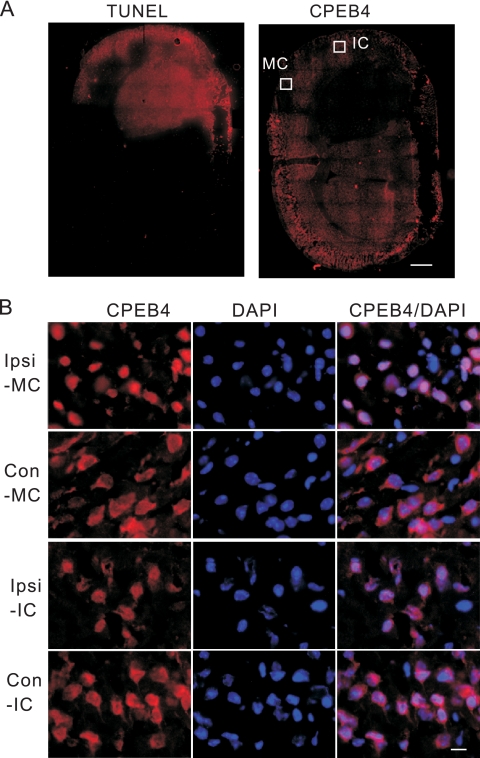
To investigate whether pathological levels of glutamate or the stress associated with it might cause CPEB4 nuclear accumulation, we turned to a mouse model for transient focal ischemia. In this paradigm, the middle cerebral artery is occluded (MCAO) for 90 min, which causes a focal deprivation of blood flow, followed by a reperfusion of blood for 24 h before sacrifice and histological preparation (47). A clear infarction was evident in the ipsilateral portion of the brain, which not only caused neuron death (as determined by TUNEL staining) but also dramatically reduced CPEB4 staining (Fig. 6A). However, in the motor cortex and insular cortex, which are in the penumbra of the severely affected area, CPEB4 staining was enriched in the nucleus. In contrast, CPEB4 staining was cytoplasmic in the corresponding contralateral regions (Fig. 6B).
FIG. 6.
Ischemia causes CPEB4 protein to become concentrated in the nucleus. (A) A frozen section of brain taken from a mouse that had a middle cerebral artery occlusion (MCAO) performed was fixed and stained for CPEB4. A consecutive section from the same animal was labeled by TUNEL staining. The white boxes refer to regions of the motor cortex (MC) and insular cortex (IC) that were examined under higher magnification, as shown in panel B. Size bar = 1 mm. (B) A section from the ischemic brain was immunostained with anti-CPEB4 antibody. DAPI staining shows nuclei. The images were taken from the motor cortex (MC) or insular cortex (IC); ipsilateral (Ipsi) and contralateral sides (Con) of these regions are shown. Size bar = 20 μm.
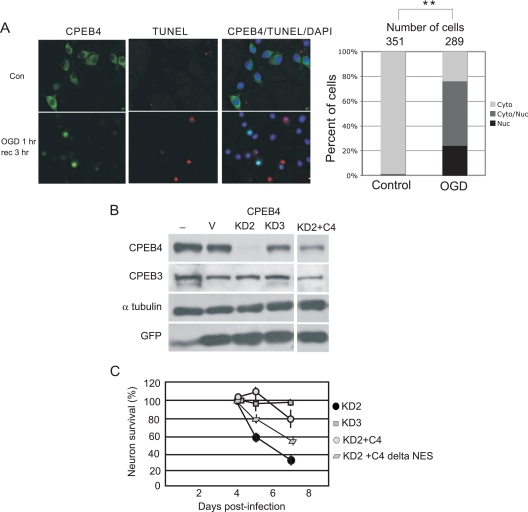
Ischemia causes not only hypoxia but also hypoglycemia in the affected part of the brain. One cell culture model that mimics these two ischemia-induced deficits is oxygen-glucose deprivation (OGD); here, neurons are cultured under conditions of reduced atmospheric oxygen and glucose. Hippocampal neurons (DIV14) were subjected to OGD treatment for 1 h and then returned to normal culture conditions for 3 h before fixation and staining for CPEB4 and apoptosis. In control cells grown under normal conditions, CPEB4 was cytoplasmic and TUNEL staining was absent (Fig. 7A). After 1 h of OGD treatment and 3 h of recovery, CPEB4 protein was undetectable in most neurons, but in others, it was concentrated in the nucleus. Interestingly, CPEB4 nuclear staining was inversely correlated with TUNEL staining (Fig. 7A, right), suggesting that nuclear CPEB4 may offer some protection against apoptosis. The results of the above-mentioned MCAO and OGD experiments suggest that excessive glutamate causes CPEB4 nuclear accumulation. However, this nuclear accumulation could be due to stress caused by the excessive glutamate.
FIG. 7.
Oxygen and glucose deprivation and CPEB4-mediated neuron survival. (A) CPEB4 nuclear localization in DIV14 hippocampal neurons after OGD treatment. Hippocampal neurons were incubated in medium without glucose in an atmosphere deprived of oxygen for 1 h (OGD 1 h), which was followed by recovery in normal culture medium in atmosphere containing oxygen for 3 h (rec 3 h). Control (Con) refers to cells without OGD treatment. The images show CPEB4 staining, TUNEL staining, and CPEB4/TUNEL/DAPI staining to show the location of nuclei. Right, quantification of the CPEB4 proteins in the nucleus and cytoplasm as described in the legend to Fig. 1. The asterisks refer to a statistically significant difference (P < 0.01, Student's t test) between the results for the indicated samples. (B) Hippocampal neurons were cultured with lentivirus expressing GFP only (V) or GFP and two different CPEB4 shRNAs (KD2 and KD3). Some neurons were also cultured with two lentiviruses expressing KD2 and CPEB4 (C4), containing mutations to prevent knockdown but still encoding the correct protein. Extracts from the cells were probed for CPEB4, CPEB3, tubulin, and GFP. (C) Survival of the neurons infected with some of the viruses noted above was determined (n = 200). Error bars represent standard errors of the means.
To investigate whether CPEB4 is important for neuron survival, cultured hippocampal neurons were infected with lentiviruses expressing GFP, as well as two different shRNAs for CPEB4. The results in Fig. 7B show that, while the KD2 shRNA (see “CPEB4 knockdown” in Materials and Methods) effectively reduced CPEB4 levels, KD3 did not. Neither shRNA affected CPEB3 or tubulin levels. Neurons were also doubly infected with viruses expressing KD2 shRNA and CPEB4 or CPEB4 ΔNES, which lacks the nuclear export signal (Fig. 7C). CPEB4 and CPEB4 ΔNES, which resides solely in the nucleus, were mutated so as to maintain the proper amino acid sequence but not to anneal with the shRNA. Most neurons died when CPEB4 was reduced (KD2 shRNA), but neurons survived up to 3 days when CPEB4 levels were restored with either full-length CPEB4 or CPEB4 ΔNES (Fig. 7C). Thus, nuclear CPEB4 is necessary for neuron survival.
CPEB4 is present on the ER, and its nuclear localization is induced by ER calcium depletion.
Transient ischemia induces protein aggregation in the ER, possibly due to inhibited folding capacity when luminal calcium levels are reduced (15). This possibility suggests that CPEB4 nuclear accumulation might also be mediated by reduced ER calcium; consequently, a membrane-permeable calcium chelator, BAPTA/AM, was used to immobilize free calcium inside the ER. Twenty minutes after the addition of BAPTA/AM, neurons were placed in fresh medium for an additional 40 min. After the membrane-permeable moiety of BAPTA/AM is cleaved by cytosolic esterases, the remaining BAPTA becomes trapped intracellularly. Because the dissociation constant of BAPTA for calcium is close to the cytosolic calcium level, BAPTA does not substantially affect cytosolic calcium levels (31). On the other hand, BAPTA targets free calcium in the ER because of its high calcium level (∼700 μM) (8). This chelation of ER calcium induced the nuclear accumulation of CPEB4 (Fig. 8A).
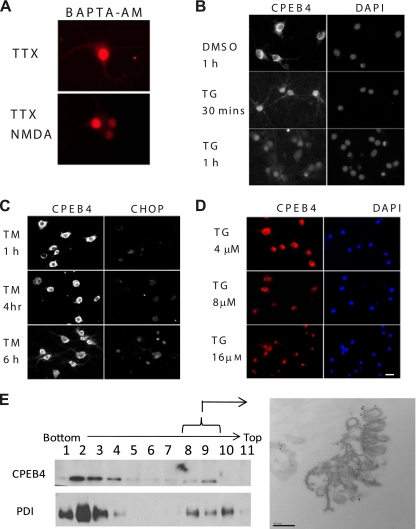
FIG. 8.
Relationship between ER calcium levels and CPEB4 nuclear localization in cultured hippocampal neurons. (A) DIV16 hippocampal neurons that had been treated with TTX for 24 h were incubated with BAPTA/AM for 20 min. The neurons were then washed, cultured in fresh medium, and then treated with either DMSO or NMDA for 40 min before fixation and immunostaining for CPEB4. (B) DIV16 hippocampal neurons were treated with DMSO as a control or 16 μM thapsigargin (TG) for 30 min or 1 h. At the end of treatment, neurons were fixed and stained with CPEB4 antibody and DAPI. (C) DIV16 hippocampal neurons were treated with tunicamycin for 1 h, 4 h, or 6 h before fixation and immunostained with CPEB4 or CHOP antibodies. (D) DIV16 hippocampal neurons were treated with 4, 8, or 16 μM thapsigargin for 1 h before fixation and immunostaining for CPEB4. DAPI staining shows location of the nucleus. Size bar = 10 μm. (E) Sucrose gradient fractionation was performed on postnuclear supernatants of brain lysates; the fractions were immunoblotted for CPEB4 and the ER marker PDI. The CPEB4-enriched fractions (fractions 8 to 10) were then analyzed by immunoelectron microscopy using CPEB4 antibody. Size bar = 200 nm.
A reduction in ER calcium diminishes protein chaperone activity in the lumen, causing the accumulation of unfolded protein that will induce the ER stress response (31). To determine whether CPEB4 nuclear accumulation is a response to ER calcium depletion or ER stress, cells were incubated with thapsigargin (TG) and tunicamycin (TM). While both agents activate the unfolded protein response (UPR), TG does so by causing ER calcium efflux, while TM disrupts protein glycosylation. In neurons treated with 16 μM TG, CPEB4 protein began to accumulate in the nucleus 30 min after application of the drug (Fig. 8B). However, CPEB4 remained cytoplasmic when cells were treated with TM, although the UPR response did occur, as determined by the expression of C/EBP homology protein (CHOP) (Fig. 8C). These data suggest that CPEB4 nuclear accumulation is induced by ER calcium depletion. The results of a dose-response experiment demonstrate that while a 1-h treatment with 4 μM TG had no effect on CPEB4 localization, 8 μM caused an even distribution between nucleus and cytoplasm and 16 μM TG caused strong nuclear CPEB4 staining (Fig. 8D). These data are consistent with the notion that the retention of CPEB4 in the nucleus is triggered by ER calcium depletion.
To investigate how ER calcium depletion might stimulate CPEB4 nuclear localization, mouse brain lysate was underlain on a discontinuous sucrose gradient and then centrifuged; molecules associated with membranes in such a flotation assay should band at a sucrose concentration with a similar density. Fig. 8E shows that protein disulfide isomerase (PDI), an ER-specific marker, banded in fractions 8 to 10; CPEB4 was most prevalent in fractions 8 and 9. The material in these fractions was then pelleted and analyzed by immunoelectron microscopy using CPEB4 antibody. Gold particles marking CPEB4 were found to colocalize with ER structures, thus confirming the presence of CPEB4 on the ER (Fig. 8E). Although we do not know what other molecules might be involved in tethering CPEB4 to the ER, we surmise that calcium depletion from this structure releases CPEB4 and facilitates its nuclear localization.
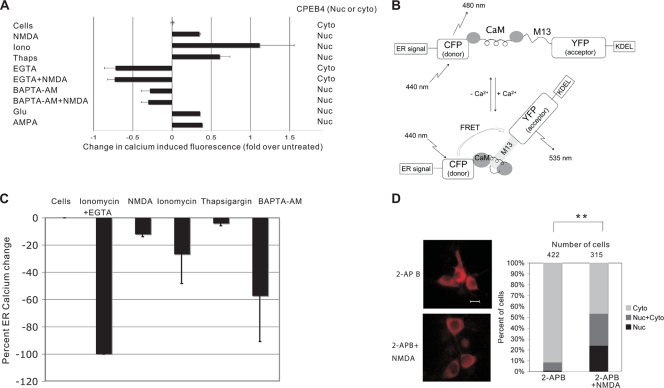
We have conducted several additional experiments to delve further into the notion that CPEB4 nuclear accumulation is driven substantially by ER calcium depletion as opposed to an increase in cytosolic calcium. Because the various agents used in this study to alter calcium levels would likely do so to varying extents, we measured relative intracellular calcium levels with Fluo-3, a calcium-sensitive fluorescent indicator. DIV16 neurons were incubated with Fluo-3 followed by treatment with each agent (NMDA, ionomycin, BAPTA/AM, thapsigargin, etc.); fluorescence intensity was then determined using a fluorescence plate reader. The results in Fig. 9A show that a high intracellular calcium concentration per se did not induce CPEB4 to become predominantly nuclear; indeed, BAPTA/AM, which reduced cytosolic calcium, resulted in nuclear CPEB4. In this case, chelation of ER calcium was most likely responsible for the nuclear accumulation of CPEB4. Taken together, these data indicate that the reduction of ER calcium stores and probably not an increase in cytosolic calcium per se is responsible for CPEB4 nuclear accumulation.
FIG. 9.
ER calcium depletion causes CPEB4 to be retained in the nucleus. (A) DIV16 neurons were loaded with 1 μM Fluo-3/AM for 30 min and then incubated for another 30 min to allow for de-esterification before being treated with the agents noted in the panel. The cells were then placed in a fluorescence plate reader to quantify Fluo-3 fluorescence, a relative measure of calcium concentration. Iono, ionomycin; Thaps, thapsigargin; Glu, glutamate. (B) Schematic structure of the D1ER cameleon construct showing an ER-targeting sequence, CFP, calmodulin (CaM; the calcium binding domains are depicted as balls with a putative flexible coiled region in between), M13 calmodulin binding peptide, YFP, and a KDEL ER retention sequence. When the calmodulin moiety binds calcium in the ER, it interacts with the M13 peptide, which in turn brings CFP and YFP into close proximity. Excitation at 440 nm elicits a 535-nm FRET emission from the YFP; this FRET signal is thus proportional to the amount of calcium in the ER. (C) DIV12 neurons were transfected with pD1ER cameleon construct, and the FRET and CFP signals were determined. The maximum and minimum FRET/CFP signals were measured when the cells were untreated or treated with ionomycin and EGTA and were set at 0 and −100, respectively. The percentage of ER calcium change was plotted (see also Palmer et al. [30]). ER calcium was determined when the cells were treated with NMDA, ionomycin, thapsigargin, or BAPTA-AM. Each experiments was performed 3 times (mean and standard error of the mean are shown). (D) DIV16 neurons were incubated with 2-APB for 45 min prior to the application of NMDA for 40 min; the cells were then fixed and stained for CPEB4. Right, quantification of the results. The asterisks refer to a statistically significant difference (P < 0.01, Student's t test) between the results for the indicated samples. Size bar = 10 μm.
We next determined the extent to which some of the agents that cause CPEB4 nuclear accumulation induce ER calcium depletion. To address this, neurons were transfected with D1ER, a plasmid devised by Palmer et al. (30) that encodes an ER localization signal (derived from calreticulin), CFP, calmodulin, M13 calmodulin-binding peptide, YFP, and a KDEL ER retention sequence. In the ER lumen, calcium binding by calmodulin will cause it to also bind the M13 peptide. This interaction will induce a conformational change in the fusion protein such that CFP and YFP will be juxtaposed. When transfected neurons are excited by 440-nm light, YFP (the acceptor) will emit a 535-nm FRET signal. The intensity of the 535-nm signal is therefore proportional to the amount of calcium that is bound to calmodulin (30) (Fig. 9B). To first “calibrate” the FRET signal, the intensity of 535-nm light emission from untreated cells was set at zero (i.e., Rmax, maximal ER calcium), and that from cells treated with ionomycin plus EGTA was set at −100 (i.e., Rmin, minimum ER calcium). The data collected from cells treated with various agents were then plotted as the percent ER calcium change (Fig. 9C). As expected, NMDA treatment indeed caused a loss of ER calcium, as did thapsigargin and BAPTA/AM, although to various extents (Fig. 9C). Thus, agents that cause CPEB4 to concentrate in the nucleus do indeed induce depletion of ER calcium (see also Fig. S4 in the supplemental material).
Finally, we addressed whether the IP3 receptor, which resides predominantly in the ER, can mediate ER calcium depletion and transduce a signal to cause CPEB4 nuclear accumulation. Neurons were treated with 2-aminoethoxydiphenyl borate (2-APB), an inhibitor of IP3 receptor signaling, with or without NMDA. The results in Fig. 9D show that 2-APB partially blocked NMDA-induced CPEB4 nuclear accumulation. Thus, these data implicate the IP3 receptor in mediating the subcellular localization of CPEB4 in response to depletion of calcium from the endoplasmic reticulum.
DISCUSSION
Most investigations of the biological functions of CPEB, the most studied member of the CPEB family of proteins, have concentrated on cytoplasmic events, such as cytoplasmic polyadenylation, translation, and RNA transport. The finding that the CPEB proteins are nucleus-cytoplasm shuttling proteins suggests new functions for these proteins, involving nuclear RNA metabolism. For example, the CPEB-interacting proteins CPSF and symplekin are involved in nuclear pre-mRNA polyadenylation, as well as cytoplasmic polyadenylation (14, 25); thus, it is possible that CPEB also modulates nuclear polyadenylation, as well as alternative splicing (22). Another possible role for CPEB proteins is RNA nuclear export and subcellular localization. Unlike Staufen2 and She2, two nucleus-cytoplasm shuttling proteins that accumulate in nucleoli when RNA binding activity is disrupted (9, 24), CPEB4 truncation mutants that have part of their RNA binding domains removed retain their shuttling activity, suggesting that CPEB4 is actively transported across the nuclear membrane instead of being passively exported by way of tethering to RNA.
The failure to identify a nuclear import signal (NLS) in CPEB4 using serial deletions suggests that there is more than one NLS. One putative NLS is probably located in an RNA binding domain, because a recombinant CPEB4 RNA binding domain alone is sufficient to induce nuclear import in the presence of HeLa cell cytosol in an in vitro import assay. On the other hand, the CPEB4 NES clearly requires leucine residues 386 and 390. Because these residues are present in CPEB2 and -3, they probably mediate the export of these proteins as well. Moreover, because they are conserved in, for example, Drosophila Orb2, we infer that they have a similar function in invertebrates as well. The observation that the two leucine residues are not present in CPEB underscores the divergent nature of these two branches of the CPEB family of proteins. Nonetheless, the fact that CPEB, like CPEB2, -3, and -4, shuttles between nucleus and cytoplasm in an NMDA-stimulated manner indicates that the CPEB family branches have retained and perhaps even share important nuclear functions.
CPEB4 is retained in the nucleus following ischemia.
The nuclear staining of CPEB4 in the penumbral region of the mouse ischemic brain demonstrates that this pathological condition causes a subcellular redistribution of this protein. The penumbra represents the area of brain that sustains secondary damage caused by the diffusion of glutamate and potassium ions from the immediate site of the infarct, as well as hypoperfusion; it is also a target of treatment that aims to reduce brain injury caused by stroke. In addition to CPEB4, other proteins have also been shown to translocate to the nucleus upon ischemia. One of them, apoptosis-inducing factor (AIF), resides in mitochondria and functions as an oxidoreductase in cells but translocates to the nucleus and induces chromatin condensation when apoptosis or necrosis is induced (7, 42). During ischemia and OGD, nuclear translocation of AIF is considered to be one of the mechanisms that cause neuron death (4, 34, 53, 54). HGF (hepatocyte growth factor), which protects neurons from ischemia-induced cell death when perfused into the brain, prevents the translocation of AIF to the nucleus (28).
Excessive NMDAR activation and ER calcium depletion.
To examine the relationship between CPEB4 nuclear accumulation and cellular calcium, we treated cells with a variety of agents and then measured the intracellular calcium concentration with Fluo-3AM, a cytosolic calcium indicator. In general, agents that induce increased calcium correlated with CPEB4 nuclear accumulation. However, BAPTA/AM, which resulted in CPEB4 nuclear accumulation, did not lead to any detectable increase in cellular calcium. On the other hand, the BAPTA/AM results are consistent with a decrease in ER calcium. Moreover, because the relationship between excessive NMDAR stimulation and (possible) ER calcium depletion is not clear, we examined ER calcium levels in neurons directly, by fluorescence resonance energy transfer (FRET). In this case, neurons were transfected with a plasmid (cameleon D1ER) encoding a fluorescent calcium sensor specifically targeted to the ER (30). Upon treatment with NMDA, ionomycin, thapsigargin, and BAPTA/AM, agents that cause CPEB4 to become concentrated in the nucleus, we observed clear decreases in ER calcium (Fig. 9). While the amount of ER depletion varied depending on the agent, the correlation between nuclear CPEB4 immunostaining and ER calcium depletion was very consistent. Thus, based on these direct measurements, we infer that ER calcium depletion is responsible for CPEB4 nuclear accumulation.
It has been shown that ER stress is induced in ischemic tissue, as demonstrated by the accumulation of misfolded proteins (15) and induction of the UPR pathway (27). The UPR is induced by decreased ER folding capacity or increased synthesis of proteins that transit through the ER. Although it has been suggested that calcium depletion may be responsible for ER stress after ischemia, no clear evidence has been provided. In our results, both ER calcium depletion and the application of NMDA caused CPEB4 nuclear accumulation; we further demonstrate that NMDAR activation causes ER calcium depletion, indicating a causal relationship. It has been reported that activation of NMDAR may induce the release of calcium from the ER through calcium-induced calcium release (10, 39). In neurons, the ER forms an extended structure that reaches synaptic spines (43) and contains two types of calcium-releasing channels, the inositol-1,4,5-triphosphate (IP3) receptor and the ryanodine receptor. Another report also suggests that the ryanodine receptor may cause ER calcium release because the application of a ryanodine receptor inhibitor, dantrolene, protects neurons from NMDA-mediated excitotoxicity (13). Caffeine, which activates the ryanodine receptor, did not cause CPEB4 nuclear accumulation, probably because it did not cause sufficient ER calcium depletion and/or because of its well-known pleiotropic effects. On the other hand, inhibition of the IP3 receptor with 2-aminoethoxydiphenyl borate (2-APB) blocked NMDA-induced CPEB4 nuclear accumulation, indicating that the IP3 receptor is of particular importance for causing CPEB4 to remain nuclear.
Mechanism for ER calcium depletion-induced nuclear retention of CPEB4.
The treatment of neurons with TG but not TM excludes ER stress per se as a possible mechanism inducing CPEB4 nuclear accumulation. The retention of CPEB4 in the nucleus after BAPTA/AM incubation suggests that ER calcium depletion plays an inhibitory role in CPEB4 nuclear export. One key extant question is that of how ER calcium depletion induces CPEB4 retention in the nucleus. Recent investigations of ER calcium homeostasis suggest that store-operated calcium entry (SOCE) replenishes ER calcium levels after depletion. SOCE involves two protein families: the stromal-interacting molecule (Stim) family and plasma membrane calcium channels, Orai. Stim proteins are located in the ER membrane and serve as ER lumen calcium level sensors (38, 52). Orai channel proteins interact with aggregated Stim proteins and induce calcium influx when ER calcium is depleted (12, 23, 26, 32, 36). The influxed cytoplasmic calcium is then transported into the ER by the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) system (20). Whether calcium influxed through SOCE triggers CPEB4 nuclear retention upon ER calcium depletion and NMDA stimulation requires further investigation.
Supplementary Material
Acknowledgments
We thank Lan Xu for advice on the in vitro nuclear import assay, Oswald Steward for providing rat brain sections, and Melissa Jungnickel and Keith Sutton for help with the calcium fluorescence assays. We also thank the UMass Medical School imaging facility and the electron microscopy core facility, Robert Singer for the N-EGFP-MS2 plasmid, Roger Tsien for the Cameleon D1ER plasmid, Arthur Mercurio for the use of his hypoxia chamber, and Rachel Groppo for reading the manuscript.
This work was supported by grants from the NIH (GM46779 and HD37267). A.C.-M. was supported by grant number 3 R01 GM046779-19S1. Core support from the Diabetes and Endocrinology Research Center Program Project (DK32520) is gratefully acknowledged.
Footnotes
Published ahead of print on 11 October 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alarcon, J. M., R. Hodgman, M. Theis, Y. S. Huang, E. R. Kandel, and J. D. Richter. 2004. Selective modulation of some forms of Schaffer collateral-CA1 synaptic plasticity in mice with a disruption of the CPEB-1 gene. Learn. Mem. 11:318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banker, G., and K. Goslin. 1988. Developments in neuronal cell culture. Nature 336:185-186. [DOI] [PubMed] [Google Scholar]
- 3.Berger-Sweeney, J., N. R. Zearfoss, and J. D. Richter. 2006. Reduced extinction of hippocampal-dependent memories in CPEB knockout mice. Learn. Mem. 13:4-7. [DOI] [PubMed] [Google Scholar]
- 4.Cao, G., R. S. Clark, W. Pei, W. Yin, F. Zhang, F. Y. Sun, S. H. Graham, and J. Chen. 2003. Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J. Cereb. Blood Flow Metab. 23:1137-1150. [DOI] [PubMed] [Google Scholar]
- 5.Choi, D. W., and S. M. Rothman. 1990. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13:171-182. [DOI] [PubMed] [Google Scholar]
- 6.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugas, E., S. A. Susin, N. Zamzami, K. F. Ferri, T. Irinopoulou, N. Larochette, M. C. Prevost, B. Leber, D. Andrews, J. Penninger, and G. Kroemer. 2000. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 14:729-739. [PubMed] [Google Scholar]
- 8.Demaurex, N., and M. Frieden. 2003. Measurements of the free luminal ER Ca(2+) concentration with targeted “cameleon” fluorescent proteins. Cell Calcium 34:109-119. [DOI] [PubMed] [Google Scholar]
- 9.Du, L., and J. D. Richter. 2005. Activity-dependent polyadenylation in neurons. RNA 11:1340-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emptage, N., T. V. Bliss, and A. Fine. 1999. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron 22:115-124. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari, F., V. Mercaldo, G. Piccoli, C. Sala, S. Cannata, T. Achsel, and C. Bagni. 2007. The fragile X mental retardation protein-RNP granules show an mGluR-dependent localization in the post-synaptic spines. Mol. Cell Neurosci. 34:343-354. [DOI] [PubMed] [Google Scholar]
- 12.Feske, S., Y. Gwack, M. Prakriya, S. Srikanth, S. H. Puppel, B. Tanasa, P. G. Hogan, R. S. Lewis, M. Daly, and A. Rao. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441:179-185. [DOI] [PubMed] [Google Scholar]
- 13.Frandsen, A., and A. Schousboe. 1992. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proc. Natl. Acad. Sci. U. S. A. 89:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, I., M. Schnolzer, I. Kaufmann, and W. W. Franke. 2002. Symplekin, a constitutive protein of karyo- and cytoplasmic particles involved in mRNA biogenesis in Xenopus laevis oocytes. Mol. Biol. Cell 13:1665-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, B. R., M. E. Martone, Y. Z. Jones, and C. L. Liu. 2000. Protein aggregation after transient cerebral ischemia. J. Neurosci. 20:3191-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, Y. S., J. H. Carson, E. Barbarese, and J. D. Richter. 2003. Facilitation of dendritic mRNA transport by CPEB. Genes Dev. 17:638-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Y. S., M. C. Kan, C. L. Lin, and J. D. Richter. 2006. CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J. 25:4865-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudmon, A., and H. Schulman. 2002. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71:473-510. [DOI] [PubMed] [Google Scholar]
- 19.Ishida, A., Y. Shigeri, Y. Tatsu, K. Uegaki, I. Kameshita, S. Okuno, T. Kitani, N. Yumoto, and H. Fujisawa. 1998. Critical amino acid residues of AIP, a highly specific inhibitory peptide of calmodulin-dependent protein kinase II. FEBS Lett. 427:115-118. [DOI] [PubMed] [Google Scholar]
- 20.Jousset, H., M. Frieden, and N. Demaurex. 2007. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J. Biol. Chem. 282:11456-11464. [DOI] [PubMed] [Google Scholar]
- 21.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. U. S. A. 96:9112-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, C. L., V. Evans, S. Shen, Y. Xing, and J. D. Richter. 2010. The nuclear experience of CPEB: implications for RNA processing and translational control. RNA 16:338-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luik, R. M., M. M. Wu, J. Buchanan, and R. S. Lewis. 2006. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174:815-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macchi, P., A. M. Brownawell, B. Grunewald, L. DesGroseillers, I. G. Macara, and M. A. Kiebler. 2004. The brain-specific double-stranded RNA-binding protein Staufen2: nucleolar accumulation and isoform-specific exportin-5-dependent export. J. Biol. Chem. 279:31440-31444. [DOI] [PubMed] [Google Scholar]
- 25.Mandel, C. R., S. Kaneko, H. Zhang, D. Gebauer, V. Vethantham, J. L. Manley, and L. Tong. 2006. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 444:953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercer, J. C., W. I. Dehaven, J. T. Smyth, B. Wedel, R. R. Boyles, G. S. Bird, and J. W. Putney, Jr. 2006. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 281:24979-24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimoto, N., Y. Oida, M. Shimazawa, M. Miura, T. Kudo, K. Imaizumi, and H. Hara. 2007. Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience 147:957-967. [DOI] [PubMed] [Google Scholar]
- 28.Niimura, M., N. Takagi, K. Takagi, R. Mizutani, N. Ishihara, K. Matsumoto, H. Funakoshi, T. Nakamura, and S. Takeo. 2006. Prevention of apoptosis-inducing factor translocation is a possible mechanism for protective effects of hepatocyte growth factor against neuronal cell death in the hippocampus after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 26:1354-1365. [DOI] [PubMed] [Google Scholar]
- 29.Nishi, K., M. Yoshida, D. Fujiwara, M. Nishikawa, S. Horinouchi, and T. Beppu. 1994. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 269:6320-6324. [PubMed] [Google Scholar]
- 30.Palmer, A. E., C. Jin, J. C. Reed, and R. Y. Tsien. 2004. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. U. S. A. 101:17404-17409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paschen, W., S. Hotop, and C. Aufenberg. 2003. Loading neurons with BAPTA-AM activates xbp1 processing indicative of induction of endoplasmic reticulum stress. Cell Calcium 33:83-89. [DOI] [PubMed] [Google Scholar]
- 32.Peinelt, C., M. Vig, D. L. Koomoa, A. Beck, M. J. Nadler, M. Koblan-Huberson, A. Lis, A. Fleig, R. Penner, and J. P. Kinet. 2006. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat. Cell Biol. 8:771-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petosa, C., G. Schoehn, P. Askjaer, U. Bauer, M. Moulin, U. Steuerwald, M. Soler-Lopez, F. Baudin, I. W. Mattaj, and C. W. Muller. 2004. Architecture of CRM1/Exportin1 suggests how cooperativity is achieved during formation of a nuclear export complex. Mol. Cell 16:761-775. [DOI] [PubMed] [Google Scholar]
- 34.Plesnila, N., C. Zhu, C. Culmsee, M. Groger, M. A. Moskowitz, and K. Blomgren. 2004. Nuclear translocation of apoptosis-inducing factor after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 24:458-466. [DOI] [PubMed] [Google Scholar]
- 35.Plum, F. 1983. What causes infarction in ischemic brain? The Robert Wartenberg Lecture. Neurology 33:222-233. [DOI] [PubMed] [Google Scholar]
- 36.Prakriya, M., S. Feske, Y. Gwack, S. Srikanth, A. Rao, and P. G. Hogan. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443:230-233. [DOI] [PubMed] [Google Scholar]
- 37.Richter, J. D. 2007. CPEB: a life in translation. Trends Biochem. Sci. 32:279-285. [DOI] [PubMed] [Google Scholar]
- 38.Roos, J., P. J. DiGregorio, A. V. Yeromin, K. Ohlsen, M. Lioudyno, S. Zhang, O. Safrina, J. A. Kozak, S. L. Wagner, M. D. Cahalan, G. Velicelebi, and K. A. Stauderman. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169:435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose, C. R., and A. Konnerth. 2001. Stores not just for storage. Intracellular calcium release and synaptic plasticity. Neuron 31:519-522. [DOI] [PubMed] [Google Scholar]
- 40.Rossi, D. J., T. Oshima, and D. Attwell. 2000. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403:316-321. [DOI] [PubMed] [Google Scholar]
- 41.Steward, O., C. S. Wallace, G. L. Lyford, and P. F. Worley. 1998. Synaptic activation causes the mRNA for the IEG arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741-751. [DOI] [PubMed] [Google Scholar]
- 42.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 43.Svoboda, K., and Z. F. Mainen. 1999. Synaptic [Ca2+]: intracellular stores spill their guts. Neuron 22:427-430. [DOI] [PubMed] [Google Scholar]
- 44.Tay, J., and J. D. Richter. 2001. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev. Cell 1:201-213. [DOI] [PubMed] [Google Scholar]
- 45.Tymianski, M., M. P. Charlton, P. L. Carlen, and C. H. Tator. 1993. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 13:2085-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tymianski, M., M. C. Wallace, I. Spigelman, M. Uno, P. L. Carlen, C. H. Tator, and M. P. Charlton. 1993. Cell-permeant Ca2+ chelators reduce early excitotoxic and ischemic neuronal injury in vitro and in vivo. Neuron 11:221-235. [DOI] [PubMed] [Google Scholar]
- 47.van Leyen, K., H. Y. Kim, S. R. Lee, G. Jin, K. Arai, and E. H. Lo. 2006. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37:3014-3018. [DOI] [PubMed] [Google Scholar]
- 48.Watterson, D. M., S. Mirzoeva, L. Guo, A. Whyte, J. J. Bourguignon, M. Hibert, J. Haiech, and L. J. Van Eldik. 2001. Ligand modulation of glial activation: cell permeable, small molecule inhibitors of serine-threonine protein kinases can block induction of interleukin 1 beta and nitric oxide synthase II. Neurochem. Int. 39:459-468. [DOI] [PubMed] [Google Scholar]
- 49.Wells, D. G., X. Dong, E. M. Quinlan, Y. S. Huang, M. F. Bear, J. D. Richter, and J. R. Fallon. 2001. A role for the cytoplasmic polyadenylation element in NMDA receptor-regulated mRNA translation in neurons. J. Neurosci. 21:9541-9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, L., D. Wells, J. Tay, D. Mendis, M. A. Abbott, A. Barnitt, E. Quinlan, A. Heynen, J. R. Fallon, and J. D. Richter. 1998. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron 21:1129-1139. [DOI] [PubMed] [Google Scholar]
- 51.Zearfoss, N. R., J. M. Alarcon, P. Trifilieff, E. Kandel, and J. D. Richter. 2008. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J. Neurosci. 28:8502-8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, S. L., Y. Yu, J. Roos, J. A. Kozak, T. J. Deerinck, M. H. Ellisman, K. A. Stauderman, and M. D. Cahalan. 2005. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437:902-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao, H., M. A. Yenari, D. Cheng, O. L. Barreto-Chang, R. M. Sapolsky, and G. K. Steinberg. 2004. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J. Cereb. Blood Flow Metab. 24:681-692. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, C., L. Qiu, X. Wang, U. Hallin, C. Cande, G. Kroemer, H. Hagberg, and K. Blomgren. 2003. Involvement of apoptosis-inducing factor in neuronal death after hypoxia-ischemia in the neonatal rat brain. J. Neurochem. 86:306-317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.