Abstract
Recent studies have revealed that innate immunity is involved in the development of adaptive immune responses; however, its role in protection is not clear. In order to elucidate the exact role of Toll-like receptor (TLR) or RIG-I-like receptor (RLR) signaling on immunogenicity and protective efficacy against influenza A virus infection (A/PR/8/34 [PR8]; H1N1), we adapted several innate signal-deficient mice (e.g., TRIF−/−, MyD88−/−, MyD88−/− TRIF−/−, TLR3−/− TLR7−/−, and IPS-1−/−). In this study, we found that MyD88 signaling was required for recruitment of CD11b+ granulocytes, production of early inflammatory cytokines, optimal proliferation of CD4 T cells, and production of Th1 cytokines by T cells. However, PR8 virus-specific IgG and IgA antibody levels in both systemic and mucosal compartments were normal in TLR- and RLR-deficient mice. To further assess the susceptibility of these mice to influenza virus infection, protective efficacy was determined after primary or secondary lethal challenge. We found that MyD88−/− and MyD88−/− TRIF−/− mice were more susceptible to primary influenza virus infection than the B6 mice but were fully protected against homologous (H1N1) and heterosubtypic (H5N2) secondary infection when primed with a nonlethal dose of PR8 virus. Taken together, these results show that MyD88 signaling plays an important role for resisting primary influenza virus infection but is dispensable for protection against a secondary lethal challenge.
Influenza A virus can cause epidemics and pandemics. The recent swine-origin H1N1 pandemic, which shows different mortality patterns, reinforces the urgency of studying immune responses to influenza viruses in order to develop better vaccines and therapeutics (38, 39, 55). Host defense against influenza A virus includes both innate and adaptive immune responses (36). The immediate innate immune responses begin after recognition of viral components by pattern recognition receptors. Two Toll-like receptors (TLRs), TLR3 and TLR7, recognize the viral RNA of influenza virus in the endosome (9, 30, 53). TLR3 utilizes the Toll-interleukin-1 (IL-1) receptor domain-containing adaptor-inducing beta interferon ([IFN-β] TRIF), while myeloid differentiation factor 88 (MyD88) is responsible for TLR7 signaling (16, 60). The cytoplasmic viral RNA is recognized by retinoic acid-inducible protein I (RIG-I), and IFN-β promoter stimulator 1 (IPS-1) mediates its signaling (20, 22, 41). TLR and RIG-I-like receptor (RLR) signaling cooperate to exert innate immune protection against influenza virus infection but their role varies by dendritic cell (DC) type (25).
A growing number of studies indicates that innate immune responses are of great importance for enhancing adaptive immune responses. The TLR signaling in antigen-presenting cells and other cell types induces the production of various cytokines to enhance subsequent adaptive immune responses (34). The intrinsic TLR signaling of T and B cells is directly engaged in the development of adaptive immune response (3, 7, 37, 42). Previously, the role of TLR signaling on systemic antibody (Ab) response after influenza virus infection was proposed (15, 25, 32). Those studies assessed the control of IgG isotype switching and the effect of type I IFN produced by plasmacytoid DCs, one of the type I IFN producers in influenza virus infection (19, 25). However, the role of TLR and RLR on induction of both systemic and mucosal immunity and protective efficacy in primary and secondary influenza virus infection was not described.
MyD88 is essential for optimal protection against various pathogens including viruses (28, 50, 62), bacteria (2, 12, 54, 61), parasites (6, 48, 49), and Candida albicans (57). In contrast, few studies have looked at the role of TRIF in protection, and one that did found its contribution minimal (59). Although MyD88 is indispensable for protection in several infection models, in some cases it induces excess inflammation and accelerates diseases (13, 47, 56, 58). For this bilaterality, MyD88 can aggravate or alleviate symptoms depending on the pathogen and experimental setting.
To our knowledge, influenza virus susceptibility in MyD88- and TRIF-deficient mice is not well characterized. Therefore, we examined the role of TLR and/or RLR for induction of immunogenicity and protective efficacy following nonlethal and lethal doses of influenza virus by using knockout mice whose TLR or RLR signaling is blocked by deletion of their adaptor molecules. We found that MyD88, but not TRIF and IPS-1, signaling is indispensable for protection against the primary influenza A virus infection but dispensable for protection in the secondary infection.
MATERIALS AND METHODS
Mice and viruses.
C57BL/6 (B6) and BALB/c mice were purchased from Charles River Laboratories (Orient Bio Inc., Sungnam, South Korea). Mice deficient in adaptor molecules that mediate TLR or RLR signaling, MyD88−/−, TRIF−/−, MyD88−/− TRIF−/− (all B6 background), and IPS-1−/− (B6/129Ola background) were generated as described previously (1, 26, 60). MyD88−/−, TRIF−/−, and MyD88−/− TRIF−/− mice were backcrossed to B6 (over 12 generations). TLR3−/− mice (BALB/c background) were crossed with TLR7−/− mice (BALB/c background) to generate TLR3−/− TLR7−/− mice. Both μMT (B6 background) and RAG2−/− mice (BALB/c background) were purchased from Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the Institutional Animal Care and Use Committee of the International Vaccine Institute. A/PR/8/34 (PR8; H1N1) and A/Aquatic bird/Korea/W81/2005 (W81; H5N2) were used to infect the mice. W81 virus was isolated from wild birds (51) and mouse adapted to enhance virulence. Viruses were injected in 11-day-old embryonated chicken eggs and incubated for 3 days at 37°C. Allantoic fluid was harvested and filtered with a 0.25-μm-pore-size syringe filter (Pall, Port Washington, NY). Virus stock was stored at −80°C before use.
T-cell analysis.
Wild-type and gene-manipulated B6 mice were immunized intranasally with A/PR/8/34 (H1N1; 5 × 102 PFU; 20 μl) and mediastinal lymph nodes (MdLN) and spleens were removed 4 weeks later. Mononuclear cells isolated from three or four mice were pooled and plated on U-bottom-shaped 96-well plates (1 × 105 cells per well). Then cells were cultured in 10% fetal bovine serum (FBS) containing RPMI 1640 medium for 3 days in the presence of 10 μg/ml formalin-inactivated PR8, which was kindly provided by Taisuke Horimoto, Tokyo University Institute of Medical Science. After cells were mixed with radiolabeled [3H]thymidine and incubated for an additional 8 h, incorporation was measured by scintillation counter (Perkin Elmer, Waltham, MA). To address cytokine levels, MdLN or spleen cells (1 × 106) from vaccinated mice were plated and cultured. The culture supernatant was collected at day 3 and analyzed for cytokine profile by a mouse Th1/Th2 Cytokine Cytometric Bead Array (CBA) kit (BD Pharmingen, San Diego, CA). To analyze nucleoprotein (NP) tetramer-specific T cells, mononuclear cells (1 × 106) were incubated with 10 μg/ml H-2Db influenza virus NP tetramer (ASNENMETM) conjugated with phycoerythrin (MBL, Nagoya, Japan) for 45 min at 4°C. After cells were washed with 1% bovine serum albumin (BSA) containing phosphate-buffered saline (PBS), CD3-fluorescein isothiocyanate (FITC), and CD8-allophycocyanin (APC; BioLegend, San Diego, CA), antibodies were incubated for 15 min at 4°C. The cells were analyzed by flow cytometry (BD Biosciences, San Jose, CA).
Sample preparation.
Mice were injected intraperitoneally with 100 μg of pilocarpine, and saliva was obtained 2 days before sacrifice. To obtain bronchoalveolar lavage fluid (BALF) and nasal wash samples, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg). Tracheas were cannulated after exsanguination, and lungs were washed with 1 ml of PBS. Then nasal passages were flushed twice with 50 μl of PBS to collect nasal wash samples. Samples were stored at −20°C before analysis.
Antigen-specific ELISA.
A 96-well microplate (Nunc, Roskilde, Denmark) was coated with 5 μg/ml inactivated PR8 virus in PBS overnight. Then wells were washed with PBS and blocked with 1% BSA in PBS for 2 h at 37°C. After a wash with PBS, serially diluted samples were incubated for 4 h. Plates were then incubated at 4°C overnight with horseradish peroxidase (HRP)-conjugated anti-mouse IgG, IgG1, IgG2b, IgG2c, IgG3, and IgA (Southernbiotech, Birmingham, AL) antibodies. HRP-conjugated polymeric immunoglobulin receptor (pIgR) (R&D Systems, Minneapolis, MN) antibody was used to detect secretory IgA (SIgA) antibody. Finally, the plates were developed by tetramethylbenzidine (TMB) solution (Moss, Pasadena, MD) before stop solution (0.5 N HCl) was added. Absorbance was measured at 450 nm on an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Sunnyvale, CA). Endpoint titers were expressed as the reciprocal log2 of the last dilution giving an optical density at 450 nm of 0.1 greater than background.
HI assay.
BALF samples were serially diluted 2-fold using PBS, and 4 hemagglutination inhibition (HI) units of virus were added in equivalent volumes and incubated for 1 h at 37°C. Then we added 50 μl of fixed chicken red blood cells and calculated the HI titer after 30 min of incubation at 4°C.
BALF analysis.
Cells were collected from BALF by centrifugation (800 × g for 5 min) and stained with the following antibodies: CD11b (M1/70), Ly6C (AL-21), and Ly6G (1A8), all purchased from BD Pharmingen. Cell populations in the BALF were classified using these surface markers: neutrophils (CD11b+ Ly6Cint Ly6G+; Ly6Cint indicates intermediate-level expression of Ly6C) and monocytes (CD11b+ Ly6Chi Ly6G−; Ly6Chi indicates high-level expression of Ly6C). The levels of IL-6, tumor necrosis factor alpha (TNF-α), and IFN-γ were measured by a Mouse Inflammatory Cytometric Bead Array Kit (BD Biosciences) according to the manufacturer's instructions.
Histology.
Lungs were removed from infected B6 and MyD88−/− mice and washed with PBS before being fixed with 4% formaldehyde for 1 h at 4°C. The tissues were embedded in paraffin and stained with hematoxylin and eosin (H&E).
Statistics.
We used a paired two-sample t test for analysis of T-cell proliferation and ELISA and HI assay results. For survival data, we used Kaplan-Meier analysis.
RESULTS
MyD88−/− TRIF−/− and TLR3−/− TLR7−/− mice are susceptible to primary H1N1 influenza virus infection.
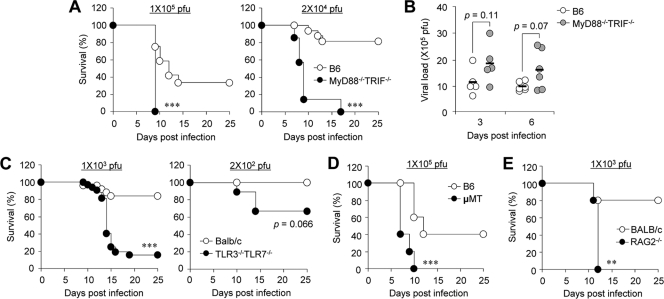
To investigate the role of TLR signaling on susceptibility after primary influenza virus infection, wild-type B6 and MyD88−/− TRIF−/− mice, in which all TLR signaling is completely disrupted, were infected with 1 × 105 PFU (approximate dose of 1× the 50% lethal dose [LD50] for B6 mice) or 2 × 104 PFU of PR8 influenza virus. All MyD88−/− TRIF−/− mice succumbed to infection while approximately 30% and 80% of B6 mice survived after infection with 1 × 105 and 2 × 104 PFU of influenza virus, respectively (Fig. 1A). Although MyD88−/− TRIF−/− mice did not show accelerated body weight loss (data not shown), they died significantly earlier than B6 mice (Fig. 1A). Since higher susceptibility of MyD88−/− TRIF−/− mice can result from increased viral burden, we next addressed viral load in the lung of infected wild-type B6 and MyD88−/− TRIF−/− mice at 3 and 6 days after infection (Fig. 1B). Although MyD88−/− TRIF−/− mice had elevated mean viral titers in the lung, these did not reach statistical significance.
FIG. 1.
Mice lacking innate and adaptive components exhibit enhanced mortality. Wild-type B6 and MyD88−/− TRIF−/− mice were infected intranasally with 1 × 105 or 2 × 104 PFU of PR8 virus, and survival rates (A) were checked daily or virus titers in lung (B) were measured at 3 and 6 days after infection. (C) Wild-type BALB/c and TLR3−/− TLR7−/− mice were infected with 1 × 103 or 2 × 102 PFU of PR8 virus, and survival rates were monitored. Survival rates are shown for μMT mice of B6 background (D) and RAG2−/− mice of BALB/c background (E) infected with 1 × 105 PFU and 1 × 103 PFU of PR8 virus, respectively. **, P < 0.01; ***, P < 0.001 (compared with wild-type controls). Results are representative of two experiments (n ≥ 5 for each group).
Since MyD88 also mediates IL-1 family signaling (1), we used TLR3−/− TLR7−/− mice to determine if TLR signaling is involved in the enhanced susceptibility observed in MyD88−/− TRIF−/− mice after influenza virus infection. In all, 15% of TLR3−/− TLR7−/− mice survived as did 85% of wild-type BALB/c mice after challenge with 1 × 103 PFU (approximate dose of 1× LD50 for BALB/c mice) of influenza virus (Fig. 1C). When a reduced dose (2 × 102 PFU) was used, 30% of TLR3−/− TLR7−/− mice died while all wild-type mice survived (Fig. 1C).
MyD88−/− TRIF−/− and TLR3−/− TLR7−/− mice may have both a defect in immediate innate immune responses and impaired adaptive immune responses that contribute to this enhanced susceptibility. To confirm earlier observations that adaptive immune responses are required for protection after primary influenza virus infection, we infected mice deficient in adaptive immune responses (Fig. 1D and E). In parallel with previous studies (14, 24), both μMT mice of B6 background and RAG2−/− mice of BALB/c background were vulnerable to influenza virus infection. When μMT of B6 background and RAG2−/− mice of BALB/c background were infected with 1 × 105 PFU or 1 × 103 PFU of PR8 virus, respectively, μMT and RAG2−/− mice succumbed to infection while approximately 40% or 80% of wild-type mice survived (Fig. 1D and E). Overall, our results indicate that TLR-mediated innate and/or acquired immunity is indispensable for efficient protection against H1N1 influenza virus infection.
TLR signaling is involved in T-cell responses to influenza virus infection.
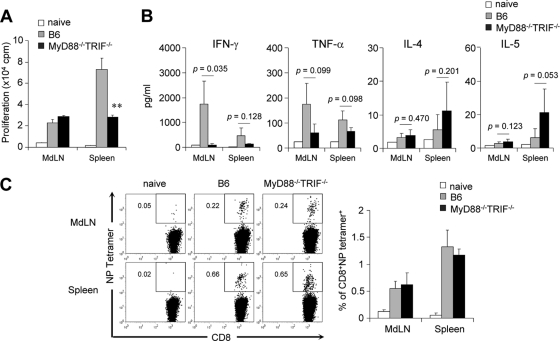
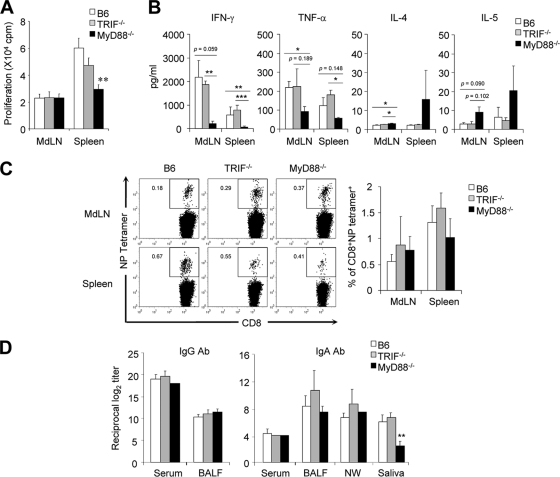
To examine the role of TLR on T-cell activation, wild-type B6 and MyD88−/− TRIF−/− mice were infected with 5 × 102 PFU of PR8 virus. After 4 weeks, mononuclear cells of MdLN and spleen were isolated and cultured in vitro for 3 days with stimulation using inactivated PR8 virus. Of note, the spleens of MyD88−/− TRIF−/− mice had significantly reduced (but detectable) CD4 T-cell proliferation compared to B6 wild-type mice, but both mouse groups had similar levels of CD4 T-cell proliferation in MdLN (Fig. 2A). We further examined Th1/Th2-related cytokines in the culture supernatant. The Th2 cytokines, IL-4 and IL-5, were increased in MyD88−/− TRIF−/− mice while TNF-α and IFN-γ were decreased, indicating that MyD88−/− TRIF−/− mice had Th2-biased T-cell differentiation but wild-type mice did not (Fig. 2B). We also tested the CD8 T-cell response using tetramer specific to NP of influenza virus. Numbers of virus-specific CD8 T cells in both MdLN and spleen were identical in MyD88−/− TRIF−/− mice and wild-type mice (Fig. 2C). Taken together, TLR signaling seems to have a role in antigen-specific CD4 proliferation and Th1 cytokine production but not in CD8 T-cell responses after influenza virus infection.
FIG. 2.
T-cell responses are reduced in MyD88−/− TRIF−/− mice. Wild-type B6 and MyD88−/− TRIF−/− mice were infected with 5 × 102 PFU of PR8 virus. As a control, wild-type B6 mice were challenged with PBS (naïve). Mononuclear cells from MdLN and spleen were isolated 4 weeks later and cocultured with 10 μg/ml inactivated PR8 virus. (A) Cell proliferation was assessed by [3H]thymidine incorporation. Data are representative of three independent experiments; values are means + standard deviations of triplicate experiments. (B) Th1/Th2 cytokine profiles were determined in culture supernatant of mononuclear cells by a CBA kit. Results are representative of three experiments (n = 3 for each group). (C) Virus-specific CD8 T cells stained with nucleoprotein-specific tetramer from MdLN and spleen of naïve or infected mice (n = 3). **, P < 0.01 versus wild-type control.
TLR signaling is dispensable for protective Ab responses following influenza virus infection.
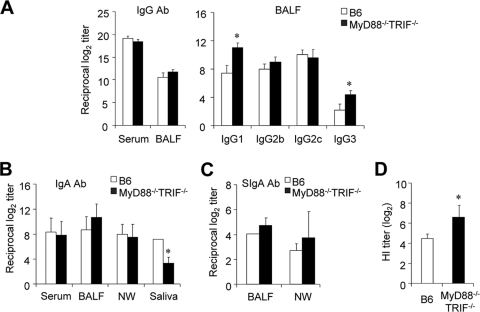
We next determined IgG Ab levels in serum and BALF of wild-type and MyD88−/− TRIF−/− mice following primary influenza virus infection. Of note, identical or slightly higher levels of virus antigen-specific IgG Ab were determined in serum and BALF of MyD88−/− TRIF−/− mice than in wild-type mice following primary infection with 5 × 102 PFU of PR8 virus (Fig. 3A). To measure Th1/Th2 balance, we examined IgG subclasses in the BALF. As reported elsewhere (18, 21) and in agreement with our Th1/Th2 cytokine analysis (Fig. 2B), IgG1 Ab was dominant in BALF of MyD88−/− TRIF−/− mice (Fig. 3A). We further examined IgA Ab levels in serum, BALF, nasal wash, and saliva following primary and secondary influenza virus infection. Of note, identical levels of IgA Ab in nasal wash, BALF, and serum were determined in both wild-type and MyD88−/− TRIF−/− mice (Fig. 3B). In contrast, virus antigen-specific IgA Ab levels were significantly downregulated in saliva of MyD88−/− TRIF−/− mice in comparison with those of wild-type mice (Fig. 3B). There were no significant differences in virus antigen-specific IgG and IgA Abs in the serum and mucosal compartments following secondary influenza virus infection (data not shown). To further assess the role of SIgA, we examined SIgA production in MyD88−/− TRIF−/− mice. SIgA levels in BALF and nasal wash from MyD88−/− TRIF−/− mice did not differ from those of wild-type B6 mice (Fig. 3C). The fact that IgA Ab levels were not affected in the BALF and nasal wash of MyD88−/− TRIF−/− mice after primary infection suggests that airway IgA secretions can be induced in a TLR-independent manner.
FIG. 3.
Comparable systemic and airway antibody responses are induced in MyD88−/− TRIF−/− mice. Wild-type B6 and MyD88−/− TRIF−/− mice were infected with 5 × 102 PFU of PR8 virus. Four weeks after primary challenge, serum and BALF were prepared. (A) PR8-specific IgG in serum and BALF and IgG subclasses in BALF were determined by ELISA. (B) IgA levels of serum, BALF, nasal wash (NW), and saliva were determined by ELISA. (C) SIgA levels in BALF and NW samples were determined using pIgR-specific antibody. (D) Hemagglutination inhibition titer was assessed using BALF. *, P < 0.05 compared with wild-type controls. Values are means + standard deviations. Results shown are representative of three experiments (n > 3 for each group).
Because we found normal levels of IgA and Th2-biased IgG responses in the BALF of MyD88−/− TRIF−/− mice (Fig. 3A and B), we next tested the inhibitory efficacy of BALF from MyD88−/− TRIF−/− mice to learn whether Ab produced after primary infection without TLR signaling can suppress hemagglutination by the hemagglutinin (HA) protein (Fig. 3D). We found that BALF isolated from MyD88−/− TRIF−/− mice was significantly more effective in inhibiting hemagglutination than that of wild-type B6 mice.
Overall, our findings show that TLR signaling is not essential for induction of protective virus antigen-specific T- and B-cell responses in either systemic or respiratory mucosa.
MyD88 plays an essential role in susceptibility after primary influenza virus infection.
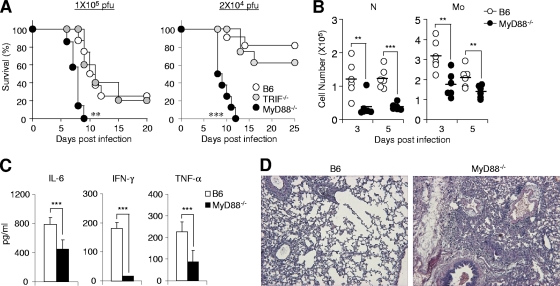
Because the MyD88−/− TRIF−/− mice exhibited high susceptibility to primary influenza virus infection, we further adapted MyD88−/− and TRIF−/− mice to learn if each adaptor molecule behaves differently. When we subsequently infected mice with 1 × 105 and 2 × 104 PFU of PR8, MyD88−/− mice showed significantly higher susceptibility than either wild-type or TRIF−/− mice (Fig. 4A). To determine if MyD88 plays a crucial role in preventing primary influenza virus infection, we observed immunological and pathological changes in wild-type and MyD88−/− mice infected with 2 × 104 PFU of PR8. Since a previous study showed that MyD88 signaling is required for Gr-1+ CD11b+ cell accumulation in the course of polymicrobial sepsis (8), we assessed cell subsets in the BALF of wild-type and MyD88−/− mice at 3 and 5 days after infection. We found that recruitment of neutrophils and monocytes was significantly reduced in MyD88−/− mice (Fig. 4B). We also measured inflammatory cytokine levels in the BALF and found that IL-6, TNF-α, and IFN-γ production was reduced in MyD88−/− mice compared to levels in wild-type mice at 5 days after infection (Fig. 4C). Importantly, infected lungs of MyD88−/− mice exhibited more severe tissue damage including edema, alveolar hemorrhage, and alveolar wall thickness than those of wild-type mice at 5 days after infection (Fig. 4D). These data indicate that MyD88 is required for optimal recruitment of immune cells and production of inflammatory cytokines which might be important for resisting primary influenza virus infection.
FIG. 4.
MyD88 is more critical for protection than TRIF. (A) Wild-type B6, MyD88−/−, and TRIF−/− mice were infected with 1 × 105 or 2 × 104 PFU of PR8 virus, and survival rates were checked daily. Cell numbers (B) at indicated time points and cytokine levels (C) at 5 days after infection (2 × 104 PFU) in BALF of wild-type B6 and MyD88−/− mice were determined. Results are pooled data from two individual experiments (n = 3). N, neutrophils; Mo, monocytes. (D) Lungs of infected wild-type B6 and MyD88−/− mice were prepared 5 days after infection (2 × 104 PFU) and stained with H&E. Pictures are representative of three mice per group. Data are means + standard deviations. **, P < 0.01; ***; P < 0.001 (compared with wild-type controls).
MyD88 signaling is involved in T-cell responses to influenza virus infection.
We further evaluated T-cell proliferation in both MyD88−/− and TRIF−/− mice and found that a defect in MyD88 but not in TRIF was responsible for reduced T-cell activation in the spleen (Fig. 5A). When the Th1/Th2 cytokine balance was compared, it was apparent that MyD88, but not TRIF, was responsible for decreased Th1 cytokines and slightly increased Th2 cytokines (Fig. 5B). Virus antigen-specific CD8 T-cell responses were comparable in wild-type, MyD88−/−, and TRIF−/− mice (Fig. 5C). As illustrated by data from the MyD88−/− TRIF−/− mice (Fig. 3A and B), IgG and IgA Ab levels were identical in wild-type, MyD88−/−, and TRIF−/− mice except for a significant decrease in the saliva of MyD88−/− mice (Fig. 5D). Overall, MyD88 signal, not TRIF, was more crucial for T-cell responses against primary influenza virus infection.
FIG. 5.
MyD88 is more critical in the adaptive immune response than TRIF. Groups of mice were infected with 5 × 102 PFU of PR8 virus, and samples were taken 4 weeks after infection. T-cell proliferation (A), Th1/Th2 cytokine profiles after in vitro culture (B), and virus antigen-specific CD8 T cells (C) were determined as described in the legend of Fig. 2. (D) IgG levels of serum and BALF and IgA levels of serum, BALF, nasal wash (NW), and saliva were determined by ELISA. Results are representative of at least two individual experiments (n ≥ 3 for each group). Data are means + standard deviations. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (compared with wild-type controls).
IPS-1-mediated RLR signaling does not influence susceptibility and adaptive immune responses to influenza virus infection.
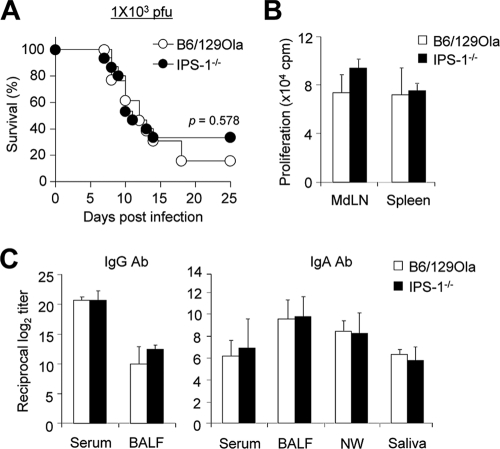
To assess the role of RLR on susceptibility of influenza virus infection, we infected wild-type B6/129Ola mice and IPS-1−/− mice of B6/129Ola background with 1 × 103 PFU (approximate dose of 1× LD50 for B6/129Ola mice) of PR8 virus. The lack of IPS-1 signaling did not affect the susceptibility to influenza virus infection (Fig. 6A). Furthermore, virus-specific T-cell proliferation levels in MdLN and spleen were comparable in both wild-type B6/129Ola and IPS-1−/− mice (Fig. 6B). In addition, similar levels of virus antigen-specific IgG and IgA Ab levels in serum and mucosal compartments were detected in wild-type B6/129Ola and IPS-1−/− mice (Fig. 6C). Taken together, these findings indicate that RLR signaling is not crucial for susceptibility and development of adaptive immunity against influenza virus infection.
FIG. 6.
IPS-1 is not involved in the development of adaptive immune responses and protection. (A) Both wild-type B6/129Ola (n = 13) and IPS-1−/− (n = 15) mice were infected with 1 × 103 PFU of PR8 virus, and survival rates were checked daily. (B and C) Groups of wild-type B6/129Ola (n = 4) and IPS-1−/− (n = 4) mice were infected with 5 × 102 PFU of PR8 virus, and samples were taken 4 weeks after infection. Values are means + standard deviations. Results shown are representative of three experiments (n ≥ 4 for each group). (B) T cell proliferation was determined as described in the legend of Fig. 2. (C) IgG levels of serum and BALF and IgA levels of serum, BALF, nasal wash (NW), and saliva were determined by ELISA.
TLR signaling is dispensable in secondary influenza virus infection.
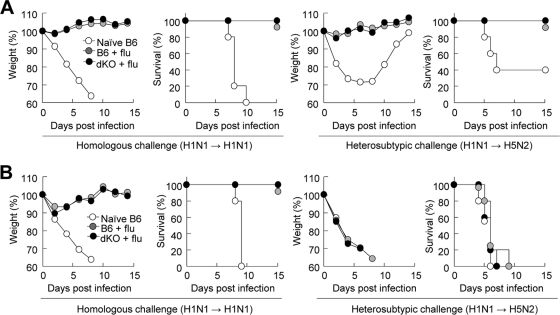
Our previous studies revealed that MyD88 signaling is essential for protection against bacterial infection irrespective of its contribution toward antibody production (23, 40). To assess how TLR signaling contributes to both homologous and heterosubtypic protection, groups of mice immunized with 2 × 104 PFU of PR8 virus (H1N1) were challenged with either 4 × 106 PFU of PR8 virus (H1N1) or 5.1 50% egg infective doses (EID50) of W81 (H5N2) virus (approximately 40× LD50 for B6 mice). Both wild-type and MyD88−/− TRIF−/− mice were completely protected against homologous and heterosubtypic challenge without body weight loss (Fig. 7A). Overall, these data clearly demonstrate that TLR signaling is dispensable for both homologous and heterosubtypic protection against secondary lethal influenza virus infection.
FIG. 7.
TLR signaling is dispensable for protection against secondary influenza virus infection (flu). Wild-type B6 and MyD88−/− TRIF−/− (dKO) mice were primed with 5 × 102 PFU of PR8 virus. (A) Mice were lethally challenged with homologous (4 × 106 PFU of PR8) or heterosubtypic (5.1 EID50 of W81) virus 4 weeks after priming. (B) Serum samples were passively transferred by intraperitoneal injection into wild-type B6 mice, and recipient mice were challenged with 4 × 106 PFU of PR8 or 5.1 EID50 of W81.
We further explored the role of serum Ab for homologous and heterosubtypic protection against lethal influenza virus infection. Both wild-type and MyD88−/− TRIF−/− mice were infected with 5 × 102 PFU of PR8. Serum samples were collected 4 weeks after infection and passively transferred to naive wild-type B6 mice. Then the recipient mice were challenged with either 4 × 106 PFU of PR8 virus (H1N1) or 5.1 EID50 of W81 (H5N2) virus. Regardless of the serum source (wild-type or MyD88−/− TRIF−/− mice), the serum Ab was effective in protecting against homologous challenge but had no effect on heterosubtypic protection (Fig. 7B). These results indicate that serum Ab that induces either a TLR signal-intact or -deficient condition is effective for homologous protection against influenza virus but that it is not sufficient for heterosubtypic protection.
DISCUSSION
The role of MyD88 signal for pathogenesis or protection during bacterial or viral infection is controversial. In previous studies using various infection models, there was less inflammation when MyD88 signaling was deleted, and the pathology associated with coxsackievirus or Burkholderia cenocepacia infection and polymicrobial septic peritonitis was also reduced (13, 56, 58). In contrast, MyD88−/− mice are much more susceptible to vesicular stomatitis virus, herpesvirus, and severe acute respiratory syndrome (SARS)-coronavirus infection than wild-type mice (28, 50, 62). After influenza virus infection, both wild-type and MyD88−/− mice have similar susceptibilities to virus-induced acute pneumonia (29). However, in the present study MyD88−/− mice but not TRIF−/− mice were significantly more susceptible to lethal primary influenza virus infection. It is possible that the differences between our results and those of other investigators are related to different experimental conditions, including virus strain (H1N1 versus H3N2) and infection volume (20 μl versus 50 μl). We extensively reviewed our experimental conditions before testing the susceptibility of MyD88−/− and MyD88−/− TRIF−/− mice to optimize the infection dose and challenge schedule and found that MyD88-deficient mice had higher susceptibility (Fig. 4A). Although we have yet to clarify these discrepancies between the different experimental designs, it is obvious that MyD88-dependent signaling has greater importance in protection than TRIF-mediated signaling against lethal influenza virus infection. In addition, reduced but sufficient adaptive immune responses in MyD88−/− and MyD88−/− TRIF−/− mice after primary sublethal influenza virus infection resulted in efficient protection against a lethal secondary influenza virus infection. As MyD88-deficient mice produce solid antibody responses in the airway mucosa after primary infection, disruption of the early innate immune response that results in a detrimental effect on the lung would be more likely to result in higher susceptibility to infection than the adaptive immune response after primary infection.
We initially expected TLR signaling to play an important role in secondary influenza virus infection as it is a major signal for inducing type I IFN signaling and NF-κB-mediated innate immunity, which are important for activating consecutive immune responses (25, 30). Thus, we were surprised that TLR was dispensable, even against heterosubtypic infection in which CD4 T-cell responses are important in cross-protection (4). We assume that minimal levels of T-cell responses and intact systemic IgG Ab under the TLR-deficient condition are sufficient to provoke efficient protection against secondary homologous and heterosubtypic influenza virus.
Other investigators reported that both TLR and RLR control innate immune response after influenza virus infection (9, 31, 33, 41). The deletion of either the MyD88 or IPS-1 gene cannot diminish the type I IFN response in the lung after influenza virus infection, but this response is shut down in MyD88−/− IPS-1−/− mice (25). TLR and RLR are also cooperative in response to poly(I:C) treatment (27). Nevertheless, in our study, despite the known contribution of IPS-1 to the immune response, IPS-1−/− mice did not show any reduced acquired immune responses (Fig. 6A) and had no altered susceptibility to lethality. Thus, it seems likely that influenza virus infection activates both TLR and RLR and that they compensate each other in induction of the innate immune response. Therefore, TRIF−/− and IPS-1−/− mice might survive with a sufficient innate immune response even if they partially lose recognition signaling (Fig. 4A and 6A). However, MyD88−/− mice were more vulnerable to influenza virus infection than were TRIF−/− and IPS-1−/− mice even though loss of MyD88 can be compensated for by other TLR or RLR signaling. Our observations and those of other investigators regarding higher susceptibility to various pathogens should be considered in the context of MyD88-dependent mechanisms other than the well-described TLR- and RLR-dependent innate immune responses (e.g., NF-κB and type I IFN), such as IL-1- and IL-18-mediated signaling (1). In an earlier study, MyD88 was important in protection while TLR did not contribute to resistance against Pseudomonas aeruginosa infection (43). Further, MyD88−/− mice had greater mortality than TLR-deficient mice when infected with Staphylococcus aureus and Mycobacterium avium (12, 54). Those results suggest the idea that the higher susceptibility of MyD88−/− mice to influenza virus infection shown in our current study may be partially attributed to mechanisms other than TLR signaling.
The lack of MyD88 signaling skews the T-cell response to Th2 dominance regardless of virus or bacterial infection. In this regard, IgG1 is much higher after influenza virus infection in MyD88−/− mice (15, 25). In our previous studies, an oral attenuated Salmonella vaccine strain resulted in Th2-type responses (dominant IgG1 subclasses) and subsequently elicited identical levels of systemic and mucosal Ab responses in MyD88−/− mice (23, 40). In the present study, higher levels of IgG1, lower levels of Th1 cytokines (i.e., IFN-γ and TNF-α), and higher levels of Th2 cytokines (i.e., IL-4 and IL-5) were found in MyD88-deficient mice than in wild-type B6 mice following primary challenge with a sublethal dose of influenza virus (Fig. 2B). These relatively Th2-biased environments could be involved in induction of normal levels of humoral immune responses under MyD88-deficient conditions that have minimal levels of CD4 T-cell responses.
A role for TLR on induction of IgA antibody in the mucosal tissues following influenza virus infection has not been well described. In general, SIgA Ab in the mucosal compartments is recognized as an important component of protection in airway infection (5). However, SIgA was dispensable for prevention of homologous influenza virus infection in one study (35), and its contribution to heterosubtypic cross-protection is unclear (4, 11). Nevertheless, SIgA Ab is important for defense in nasal and respiratory compartments and protective when passively transferred (44-46). Our results in the current study show that TLR- or RLR- or MyD88-mediated innate immunity is not essential for induction of SIgA Ab in the respiratory mucosal compartments (i.e., lung and nasal).
Members of TLR family recognize their own ligand, but their responses vary (52). Furthermore, TLR signals from different cell types cooperate to develop TLR-induced adaptive immune responses (3, 15, 42). Interestingly, this variation seems to lead the host to develop different responses against different antigens. Our previous studies revealed that the Ab response to the Salmonella lipopolysaccharide (LPS) or Salmonella expressing Streptococcus antigen (PspA) was accelerated in MyD88−/− mice (23, 40). We postulated that this unexpected augmentation of the B-cell response is responsible for the increasing numbers of CD11b+ Gr-1+ myeloid cells (submitted for publication). Likewise, oral Salmonella administration and intranasal challenge of influenza virus recruit myeloid cells at the site of infection; however, influenza virus fails to induce hyperactive IgG and IgA responses in MyD88−/− and MyD88−/− TRIF−/− mice. In addition, MyD88 signal is critical for protection against both Salmonella and Streptococcus pneumoniae and noninvasive bacterial infection, regardless of the importance of mucosal Ab for protection (23, 40). However, in the current study, TLR signaling was dispensable for protection against homologous and heterosubtypic influenza virus infection. Although the underlying mechanism for the role of MyD88 in bacterial infection remains under investigation, our new findings clearly suggest that MyD88 may have a reciprocal role against bacterial and viral infections.
For development of more efficient vaccines, various TLR agonists have been suggested as adjuvant candidates (34). The aim of stimulation of TLR signaling is to induce stronger and/or optimal immune responses and to manipulate the type of response required for better protection. The influenza virus, which has pandemic potential, must have a broad-spectrum vaccine because it can readily mutate, and outbreaks are unpredictable (17). Therefore, to preempt a future pandemic a vaccine must provide strong heterosubtypic immunity (10). When we examined the contribution of TLR signaling on secondary heterosubtypic protection, we found that sublethal live virus infection can induce efficient heterosubtypic protection independent of TLR signaling. This suggests that live vaccine could achieve immunity in a TLR-independent manner, which would be advantageous over an inactivated vaccine with TLR-dependent protection (25).
To extend our current findings, we anticipate further studies to find TLR-dependent and -independent factors that play key roles in protection during primary virus infection. We will also explore the underlying mechanisms of TLRs against bacteria and virus infections.
Acknowledgments
The W81 H5N2 strain was kindly provided by Young Ki Choi, Chungbuk University, South Korea.
This study was supported by the governments of the Republic of Korea, Sweden, and Kuwait, by a National Research Foundation of Korea (NRH) grant funded by the Korean government (MEST, 2010-0029133), and by a Top Brand Project grant from the Korea Research Council of Fundamental Science and Technology and KRIBB Initiative program (KGM3110912).
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Archer, K. A., L. Alexopoulou, R. A. Flavell, and C. R. Roy. 2009. Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cell Microbiol. 11:21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr, T. A., S. Brown, P. Mastroeni, and D. Gray. 2009. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J. Immunol. 183:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. A. Misplon, C. Y. Lo, R. R. Brutkiewicz, S. A. Prasad, and S. L. Epstein. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J. Immunol. 166:7437-7445. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., F. L. Jahnsen, I. N. Farstad, and G. Haraldsen. 1997. Mucosal immunology of the upper airways: an overview. Ann. N. Y. Acad. Sci. 830:1-18. [DOI] [PubMed] [Google Scholar]
- 6.Campos, M. A., M. Closel, E. P. Valente, J. E. Cardoso, S. Akira, J. I. Alvarez-Leite, C. Ropert, and R. T. Gazzinelli. 2004. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J. Immunol. 172:1711-1718. [DOI] [PubMed] [Google Scholar]
- 7.Cottalorda, A., B. C. Mercier, F. M. Mbitikon-Kobo, C. Arpin, D. Y. Teoh, A. McMichael, J. Marvel, and N. Bonnefoy-Berard. 2009. TLR2 engagement on memory CD8+ T cells improves their cytokine-mediated proliferation and IFN-gamma secretion in the absence of Ag. Eur. J. Immunol. 39:2673-2681. [DOI] [PubMed] [Google Scholar]
- 8.Delano, M. J., P. O. Scumpia, J. S. Weinstein, D. Coco, S. Nagaraj, K. M. Kelly-Scumpia, K. A. O'Malley, J. L. Wynn, S. Antonenko, S. Z. Al-Quran, R. Swan, C. S. Chung, M. A. Atkinson, R. Ramphal, D. I. Gabrilovich, W. H. Reeves, A. Ayala, J. Phillips, D. Laface, P. G. Heyworth, M. Clare-Salzler, and L. L. Moldawer. 2007. MyD88-dependent expansion of an immature GR-1+ CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. C., S. J. Turner, R. G. Webby, and P. G. Thomas. 2006. Influenza and the challenge for immunology. Nat. Immunol. 7:449-455. [DOI] [PubMed] [Google Scholar]
- 11.Epstein, S. L., C. Y. Lo, J. A. Misplon, C. M. Lawson, B. A. Hendrickson, E. E. Max, and K. Subbarao. 1997. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, β2-microglobulin-deficient, and J. chain-deficient mice. J. Immunol. 158:1222-1230. [PubMed] [Google Scholar]
- 12.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758-4764. [DOI] [PubMed] [Google Scholar]
- 13.Fuse, K., G. Chan, Y. Liu, P. Gudgeon, M. Husain, M. Chen, W. C. Yeh, S. Akira, and P. P. Liu. 2005. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation 112:2276-2285. [DOI] [PubMed] [Google Scholar]
- 14.Graham, M. B., and T. J. Braciale. 1997. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 186:2063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heer, A. K., A. Shamshiev, A. Donda, S. Uematsu, S. Akira, M. Kopf, and B. J. Marsland. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178:2182-2191. [DOI] [PubMed] [Google Scholar]
- 16.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 17.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3:591-600. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki, A., and R. Medzhitov. 2010. Regulation of adaptive immunity by the innate immune system. Science 327:291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewell, N. A., N. Vaghefi, S. E. Mertz, P. Akter, R. S. Peebles, Jr., L. O. Bakaletz, R. K. Durbin, E. Flano, and J. E. Durbin. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J. Virol. 81:9790-9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, T., and S. Akira. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373-384. [DOI] [PubMed] [Google Scholar]
- 22.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 23.Ko, H. J., J. Y. Yang, D. H. Shim, H. Yang, S. M. Park, R. Curtiss, 3rd, and M. N. Kweon. 2009. Innate immunity mediated by MyD88 signal is not essential for induction of lipopolysaccharide-specific B cell responses but is indispensable for protection against Salmonella enterica serovar Typhimurium infection. J. Immunol. 182:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopf, M., F. Brombacher, and M. F. Bachmann. 2002. Role of IgM antibodies versus B cells in influenza virus-specific immunity. Eur. J. Immunol. 32:2229-2236. [DOI] [PubMed] [Google Scholar]
- 25.Koyama, S., K. J. Ishii, H. Kumar, T. Tanimoto, C. Coban, S. Uematsu, T. Kawai, and S. Akira. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179:4711-4720. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, H., T. Kawai, H. Kato, S. Sato, K. Takahashi, C. Coban, M. Yamamoto, S. Uematsu, K. J. Ishii, O. Takeuchi, and S. Akira. 2006. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203:1795-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, H., S. Koyama, K. J. Ishii, T. Kawai, and S. Akira. 2008. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180:683-687. [DOI] [PubMed] [Google Scholar]
- 28.Lang, K. S., A. A. Navarini, M. Recher, P. A. Lang, M. Heikenwalder, B. Stecher, A. Bergthaler, B. Odermatt, S. Akira, K. Honda, H. Hengartner, and R. M. Zinkernagel. 2007. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur. J. Immunol. 37:2434-2440. [DOI] [PubMed] [Google Scholar]
- 29.Le Goffic, R., V. Balloy, M. Lagranderie, L. Alexopoulou, N. Escriou, R. Flavell, M. Chignard, and M. Si-Tahar. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Goffic, R., J. Pothlichet, D. Vitour, T. Fujita, E. Meurs, M. Chignard, and M. Si-Tahar. 2007. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178:3368-3372. [DOI] [PubMed] [Google Scholar]
- 31.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 33.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manicassamy, S., and B. Pulendran. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbawuike, I. N., S. Pacheco, C. L. Acuna, K. C. Switzer, Y. Zhang, and G. R. Harriman. 1999. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J. Immunol. 162:2530-2537. [PubMed] [Google Scholar]
- 36.McGill, J., J. W. Heusel, and K. L. Legge. 2009. Innate immune control and regulation of influenza virus infections. J. Leukoc. Biol. 86:803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercier, B. C., A. Cottalorda, C. A. Coupet, J. Marvel, and N. Bonnefoy-Berard. 2009. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J. Immunol. 182:1860-1867. [DOI] [PubMed] [Google Scholar]
- 38.Neumann, G., T. Noda, and Y. Kawaoka. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, K. G., J. M. Wood, and M. Zambon. 2003. Influenza. Lancet 362:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, S. M., H. J. Ko, D. H. Shim, J. Y. Yang, Y. H. Park, R. Curtiss, 3rd, and M. N. Kweon. 2008. MyD88 signaling is not essential for induction of antigen-specific B cell responses but is indispensable for protection against Streptococcus pneumoniae infection following oral vaccination with attenuated Salmonella expressing PspA antigen. J. Immunol. 181:6447-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 42.Rahman, A. H., D. K. Taylor, and L. A. Turka. 2009. The contribution of direct TLR signaling to T cell responses. Immunol. Res. 45:25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramphal, R., V. Balloy, M. Huerre, M. Si-Tahar, and M. Chignard. 2005. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J. Immunol. 175:3927-3934. [DOI] [PubMed] [Google Scholar]
- 44.Renegar, K. B., and P. A. Small, Jr. 1991. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J. Virol. 65:2146-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renegar, K. B., and P. A. Small, Jr. 1991. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 146:1972-1978. [PubMed] [Google Scholar]
- 46.Renegar, K. B., P. A. Small, Jr., L. G. Boykins, and P. F. Wright. 2004. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173:1978-1986. [DOI] [PubMed] [Google Scholar]
- 47.Richer, M. J., D. J. Lavallee, I. Shanina, and M. S. Horwitz. 2009. Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PLoS One 4:e4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers, K. A., A. B. Rogers, B. A. Leav, A. Sanchez, E. Vannier, S. Uematsu, S. Akira, D. Golenbock, and H. D. Ward. 2006. MyD88-dependent pathways mediate resistance to Cryptosporidium parvum infection in mice. Infect. Immun. 74:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 50.Sheahan, T., T. E. Morrison, W. Funkhouser, S. Uematsu, S. Akira, R. S. Baric, and M. T. Heise. 2008. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 4:e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song, M. S., P. N. Pascua, J. H. Lee, Y. H. Baek, O. J. Lee, C. J. Kim, H. Kim, R. J. Webby, R. G. Webster, and Y. K. Choi. 2009. The polymerase acidic protein gene of influenza a virus contributes to pathogenicity in a mouse model. J. Virol. 83:12325-12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takada, A., S. Matsushita, A. Ninomiya, Y. Kawaoka, and H. Kida. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212-3218. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi, O., and S. Akira. 2010. Pattern recognition receptors and inflammation. Cell 140:805-820. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 55.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 56.Ventura, G. M., V. Balloy, R. Ramphal, H. Khun, M. Huerre, B. Ryffel, M. C. Plotkowski, M. Chignard, and M. Si-Tahar. 2009. Lack of MyD88 protects the immunodeficient host against fatal lung inflammation triggered by the opportunistic bacteria Burkholderia cenocepacia. J. Immunol. 183:670-676. [DOI] [PubMed] [Google Scholar]
- 57.Villamon, E., D. Gozalbo, P. Roig, C. Murciano, J. E. O'Connor, D. Fradelizi, and M. L. Gil. 2004. Myeloid differentiation factor 88 (MyD88) is required for murine resistance to Candida albicans and is critically involved in Candida-induced production of cytokines. Eur. Cytokine Netw. 15:263-271. [PubMed] [Google Scholar]
- 58.Weighardt, H., S. Kaiser-Moore, R. M. Vabulas, C. J. Kirschning, H. Wagner, and B. Holzmann. 2002. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 169:2823-2827. [DOI] [PubMed] [Google Scholar]
- 59.Wiersinga, W. J., C. W. Wieland, J. J. Roelofs, and T. van der Poll. 2008. MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei. PLoS One 3:e3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, X., L. Gao, L. Lei, Y. Zhong, P. Dube, M. T. Berton, B. Arulanandam, J. Zhang, and G. Zhong. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 183:1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zucchini, N., G. Bessou, S. Traub, S. H. Robbins, S. Uematsu, S. Akira, L. Alexopoulou, and M. Dalod. 2008. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180:5799-5803. [DOI] [PubMed] [Google Scholar]