Abstract
The high-risk human papillomavirus (HPV) E6 and E7 oncoproteins are critical to the immortalization of keratinocytes. HPV type 16 (HPV16) E6 interacts with endogenous proteins to activate hTERT, the catalytic subunit of telomerase, thus avoiding cellular senescence signals. NFX1-123, the longer splice variant of NFX1, interacts with HPV16 E6, as well as cytoplasmic poly(A) binding proteins 1 and 4 (PABPC1 and PABPC4). HPV16 E6 affects hTERT expression posttranscriptionally through NFX1-123, as NFX1-123 interacts with hTERT mRNA and stabilizes it, leading to greater telomerase activity. The PAM2 motif of NFX1-123, with which it binds PABPCs, is required for the posttranscriptional regulation of hTERT by HPV16 E6 and NFX1-123. There is increasing evidence that RNA and DNA viruses utilize RNA-processing proteins, and specifically PABPCs, in the normal virus life cycle, and there is also evidence that RNA-processing proteins are perturbed in cancers. Here, we show that PABPCs are critical in hTERT regulation by HPV16 E6. Although the amount and cellular localization of PABPCs were largely unchanged in cervical cancer cell lines with or without HPV16 and in human foreskin keratinocytes (HFKs) with or without HPV16 E6, knockdown of PABPCs decreased hTERT mRNA and telomerase activity and overexpression of PABPC4 increased these in HPV16 E6-expressing HFKs. In contrast, knockdown of PABPCs in C33A cells had no effect on hTERT mRNA or telomerase activity. Additionally, overexpression of PABPC4 and hTERT led to greater growth of cultured HPV16 E6-expressing HFKs. This is the first evidence that PABPCs have a targeted role in hTERT regulation leading to a growth advantage in cells expressing HPV16 E6.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection, and high-risk HPV types are associated with anogenital and cervical cancers (12, 13, 38, 39, 47). The oncoproteins of high-risk HPV, E7 and E6, are universally expressed in HPV-associated cancers. E7 targets retinoblastoma protein for degradation and allows S-phase genes to be expressed and DNA synthesis to occur (11, 14, 22, 35). E6 with E6-associated protein (E6AP) activates the catalytic subunit of telomerase, hTERT, blocking cellular senescence signals (18, 36, 45, 46). Most studies of hTERT derepression by E6/E6AP have focused on both cis and trans elements at the promoter, as well as chromatin remodeling (23, 49). However, we have found that in HPV type 16 (HPV16) E6-expressing human foreskin keratinocytes (HFKs), hTERT has both its promoter and its mRNA regulated by two endogenous splice variant isoforms of NFX1, NFX1-91 and NFX1-123 (19, 25, 26, 49).
NFX1-91 is a constitutive transcriptional repressor of hTERT. It binds to the hTERT promoter at a proximal X1 box and recruits histone deacetylase activity to the hTERT promoter (19, 49). As a repressor, NFX1-91 is targeted for ubiquitin-mediated degradation by HPV16 E6/E6AP to allow for transcriptional activation of hTERT (19). NFX1-123 is a cytoplasmic protein, and it is required for augmentation of hTERT expression once the hTERT mRNA is produced. NFX1-123 associates with hTERT mRNA, increases its stability, and leads to greater telomerase activity in HPV16 E6-expressing HFKs (25, 26). NFX1-123 interacts with multiple RNA-processing proteins (25), so understanding the roles of NFX1-123 and HPV in RNA regulation is important.
Once a gene is transcribed, the RNA product is most often spliced and the guanidine is methylated (yielding m7G) at the start of the RNA 5′ untranslated region (5′ UTR). The 5′ cap structure is bound by the eukaryotic initiation factor 4F (eIF4F) complex, which includes eIF4E (which binds the 5′ m7G cap), eIF4A (RNA helicase), and eIF4G (a scaffold protein for eIF4E and eIF4A). A poly(A) tail is added to the 3′ UTR of mRNA, and cytoplasmic poly(A) binding proteins (PABPCs) bind to this repetitive sequence. The scaffold protein eIF4G binds to PABPCs, creating a closed-loop formation of mRNA between the 5′ and 3′ ends and a bridge between eIF4E and PABPCs (2, 48).
PABPCs have critical roles in RNA processing beyond simply binding to poly(A) sequences. They shuttle from the nucleus to the cytoplasm with mRNAs, increase eIF4F assembly at caps, form closed-looped RNA, aid in recruitment of ribosomal subunits to 5′ UTRs, and increase reuse of translational machinery after polypeptide synthesis (for a review, see references 31 and 34). PABPCs bind to RNA through their four N-terminal RNA recognition motifs (RRMs), and they interact with other proteins that contain a poly(A) binding protein-interacting motif (PAM2) through their C-terminal poly(A) binding (PAB) domain (the PABC or MLLE domain) (1, 27-30). NFX1-123 has a PAM2 motif at its N terminus that is key to both its interaction with PABPCs and its regulation of hTERT (26).
As evidence of their importance, there are four PABPCs. PABPC1 is the most abundant PABPC and has been studied most widely. PABPC3 is encoded by an intronless gene, with significant homology to the PABPC1 gene, that is expressed only in the testis (16). PABPC4, also known as inducible poly(A) binding protein (iPABP), is widely expressed like PABPC1 and is upregulated during T-cell activation (41, 50). The PABPC5 gene is found on the X chromosome, but unlike PABPC1, PABPC3, or PABPC4, PABPC5 lacks the C-terminal domain (31).
Just as genetic and epigenetic changes during oncogenesis have been studied, increasing evidence that RNA-processing proteins have a role in cell proliferation and cancer development has been obtained. The initiation factor eIF4B increases ribosomal recruitment and is critical for normal cell cycle regulation, as its knockdown blunts translation and leads to apoptosis (40). The cap protein eIF4E has been identified as being upregulated in prostate cancer (20), and RNA binding and -processing proteins are increased in cervical cancer models and in cervical dysplasia samples (15, 37).
Cellular RNA-processing proteins are often targets of RNA and DNA viruses. PABPCs can be recruited to translate viral RNA and disrupt normal cellular protein production (for a review, see reference 43). Rotavirus can displace PABPCs from cellular mRNA, shifting PABPCs to the nucleus (21). Poliovirus can cleave PABPCs and other initiation factors to decrease normal cellular protein production, increase its own viral translation, and switch from protein production to RNA replication as a part of its viral life cycle (10, 32, 44). Kaposi's sarcoma-associated herpesvirus protein K10/10.1 interacts with PABPC, and the herpes simplex virus type 1 immediate early ICP27 protein binds PABPC as well as other initiation factors (17, 24).
In this study, we wanted to determine the direct role of PABPCs in hTERT regulation, key in HPV-associated oncogenic progression. We found that cervical cancer cell lines did not affect gross localization or expression of PABPCs and neither did expression of HPV16 E6 alone in HFKs. However, PABPCs did play a specific role in hTERT regulation, as PABPC expression levels affected the amounts of hTERT mRNA produced and telomerase activity detected when HPV16 E6 was expressed. Additionally, PABPC4 expression levels affected the growth of HPV16 E6-expressing HFKs in culture in parallel to the increases in hTERT mRNA and telomerase activity. This is the first evidence that PABPCs can have a targeted effect on specific mRNAs and that PABPCs can affect the growth of HPV16 E6-expressing HFKs.
MATERIALS AND METHODS
Plasmids.
The scramble and PABPC1 short hairpin 1 (sh1PABPC1) plasmids have been described previously (25, 52). PABPC1 short hairpin 2 (sh2PABPC1), PABPC4 short hairpin 2 (sh2PABPC4), and PABPC4 short hairpin 3 (sh3PABPC4) plasmids were made with the following phosphorylated and annealed oligonucleotides: for sh2PABC1, 5′ GATCCAACCCCTACCAGCCAGCACCTTTCAAGAGAAGGTGCTGGCTGGTAGGGGTTTTTTTTGG 3′ (forward) and 5′ CAACCAACCCCTACCAGCCAGCACCTTTCAAGAGAAGGTGCTGGCTGGTAGGGGTTTTTTTTGGAATT 3′ (reverse); for sh2PABPC4, 5′ GATCCAAGACTTCCTAGCTTGTCCTATTCAAGAGATAGGACAAGCTAGGAAGTCTTTTTTTTGG 3′ (forward) and 5′ CAACCAAGACTTCCTAGCTTGTCCTATTCAAGAGATAGGACAAGCTAGGAAGTCTTTTTTTTGGAATT 3′ (reverse); and for sh3PABPC4, 5′ GATCCAATATGTTCTTTGGGTTCTGCTTCAAGAGAGCAGAACCCAAAGAACATATTTTTTTTGG 3′ (forward) and 5′ CAACCAATATGTTCTTTGGGTTCTGCTTCAAGAGAGCAGAACCCAAAGAACATATTTTTTTTGGAATT 3′ (reverse). These oligonucleotides were ligated into the C-FUGW vector BamHI and EcoRI sites.
Tissue culture and cell count.
Primary human keratinocytes (HFKs) were derived from neonatal human foreskins and grown in EpiLife medium supplemented with calcium chloride (60 μM), human keratinocyte growth supplement (Invitrogen, Carlsbad, CA), and penicillin-streptomycin. 293T, C33A, and SiHa cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum and penicillin-streptomycin.
Retroviruses were produced transiently in 293T cells by a vesicular stomatitis virus G-pseudotyped virus production protocol as described previously (5) or by a lentivirus protocol as described previously (33). The hTERT cDNA was cloned by PCR from a HeLa cDNA library (Clontech) and was subsequently transferred into control vector LXSN for retroviral expression (7). All retrovirus was concentrated by ultracentrifugation, mixed with 8 μg/ml Polybrene, and incubated with HFKs at 50 to 60% confluence. Three hours later, EpiLife medium (Cascade Biologics, Portland, OR) was replaced. Twenty-four hours later, the cells were expanded, and 48 h later, cells were subjected to selection with neomycin/G418 (50 μg/ml) or puromycin (0.5 μg/ml) if required. All lentivirus infections were confirmed by green fluorescent protein (GFP) expression, and cells were collected at 72 h without selection.
For daily cell counts, HFKs were seeded at a density of 1.5 × 105 to 2.0 × 105 cells per 60-mm plate. Cells on triplicate plates were counted every 24 h for 4 days.
Quantitative real-time PCR.
HFK cells were lysed with 1 ml TRIzol reagent (Invitrogen, Carlsbad, CA) and purified by chloroform extraction and isopropanol precipitation. One microgram of total RNA was used to generate cDNA with random hexamer primers and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative real-time PCR (qPCR) was conducted with Sybr green (ABI, Foster City, CA) on an ABI Prism 9700 instrument, and results were analyzed with SDS 2.2.2 software. Samples were amplified in triplicate and normalized to the level of mRNA for 36B4, an endogenous ribosomal protein which served as the control. Primer sequences for PABPC1 were 5′ GCAGACGGAACTTAAGCGC 3′ (forward) and 5′ CTGGGGAGGAGAAACATACAAAAC 3′ (reverse), and those for PABPC4 were 5′ CTCAGGGAAGGCCTCCAT 3′ (forward) and 5′ GAGCAGCAGCAGCAACAG 3′ (reverse). Primer sequences for hTERT and 36B4 were described previously (18). Error bars represent 95% confidence intervals (95% CI) for the following quotient: result for gene of interest/result for 36B4 gene. Statistical significance was calculated by using a two-tailed unpaired t test.
Reverse transcription-PCR (RT-PCR).
cDNAs were produced as described above. PCR amplification was then performed to identify the 254-bp amplicon with the following hemagglutinin (HA)-PABPC4 primers: 5′ GGAACAGAAGCAGATGCTGG 3′ (forward) and 5′ GAACATCATATGGATAAGAGGTAGCA 3′ (reverse). The primers for 36B4 were described previously (18).
Western blot analyses.
Western blots were probed with the following antibodies: anti-PABPC1 [designated (pan) PABPC in the figures; 1:1,000 (Cell Signaling, Danvers, MA)], anti-PABPC1 (designated PABPC1 in the figures; 1:5,000 [Abcam, Cambridge, MA]), anti-PABPC4 (1:2,000; Bethyl, Montgomery, TX), anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; 1:100,000 [Abcam, Cambridge, MA]), and anti-HA.11 clone 16B12 (1:1,000; Covance, Emeryville, CA).
Telomerase activity.
Telomerase activity was detected using the TRAPeze detection kit (Millipore, Billerica, MA) as described previously (19).
Immunofluorescence and deconvolution microscopy.
HFKs and SiHa and C33A cells were plated onto coverslips, fixed with 4% paraformaldehyde, and stored in phosphate-buffered saline (PBS). Cells were permeabilized (using 0.5% Triton X-100 in PBS) for 5 min, blocked (using 0.2% Tween 20 and 5% goat serum in PBS) for 30 min at 37°C, and washed twice in PBS. Cells were probed for 1 to 2 h at 37°C with anti-PABPC1 (1:310; Abcam, Cambridge, MA) and then washed four times with PBS. Cells were probed for 1 h at 37°C with goat anti-rabbit Alexa Fluor 488 secondary antibody (1:1,000; Invitrogen, Carlsbad, CA), washed four times in PBS, and stained with DAPI (4′,6-diamidino-2-phenylindole).
Deconvolution analysis was described previously (26).
RESULTS
PABPC1 and PABPC4 are expressed in cervical cancer cell lines.
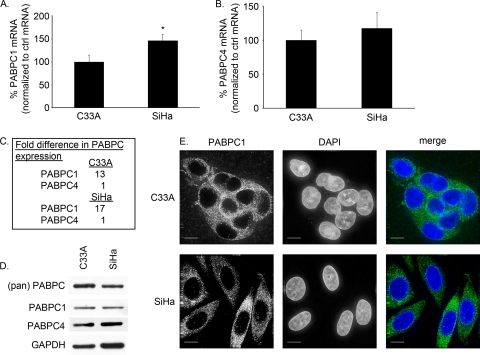
As viruses can perturb the amount of PABPCs expressed in cells, we wanted to determine whether this occurred in HPV-positive versus HPV-negative cell lines. SiHa cells (an HPV16-positive cervical cancer cell line) (4) had approximately 150% the amount of PABPC1 mRNA found in C33A cells (an HPV-negative cervical cancer cell line) (3, 51) by qPCR (Fig. 1A). The amount of PABPC4 mRNA was slightly increased in SiHa cells, at 117% the amount in C33A cells; however, this difference was not significant (Fig. 1B).
FIG. 1.
PABPC1 and PABPC4 were expressed in HPV16-negative and -positive cell lines C33A and SiHa. (A to C) mRNA levels were detected by qPCR, normalized to the level of mRNA for 36B4, an endogenous ribosomal protein (control [ctrl]), and then expressed as a ratio. (A) PABPC1 mRNA levels were detected by qPCR, and in SiHa cells PABPC1 was expressed at 150% the amount found in C33A cells (*, P = 0.01; error bars indicate 95% CI). (B) PABPC4 mRNA levels were detected by qPCR and found to be similar in C33A and SiHa cells (P = 0.29; error bars indicate 95% CI). (C) PABPC1 and PABPC4 mRNAs were expressed in C33A and SiHa cells at ratios of 13:1 and 17:1, respectively. (D) Western blot analysis of whole-cell extracts showed equal levels of expression of all PABPCs and specifically PABPC1 and PABPC4 in C33A and SiHa cells. (pan) PABPC, antibody to region common to all PABPCs; PABPC1, antibody to unique region of PABPC1; PABPC4, antibody to unique region of PABPC4; GAPDH, loading control. (E) C33A and SiHa cells expressed PABPC1 primarily in the cytoplasm, although there was a minimal amount of PABPC1 found in the nuclei of SiHa cells, in contrast to C33A cells. (Green, PABPC1; blue, DAPI; white bars, 10 μm.)
PABPC1 has been the most studied of the PABPCs; however, the expression level of PABPC1 relative to other PABPCs in primary cancers or cancer cell lines has not been examined. By qPCR, C33A cells were found to have a ratio of PABPC1 mRNA to PABPC4 mRNA of 13:1, and SiHa cells had a ratio of 17:1 (Fig. 1C). Thus, PABPC1 is the predominant PABPC expressed in these cell lines, although both PABPC1 and PABPC4 RNAs are expressed abundantly compared to other common RNAs (data not shown).
PABPC1 and PABPC4 levels in these cervical cancer cell lines were also studied. Despite the differences in PABPC1 mRNA in SiHa and C33A cells, the total amounts of PABPC1 in the two cervical cancer cell lines were relatively similar, and PABPC4 levels remained equivalent (Fig. 1D).
To determine whether HPV affected the localization of PABPCs, cells were stained for immunofluorescence of PABPC1. In C33A cells, PABPC1 was primarily in the cytoplasm. In SiHa cells, PABPC1 was also primarily cytoplasmic, although there was some punctate staining of PABPC1 in the nucleus not seen in C33A cells (Fig. 1E). In Western blot analyses of cytoplasmic and nuclear protein extracts, PABPC1 and PABPC4 remained in the cytoplasm of C33A cells but, again, a small shift of PABPC1 and PABPC4 in SiHa cells could be detected (data not shown). Thus, although SiHa cells did have a subtle shift in expression of PABPC1 mRNA and of PABPCs found in the nucleus compared to C33A cells, this contrast was subtle.
PABPC1 and PABPC4 are expressed in HFKs.
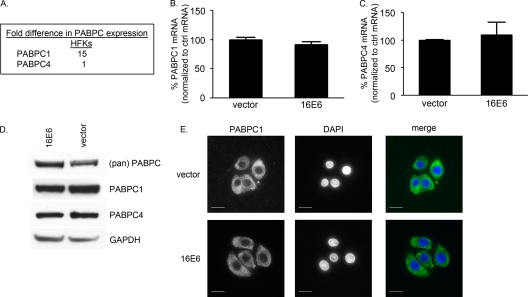
C33A and SiHa cells are cervical cancer cell lines, which may have additional changes in their RNA and protein expression levels that do not necessarily reflect the normal expression pattern in HFKs. Additionally, since we know that both HPV16 E6 and PABPCs partner with NFX1-123, we wanted to study any targeted changes in PABPC expression and localization in HFKs when HPV16 E6 was expressed without other HPV proteins. Therefore, the typical expression pattern of PABPCs in HFKs was examined.
PABPC1 and PABPC4 mRNAs were expressed at a 15:1 ratio in HFKs (Fig. 2A). This was within the range for C33A and SiHa cells. The ratio of PABPC1 and PABPC4 mRNAs was not altered by expression of HPV16 E6 (data not shown), nor was the total amount of PABPC1 or PABPC4 mRNA changed (Fig. 2B and C). By Western blot analysis, PABPC1 and PABPC4 expression was unchanged by expression of HPV16 E6 (Fig. 2D), and the cellular localization determined by immunofluorescent staining for PABPC1 was also unchanged (Fig. 2E). Therefore, in keratinocytes, PABPC1 and PABPC4 were normally expressed in a manner similar to that in cervical cancer cell lines, regardless of whether the cells were HPV16 positive or negative, and this expression pattern was unchanged with expression of HPV16 E6 alone. Although the regulation of hTERT by HPV16 E6 requires NFX1-123 expression and NFX1- 123 binds to PABPCs, HPV16 E6 did not directly perturb the expression or localization of these RNA binding proteins.
FIG. 2.
PABPC1 and PABPC4 were expressed in HFKs with and without HPV16 E6. (A to C) mRNA levels were detected by qPCR, normalized to the level of mRNA for 36B4, an endogenous ribosomal protein (control [ctrl]), and then expressed as a ratio. (A) PABPC1 and PABPC4 were expressed in HFKs. Their relative mRNA expression levels were approximately 15:1. (B) PABPC1 mRNA levels were detected by qPCR and were similar in HFKs with and without HPV16 E6 (P = 0.14; error bars indicate 95% CI). (C) PABPC4 mRNA levels were detected by qPCR and were similar in HFKs with and without HPV16 E6 (P = 0.35; error bars indicate 95% CI). (D) Western blot analysis of whole-cell extracts showed equal levels of expression of all PABPCs and specifically PABPC1 and PABPC4 in HFKs with and without HPV16 E6. (pan) PABPC, antibody to region common to all PABPCs; PABPC1, antibody to unique region of PABPC1; PABPC4, antibody to unique region of PABPC4; GAPDH, loading control. (E) HFKs with and without HPV16 E6 expressed PABPC1 in the cytoplasm. (Green, PABPC1; blue, DAPI; white bars, 10 μm.)
PABPC1 and PABPC4 are required for hTERT expression in HPV16 E6-expressing HFKs.
Despite the fact that PABPCs are similarly expressed in HFKs with and without HPV16 E6, it was important to understand whether HPV16 E6-expressing HFKs directly utilized PABPC1 or PABPC4, or both, to regulate hTERT. We previously found that NFX1-123 expression levels affect hTERT mRNA and telomerase activity and that the PAM2 motif of NFX1-123, to which it binds PABPC1 and PABPC4, is critical for this effect (25, 26). Therefore, we wanted to determine whether reducing the endogenous levels of PABPC1 or PABPC4, or both, would have targeted effects on hTERT mRNA and telomerase activity.
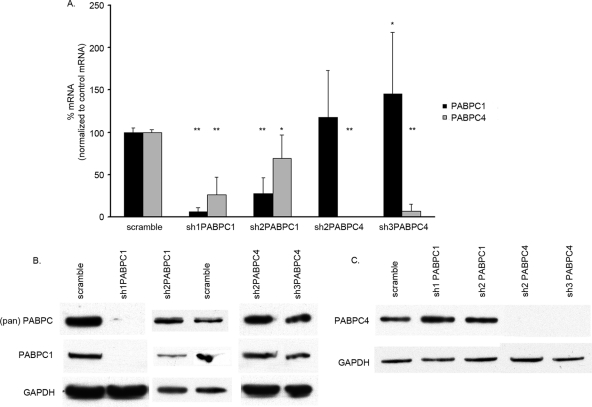
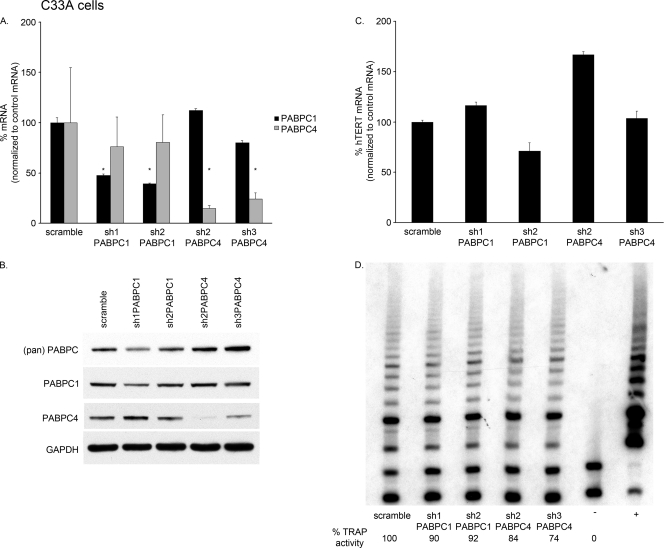
We designed a series of short hairpin RNAs (shRNAs) against specific sequences of PABPC1, PABPC4, or regions common to the two. By using qPCR and Western blot analysis, we then studied the effects of these shRNAs in reducing each PABPC 72 h after infection. Although the degree of sequence homology between PABPC1 and PABPC4 is high, we were able to target the individual PABPCs in different manners with the four shRNAs (Fig. 3). Each shRNA was named based on the PABPC mRNA on which it had greater effect, although most shRNAs affected both PABPC1 and PABPC4. sh1PABPC1 (52) knocked down PABPC1 to less than 10% and PABPC4 to 27% of the levels in the presence of control (scramble) shRNA. sh2PABPC1 knocked down PABPC1 to 27% and PABPC4 to approximately 70% of the control levels. sh2PABPC4 knocked down PABPC4 to only 1% of the control level but had no effect on PABPC1. sh3PABPC4 knocked down PABPC4 to less than 10% of the control level but increased PABPC1 to nearly 1.5-fold the control level (Fig. 3A).
FIG. 3.
PABPC1 and PABPC4 knockdown by shRNA in HPV16 E6-expressing HFKs. (A) PABPC1 and PABPC4 mRNA levels were detected by qPCR and graphed as percentages after normalization to levels in the presence of the scramble control. All P values were calculated by comparing results for an shRNA to results for the scramble control. sh1PABPC1 knocked down PABPC1 mRNA to 7% and PABPC4 to 27% (**, P < 0.0001 for both). sh2PABPC1 knocked down PABPC1 mRNA to 27% and PABPC4 to 69% (**, P < 0.0001; *, P = 0.0029). sh2PABPC4 knocked down PABPC4 to 1% (**, P < 0.0001). sh3PABPC4 knocked down PABPC4 mRNA to 8% and increased PABPC1 mRNA levels to 146% (**, P < 0.0001; *, P = 0.0421). These data are a summary of results from triplicate qPCRs conducted with three HPV16 E6-expressing HFK cell lines, with values normalized to 36B4 control mRNA levels. (Error bars, 95% CI.) (B) Western blot analysis of whole-cell extracts showed knockdown of PABPC1 by sh1PABPC1 and reduction of PABPC1 by sh2PABPC1 compared to levels in the presence of the scramble vector and a loading control. (pan) PABPC, antibody to region common to all PABPCs; PABPC1, antibody to unique region of PABPC1; GAPDH, loading control. (C) Western blot analysis of whole-cells extracts showed knockdown of PABPC4 by sh2PABPC4 and sh3PABPC4 compared to levels in the presence of the scramble shRNA and the loading control. PABPC4, antibody to unique region of PABPC4; GAPDH, loading control.
The knockdown of PABPC1 and PABPC4 in HPV16 E6-expressing HFKs was also confirmed by Western blot analysis (Fig. 3B). sh1PABPC1 caused a marked reduction and sh2PABPC1 caused a subtle reduction in PABPC1 levels (Fig. 3B). Lysates containing sh2PABPC4 and sh3PABPC4 showed no expression of PABPC4 (Fig. 3C). The reduction of PABPC4 by sh1PABPC1 and sh2PABPC1 seen at the mRNA level was not detected by Western blot analysis, nor was the increase in PABPC1 mRNA seen with sh3PABPC4 expression. The lack of differences may be due to the only semiquantitative nature of Western blot analysis.
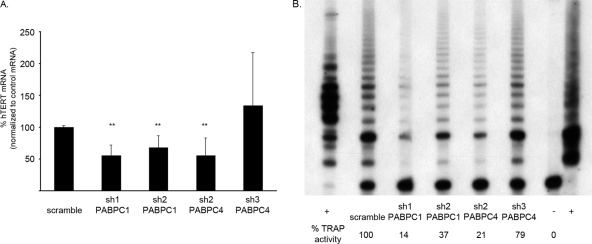
In HPV16 E6-expressing HFKs in which both PABPC1 and PABPC4 were reduced, there was a decrease in hTERT mRNA levels by approximately half compared to those in the presence of the shRNA scramble control (Fig. 4A). Additionally, when the level of PABPC4 was reduced to only 1%, hTERT mRNA levels were also cut in half (Fig. 4A). However, when PABPC4 was decreased by shRNA to 8% but PABPC1 mRNA levels were increased, hTERT mRNA levels were unaffected (Fig. 4A). These data show that despite the fact that PABPCs bind to a repetitive sequence common to all mRNAs, in HPV16 E6-expressing HFKs there is some selectivity in their function. Additionally, although PABPC4 is much less abundant than PABPC1, its expression is critical. Removal of PABPC4 from these cells, without an additional increase in PABPC1, led to a decrease in hTERT mRNA levels. These findings were consistently seen in three different HPV16 E6-expressing HFK cell lines.
FIG. 4.
Knockdown of PABPC1 or PABPC4 decreased hTERT expression in HPV16 E6-expressing HFKs. (A) shRNA expressed in HPV16 E6-expressing HFKs knocked down PABPC1, PABPC4, or both. When PABPC1, PABPC4, or both were reduced by shRNA, hTERT mRNA levels were also reduced relative to those in the presence of the scramble control. When PABPC4 was reduced but PABPC1 was increased, there was no difference in hTERT mRNA levels. These data are a summary of results from triplicate qPCRs conducted with three HPV16 E6-expressing HFK cell lines, with values normalized to 36B4 control mRNA levels. (**, P ≤ 0.002; error bars indicate 95% CI.) (B) Telomerase activity in HPV16 E6-expressing HFKs was reduced when PABPC1, PABPC4, or both were knocked down by shRNA. These data are representative of results for three HPV16 E6-expressing HFK cell lines. (+, TSR8 and HeLa positive controls; −, negative control.)
As hTERT mRNA levels can correlate with telomerase activity in cells, we wanted to determine whether a decrease in hTERT mRNA led to a decrease in telomerase activity in these cells. Indeed, in HPV16 E6-expressing HFKs with the knockdown of endogenous PABPC1, PABPC4, or both, there was a significant decrease in telomerase activity detected by a telomeric repeat amplification protocol (TRAP) assay (Fig. 4B). Thus, a decrease in PABPCs and a targeted reduction in hTERT mRNA led to a parallel decrease in telomerase activity in HPV16 E6-expressing HFKs.
PABPC1 and PABPC4 are not required for hTERT expression in HPV-negative cervical cancer cell line C33A.
As PABPC1 and PABPC4 levels were important for full expression of hTERT mRNA and telomerase activity in cells with HPV16 E6, we wanted to determine if they were also important in cells that express telomerase but not HPV16 E6. Therefore, PABPC1 and PABPC4 were knocked down by shRNA in C33A cells. Although the reductions in mRNA were not as dramatic as those in HPV16 E6-expressing HFKs, PABPC1 was reduced by more than half with sh1PABPC1 and sh2PABPC1 compared to the control (scramble) shRNA, and PABPC4 was reduced to 15 and 24% compared to the control levels (Fig. 5A). There was little cross-reactivity between PABPC1 and PABPC4 knockdown seen with our shRNA library in C33A cells. A reduction in expression of PABPC1 and PABPC4 compared to that in the presence of the scramble control was also seen by Western blot analysis of whole-cell extracts (Fig. 5B).
FIG. 5.
Knockdown of PABPC1 or PABPC4 in C33A cells did not affect hTERT expression. (A) PABPC1 and PABPC4 mRNA levels were detected by qPCR and graphed as percentages after normalization to levels in the presence of the scramble control. sh1PABPC1 knocked down PABPC1 mRNA to 47%, and sh2PABPC1 knocked down PABPC1 mRNA to 39%. sh2PABPC4 knocked down PABPC4 to 15%, and sh3PABPC4 knocked down PABPC4 mRNA to 24%. sh2PABPC4 and sh3PABPC4 had minor effects on PABPC1 levels, and sh1PABPC1 and sh2PABPC1 had no effect on PABPC4 levels. These data are from triplicate qPCRs and were normalized to 36B4 control mRNA levels. All P values were calculated by comparing results for an shRNA to the results for the scramble control. (*, P ≤ 0.05; error bars, 95% CI.) (B) Western blot analysis of whole-cell extracts showed some decrease in PABPC1 by sh1PABPC1 and sh2PABPC1 and larger decreases in PABPC4 by sh2PABPC4 and sh3PABPC4 compared to levels in the presence of the scramble vector and a loading control. (pan) PABPC, antibody to region common to all PABPCs. PABPC1, antibody to unique region of PABPC1; PABPC4, antibody to unique region of PABPC4; GAPDH, loading control. (C) When PABPC1 or PABPC4 was knocked down by shRNA, hTERT mRNA levels were not consistently reduced relative to those in the presence of the scramble control. Error bars, 95% CI. (D) Telomerase activity in C33A cells was not consistently reduced when PABPC1 or PABPC4 was knocked down by shRNA. These data are representative of results from three separate experiments. (+, HeLa positive control; −, negative control.)
In C33A cells in which PABPC1 or PABPC4 was reduced, there was no consistent change in hTERT mRNA levels (Fig. 5C) or in telomerase activity (Fig. 5D). These findings were consistently seen in three separate experiments. Thus, PABPCs had an effect on hTERT mRNA and telomerase activity only when they were dramatically decreased in cells with HPV16 E6.
PABPC4 overexpression increased hTERT expression in HPV16 E6-expressing HFKs.
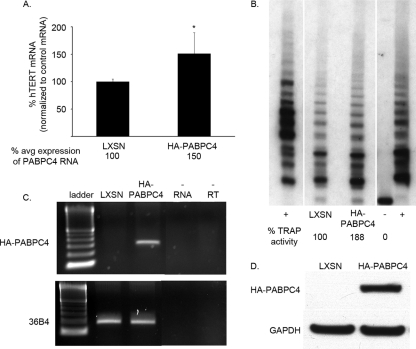
As the level of PABPCs appeared to be important in the expression of hTERT in HPV16 E6-expressing HFKs and PABPC4 was critical despite the fact that its endogenous expression level was only 1/15th that of PABPC1, we wanted to determine whether overexpression of PABPC4 could affect hTERT and telomerase. HPV16 E6-expressing HFKs that expressed HA-tagged PABPC4 increased their total level of PABPC4 expression to 150% of that in HPV16 E6-expressing vector control cells (Fig. 6A). HA-PABPC4 expression was confirmed by RT-PCR and by Western blot analysis (Fig. 6C and D). In HPV16 E6/HA-PABPC4-expressing cells, hTERT mRNA levels also increased to 150% of those in HPV16 E6-expressing vector control cells (Fig. 6A). In the TRAP assay, HPV16 E6/HA-PABPC4-expressing cells had increased telomerase activity compared to HPV16 E6-expressing vector control cells (Fig. 6B). These findings were similar for three different HPV16 E6-expressing HFK cell lines.
FIG. 6.
Overexpression of PABPC4 increased hTERT expression in HPV16 E6-expressing HFKs. (A) HA-tagged PABPC4 expressed in HPV16 E6-expressing HFKs increased total PABPC4 mRNA levels to 150% of endogenous levels by qPCR. Overexpression of PABPC4 increased hTERT to 150% that found in LXSN (vector control) HPV16 E6-expressing cells. These data are a summary of results from triplicate qPCRs conducted with three HPV16 E6-expressing HFK cell lines, with values normalized to 36B4 control mRNA levels. (*, P < 0.05; error bars indicate 95% CI.) (B) Telomerase activity in HPV16 E6-expressing HFKs overexpressing HA-tagged PABPC4 was increased relative to that in LXSN- and HPV16 E6-expressing cells. These data are representative of results for three HPV16 E6-expressing HFK cell lines. (+, TSR8 and HeLa positive controls; −, negative control.) (C) HA-tagged PABPC4 was detected by RT-PCR. (36B4, positive RNA control; −RNA, no RNA template; −RT, no reverse transcriptase.) (D) HA-tagged PABPC4 was detected by Western blot analysis of whole-cell extracts. (GAPDH, loading control.)
PABPC4 overexpression increased growth of HPV16 E6-expressing HFKs in culture.
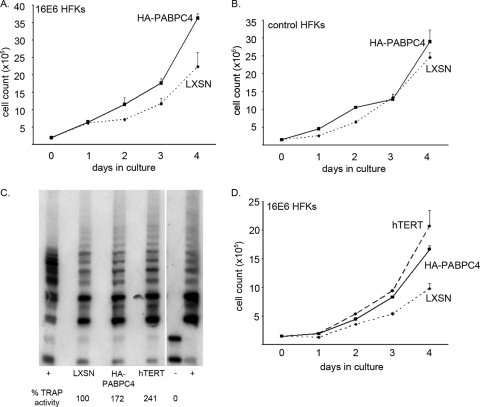
Previous studies have shown that hTERT can affect cell growth in culture, both under normal conditions and under stress (6, 9, 42). As the level of PABPC4 was important in the regulation of hTERT mRNA and telomerase activity in HPV16 E6-expressing HFKs, we wanted to determine whether HPV16 E6-expressing HFKs that overexpressed HA-PABPC4, and therefore had more telomerase activity, would grow better in culture than HPV16 E6/LXSN-expressing cells. In four separate experiments, HPV16 E6/HA-PABPC4-expressing HFKs and HPV16 E6/LXSN-expressing HFKs were plated in equal numbers and daily cell counts were conducted until the cells reached confluence. Over 4 days in culture, HPV16 E6/HA-PABPC4-expressing cells grew to greater numbers than the HPV16 E6/LXSN-expressing controls (Fig. 7A).
FIG. 7.
Overexpression of PABPC4 and hTERT increased daily growth of HPV16 E6-expressing HFKs in culture. (A) HPV16 E6-expressing HFKs overexpressing HA-tagged PABPC4 and LXSN (vector control)/HPV16 E6-expressing cells were plated in equal numbers and counted daily for 4 days. HPV16 E6-expressing HFKs overexpressing HA-PABPC4 grew to greater numbers over 4 days. (Error bars, 95% CI.) (B) pBABEpuro HFKs (control HFKs) overexpressing HA-PABPC4 and LXSN control HFKs were plated in equal numbers and counted daily for 4 days. Control HFKs overexpressing HA-PABPC4 did not grow to greater numbers over 4 days. (Error bars, 95% CI.) (A and B) These data are representative of results for two different HPV16 E6- and pBABEpuro-expressing HFK cell lines counted in triplicate in two different experiments. (C) Telomerase activity in HPV16 E6-expressing HFKs overexpressing HA-tagged PABPC4 or hTERT was increased relative to that in LXSN/HPV16 E6-expressing cells. (+, TSR8 and HeLa positive controls; −, negative control.) (D) HPV16 E6-expressing HFKs overexpressing HA-PABPC4 or hTERT and LXSN (vector control)/HPV16 E6-expressing cells were plated in equal numbers and counted daily for 4 days. HPV16 E6-expressing HFKs overexpressing HA-PABPC4 grew as well as those overexpressing hTERT, and both grew to greater numbers than LXSN/HPV16 E6-expressing cells. (Error bars, 95% CI.) These data are representative of results for two HPV16 E6-expressing cell lines counted in triplicate.
This difference was not due to the overexpression of PABPC4's leading to a general increase in mRNA stability, protein expression, and cell growth in culture. Vector control (pBABEpuro)-expressing HFKs that overexpressed either HA-PABPC4 or a second vector control (LXSN) were also plated in equal numbers, and daily cell counts were conducted. There was no difference in culture between control HFKs overexpressing HA-PABPC4 and control/LXSN cells when there was no coexpression of HPV16 E6 (Fig. 7B). Thus, in the context of HPV16 E6, when telomerase is active, the levels of PABPC4 could drive increased cell proliferation in culture.
Finally, as HPV16 E6-expressing HFKs also expressing HA-PABPC4 had more hTERT mRNA and telomerase activity and these cells increased in number more quickly, further overexpression of hTERT itself in HPV16 E6-expressing HFKs should also lead to increased cell numbers. HPV16 E6-expressing HFKs were secondarily transduced and selected to overexpress exogenous hTERT. hTERT mRNA levels and telomerase activity were increased more than 2-fold in HPV16 E6/hTERT-expressing cells compared to HPV16 E6/LXSN-expressing cells (Fig. 7C and data not shown), and HPV16 E6/hTERT-expressing cells had growth that paralleled that of HPV16 E6/HA-PABPC4-expressing cells (Fig. 7D). Thus, HPV16 E6-expressing HFKs with greater hTERT mRNA and telomerase activity grow better than HPV16 E6/LXSN-expressing cells.
DISCUSSION
We have demonstrated the presence and the importance of PABPCs in epithelial cells. First, we confirmed the expression of PABPC1 and PABPC4 in primary keratinocytes as well as cervical cancer cell lines and verified that PABPC1 is the most abundant PABPC expressed in these cells. Second, we also found that the levels and localization patterns of PABPC1 and PABPC4 are not changed by HPV16 E6 or by the full HPV16 genome. We did see a subtle nuclear shift, by immunofluorescent staining of PABPC1, in SiHa cells, and this paralleled PABPC1 and PABPC4 nuclear and cytoplasmic localization patterns in other HPV-expressing cervical cancer cell lines as revealed by Western blotting; however, these changes were not dramatic, nor were they due to HPV16 E6, as there was no change in localization of PABPC1 in HFKs expressing HPV16 E6. Thus, in cells expressing HPV16, unlike other DNA or RNA viruses, PABPCs are not grossly affected in their expression levels or localization.
However, there is a role of PABPCs in targeted RNA regulation in cells with HPV16 E6. Importantly, although PABPC4 was expressed at a fraction of the amount of PABPC1, its role in regulation of hTERT by HPV16 E6 was critical. When PABPC4 was almost fully knocked down by shRNA, hTERT mRNA and telomerase activity were decreased. When PABPC4 expression was increased (although to still just 1/10th the amount of PABPC1), we detected more hTERT mRNA and telomerase activity. Thus, hTERT regulation was sensitive specifically to the level of PABPC4. Reductions in PABPC1 by shRNA also decreased hTERT mRNA and telomerase activity, but we were unable to decrease PABPC1 selectively without also affecting PABPC4 in HFKs despite attempts with additional novel shRNAs. The specificity of PABPC4 for hTERT mRNA is due likely to the greater affinity of NFX1-123 for PABPC4 than for PABPC1 (25) and the role of NFX1-123 in hTERT mRNA stabilization and posttranscriptional regulation (25) in HPV16 E6-expressing HFKs.
This study adds to prior published work on RNA-processing proteins affecting cancer cell growth and disease progression (15, 20, 37). Previous studies have focused on cap binding proteins, RNA helicases, and splicing factors affecting oncogenic progression. Now we show that PABPCs are also important in the activation of telomerase, a key step in cellular immortalization, in HPV16 E6-expressing HFKs.
Telomerase allows cells to avoid senescence signals in cancer progression, but telomerase has also been shown to affect growth of nontransformed cells in tissue culture. Smith et al. showed that overexpression of hTERT in mouse embryonic fibroblasts (MEFs) could allow for continued cell growth, even under stress (42). Additionally, hTERT-expressing MEFs had increased daily growth compared to controls in culture (42). We have shown previously that hTERT expression activated by different alphaherpesvirus or betaherpesvirus genus HPV E6 genes in HFKs can lead to shifts in population doubling times (6) and longer growth in culture based on the expression level of hTERT and the activity level of telomerase (6; K. M. Bedard et al., unpublished data). This dose-dependent response in cell growth in culture has also been seen for human foreskin fibroblasts (9; J. A. Benanti et al., unpublished data).
There are two possible explanations for the differences in daily cell growth we saw among HPV16 E6-expressing HFKs when PABPC4 was overexpressed. First, there was higher telomerase activity in PABPC4-overexpressing cells. Although we did not see a large extension in passage doublings in HPV16 E6-expressing HFKs overexpressing PABPC4, with more telomerase expression, cells grew more quickly in culture. Second, the HFKs used in our studies had gone through two rounds of transduction and selection in order to express both HPV16 E6 and PABPC4 or matched vector controls. Given the number of population doublings that untransduced HFKs can complete in culture before entering senescence, these doubly transduced cells were nearing passages when they would senesce in culture without HPV16 E6 expression. As growth in culture can induce stress responses in cells over time (7, 8), it is also likely that the daily increase in cell number, driven by the level of hTERT expression, was further amplified by the age and stress of the HFKs when counting occurred. HPV16 E6-expressing HFKs that had knockdown of PABPC1 or PABPC4 by shRNA grew qualitatively more slowly in culture at 72 h after transduction; however, the PABPC1 and PABPC4 levels rebounded by 7 days, with a return to normal growth in culture.
In the context of HPV infection and specifically HPV16 E6 expression, telomerase is affected by the level of PABPC1 or PABPC4 in the cells and cell growth is affected as well. PABPCs, especially PABPC4, could potentiate immortalization and increase transformation of HFKs that are expressing HPV16 E6. This may be important in HPV-associated cancers, just as other RNA-processing proteins have been identified as important in other cancers.
Acknowledgments
This work was supported by Public Health Service grant R01 CA064795 to D.A.G. and by Public Health Service developmental grants K08 CA131171-01 and U19 AI 31448 to R.A.K.
We thank Kristin Robinson for help with tissue culture and microscopy and Kristin Bedard for helpful discussions.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Albrecht, M., and T. Lengauer. 2004. Survey on the PABC recognition motif PAM2. Biochem. Biophys. Res. Commun. 316:129-138. [DOI] [PubMed] [Google Scholar]
- 2.Amrani, N., S. Ghosh, D. A. Mangus, and A. Jacobson. 2008. Translation factors promote the formation of two states of the closed-loop mRNP. Nature 453:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auersperg, N. 1964. Long-term cultivation of hypodiploid human tumor cells. J. Natl. Cancer Inst. 32:135-163. [PubMed] [Google Scholar]
- 4.Baker, C. C., W. C. Phelps, V. Lindgren, M. J. Braun, M. A. Gonda, and P. M. Howley. 1987. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 61:962-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. [DOI] [PubMed] [Google Scholar]
- 6.Bedard, K. M., M. P. Underbrink, H. L. Howie, and D. A. Galloway. 2008. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J. Virol. 82:3894-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benanti, J. A., and D. A. Galloway. 2004. Normal human fibroblasts are resistant to RAS-induced senescence. Mol. Cell. Biol. 24:2842-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benanti, J. A., and D. A. Galloway. 2004. The normal response to RAS: senescence or transformation? Cell Cycle 3:715-717. [PubMed] [Google Scholar]
- 9.Benanti, J. A., M. L. Wang, H. E. Myers, K. L. Robinson, C. Grandori, and D. A. Galloway. 2007. Epigenetic down-regulation of ARF expression is a selection step in immortalization of human fibroblasts by c-Myc. Mol. Cancer Res. 5:1181-1189. [DOI] [PubMed] [Google Scholar]
- 10.Bonderoff, J. M., J. L. Larey, and R. E. Lloyd. 2008. Cleavage of poly(A)-binding protein by poliovirus 3C proteinase inhibits viral internal ribosome entry site-mediated translation. J. Virol. 82:9389-9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng, S., D. C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335-2349. [DOI] [PubMed] [Google Scholar]
- 12.Daling, J. R., M. M. Madeleine, L. G. Johnson, S. M. Schwartz, K. A. Shera, M. A. Wurscher, J. J. Carter, P. L. Porter, D. A. Galloway, J. K. McDougall, and J. N. Krieger. 2005. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int. J. Cancer 116:606-616. [DOI] [PubMed] [Google Scholar]
- 13.Daling, J. R., M. M. Madeleine, S. M. Schwartz, K. A. Shera, J. J. Carter, B. McKnight, P. L. Porter, D. A. Galloway, J. K. McDougall, and H. Tamimi. 2002. A population-based study of squamous cell vaginal cancer: HPV and cofactors. Gynecol. Oncol. 84:263-270. [DOI] [PubMed] [Google Scholar]
- 14.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 15.Fay, J., P. Kelehan, H. Lambkin, and S. Schwartz. 2009. Increased expression of cellular RNA-binding proteins in HPV-induced neoplasia and cervical cancer. J. Med. Virol. 81:897-907. [DOI] [PubMed] [Google Scholar]
- 16.Feral, C., G. Guellaen, and A. Pawlak. 2001. Human testis expresses a specific poly(A)-binding protein. Nucleic Acids Res. 29:1872-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontaine-Rodriguez, E. C., T. J. Taylor, M. Olesky, and D. M. Knipe. 2004. Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330:487-492. [DOI] [PubMed] [Google Scholar]
- 18.Gewin, L., and D. A. Galloway. 2001. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J. Virol. 75:7198-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gewin, L., H. Myers, T. Kiyono, and D. A. Galloway. 2004. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes Dev. 18:2269-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graff, J. R., B. W. Konicek, R. L. Lynch, C. A. Dumstorf, M. S. Dowless, A. M. McNulty, S. H. Parsons, L. H. Brail, B. M. Colligan, J. W. Koop, B. M. Hurst, J. A. Deddens, B. L. Neubauer, L. F. Stancato, H. W. Carter, L. E. Douglass, and J. H. Carter. 2009. eIF4E activation is commonly elevated in advanced human prostate cancers and significantly related to reduced patient survival. Cancer Res. 69:3866-3873. [DOI] [PubMed] [Google Scholar]
- 21.Harb, M., M. M. Becker, D. Vitour, C. H. Baron, P. Vende, S. C. Brown, S. Bolte, S. T. Arold, and D. Poncet. 2008. Nuclear localization of cytoplasmic poly(A)-binding protein upon rotavirus infection involves the interaction of NSP3 with eIF4G and RoXaN. J. Virol. 82:11283-11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helt, A. M., J. O. Funk, and D. A. Galloway. 2002. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. J. Virol. 76:10559-10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James, M. A., J. H. Lee, and A. J. Klingelhutz. 2006. HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int. J. Cancer 119:1878-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno, T., Y. Sato, T. Sata, and H. Katano. 2006. Expression of Kaposi's sarcoma-associated herpesvirus-encoded K10/10.1 protein in tissues and its interaction with poly(A)-binding protein. Virology 352:100-109. [DOI] [PubMed] [Google Scholar]
- 25.Katzenellenbogen, R. A., E. M. Egelkrout, P. Vliet-Gregg, L. C. Gewin, P. R. Gafken, and D. A. Galloway. 2007. NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells. J. Virol. 81:3786-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzenellenbogen, R. A., P. Vliet-Gregg, M. Xu, and D. A. Galloway. 2009. NFX1-123 increases hTERT expression and telomerase activity posttranscriptionally in human papillomavirus type 16 E6 keratinocytes. J. Virol. 83:6446-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlov, G., G. De Crescenzo, N. S. Lim, N. Siddiqui, D. Fantus, A. Kahvejian, J. F. Trempe, D. Elias, I. Ekiel, N. Sonenberg, M. O'Connor-McCourt, and K. Gehring. 2004. Structural basis of ligand recognition by PABC, a highly specific peptide-binding domain found in poly(A)-binding protein and a HECT ubiquitin ligase. EMBO J. 23:272-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlov, G., and K. Gehring. 2010. Molecular basis of eRF3 recognition by the MLLE domain of poly(A)-binding protein. PLoS One 5:e10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozlov, G., M. Menade, A. Rosenauer, L. Nguyen, and K. Gehring. 2010. Molecular determinants of PAM2 recognition by the MLLE domain of poly(A)-binding protein. J. Mol. Biol. 397:397-407. [DOI] [PubMed] [Google Scholar]
- 30.Kozlov, G., J. F. Trempe, K. Khaleghpour, A. Kahvejian, I. Ekiel, and K. Gehring. 2001. Structure and function of the C-terminal PABC domain of human poly(A)-binding protein. Proc. Natl. Acad. Sci. U. S. A. 98:4409-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn, U., and E. Wahle. 2004. Structure and function of poly(A) binding proteins. Biochim. Biophys. Acta 1678:67-84. [DOI] [PubMed] [Google Scholar]
- 32.Kuyumcu-Martinez, N. M., M. E. Van Eden, P. Younan, and R. E. Lloyd. 2004. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 24:1779-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 34.Mangus, D. A., M. C. Evans, and A. Jacobson. 2003. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 4:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin-Drubin, M. E., K. W. Huh, and K. Munger. 2008. Human papillomavirus type 16 E7 oncoprotein associates with E2F6. J. Virol. 82:8695-8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMurray, H. R., and D. J. McCance. 2003. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J. Virol. 77:9852-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mole, S., M. McFarlane, T. Chuen-Im, S. G. Milligan, D. Millan, and S. V. Graham. 2009. RNA splicing factors regulated by HPV16 during cervical tumour progression. J. Pathol. 219:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518-527. [DOI] [PubMed] [Google Scholar]
- 39.Munoz, N., F. X. Bosch, S. de Sanjosé, L. Tafur, I. Izarzugaza, M. Gili, P. Viladiu, C. Navarro, C. Martos, N. Ascunce, et al. 1992. The causal link between human papillomavirus and invasive cervical cancer: a population-based case-control study in Colombia and Spain. Int. J. Cancer 52:743-749. [DOI] [PubMed] [Google Scholar]
- 40.Shahbazian, D., A. Parsyan, E. Petroulakis, I. Topisirovic, Y. Martineau, B. F. Gibbs, Y. Svitkin, and N. Sonenberg. 2010. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol. Cell. Biol. 30:1478-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sladic, R. T., C. A. Lagnado, C. J. Bagley, and G. J. Goodall. 2004. Human PABP binds AU-rich RNA via RNA-binding domains 3 and 4. Eur. J. Biochem. 271:450-457. [DOI] [PubMed] [Google Scholar]
- 42.Smith, L. L., H. A. Coller, and J. M. Roberts. 2003. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 5:474-479. [DOI] [PubMed] [Google Scholar]
- 43.Smith, R. W., and N. K. Gray. 2010. Poly(A)-binding protein (PABP): a common viral target. Biochem. J. 426:1-12. [DOI] [PubMed] [Google Scholar]
- 44.Svitkin, Y. V., H. Imataka, K. Khaleghpour, A. Kahvejian, H. D. Liebig, and N. Sonenberg. 2001. Poly(A)-binding protein interaction with elF4G stimulates picornavirus IRES-dependent translation. RNA 7:1743-1752. [PMC free article] [PubMed] [Google Scholar]
- 45.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 75:4467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldman, T., X. Liu, H. Yuan, and R. Schlegel. 2003. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc. Natl. Acad. Sci. U. S. A. 100:8211-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 48.Wells, S. E., P. E. Hillner, R. D. Vale, and A. B. Sachs. 1998. Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2:135-140. [DOI] [PubMed] [Google Scholar]
- 49.Xu, M., W. Luo, D. J. Elzi, C. Grandori, and D. A. Galloway. 2008. NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes. Mol. Cell. Biol. 28:4819-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, H., C. S. Duckett, and T. Lindsten. 1995. iPABP, an inducible poly(A)-binding protein detected in activated human T cells. Mol. Cell. Biol. 15:6770-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yee, C., I. Krishnan-Hewlett, C. C. Baker, R. Schlegel, and P. M. Howley. 1985. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 119:361-366. [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida, M., K. Yoshida, G. Kozlov, N. S. Lim, G. De Crescenzo, Z. Pang, J. J. Berlanga, A. Kahvejian, K. Gehring, S. S. Wing, and N. Sonenberg. 2006. Poly(A) binding protein (PABP) homeostasis is mediated by the stability of its inhibitor, Paip2. EMBO J. 25:1934-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]