Abstract
Elucidating mechanisms leading to the natural control of HIV-1 infection is of great importance for vaccine design and for understanding viral pathogenesis. Rare HIV-1-infected individuals, termed HIV-1 controllers, have plasma HIV-1 RNA levels below the limit of detection by standard clinical assays (<50 to 75 copies/ml) without antiretroviral therapy. Although several recent studies have documented persistent low-grade viremia in HIV-1 controllers at a level not significantly different from that in HIV-1-infected individuals undergoing treatment with combination antiretroviral therapy (cART), it is unclear if plasma viruses are undergoing full cycles of replication in vivo or if the infection of new cells is completely blocked by host immune mechanisms. We studied a cohort of 21 HIV-1 controllers with a median level of viremia below 1 copy/ml, followed for a median of 11 years. Less than half of the cohort carried known protective HLA types (B*57/27). By isolating HIV-1 RNA from large volumes of plasma, we amplified single genome sequences of both pro-rt and env longitudinally. This study is the first to document that HIV-1 pro-rt and env evolve in this patient group, albeit at rates somewhat lower than in HIV-1 noncontrollers, in HLA B*57/27-positive, as well as HLA B*57/27-negative, individuals. Viral diversity and adaptive events associated with immune escape were found to be restricted in HIV-1 controllers, suggesting that replication occurs in the face of less overall immune selection.
Rare HIV-infected individuals, termed HIV-1 controllers, natural HIV-1 suppressors, HIV-1 elite suppressors, or elite controllers (1, 5, 33, 52, 59), have plasma HIV-1 RNA levels below the limit of detection of standard clinical assays (<50 to 75 copies/ml) without combination antiretroviral therapy (cART). HIV-1 controllers are a subgroup of long-term nonprogressors (LTNP) and are defined much more stringently by having undetectable levels of HIV-1 RNA, whereas LTNP are defined by having normal CD4+ T-cell counts for >7 to 10 years without therapy, independent of the level of viremia. HIV-1 controllers comprise about 0.5 to 1% of well-described cohorts (9) and rarely progress to AIDS (24). These individuals are of special interest because they may reveal mechanisms that control HIV-1 infection and thus be of great importance for future vaccine and drug design.
Recent studies have demonstrated that HIV-1 controllers have persistent low-grade viremia at levels not significantly different from those of HIV-1-infected individuals who achieve control through the use of cART (<1 copy/ml) (11, 22, 27, 42, 43, 53). Although the topic remains controversial (6), many studies have found that combination therapy effectively blocks the infection of new cells, as confirmed both by the absence of sequence evolution (2, 7, 41, 60) and by the lack of reduction in HIV-1 RNA when cART is intensified with a fourth drug (12, 17, 18, 40). Thus, the persistent viremia in patients on successful cART stems from a reservoir of long-lived cells in the absence of de novo infection. In contrast, the source of viremia in HIV-1 controllers is unclear.
Although infection with a replication-impaired virus, such as a strain of HIV-1 with nef deleted (34, 35), can lead to reduced levels of viremia, several recent studies have suggested that most HIV-1 controllers are infected with replication-competent strains. A large cross-sectional study of 95 controllers did not identify any apparent genetic defects in gag, pol, env, nef, and vif, vpr, and vpu from plasma viruses (45). In addition, replication-competent virus has been cultured from controllers ex vivo and has been shown to reproduce at a rate not significantly different from that of known laboratory strains (5, 25, 33). However, it is still possible that subtle genetic changes in the virus of controllers may affect viral replication in vivo or that replication is blocked by host immune mechanisms.
Several studies have associated properties of the cellular immune response with controller status, indicating that host immune responses control infection in HIV-1 controllers. Certain rare human leukocyte antigen (HLA) class 1 types, such as B*57 and B*27, have been shown to be overrepresented among controllers at frequencies up to 85% (1, 31, 44, 52). These HLA types are not found in all HIV-1 controllers and are thus not necessary for controller status. In addition, factors related to cytotoxic-T-lymphocyte (CTL) function have also been linked with natural HIV-1 control (4, 42, 59). In contrast to the cellular immune system, no unique characteristics of the humoral immune response have yet been identified among controllers (1, 13, 32), and it is thus uncertain if neutralizing antibodies are important for achieving or maintaining controller status.
To date, it remains unclear if HIV-1 undergoes productive cycles of replication in vivo leading to virus evolution in controllers or if replication is absent or completely blocked. To answer these questions, we applied single-genome sequencing (SGS) (50) of the pro-rt and env genes to longitudinally sampled plasma samples from a cohort of 21 HIV-1 controllers with a median minimum duration of infection of 11 years and HIV-1 RNA levels of 0.3 to 0.8 copies/ml. Our results indicate that the pol and env sequences isolated from HIV-1 controllers are evolving at rates somewhat lower than, but not significantly different from, those of HIV-1 noncontrollers but under different selective pressure from the immune system.
MATERIALS AND METHODS
Study population.
Study subjects were identified from the Danish HIV Cohort Study (48) and by physician referral. Inclusion criteria were (i) HIV-1 enzyme-linked immunosorbent assay (ELISA) reactive and Western blot positive, (ii) at least 3 HIV-RNA test results available without cART, (iii) a span of at least 1 year between the first and the last HIV-RNA test results, and (iv) no test showing >50 HIV-1 RNA copies/ml.
Informed consent was obtained. The protocol was approved by the Danish National Committee on Biomedical Research (H-B-2007-019) and the Institutional Review Board and the Office of Human Subjects Research at the National Institutes of Health (NIH).
A flowchart of the patient identification and amplification strategy can be found in Fig. S1 in the supplemental material.
As controls, we used single genome sequences from a total of 25 HIV-1 noncontrollers (28, 29). Noncontrol was defined as having HIV-1 RNA levels of >1,000 copies/ml without antiretroviral therapy. The control data set from chronically infected noncontrollers (with chronic infection defined as >1 year of infection) contained a total of 1,206 pro-rt sequences from 20 patients (10 with longitudinal data) and a total of 778 env sequences from 12 patients (9 with longitudinal data). The control data set from early infected patients (with early infection defined as <1 year of infection) contained a total of 715 pro-rt sequences from 11 patients (6 with longitudinal data) and 548 env sequences from 9 patients (7 with longitudinal data). For comparison of V1/V2, we used 2,119 single genome sequences from 78 early infected patients (Fiebig stages 1 to 6 [15]), in which infection was likely established by a single variant, from Keele et al. (30).
HIV-1 extraction, SGS, and SCA.
HIV-1 RNA was extracted from 1 to 7 ml of plasma using ultracentrifugation and analyzed using single-copy assay (SCA) and SGS, as described previously (28, 29, 51). Briefly, SCA is a real-time quantitative assay for HIV-1 using a short conserved sequence within gag. The lower limit of detection is estimated based on the volume of plasma analyzed (51). SGS is a limiting-dilution assay performed as described previously (28, 29, 50) for amplification of 1.5 kb of genomic RNA encoding p6, protease, and reverse transcriptase (RT) (p6-rt) or an approximately 1-kb fragment of gp120 including the V1/V2 and V3 regions and sequenced bidirectionally.
Genetic analyses.
Sequence contigs were constructed using SeqMan (DNAStar, Madison, WI) and aligned using the clustalW algorithm in Bioedit 7.0.9 (21). Alignments of env sequences were constructed after translation into amino acids and subsequent stripping of gaps. The sequences covered nucleotide positions 2252 to 3233 (pro-rt) and 6565 to 7246 (env) according to HXB2, the reference strain of HIV-1. The number of recombination events in alignments was detected by a method described by Kaplan and Hudson (26) as implemented in dnaSP (57), and sequences with extensive recombination were excluded from divergence analyses (PID 5003). The program Modeltest v. 3.7 (56) was used to determine the most appropriate nucleotide substitution model; the HKY + G + I model was the most appropriate in most cases. PAUP* version 4.0b (61) was used to calculate maximum-likelihood (ML) trees incorporating the optimal evolutionary model and its parameters from Modeltest in a heuristic search. Trees were rooted using outgroup rooting; estimates of the root of the tree were carried out without any clock assumptions. Root-to-tip distances were extracted from phylogenetic trees using TreeStat v. 1.2 (http://www.tree.bio.ed.ac.uk/software/treestat) and plotted against sampling time. Linear regression was used to determine if slopes were significantly different from zero. Estimation of mutation rates was carried out using the Bayesian Evolutionary Analyses Sampling Trees (BEAST) v. 1.5.4 software (14). The nucleotide substitution model was set to HKY with gamma and invariant sites, because this was the best-fitting nucleotide substitution model in most cases. Estimations were determined under a relaxed clock model and constant population size.
Parameters with their confidence intervals were estimated by using Bayesian Markov chain Monte Carlo inference with a chain of 10 million states sampled every 100th generation. Two independent runs were carried out in parallel to ensure convergence of results. Absolute rates of expected synonymous and nonsynonymous divergence were extracted from the phylogenetic trees constructed by BEAST by the use of the program HYPHY (55), as described by Lemey et al. (36). Sequence diversity was estimated using average pairwise distances (APD), as described by Nei and Kumar (47), implemented in MEGA 4.1 (62). Known CTL epitopes in Pro and RT were determined using the Epitope Location Finder (ELF) web tool from the Los Alamos HIV database. Sequences from controllers were compared to a consensus of subtype B sequences obtained from 60 HIV-1 seroconverters in Denmark sampled from 2000 to 2007. A1 subtypes (n = 3) were compared to an A1 consensus sequence constructed using the 361 available A1 sequences from single individuals from Uganda available in the Los Alamos HIV database. Sequences from noncontrollers were compared to a U.S. subtype B consensus sequence (28). Potential N-linked glycosylation sites (PNLGS) were predicted using the N-GLYCOSITE web tool from the Los Alamos HIV database (67).
HLA typing.
Genomic DNA was extracted from whole blood or peripheral blood mononuclear cells (PBMCs) and typed for HLA class I alleles using direct sequencing of PCR products (19).
Statistics.
All values are shown as median and interquartile range. Linear regression analyses were used to determine the significance of slopes derived from root-to-tip distances from rooted maximum-likelihood trees. The Mann-Whitney U test was used to test differences between groups. Statistical analysis was performed with GraphPad Prism version 4 (GraphPad Software, San Diego, CA). General linear mixed-effects models of varying complexity were fitted to the diversity values of the pro-rt region. The Wald test was used to determine the significance of variables within the general linear mixed-effects models. Wald test P values of >0 signified that the restricted hypothesis was rejected. Mixed-effects modeling was carried out using the Mathworks program.
Nucleotide sequence accession numbers.
The pro-rt and env sequences were submitted to GenBank and assigned accession numbers HQ337101 through HQ337433.
RESULTS
Characteristics of HIV-1 controllers and noncontrollers.
The characteristics of the HIV-1 controllers and noncontrollers are listed in Table 1, and more details can be found in Table S1 in the supplemental material. As expected, HIV-1 controllers had lower viremia (<20 copies/ml versus 264,980 copies/ml; P < 0.0001) and higher peripheral CD4+ T-cell counts (744 cells/μl versus 402 cells/μl; P = 0.0003) than noncontrollers. Controllers were older at follow up (43 years versus 36 years; P = 0.0014) and had been infected longer (11.0 years versus 3.2 years; P = 0.0007) than HIV-1 noncontrollers.
TABLE 1.
Characteristics of HIV-1 controllers and noncontrollers
| Characteristica | Value |
|
|---|---|---|
| Controllers | Noncontrollers | |
| n | 21 | 25 |
| Age at follow-up [yr (range)] | 43 (39.5-58.0) | 36 (31-39) |
| No. of males (%) | 13 (62) | 23 (92) |
| Transmission group [no. (%)] | ||
| MSM | 8 (38) | 18 (72) |
| IDU | 5 (24) | 1 (4) |
| Heterosexual | 7 (33) | 4 (16) |
| Other | 1 (5) | 2 (8) |
| HIV-1 RNA [copies/ml (range)], baseline | <20 (<20-129.5) | 264,980 (48,406-451,000) |
| CD4 [cells/μl (range)], baseline | 744 (555-986) | 402 (254-600) |
| Minimum duration of infection [yr (range)] | 11.0 (7.3-17.5) | 3.2 (1.8-11.3) |
MSM, men who have sex with men; IDU, intravenous drug users.
The HLA class I alleles of HIV-1 controllers are listed in Table 2. DNA for HLA typing was available from 16 of the 21 controllers. Six of the 16 (38%) carried protective B*57 and/or B*27 alleles, a frequency much lower than that reported for other small cohorts (31, 44) but similar to the level reported in the HIV controller consortium study (52). Interestingly, 9/16 (56%) carried homozygous HLA alleles, 2 of which were common alleles (A*0201 and A*1101). Due to the relatively small number of controllers in this cohort, it is difficult to determine whether this distribution reflects a true overrepresentation of homozygotes. However, compared to a total of 44 local controls (matched for age, sex, and year of diagnosis), homozygosity in the A allele was significantly overrepresented among controllers (P = 0.01; Fisher's exact test) (data not shown).
TABLE 2.
HLA class 1 types and mutations in known CTL epitopes in HIV-1 controllers
| Identifier | Individual HLA typea |
No. of sensitive epitopes in reference |
No. of epitopes with mutation (codon position) |
% Mutated epitopes |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | C1 | C2 | Pro | RT | Pro | RT | Pro | RT | |
| C01 | 0201 | 0201 | 0702 | 4001 | 0304 | 0702 | 7 | 13 | 2 (37, 41) | 2 (207) | 29 | 15 |
| C02 | 0301 | 2402 | 2705 | 5701 | 0202 | 0602 | 2 | 8 | 0 | 3 (35, 197) | 0 | 38 |
| C04 | 0201 | 0201 | 0702 | 5201 | 0702 | 1202 | 5 | 6 | 1 (44) | 5 (11, 126, 135, 162) | 20 | 83 |
| C05 | 0301 | 6801 | 2705 | 3503 | 0102 | 1203 | 0 | 4 | 0 | 2 (177, 123) | 0 | 50 |
| C06 | NA | NA | NA | NA | NA | NA | ||||||
| C07 | 0201 | 0201 | 2705 | 5101 | 0102 | 1502 | 4 | 6 | 2 (16, 25) | 5 (11, 50, 135) | 50 | 83 |
| C08 | NA | NA | NA | NA | NA | NA | ||||||
| C10 | NA | NA | NA | NA | NA | NA | ||||||
| C11 | 0201 | 1101 | 1501 | 3501 | 0303 | 0401 | 7 | 22 | 0 | 2 (105, 207) | 0 | 9 |
| C12 | NA | NA | NA | NA | NA | NA | ||||||
| C13 | NA | NA | NA | NA | NA | NA | ||||||
| C14 | 0101 | 3002 | 1801 | 4402 | 0501 | 0501 | ||||||
| C15 | 0205 | 3101 | 0702 | 2705 | 0202 | 0702 | 5 | 14 | 0 | 2 (64, 135) | 0 | 14 |
| C16 | 0101 | 0201 | 1501 | 5701 | 0304 | 0602 | ||||||
| C17 | 1101 | 1101 | 0702 | 3501 | 0401 | 0702 | 1 | 18 | 1 (15) | 0 | 100 | 0 |
| C18 | 0301 | 0301 | 2705 | 3501 | 0202 | 0401 | 2 | 12 | 0 | 2 (123, 177) | 0 | 17 |
| C19 | 3101 | 3303 | 3901 | 5801 | 0302 | 1203 | 3 | 2 | 2 (16, 72, 77) | 2 (166) | 67 | 100 |
| C20 | 0101 | 2902 | 4201 | 8101 | 1700 | 1800 | ||||||
| C22 | 1101 | 1101 | 4403 | 5101 | 1502 | 1601 | 1 | 11 | 0 | 2 (20, 21, 135) | 0 | 18 |
| C23 | 0201 | 0201 | 4402 | 5701 | 0501 | 0602 | 6 | 9 | 4 (37, 41, 63, 75) | 0 | 67 | 0 |
| C24 | 0201 | 2402 | 1501 | 1501 | 0303 | 0304 | ||||||
NA, not applicable.
Viremia in HIV-1 controllers.
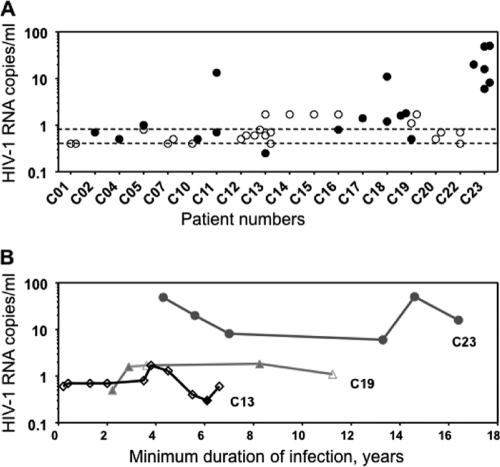
Previous reports have described low levels of viremia in HIV-1 controllers, detectable only by ultrasensitive assays for viral RNA (11, 22, 27, 42, 53). To characterize the present cohort, the level of viremia was assessed using the SCA. An overview of samples and outcome of SGS and SCA are listed in Table S2 and Fig. S1 in the supplemental material. A total of 44 samples from 18 HIV-1 controllers were assayed by SCA (Fig. 1). Twenty of these (45%) contained measurable viremia, and most (11 of 18) controllers had at least one episode with measurable but low-level viremia (Fig. 1A). Median HIV-1 RNA was estimated in two ways: (i) the maximum median, in which values below the limit of detection were registered as the limit of detection, and (ii) the minimum median, in which values below the limit of detection were registered as 0. The maximum median of the entire cohort was 0.8 copies/ml, and the minimum median was 0.3 copies/ml (Fig. 1A). Longitudinal SCA measures from 3 controllers showed that HIV-1 RNA values remained constant over time, with values near an individual set point for up to 17 years after diagnosis (Fig. 1B).
FIG. 1.
Viremia in HIV-1 controllers measured by SCA. (A) HIV-1 RNA levels in 18 controllers. The open symbols indicate undetectable values plotted at the limit of detection; the closed symbols indicate measurable HIV-1 RNA values. The detection limit of SCA varied with the volume of plasma assayed. The minimum median was 0.3 copies/ml, and the maximum median was 0.8 copies/ml, shown with dashed lines. (B) Longitudinal HIV-1 RNA values from 3 controllers.
Amplification of pro-rt and env by SGS.
SGS has the advantage of not being biased by PCR-introduced recombination or resampling of the same sequence if the amount of template is small (37). SGS was performed on a total of 214 plasma samples from 21 HIV-1 controllers; 158 samples from 20 HIV-1 controllers were assayed for pro-rt. Of these, 47 samples (30%) yielded amplification products. Fifty-six plasma samples were assayed for env; 23 of these yielded products (40%). In all, pro-rt or env sequences were amplified from a total of 18 of 21 HIV-1 controllers. Products from more than 1 time point were obtained from 15 individuals (pro-rt from 13 individuals and env from 9 individuals). An average of 5 (range, 1 to 20) single genomes were obtained per plasma sample. In 3 controllers (C06, C08, and C12), neither quantitative HIV-1 RNA nor sequence data were obtained. These low yields were primarily due to low volumes of available plasma, although polymorphisms within primer binding sites may also have contributed to lack of amplification in these samples. Study participants with no amplification did not differ in nadir CD4+ T-cell counts or CD4+ T-cell slopes from other controllers, suggesting that pathogenesis was not significantly different in the two groups (Table 1).
Evolution of pro-rt and env in controllers.
The presence of low-grade viremia could reflect ongoing, complete cycles of replication or expression of a nonreplicating reservoir of long-lived cells, as is found in patients on successful cART (13-16). To differentiate between these possibilities, detailed phylogenetic analyses were applied to the 15 HIV-1 controllers from whom single genomes from >1 point in time were amplified.
To test for evolution of viral genes over time, individual rooted ML phylogenetic trees were constructed. Root-to-tip distances were extracted from the trees and plotted against sampling time. Linear regression was used to determine if slopes were significantly different from zero. As a result of the small amount of sequence data obtained at some time points (<2 sequences) or a narrow sampling interval (less than 3 months), we were not able to determine if there was evolution in some phylogenetic trees, and these were categorized as undetermined. An overview of the maximum-likelihood analyses can be found in Table 3 . As an example, rooted maximum-likelihood trees of pro-rt and env and the corresponding root-to-tip distances of virus in an HIV-1 controller (C23) are shown in Fig. 2. Evidence of evolution in the form of increasing root-to-tip distances was found in pro-rt from C02, C05, C07, C10, C19, C22, and C23 and in env from C05, C07, C11, C16, C22, and C23. Evidence of evolution was found in patients both with and without known protective HLA types (HLA*B-27 and -5701). C11, C19, and C22 did not carry protective HLA types. We were not able to determine pro-rt evolution in 5 patients (C01, C04, C11, C17, and C18) and env evolution in 2 patients (C17 and C24). In one case (C15), sequences consistently and across time points formed 2 separate clusters. This patient was most likely infected with 2 strains of HIV-1 and was therefore excluded for the analyses of divergence.
TABLE 3.
Analyses of root-to-tip distances and rates of evolution in HIV-1 controllers and noncontrollers
| Identifier | Analyses of root-to-tip distances in rooted ML trees |
Rate of evolution (10−6/day) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | P valuea | Observation (days) | Evolution | Comment | Overall ratec | SEM | dSd | SD | dNe | SD | |
| C01 | pol | 0.45 | 329 | NDb | Few sequences | 8.30 | 0.31 | 0.67 | 0.95 | 6.89 | 5.60 |
| C02 | pol | <0.01 | 2,828 | Yes | 3.84 | 0.03 | 2.57 | 0.70 | 1.28 | 0.35 | |
| C04 | pol | 0.67 | 645 | ND | Few sequences | 8.30 | 5.49 | 1.88 | 1.73 | 0.41 | 0.36 |
| C05 | pol | 0.02 | 3,496 | Yes | 2.60 | 0.03 | 2.73 | 0.85 | 0.81 | 0.28 | |
| C07 | pol | 0.01 | 3,807 | Yes | 2.55 | 0.02 | 0.73 | 0.32 | 1.79 | 0.70 | |
| C10 | pol | <0.01 | 1,873 | Yes | 28.2 | 0.64 | 24.7 | 27.8 | 4.23 | 1.43 | |
| C11 | pol | 0.11 | 2,630 | ND | Few sequences | 9.50 | 0.05 | 6.61 | 1.91 | 2.91 | 0.86 |
| C15 | pol | 0.97 | 991 | ND | Dually infected | 1.16 | 0.03 | 3.15 | 1.96 | 3.70 | 2.23 |
| C17 | pol | 0.72 | 313 | ND | Few sequences | 7.80 | 0.21 | 3.47 | 3.83 | 4.35 | 4.62 |
| C18 | pol | 0.03 | 1,053 | ND | Few sequences | 3770 | 3240 | 42.9 | 209 | 6.61 | 32.8 |
| C19 | pol | 0.01 | 3,538 | Yes | 5.32 | 0.09 | 3.76 | 2.96 | 2.01 | 1.05 | |
| C22 | pol | <0.01 | 2,577 | Yes | 2.87 | 0.07 | 2.04 | 1.09 | 0.90 | 0.44 | |
| C23 | pol | <0.01 | 3,695 | Yes | 3.64 | 0.02 | 3.23 | 0.89 | 0.48 | 0.18 | |
| C05 | env | 0.04 | 1,903 | Yes | 21.9 | 0.78 | 7.43 | 4.61 | 15.0 | 8.58 | |
| C07 | env | <0.01 | 918 | Yes | 6.17 | 0.08 | 1.71 | 0.90 | 4.18 | 2.21 | |
| C11 | env | 0.03 | 371 | Yes | 78.7 | 0.80 | 19.6 | 9.62 | 58.2 | 29.0 | |
| C15 | env | 0.89 | 897 | ND | Dually infected | 10.1 | 0.17 | 0.92 | 0.92 | 0.19 | 0.18 |
| C16 | env | <0.01 | 1,441 | Yes | 35.0 | 1.13 | 22.4 | 16.5 | 11.4 | 2.95 | |
| C17 | env | ND | 176 | ND | Few sequences | 2.29 | ND | ND | ND | ND | ND |
| C22 | env | <0.01 | 764 | Yes | 6.21 | 0.15 | 2.29 | 1.20 | 3.76 | 2.05 | |
| C23 | env | <0.01 | 2,581 | Yes | 12.7 | 0.24 | 2.82 | 0.96 | 10.1 | 3.28 | |
| C24 | env | 0.95 | 98 | ND | Short observation | 77.8 | 10.7 | 53.0 | 71.8 | 24.5 | 31.4 |
| 3021 | pol | <0.01 | 1,259 | Yes | 26.7 | 0.61 | 18.9 | 4.35 | 7.40 | 1.64 | |
| 3024 | pol | <0.01 | 531 | Yes | 19.1 | 0.20 | 16.5 | 5.20 | 11.2 | 3.18 | |
| 3037 | pol | <0.01 | 747 | Yes | 9.03 | 0.20 | 5.83 | 1.83 | 3.45 | 1.21 | |
| 3041 | pol | <0.01 | 590 | Yes | 0.718 | 0.07 | 5.57 | 1.12 | 1.57 | 0.364 | |
| 3062 | pol | <0.01 | 750 | Yes | 18.3 | 0.35 | 13.5 | 2.93 | 8.19 | 5.86 | |
| 3077 | pol | <0.01 | 310 | Yes | 11.3 | 0.89 | 11.5 | 8.19 | 8.14 | 2.35 | |
| 3088 | pol | <0.01 | 133 | Yes | 22.7 | 0.33 | 14.2 | 3.87 | 4.61 | 1.03 | |
| 5001 | pol | <0.01 | 1,885 | Yes | 9.37 | 0.51 | 7.47 | 2.75 | 1.80 | 0.65 | |
| 5002 | pol | <0.01 | 1,750 | Yes | 11.0 | 0.22 | 9.14 | 2.34 | 1.57 | 0.41 | |
| 5003 | pol | 0.24 | 1,430 | No | Recombination | 3.32 | 0.12 | 2.44 | 0.84 | 0.81 | 0.27 |
| 3021 | env | <0.01 | 1,259 | Yes | 39.3 | 0.43 | 20.9 | 4.39 | 23.4 | 4.76 | |
| 3024 | env | 0.04 | 531 | Yes | 49.0 | 0.70 | 21.2 | 5.83 | 42.2 | 10.5 | |
| 3037 | env | <0.01 | 747 | Yes | 25.0 | 0.28 | 12.3 | 2.92 | 11.9 | 2.96 | |
| 3041 | env | <0.01 | 681 | Yes | 42.8 | 1.04 | 11.0 | 2.34 | 33.1 | 6.69 | |
| 3077 | env | <0.01 | 456 | Yes | 231 | 2.50 | 43.4 | 7.52 | 131 | 21.1 | |
| 3088 | env | 0.55 | 70 | ND | Short observation | 86.1 | 5.30 | 16.3 | 10.0 | 68.3 | 41.4 |
| 4001 | env | <0.01 | 1,166 | Yes | 172 | 1.24 | 28.2 | 8.24 | 55.6 | 15.7 | |
| 5002 | env | <0.01 | 2,085 | ND | 89.2 | 1.49 | 21.5 | 3.34 | 68.3 | 10.3 | |
| 5003 | env | 0.60 | 1,373 | No | Recombination | 23.2 | 0.651 | 5.64 | 1.69 | 17.9 | 5.14 |
P value of the linear regression analyses of the root-to-tip distances from the rooted maximum likelihood trees.
ND, not determined.
Substitutions per site per day.
Expected synonymous substitutions per synonymous site per day.
Expected nonsynonymous substitutions per nonsynonymous site per day.
FIG. 2.
Sequence trees from two HIV-1 controllers. (A and B) Rooted maximum-likelihood phylogenetic trees of pro-rt (A) and env (B) from HIV-1 controller C23. The trees were rooted using HXB2 as an outgroup. (C and D) Root-to-tip distances plotted against sampling time.
Among HIV-1 noncontrollers, evidence of evolution in the form of increasing root-to-tip distances was found in all patients, except patient 3088 (env), most likely because of a short observation time (70 days), and patient 5003 (pro-rt and env), who exhibited extensive recombination, causing genes to evolve in a nonclock fashion.
Relative rates of evolution in HIV-1 controllers and noncontrollers.
We then went on to estimate the rates of evolution in those controllers and noncontrollers who showed evidence of evolution in phylogenetic trees. The rates were estimated by the use of Bayesian statistics and under the assumption of a relaxed clock model. The results of the rate analysis for each patient are summarized in Table 3, and the median values for the two groups and regions analyzed are shown in Table 4. Overall rates of nucleotide substitution were 3- to 4-fold lower in controllers than in noncontrollers in both pro-rt and env, although statistical significance varied, due to low sample numbers. These results did not change if alignments with undetermined or no evolution were included in the analyses (data not shown).
TABLE 4.
Summary of evolutionary parameters in controllers and noncontrollers
| Gene | Group | Evolution ratea,b |
Substitution ratea,b |
Diversity (%)d,e |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Pc | dS | P | dN | P | Total | P | ||
| pro-rt | Controller | 1.8 (1.3-2.7) | 0.09 | 1.8 (1.6-3.4) | 0.02 | 0.82 (0.54-1.5) | 0.03 | 0.31 (0.06-0.77) | 0.005 |
| Noncontroller | 6.1 (4.8-11) | 8.2 (4.5-10) | 2.8 (1.1-6.1) | 0.83 (0.60-1.41) | |||||
| env | Controller | 8.9 (3.2-23) | 0.02 | 3.9 (1.6-15) | 0.18 | 6.9 (3.1-20) | 0.05 | 0.81 (0.2-1.92) | <0.0001-0.03 |
| Noncontroller | 35 (20-76) | 13 (7.6-18) | 26 (14-39) | 0.05 (0.02-0.1) E; 1.84 (91.83-2.86) C | |||||
Median (range).
Substitutions/site × 10−6/day.
P value (Mann-Whitney) for the difference between controllers and noncontrollers.
E, early infection; C, chronic infection.
At the earliest time sampled.
Expansion of diversity in pro-rt is restricted in HIV-1 controllers.
HIV-1 populations during early infection are most often homogeneous (10, 28, 30, 65, 66), and diversity accumulates over time as a result of the high replication rate (23), the high error rate of replication (38), and selection. Thus, the accumulation of diversity reflects the interplay between replication and selection. We were interested, therefore, in characterizing how genetic diversity accumulated with time in controllers and noncontrollers.
Figure 3A to C shows the accumulation of diversity in pro-rt during chronic infection in 12 controllers and 21 noncontrollers. Because the analysis included multiple observations from the same individuals, general mixed-effects linear models were used to analyze the data. Overall, we observed similar patterns of expansion in the diversity of pro-rt in the two groups, but at much lower levels in the controllers. The expansion of the total diversity in HIV-1 controllers and noncontrollers is shown in Fig. 3A. The total diversity increased with time in noncontrollers (P > 0; Wald test) but did not increase or increased at a very low rate in controllers (P = 0.33). The increase in diversity among HIV-1 noncontrollers was estimated to be 1.07% per year (95% confidence interval [CI], 1.03 to 1.11%). As with the total diversity, synonymous diversity kept increasing with time in HIV-1 noncontrollers (P > 0; Wald test) and remained stable or increased at a very low rate in controllers (P = 0.77) (Fig. 3B). The nonsynonymous diversity was not found to be significantly different between groups (P = 0.34) and was stable over time in both controllers and noncontrollers (P = 0.45), consistent with the occurrence of a mutation-selection balance (Fig. 3C).
FIG. 3.
Diversity in HIV-1 controllers and noncontrollers. (A to C) Comparison of diversity, length (measured in amino acids), and numbers of PNLGS of the V1/V2 region of env between controllers (white circles) and noncontrollers during early infection (black circles) and chronic infection (black diamonds). (D to F) Diversity of pro-rt during chronic infection in controllers (white circles) and noncontrollers (black diamonds), with linear regression lines. AA, amino acids.
Adaptation is restricted in HIV-1 controllers.
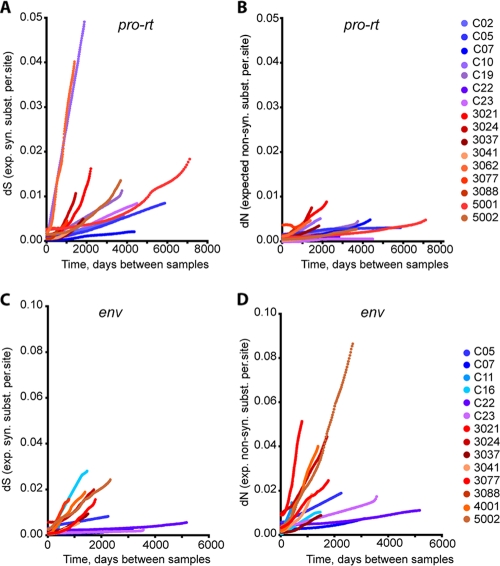
To obtain information on the role of selective forces in driving HIV-1 evolution in controllers, absolute rates of nonsynonymous substitutions (dN) and synonymous substitutions (dS) were estimated by the use of Bayesian statistics, under the assumption of a relaxed clock model. The results from the analyses are shown in Tables 3 and 4 and Fig. 4. Individual plots of dN and dS can be found in Fig. S2 to S5 in the supplemental material. Overall, similar ratios of dN and dS were found in pro-rt and env in the two patient groups, suggesting that these genes evolve in a similar fashion in both patient populations. In both groups, the evolution of pro-rt was driven by purifying selection (dN < dS), whereas the evolution of env was driven by positive selection (dN > dS). In general, the absolute rates of both synonymous and nonsynonymous substitutions in pro-rt and env were 3- to 4-fold lower in HIV-1 controllers than in noncontrollers (Table 4). These results imply similar effects of both positive and purifying selection in the two groups, imposed on a lower rate of overall substitution in the controller group.
FIG. 4.
Synonymous and nonsynonymous divergence in HIV-1 controllers and noncontrollers. The accumulation of synonymous (syn.) (dS) (A and C) and nonsynonymous (non-syn.) substitutions (subst.) (dN) (B and D) in controllers (blue) and noncontrollers (red) in pro-rt (A and B) and env (C and D) relative to the earliest sample time is plotted for each patient. Absolute values of dS and dN were estimated using maximum-likelihood codon models under the assumption of a relaxed molecular clock.
The primary mechanisms of HIV-1 escape from immune mechanisms are different in env and pro-rt. Escape from neutralizing antibodies is thought to be primarily conferred by changes in N-linked glycosylation patterns combined with variation in the lengths of the hypervariable loop regions. Since indels are stripped from env alignments before rates of divergence are estimated, most such adaptive changes may be overlooked in phylogenetic analyses. To determine the role of such changes in env, we examined diversity, loop lengths, and the number of potential N-linked glycosylation sites in env sequences from HIV-1 controllers and noncontrollers (Fig. 3D to F). In the subsequent analyses, we selected the first time point amplified by SGS in the 12 HIV-1 controllers with env sequence data. env sequences from HIV-1 controllers were compared to a cross-sectional data set consisting of sequences from 87 early HIV-1-infected patients (Fiebig stages 1 to 6) (28, 30) and 12 chronically infected patients (>1 year of infection). The diversity of env sequences from HIV-1 controllers was found to be significantly higher than that of sequences taken from noncontrollers during early infection but significantly lower than that of sequences from noncontrollers during chronic infection (Table 4). This result suggests that, assuming that infection in controllers is also caused by a single variant, some diversity must have accumulated compared to early infected individuals but that diversity in controllers did not generally reach the same high levels as in chronically infected noncontrollers. V1/V2 loop lengths were found to be significantly shorter in controllers than in noncontrollers during both early and chronic infections (Fig. 3E). Similarly, the number of PNLGS was found to be significantly lower in controllers than in early and chronic infections in noncontrollers (Fig. 3F). Taken together, these analyses suggest that adaptive events that are of importance to antibody escape are limited in HIV-1 controllers relative to noncontrollers.
Presences and selection of CTL escape mutations in HIV-1 controllers.
HLA sequences and full types were available from 12 controllers (Table 2) and 5 chronically infected noncontrollers (data not shown). An estimate of the percentage of CTL epitopes containing mutations in each individual was obtained by dividing the number of mutated epitopes in the autologous sequence by the number of sensitive epitopes present in a local consensus sequence. In PR from HIV-1 controllers, a median of 10% (range, 0 to 58.5%) of epitopes carried escape mutations, compared to a median of 75% (range, 56 to 90%) of epitopes in noncontrollers (P = 0.03). In RT, a median of 17.5% (range, 11.5 to 66.5%) of epitopes from controllers carried mutations compared to a median of 27% (range, 19 to 33%) in noncontrollers (P = 0.79). A complete list of epitopes and mutations in HIV-1 controllers is shown in Table S3 in the supplemental material. Escape mutations can arise in the infected host or can be acquired along the chain of transmission. The donor of one HIV-1 controller (C17) is known and is discordant with the recipient at all HLA loci. In the virus from C17, we observed a mutation (I15V) in a well-described A*1101 epitope located in Pro (VTIKIGGQLK). This mutation has been shown to affect T-cell receptor binding affinity (39) and was not present in any of the 20 sequences obtained from the donor. A Highlighter plot and the phylogenetic relationship of the Pro-RT region from the transmission pair can be found in Fig. S6 and S7 in the supplemental material. In addition, the virus in C07 and C11 acquired mutations in or adjacent to one of the anchor positions of well-described CTL epitopes during the observation period. These results imply the selection for specific CTL escape mutants in at least these three individuals.
DISCUSSION
In this report, we studied the evolution of pro-rt and env amplified by single-genome sequencing in a cohort of 21 HIV-1 controllers, infected for a median of 11 years, with persistent low-level viremia at a median level of 0.3 to 0.8 copies/ml.
Evidence of ongoing viral replication based on increasing divergence over time was found in a substantial number of HIV-1 controllers. Because of the extremely low viral loads in HIV-1 controllers, we were able to amplify sequences from only 30 to 40% of samples. Despite the low success rate, we were able to obtain sequences from more than one point in time in 15 of 21 HIV-1 controllers.
Phylogenetic analyses of both pro-rt and env from these 15 individuals with longitudinal data showed that viral divergence increased significantly with time in 8 HIV-1 controllers. In the remaining 6 controllers, we were not able to determine if there was evolution over time because of the small amount of sequence data or a narrow sampling interval (less than 3 months of observation). However, these results suggest that HIV-1 is undergoing full cycles of replication in at least 40% of the HIV-1 controllers in this cohort, and they do not exclude this possibility in any of the others. Evolution of plasma viruses in HIV-1 controllers was found in HLA B*57/27-positive, as well as HLA B*57/27-negative, individuals, suggesting that virus replication was not dependent on the absence of protective HLA alleles.
Some case studies have previously reported the absence of in vivo evolution in HIV-1 controllers (63, 64), as well as infection with replication-impaired strains of HIV-1 (34, 35). Although it is possible that some HIV-1 controllers are infected with replication-incompetent strains, such results must be interpreted with caution when phylogenetic analyses are based on viral DNA sequences obtained by analysis of the total PCR product. Such “bulk sequencing” may not be sufficiently sensitive to detect evolution over time, because it can detect only sequences present in about 20% or more of the virus population. In the present study, we used SGS to amplify viral sequences from plasma virus RNA, an approach that is far more sensitive than bulk sequencing (50) and unbiased by PCR-introduced recombination or resampling of sequences. Additionally, we prefer plasma RNA over viral DNA to assess viral evolution, since the latter is known to contain a significant fraction of sequences “archived” from much earlier times due to mutation or latent infection and may not give as accurate a picture of the population at the time of sampling (3, 58).
It was surprising to find that, although the level of viremia in HIV-1 controllers was much lower than in HIV-1 noncontrollers, pro-rt and env evolved at rates that were not greatly different. The rate of divergence primarily reflects the number of replication cycles the virus undergoes over a given period of time. In contrast, the level of viremia in an infected host reflects the steady state between the influx of virus into the plasma population, which is primarily determined by the number of virus-producing cells, and viral clearance. Thus, our observations suggest that, while viral generation times are not greatly different between controllers and noncontrollers, the number of infected cells, the number of progeny produced from each infected cell, and/or the rate of viral clearance differs greatly between them. Our results thus suggest that the efficiency at which the virus replicates in HIV-1 controllers is reduced to a much lesser extent (3- to 4-fold) than the level of viremia (ca. 10,000-fold) in the two groups, implying that factors other than slower replication must account for the difference in viremia.
Viral adaptation was found to be restricted in HIV-1 controllers. Because of the high evolutionary rate in HIV-1 in vivo, selection in viral genes can be interpreted as footprints of host immune pressure. The highest rates of adaptation (dN) are found in viruses replicating under moderate immune pressure, whereas low rates of adaptation are observed under particularly strong or weak immune responses (20).
Overall, the modes of selection were found to be similar regardless of controller status. In both patient groups, pro-rt evolved under purifying selection (dN/dS ≈ 0.5), whereas positive selection acted on env (dN/dS ≈ 2).
The similarity of the dN/dS ratios in the two groups implies that both were influenced by similar balances of adaptive and purifying selection forces. When examined in detail, however, different patterns of adaptation to the immune response in controllers and noncontrollers emerged. In Pro and RT, all HIV-1 controllers carried significantly fewer mutations within known CTL epitopes than did noncontrollers. The overall frequency of mutant epitopes in controllers was ∼25% that of sensitive CTL epitopes, which is at the same level that has been observed by others (3, 46) but significantly lower than that of noncontrollers, who carried escape mutations in as many as 50 to 100% of sensitive sites. As we reported previously (29), some of these mutations may have accumulated along the chain of transmission prior to infection of the patients studied, but we also found evidence for adaptation to specific immune responses in the infected controllers in at least three cases. These results suggest that, in both controllers and noncontrollers, the virus is replicating and adapting to a specific cellular immune response, although the lower overall rate of replication may reduce the extent of stimulation of the CTL response and, as a consequence, reduce the extent of selection of escape mutants.
In env, we found that adaptive events that are thought to be major determinants of escape from antibody neutralization—N-linked glycosylation sites and V1/V2 loop extensions (16, 54)—were much lower in HIV-1 controllers than in both early and chronically infected noncontrollers. As with the CTL response to PR-RT, these results may suggest that replication in controllers occurs in the face of relatively weak antibody responses that do not select for adaptive events and that in the absence of a specific selection pressure, the virus reverts to a form in which it replicates more efficiently. In support of this conclusion, no unique characteristic of the humoral immune response has been associated with controller status (1, 13, 32).
The relationship between the occurrence of CTL escape mutations, viremia, and disease progression is presently incompletely understood. It is beyond the scope of this study to directly address why viral suppression persists despite escape. Nevertheless, it is tempting to speculate that a hierarchy of epitopes exists—some provoke a very strong immune response that can effectively control (but not eliminate) the infection and thus remain sensitive and from which the virus cannot readily escape (at least not without a large fitness cost), whereas other epitopes, under weaker selection pressure, can escape without affecting control, and still other epitopes are not recognized at all because the low levels of virus replication preclude adequately efficient presentation. Under this hypothesis, the less potent CTL and humoral immune response in controllers is a consequence of the lower levels of virus replication, which may result from a potent CTL response to one or a small number of epitopes. Alternatively, some other restriction factor operating in controllers may lead to their low levels of viremia. We were not able to differentiate between these two scenarios, but further studies of host immune mechanisms may clarify this. The recent observation by Clerc et al. (8) of high-level viremia in two HIV controllers following superinfection with a distinct viral subtype is consistent with the absence of a generalized restriction on HIV infection in these individuals, lending support to the idea that control may reflect immunity directed at a specific epitope or epitopes.
We can also conclude that natural control of HIV-1 is not analogous to combination antiviral treatment. Although the level of viremia is significantly reduced in both cases, control persists despite ongoing full cycles of replication in HIV-1 controllers whereas replication, in many studies, has been found to be completely blocked in patients on a fully suppressive cART regimen (2, 7, 41, 60).
While this study was under review, O'Connell et al. (49) published their observation of evolution in the gag genes of at least 3 of 6 HIV-1 controllers, all of whom were HLA-B*57/27 positive. In our study, we were able to confirm these observations, and we have extended our understanding of viral selection of 2 different gene regions and of the replicative dynamics of HIV-1 in controllers compared to virus from noncontrollers. In addition, many of the patients in our study did not have HLA B27 or B57 alleles but still had evidence of low-level ongoing replication with naturally controlled viremia, suggesting that the mechanism of control does not require either of these two specific HLA alleles.
In conclusion, our examination of genetic changes in single-genome sequences in a cohort of 21 HIV-1 controllers, infected for a median of 11 years, has revealed that viral genes are evolving, implying underlying full cycles of viral replication. These results indicate that natural control is very different from control obtained through the use of antiretroviral drugs, and they raise hope for development of effective vaccines/therapeutics that can delay disease progression and prevent transmission by reducing the viral burden, but not necessarily blocking HIV-1 replication completely.
Supplementary Material
Acknowledgments
We thank the patients for participating in this study. We thank Mary Carrington and Maureen P. Martin for providing HLA genotypes; Jens Ole Nielsen for supporting the study; and Louise Nygaard Clausen, Anna-Louise Sørensen, Magrethe Lyneborg-Nielsen, and Dorthe Hass for help in the laboratory. Thanks are due to Matt Fivash for help with general mixed linear models. We also thank Bente Baadegaard and Lene Pors Jensen for including patients in the study. Special thanks are due to Andrea Galli for helping with graphics. We thank Brandon Keele for sharing sequences from early infected patients and Louise Bruun Jørgensen for providing sequences from local seroconverters.
The study was partly funded by The Danish Medical Research Council (271-07-0371). J.M.C. was a Research Professor of the American Cancer Society with support from the F.M. Kirby Foundation.
Footnotes
Published ahead of print on 6 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Bailey, J. R., K. G. Lassen, H. C. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 80:4758-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, J. R., A. R. Sedaghat, T. Kieffer, T. Brennan, P. K. Lee, M. Wind-Rotolo, C. M. Haggerty, A. R. Kamireddi, Y. Liu, J. Lee, D. Persaud, J. E. Gallant, J. Cofrancesco, Jr., T. C. Quinn, C. O. Wilke, S. C. Ray, J. D. Siliciano, R. E. Nettles, and R. F. Siliciano. 2006. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80:6441-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 203:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankson, J. N., J. R. Bailey, S. Thayil, H. C. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2006. Isolation and characterization of replication-competent HIV-1 from a subset of elite suppressors. J. Virol. 81:2508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzón, M. J., M. Massanella, J. M. Llibre, A. Esteve, V. Dahl, M. C. Puertas, J. M. Gatell, P. Domingo, R. Paredes, M. Sharkey, S. Palmer, M. Stevenson, B. Clotet, J. Blanco, and J. Martinez-Picado. 2010. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 16:460-465. [DOI] [PubMed] [Google Scholar]
- 7.Chomont, N., M. El-Far, P. Ancuta, L. Trautmann, F. A. Procopio, B. Yassine-Diab, G. Boucher, M. R. Boulassel, G. Ghattas, J. M. Brenchley, T. W. Schacker, B. J. Hill, D. C. Douek, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2009. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerc, O., S. Colombo, S. Yerly, A. Telenti, and M. Cavassini. 2010. HIV-1 elite controllers: beware of super-infections. J. Clin. Virol. 47:376-378. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 10.Delwart, E. L., H. W. Sheppard, B. D. Walker, J. Goudsmit, and J. I. Mullins. 1994. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J. Virol. 68:6672-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinoso, J. B., S. Y. Kim, R. F. Siliciano, and J. N. Blankson. 2008. A comparison of viral loads between HIV-1-infected elite suppressors and individuals who receive suppressive highly active antiretroviral therapy. Clin. Infect. Dis. 47:102-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinoso, J. B., S. Y. Kim, A. M. Wiegand, S. E. Palmer, S. J. Gange, L. Cranmer, A. O'Shea, M. Callender, A. Spivak, T. Brennan, M. F. Kearney, M. A. Proschan, J. M. Mican, C. A. Rehm, J. M. Coffin, J. W. Mellors, R. F. Siliciano, and F. Maldarelli. 2009. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 106:9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doria-Rose, N. A., R. M. Klein, M. M. Manion, S. O'Dell, A. Phogat, B. Chakrabarti, C. W. Hallahan, S. A. Migueles, J. Wrammert, R. Ahmed, M. Nason, R. T. Wyatt, J. R. Mascola, and M. Connors. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond, A. J., and A. Rambaut. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiebig, E. W., D. J. Wright, B. D. Rawal, P. E. Garrett, R. T. Schumacher, L. Peddada, C. Heldebrant, R. Smith, A. Conrad, S. H. Kleinman, and M. P. Busch. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871-1879. [DOI] [PubMed] [Google Scholar]
- 16.Frost, S. D., T. Wrin, D. M. Smith, S. L. Kosakovsky Pond, Y. Liu, E. Paxinos, C. Chappey, J. Galovich, J. Beauchaine, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. U. S. A. 102:18514-18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi, R. T., R. J. Bosch, E. Aga, M. Albrecht, L. M. Demeter, C. Dykes, B. Bastow, M. Para, J. Lai, R. F. Siliciano, J. D. Siliciano, and J. J. Eron. 2010. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J. Infect. Dis. 201:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi, R. T., L. Zheng, R. J. Bosch, E. S. Chan, D. M. Margolis, S. Read, B. Kallungal, S. Palmer, K. Medvik, M. M. Lederman, N. Alatrakchi, J. M. Jacobson, A. Wiegand, M. Kearney, J. M. Coffin, J. M. Mellors, and J. J. Eron on behalf of the AIDS Clinical Trials Group A5244 team. 2010. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 7:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao, X., G. W. Nelson, P. Karacki, M. P. Martin, J. Phair, R. Kaslow, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, S. J. O'Brien, and M. Carrington. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344:1668-1675. [DOI] [PubMed] [Google Scholar]
- 20.Grenfell, B. T., O. G. Pybus, J. R. Gog, J. L. Wood, J. M. Daly, J. A. Mumford, and E. C. Holmes. 2004. Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303:327-332. [DOI] [PubMed] [Google Scholar]
- 21.Hall, T. 2007. Bioedit, 7.0.9 ed. Ibis Biosciences, Carlsbad, CA.
- 22.Hatano, H., E. L. Delwart, P. J. Norris, T. H. Lee, J. Dunn-Williams, P. W. Hunt, R. Hoh, S. L. Stramer, J. M. Linnen, J. M. McCune, J. N. Martin, M. P. Busch, and S. G. Deeks. 2009. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J. Virol. 83:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 24.Hunt, P. W., J. Brenchley, E. Sinclair, J. M. McCune, M. Roland, K. Page-Shafer, P. Hsue, B. Emu, M. Krone, H. Lampiris, D. Douek, J. N. Martin, and S. G. Deeks. 2008. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 197:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julg, B., F. Pereyra, M. J. Buzon, A. Piechocka-Trocha, M. J. Clark, B. M. Baker, J. Lian, T. Miura, J. Martinez-Picado, M. M. Addo, and B. D. Walker. 2010. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin. Infect. Dis. 51:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan, N., and R. R. Hudson. 1985. The use of sample genealogies for studying a selectively neutral m-loci model with recombination. Theor. Popul. Biol. 28:382-396. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann, D. E., D. G. Kavanagh, F. Pereyra, J. J. Zaunders, E. W. Mackey, T. Miura, S. Palmer, M. Brockman, A. Rathod, A. Piechocka-Trocha, B. Baker, B. Zhu, G. S. Le, M. T. Waring, R. Ahern, K. Moss, A. D. Kelleher, J. M. Coffin, G. J. Freeman, E. S. Rosenberg, and B. D. Walker. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8:1246-1254. [DOI] [PubMed] [Google Scholar]
- 28.Kearney, M., F. Maldarelli, W. Shao, J. B. Margolick, E. S. Daar, J. W. Mellors, V. Rao, J. M. Coffin, and S. Palmer. 2009. HIV-1 population genetics and adaptation in newly infected individuals. J. Virol. 83:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearney, M., S. Palmer, F. Maldarelli, W. Shao, M. A. Polis, J. Mican, D. Rock-Kress, J. B. Margolick, J. M. Coffin, and J. W. Mellors. 2008. Frequent polymorphism at drug resistance sites in HIV-1 protease and reverse transcriptase. AIDS 22:497-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambotte, O., F. Boufassa, Y. Madec, A. Nguyen, C. Goujard, L. Meyer, C. Rouzioux, A. Venet, and J. F. Delfraissy. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053-1056. [DOI] [PubMed] [Google Scholar]
- 32.Lambotte, O., G. Ferrari, C. Moog, N. L. Yates, H. X. Liao, R. J. Parks, C. B. Hicks, K. Owzar, G. D. Tomaras, D. C. Montefiori, B. F. Haynes, and J. F. Delfraissy. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamine, A., A. Caumont-Sarcos, M. L. Chaix, A. Saez-Cirion, C. Rouzioux, J. F. Delfraissy, G. Pancino, and O. Lambotte. 2007. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study). AIDS 21:1043-1045. [DOI] [PubMed] [Google Scholar]
- 34.Learmont, J., B. Tindall, L. Evans, A. Cunningham, P. Cunningham, J. Wells, R. Penny, J. Kaldor, and D. A. Cooper. 1992. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340:863-867. [DOI] [PubMed] [Google Scholar]
- 35.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 36.Lemey, P., S. L. Kosakovsky Pond, A. J. Drummond, O. G. Pybus, B. Shapiro, H. Barroso, N. Taveira, and A. Rambaut. 2007. Synonymous substitution rates predict HIV disease progression as a result of underlying replication dynamics. PLoS. Comput. Biol. 3:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, S. L., A. G. Rodrigo, R. Shankarappa, G. H. Learn, L. Hsu, O. Davidov, L. P. Zhao, and J. I. Mullins. 1996. HIV quasispecies and resampling. Science 273:415-416. [DOI] [PubMed] [Google Scholar]
- 38.Mansky, L. M., and H. M. Temin. 1995. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 69:5087-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney, D. M., R. Skvoretz, B. D. Livingston, C. C. Wilson, M. Anders, R. W. Chesnut, A. Sette, M. Essex, V. Novitsky, and M. J. Newman. 2004. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J. Immunol. 173:1941-1950. [DOI] [PubMed] [Google Scholar]
- 40.McMahon, D., J. Jones, A. Wiegand, S. J. Gange, M. Kearney, S. Palmer, S. McNulty, J. A. Metcalf, E. Acosta, C. Rehm, J. M. Coffin, J. W. Mellors, and F. Maldarelli. 2010. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin. Infect. Dis. 50:912-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mens, H., A. G. Pedersen, L. B. Jorgensen, S. Hue, Y. Yang, J. Gerstoft, and T. L. Katzenstein. 2007. Investigating signs of recent evolution in the pool of proviral HIV type 1 DNA during years of successful HAART. AIDS Res. Hum. Retroviruses 23:107-115. [DOI] [PubMed] [Google Scholar]
- 42.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, B. D. Van, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 43.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miura, T., M. A. Brockman, C. J. Brumme, Z. L. Brumme, J. M. Carlson, F. Pereyra, A. Trocha, M. M. Addo, B. L. Block, A. C. Rothchild, B. M. Baker, T. Flynn, A. Schneidewind, B. Li, Y. E. Wang, D. Heckerman, T. M. Allen, and B. D. Walker. 2008. Genetic characterization of human immunodeficiency virus type 1 in elite controllers: lack of gross genetic defects or common amino acid changes. J. Virol. 82:8422-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miura, T., C. J. Brumme, M. A. Brockman, Z. L. Brumme, F. Pereyra, B. L. Block, A. Trocha, M. John, S. Mallal, P. R. Harrigan, and B. D. Walker. 2009. HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. J. Virol. 83:3407-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nei, M., and S. Kumar. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY.
- 48.Obel, N., F. N. Engsig, L. D. Rasmussen, M. V. Larsen, L. H. Omland, and H. T. Sorensen. 2009. Cohort profile: the danish HIV cohort study. Int. J. Epidemiol. 38:1202-1206. [DOI] [PubMed] [Google Scholar]
- 49.O'Connell, K. A., T. P. Brennan, J. R. Bailey, S. C. Ray, R. F. Siliciano, and J. N. Blankson. 2010. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J. Virol. 84:7018-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer, S., M. Kearney, F. Maldarelli, E. K. Halvas, C. J. Bixby, H. Bazmi, D. Rock, J. Falloon, R. T. Davey, Jr., R. L. Dewar, J. A. Metcalf, S. Hammer, J. W. Mellors, and J. M. Coffin. 2005. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J. Clin. Microbiol. 43:406-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer, S., A. P. Wiegand, F. Maldarelli, H. Bazmi, J. M. Mican, M. Polis, R. L. Dewar, A. Planta, S. Liu, J. A. Metcalf, J. W. Mellors, and J. M. Coffin. 2003. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J. Clin. Microbiol. 41:4531-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 53.Pereyra, F., S. Palmer, T. Miura, B. L. Block, A. Wiegand, A. C. Rothchild, B. Baker, R. Rosenberg, E. Cutrell, M. S. Seaman, J. M. Coffin, and B. D. Walker. 2009. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 200:984-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pond, S. L., S. D. Frost, and S. V. Muse. 2005. HyPhy: hypothesis testing using phylogenies. Bioinformatics 21:676-679. [DOI] [PubMed] [Google Scholar]
- 56.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 57.Rozas, J., J. C. Sanchez-DelBarrio, X. Messeguer, and R. Rozas. 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496-2497. [DOI] [PubMed] [Google Scholar]
- 58.Russell, R. A., M. D. Moore, W. S. Hu, and V. K. Pathak. 2009. APOBEC3G induces a hypermutation gradient: purifying selection at multiple steps during HIV-1 replication results in levels of G-to-A mutations that are high in DNA, intermediate in cellular viral RNA, and low in virion RNA. Retrovirology 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sáez-Cirión, A., C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barre-Sinoussi, J. F. Delfraissy, M. Sinet, G. Pancino, and A. Venet. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sedaghat, A. R., J. D. Siliciano, T. P. Brennan, C. O. Wilke, and R. F. Siliciano. 2007. Limits on replenishment of the resting CD4+ T cell reservoir for HIV in patients on HAART. PLoS Pathog. 3:e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swofford, D. L. 2003. PAUP—phylogenetic analysis using parsimony, 4 ed. Sinauer Associates, Sunderland, MA.
- 62.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 63.Wang, B., W. B. Dyer, J. J. Zaunders, M. Mikhail, J. S. Sullivan, L. Williams, D. N. Haddad, G. Harris, J. A. Holt, D. A. Cooper, M. Miranda-Saksena, R. Boadle, A. D. Kelleher, and N. K. Saksena. 2002. Comprehensive analyses of a unique HIV-1-infected nonprogressor reveal a complex association of immunobiological mechanisms in the context of replication-incompetent infection. Virology 304:246-264. [DOI] [PubMed] [Google Scholar]
- 64.Wang, B., M. Mikhail, W. B. Dyer, J. J. Zaunders, A. D. Kelleher, and N. K. Saksena. 2003. First demonstration of a lack of viral sequence evolution in a nonprogressor, defining replication-incompetent HIV-1 infection. Virology 312:135-150. [DOI] [PubMed] [Google Scholar]
- 65.Wolfs, T. F., G. Zwart, M. Bakker, and J. Goudsmit. 1992. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology 189:103-110. [DOI] [PubMed] [Google Scholar]
- 66.Wolinsky, S. M., C. M. Wike, B. T. Korber, C. Hutto, W. P. Parks, L. L. Rosenblum, K. J. Kunstman, M. R. Furtado, and J. L. Munoz. 1992. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255:1134-1137. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, M., B. Gaschen, W. Blay, B. Foley, N. Haigwood, C. Kuiken, and B. Korber. 2004. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology 14:1229-1246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.