Abstract
A safe and potent adjuvant is needed for development of mucosal vaccines against etiological agents, such as influenza virus, that enter the host at mucosal surfaces. Cytokines are potential adjuvants for mucosal vaccines because they can enhance primary and memory immune responses enough to protect against some infectious agents. For this study, we tested 26 interleukin (IL) cytokines as mucosal vaccine adjuvants and compared their abilities to induce antigen (Ag)-specific immune responses against influenza virus. In mice intranasally immunized with recombinant influenza virus hemagglutinin (rHA) plus one of the IL cytokines, IL-1 family cytokines (i.e., IL-1α, IL-1β, IL-18, and IL-33) were found to increase Ag-specific immunoglobulin G (IgG) in plasma and IgA in mucosal secretions compared to those after immunization with rHA alone. In addition, high levels of both Th1- and Th2-type cytokines were observed in mice immunized with rHA plus an IL-1 family cytokine. Furthermore, mice intranasally immunized with rHA plus an IL-1 family cytokine had significant protection against a lethal influenza virus infection. Interestingly, the adjuvant effects of IL-18 and IL-33 were significantly decreased in mast cell-deficient W/Wv mice, indicating that mast cells have an important role in induction of Ag-specific mucosal immune responses induced by IL-1 family cytokines. In summary, our results demonstrate that IL-1 family cytokines are potential mucosal vaccine adjuvants and can induce Ag-specific immune responses for protection against pathogens like influenza virus.
Because most pathogenic viruses, including influenza virus, enter through a mucosal surface (18), preventing infection at the viral entry site by inducing mucosal immunity should be an effective strategy for combating such pathogens. A key aspect of mucosal immunity is production of secretory immunoglobulin A (sIgA), as well as induction of cytolytic T lymphocytes (CTLs) against epithelium-transmitted pathogens (5, 21). Therefore, it is important to develop mucosal vaccines that induce effective immune responses at mucosal surfaces (31).
However, protein subunit antigens (Ags) generally evoke only a weak or undetectable adaptive immune response when administered intramucosally (1). Therefore, to produce effective mucosal vaccines, it is necessary to develop an appropriate mucosal vaccine adjuvant (34). Cholera toxin (CT) and Escherichia coli heat-labile enterotoxin are known potent mucosal vaccine adjuvants and have been used in nonclinical experimental systems (9, 27). However, their clinical application as nasal adjuvants had to be discontinued because of side effects such as Bell's palsy (29). Therefore, mucosal vaccine adjuvants with high efficacy and safety for clinical application continue to be urgently required.
Cytokines are key molecules that trigger the innate and adaptive immune responses (including maturation of Ag-presenting cells, differentiation of Th1 and Th2 cells, and induction of cytotoxic natural killer [NK] cells and CTLs), resulting in protective layers against virus infection (11, 41, 43). Therefore, cytokines are promising vaccine adjuvants for enhancing the immune response against infectious pathogens. At present, more than 30 members of the interleukin (IL) cytokine/IL receptor family have been identified and found to be involved in regulating and maintaining homeostasis of the immune system (3, 14). Specific IL cytokines have been used as vaccine adjuvants to enhance primary and memory immune responses against some cancers and infectious diseases (2, 6). However, there has been no comparative study of IL cytokines as mucosal vaccine adjuvants.
Recently, it was pointed out that identification of the cellular targets of vaccine adjuvants is an important issue (12). Dendritic cells (DCs) are responsible for Ag uptake and presentation to naive T cells and represent a key target for adjuvant activity (22, 33). Recent reports have demonstrated that other accessory cells, such as mast cells (MCs) and NKT cells, act as immunosensors to initiate and modulate innate and adaptive immune responses (16, 40). It has been reported that MCs contribute to the induction of an adaptive immune response or accessory function and that the synthetic Toll-like receptor 7 ligand imiquimod acts as a mucosal vaccine adjuvant in an MC-dependent manner (19). However, it is still not clear whether MCs are promising cellular targets for cytokine adjuvants in mucosal vaccines.
In this study to develop effective and safe mucosal vaccine adjuvants, we identified promising cytokines with mucosal vaccine adjuvant activity by screening 26 different IL cytokines. We also investigated the mucosal and systemic immune responses induced by these cytokines in normal and MC-deficient mice. The IL-1 family cytokines (IL-1α, IL-1β, IL-18, and IL-33) were found to be effective mucosal vaccine adjuvants for induction of protective sIgA and CTL immunity against influenza virus. In addition, the adjuvant activities of IL-18 and IL-33 were MC dependent.
MATERIALS AND METHODS
Cytokines and Ags.
CT was purchased from List Biological Laboratories (Campbell, CA). Twenty-six types of mouse recombinant IL cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-17, IL-18, IL-19, IL-20, IL-21, IL-22, IL-23, IL-27, IL-28A, IL-28B, IL-31, and IL-33) were purchased from R&D Systems (Minneapolis, MN). Baculovirus-expressed recombinant influenza virus hemagglutinin (rHA) derived from influenza virus A/New Caledonia/20/1999 (Protein Sciences, Meriden, CT) was used as the vaccine Ag.
Mice and immunization protocols.
Female BALB/c mice and MC-deficient (WBB6F1 W/Wv) and congenic littermate control (WBB6F1 WT) mice were purchased from Japan SLC (Hamamatsu, Japan) and used at 6 weeks of age. All animal experimental procedures used in this study were performed in accordance with our institutional guidelines for animal experiments. Mice were immunized intranasally with rHA alone (1 μg/mouse), rHA (1 μg/mouse) plus CT (1 μg/mouse), or rHA (1 μg/mouse) plus one of the IL cytokines (0.1 μg, 0.3 μg, or 1.0 μg/mouse) on days 0 and 28.
Sample collection.
Fourteen days after the final immunization, plasma and mucosal secretions (nasal washes, saliva, vaginal washes, and fecal extracts) were obtained as previously described (24).
Detection of Ab responses by ELISA.
rHA-specific antibody (Ab) levels in plasma and mucosal secretions were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (24). Briefly, ELISA plates were coated with 2 μg rHA/ml of 0.1 M carbonate buffer and incubated overnight at 4°C. The plates were then incubated with blocking solution (Block Ace; DS Pharma Biomedical, Osaka, Japan) at 37°C for 2 h. Diluted plasma or mucosal secretions were added. After incubation at 37°C for 2 h, the coated plates were washed with phosphate-buffered saline (PBS)-polyoxyethylene sorbitan monolaurate (Tween 20; Wako Pure Chemical, Tokyo, Japan) and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG solution to detect IgG in plasma or with a biotin-conjugated goat anti-mouse IgA detection Ab (Southern Biotechnology Associates, Birmingham, AL) solution to detect sIgA in mucosal secretions, at 37°C for 2 h. For detection of sIgA, the plates were incubated with HRP-coupled streptavidin (Zymed Laboratories, South San Francisco, CA) for 1 h at room temperature. After incubation, a color reaction was developed with tetramethylbenzidine (Moss, Inc., Pasadena, MD), stopped with 2 N H2SO4, and measured as the optical density at 450 to 655 nm (OD450-655) in a microplate reader.
Multiplex cytokine assay.
Splenocytes from immunized BALB/c, WBB6F1 W/Wv, or WBB6F1 WT mice were harvested 14 days after the final immunization and stimulated in vitro with 10 μg rHA/ml. After 72 h, culture supernatants from in vitro unstimulated and rHA-stimulated cells were analyzed by a Bio-Plex multiplex cytokine assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Samples were analyzed on a Luminex 100 analyzer (Luminex, Austin, TX). The mean concentrations of cytokines in supernatants from rHA-stimulated cells were calculated relative to those in unstimulated cells.
IFN-γ ELISPOT assay.
Splenocytes from immunized mice were harvested 14 days after the final immunization and stimulated at a cell density of 1 × 107 cells/ml with a mixture of two H-2Kd-restricted class I HA peptides, HA240-248 (IYSTVASSL) and HA462-470 (LYEKVKSQL) (MBL, Nagoya, Japan), at a final concentration of 10 μg total peptide/ml complete RPMI (25). After 24 h of incubation at 37°C, plates were washed, and gamma interferon (IFN-γ)-producing cells were measured by use of an enzyme-linked immunospot (ELISPOT) assay kit (BD Biosciences, San Diego, CA) according to the manufacturer's instructions.
Tetramer assay.
Splenocytes from immunized mice were harvested 14 days after the final immunization and used as effector cells to determine HA240-248-specific CTL responses. Splenocytes (7 × 106 cells) were added to wells in a 24-well plate, followed by addition of 1 ml of medium containing a CTL epitope peptide (HA240-248; IYSTVASSL) at a final concentration of 1 μg/ml. After incubation at 37°C for 2 days, medium containing human recombinant IL-2 (rIL-2) (Shionogi Co., Osaka, Japan) was added to each well of CTL effector cells, to a final concentration of 10 U human rIL-2/ml. Effector cells were stained for tetramers after restimulation for 7 days. For analysis, 1 × 106 cells were treated with purified anti-mouse CD16/CD32 Ab (Fc-γ III/II receptor Ab; BD Biosciences Pharmingen, San Diego, CA) and then stained with phycoerythrin (PE)-conjugated H-2Kd-HA240-248 peptide tetramer (MBL, Nagoya, Japan) for 20 min at room temperature. Fluorescein isothiocyanate (FITC)-conjugated CD8α (clone KT15; MBL, Nagoya, Japan) was added for an additional 20 min. Cells were analyzed with a FACS Canto flow cytometer (BD Biosciences Pharmingen). Data analysis was done with FlowJo (TreeStar, Eugene, OR) software.
Histopathological analysis.
BALB/c mice were immunized intranasally with rHA (1 μg/mouse), with or without IL-1α, IL-1β, IL-18, or IL-33 (1 μg/mouse), on days 0 and 28. Fourteen days after the final immunization, the heads of the mice were severed from the bodies and placed in fixative solution (4% paraformaldehyde). The samples then were sectioned and stained with hematoxylin and eosin (H&E) or Luna stain and examined for pathological changes under a light microscope. Histopathological examination was performed by the Applied Medical Research Laboratory (Osaka, Japan).
Influenza virus infection in vivo.
To examine the prophylactic effect of IL cytokine treatment against influenza virus, mice were immunized intranasally on days 0 and 28 with 1 μg PR8 HA vaccine (inactivated-product vaccine with influenza virus A/Puerto Rico/8/34) (Charles River, North Franklin, CT)/mouse plus 1 μg CT or IL-1 family cytokine/mouse. Fourteen days after the final immunization, mice were fully anesthetized by intraperitoneal injection of pentobarbital, and each was infected by intranasal application of 25 μl PBS containing 256 hemagglutinating units (HAU) of influenza virus A/PR/8/34 (H1N1) (kindly provided by the Research Institute for Microbial Diseases of Osaka University, Osaka, Japan) per mouse. This procedure produced upper and lower respiratory tract infections.
Statistical analysis.
All results are expressed as means ± standard errors of the means (SEM). Differences were compared using Bonferroni analysis of variance (ANOVA).
RESULTS
Comparative analysis of rHA-specific Ab responses induced by 26 different IL cytokines.
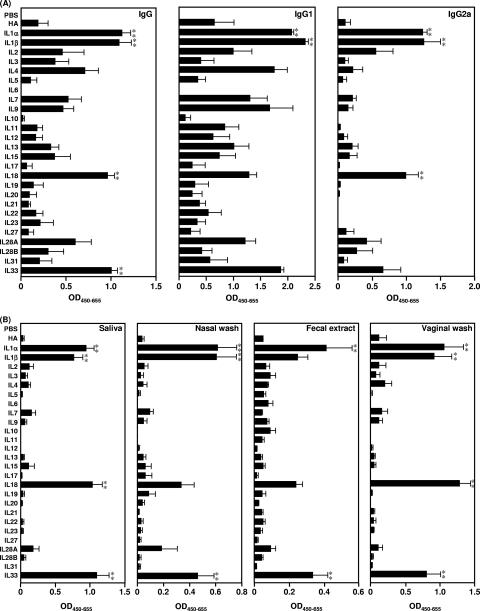
One potential advantage of successful mucosal immunization is the possibility of eliciting both systemic IgG and mucosal sIgA Ab responses against invading pathogens. Therefore, in this study, we tested 26 different IL cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-17, IL-18, IL-19, IL-20, IL-21, IL-22, IL-23, IL-27, IL-28A, IL-28B, IL-31, and IL-33) as mucosal vaccine adjuvants. To examine the potential of these IL cytokines as mucosal vaccine adjuvants, BALB/c mice were immunized intranasally with 1 μg rHA plus 1 μg of an IL cytokine on days 0 and 28. Fourteen days after the final immunization, we examined the level of anti-rHA IgG in plasma by ELISA (Fig. 1A). Intranasal immunization with rHA plus 11 of the IL cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-7, IL-9, IL-13, IL-15, IL-18, IL-28A, and IL-33) induced higher rHA-specific IgG responses in plasma than those for mice immunized with rHA alone (Fig. 1A). In particular, immunization with rHA plus IL-1α, IL-1β, IL-18, or IL-33, referred to as IL-1 family cytokines, resulted in the highest rHA-specific IgG responses among the IL cytokines. The IgG subclass of the rHA-specific responses was then examined to assess the type of immune response induced by the 26 IL cytokines (Fig. 1A). Plasma Ag-specific IgG subclasses reflect the subset of CD4+ T-helper cells induced by vaccination, with IgG1 and IgG2a corresponding to Th2 and Th1 responses, respectively. Consistent with the rHA-specific IgG responses, intranasal immunization with rHA plus IL-2, IL-3, IL-4, IL-7, IL-9, IL-13, IL-15, or IL-28A generally produced a greater rHA-specific IgG1 subclass response than immunization with rHA alone but a similar IgG2a response to that with rHA alone. In contrast, mice immunized with rHA plus IL-1 family cytokines showed significantly higher IgG1 and IgG2a Ab responses than those immunized with rHA alone. These results indicate that nasal administration of IL-1 family cytokines has the potential to induce potent rHA-specific systemic IgG Abs, as well as IgG1 and IgG2a Ab responses. We then studied the rHA-specific sIgA response in mucosal secretions (i.e., in saliva, nasal washes, fecal extracts, and vaginal washes) induced by the 26 IL cytokines (Fig. 1B). For these 26 IL cytokines, IL-1 family cytokines induced the highest mucosal sIgA Ab responses in salivary, nasal, fecal, and vaginal mucosal secretions (Fig. 1B). Taken together, these results indicate that nasal immunization with IL-1 family cytokines effectively induced rHA-specific Ab responses in both systemic and mucosal immune compartments, suggesting that IL-1 family cytokines might be effective mucosal vaccine adjuvants.
FIG. 1.
Ab responses induced by IL-1 family cytokines. BALB/c mice were immunized intranasally at 0 and 28 days with rHA alone or rHA plus each interleukin. (A) Plasma was collected 14 days after the final immunization and analyzed by ELISA for rHA-specific IgG, IgG1, and IgG2a. (B) Saliva, nasal washes, fecal extracts, and vaginal washes were collected 14 days after the final immunization and analyzed by ELISA for rHA-specific sIgA. Data are presented as means ± SEM (n = 5). **, P < 0.01 compared to the value for the rHA-treated group.
Dose-response relationship of IL-1 family cytokines as mucosal vaccine adjuvants for induction of rHA-specific Ab responses.
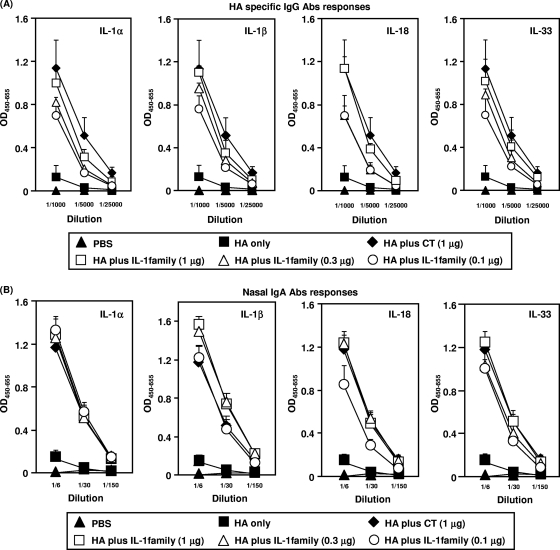
To determine the dose-response relationship of IL-1 family cytokines as mucosal vaccine adjuvants to induce rHA-specific IgG and sIgA Ab responses, mice were immunized intranasally with rHA plus 0.1, 0.3, or 1 μg of each IL-1 family cytokine (Fig. 2). Immunization with rHA plus the IL-1 family cytokines induced rHA-specific IgG in plasma in a dose-dependent manner. Even rHA plus the lowest dose (0.1 μg) of IL-1 family cytokines induced IgG to levels significantly higher than those induced by rHA alone (Fig. 2A). Importantly, the use of 1 μg of IL-1 family cytokines as an adjuvant resulted in strong rHA-specific IgG Ab responses equivalent to those elicited by CT, which is one of the most potent mucosal vaccine adjuvants (Fig. 2A). Furthermore, the level of rHA-specific sIgA induced by rHA plus 0.1 μg of each IL-1 family cytokine in nasal secretions was significantly higher than that induced by rHA alone (Fig. 2B). The level of rHA-specific nasal sIgA induced in mice immunized intranasally with rHA plus 0.3 μg of each IL-1 family cytokine was equivalent to that observed in mice treated with 1 μg CT. Taken together, these results clearly indicate that nasal immunization with an IL-1 family cytokine as a mucosal vaccine adjuvant induced dose-dependent levels of both rHA-specific IgG and sIgA Abs in the mucosal and systemic immune compartments.
FIG. 2.
Dose-response relationship for induction of rHA-specific Ab responses by nasal immunization with rHA plus an IL-1 family cytokine. BALB/c mice were immunized intranasally at 0 and 28 days with rHA alone, rHA plus CT (1 μg/mouse), or rHA plus an IL-1 family cytokine (0.1, 0.3, or 1 μg/mouse). (A) Plasma was collected 14 days after the final immunization and analyzed by ELISA for rHA-specific IgG, at dilutions of 1/1,000, 1/5,000, and 1/250,000. (B) Nasal washes were collected 14 days after the final immunization and analyzed by ELISA for rHA-specific sIgA, at dilutions of 1/6, 1/30, and 1/150. Data are presented as means ± SEM (n = 5).
Induction of rHA-specific Th1- and Th2-type responses after nasal administration of IL-1 family cytokines as mucosal vaccine adjuvants.
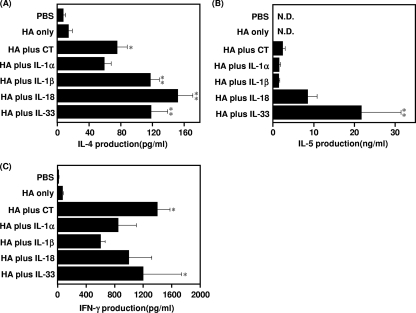
To evaluate the ability of IL-1 family cytokines to boost rHA-specific cytokine responses induced by mucosal immunization, splenocytes from mice that had been immunized intranasally with rHA alone, rHA plus CT, or rHA plus an IL-1 family cytokine were restimulated in vitro with rHA and then assayed for Th1 (IFN-γ) and Th2 (IL-4 and IL-5) cytokines (Fig. 3). Splenocytes from mice immunized with rHA alone did not show significant cytokine production compared to those from PBS-treated mice. Consistent with the IgG subclass results (Fig. 1A), mice immunized with IL-1 family cytokines had higher levels of IL-4 and IL-5 (Th2-associated sIgA-enhancing cytokines) than mice given rHA alone. In particular, the highest levels of IL-4 and IL-5 were detected in splenocytes of mice immunized with rHA plus IL-18 or IL-33, and these responses were significantly higher than those in splenocytes of mice immunized with CT. It was also noteworthy that IFN-γ, a Th1 cytokine, was induced in mice immunized intranasally with rHA plus an IL-1 family cytokine. Thus, IL-1 family cytokines might induce CTL responses when administered nasally. These results show that as mucosal vaccine adjuvants, IL-1α, IL-1β, IL-18, and IL-33 elicit both Th1- and Th2-type cytokine responses.
FIG. 3.
Cytokine responses induced by nasal immunization with rHA plus IL-1 family cytokines. BALB/c mice were immunized intranasally at 0 and 28 days with rHA alone, rHA plus CT, or rHA plus an IL-1 family cytokine. Fourteen days after the final immunization, splenocytes from each group were cultured with 10 μg rHA/ml. Culture supernatants were harvested after a 3-day incubation and then assayed for rHA-specific IL-4 (A), IL-5 (B), and IFN-γ (C), using a Bio-Plex multiplex cytokine assay. Data are presented as means ± SEM (n = 5). *, P < 0.05; **, P < 0.01 compared to the value for the rHA-treated group. N.D., not done.
In vivo CTL induction by nasal immunization with rHA plus IL-1 family cytokines as mucosal vaccine adjuvants.
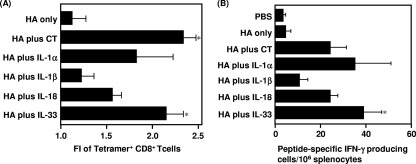
Virus clearance is known to require strong Th1-polarized immune responses characterized by IFN-γ production and CTL responses in the systemic compartment. To investigate the ability of IL-1 family cytokines to act as mucosal vaccine adjuvants and to induce rHA-specific Th1/CTL immune responses, we measured H-2Kd/HA240-248 tetramer+ CD8+ T cells (Fig. 4A) and H-2Kd/HA240-248-specific IFN-γ-secreting cells (Fig. 4B) in splenocytes from mice that had been immunized intranasally with rHA alone, rHA plus CT, or rHA plus an IL-1 family cytokine. The level of H-2Kd/HA240-248 tetramer+ CD8+ T cells induced by rHA plus IL-1β was found to be similar to that induced by rHA alone, but the level induced by rHA plus IL-1α, IL-18, or IL-33 was significantly greater than that induced by rHA alone (Fig. 4A). Furthermore, the level of functionally active H-2Kd/HA240-248-specific IFN-γ-secreting cells induced by rHA plus IL-1α, IL-18, or IL-33 was the same as or greater than that in mice intranasally immunized with rHA plus CT (Fig. 4B). Taken together, these results indicate that the IL-1 family cytokines IL-1α, IL-18 and IL-33 induce high-avidity CD8+ CTLs. Therefore, intranasally administered IL-1α, IL-18, and IL-33 might be useful adjuvants for development of an effective mucosal influenza vaccine.
FIG. 4.
Measurement of H-2Kd/HA240-248 tetramer+ CD8+ T cells and H-2Kd/HA240-248-specific IFN-γ-secreting cells in the spleen after nasal immunization with rHA plus an IL-1 family cytokine. BALB/c mice were immunized intranasally at 0 and 28 days with rHA alone, rHA plus CT, or rHA plus an IL-1 family cytokine. Fourteen days after the final immunization, splenocytes from immunized mice were harvested and stimulated with H-2Kd-restricted class I HA peptide at a final concentration of 10 μg total peptide/ml. (A) For detection of H-2Kd/HA240-248 tetramer+ CD8+ T cells, splenocytes from immunized mice were cultured in medium containing a CTL epitope peptide (HA240-248; IYSTVASSL) plus 10 U human IL-2/ml for 7 days, stained for CD8, and analyzed for tetramer-binding cells by flow cytometry. FI, fluorescence intensity. (B) After 24 h of incubation, IFN-γ-producing cells were measured by an ELISPOT assay. Data are presented as means ± SEM (n = 5). *, P < 0.05 compared to the value for the rHA-treated group.
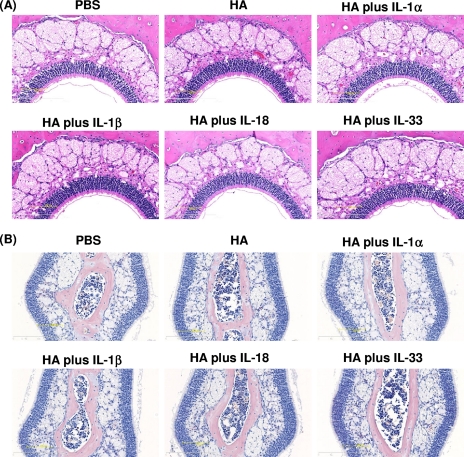
Histopathological changes due to IL-1 family cytokines administered intranasally as mucosal vaccine adjuvants.
Although enterotoxin-based adjuvants show strong mucosal immunity-inducing ability, they have significant toxic side effects on the central nervous system due to the presence of a specific receptor, GM1 ganglioside, which is highly expressed in neuronal tissue (39). To evaluate the in vivo toxicity of IL-1 family cytokines, histopathological changes in nasal tissues of mice given 1 μg of IL-1 family cytokines were investigated. No histological changes indicative of severe inflammation or membrane barrier disruption were observed in the nasal cavities of mice nasally administrated 1 μg of an IL-1 family cytokine (Fig. 5A). In particular, there was no evidence of massive accumulations of mononuclear cells around the airways and blood vessels or of infiltrates in the nasal tissues for all mice examined. Importantly, mice immunized intranasally with IL-1 family cytokines did not induce the goblet cell hyperplasia observed in patients with asthma and chronic obstructive pulmonary disease. Furthermore, Luna staining revealed that IL-1 family cytokine-treated mice did not develop infiltration of Luna-stained eosinophils into the nasal septum (Fig. 5B). Although further evaluation is required, these results indicate that the toxicity of IL-1 family cytokines is likely to be relatively low.
FIG. 5.
Histopathological analysis of the nasal cavities of mice immunized intranasally with IL-1 family cytokines. Frontal cross sections of the nasal cavities of mice were taken after two administrations of PBS, rHA alone, or rHA plus an IL-1 family cytokine. Sections were prepared and stained with H&E (A) or Luna stain (B) to assess pathological changes. Overall views of the nasal epithelium (A) and of Luna-stained eosinophils in the nasal septum (B) are shown.
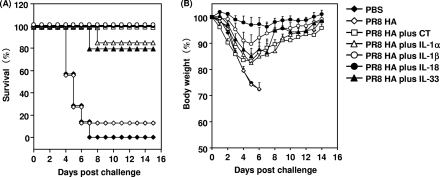
Antiviral immune response to influenza virus infection in mice after nasal immunization with IL-1 family cytokines as mucosal vaccine adjuvants.
To determine the level of protection against viral infection provided by IL-1 family cytokines, BALB/c mice were immunized intranasally with 1 μg PR8 HA alone or with 1 μg of an IL-1 family cytokine on days 0 and 28. The immunized mice were then challenged with 256 HAU of mouse-adapted PR8 virus 14 days after the final immunization. The survival and weight of the infected mice were observed every other day (Fig. 6). All mice in the group receiving PBS alone and 86% of the mice immunized with PR8 HA alone died within 7 days of infection. In contrast, mice immunized intranasally with PR8 HA plus an IL-1 family cytokine showed a marked increase in survival (Fig. 6A). Notably, mice immunized with PR8 HA plus IL-1β or IL-18 had 100% survival 14 days after challenge, though with a slight loss of body weight (Fig. 6B). These results indicate that IL-1 family cytokines are potent nasal vaccine adjuvants for providing protection against viral infection.
FIG. 6.
Protection of BALB/c mice against lethal influenza virus infection by IL-1 family cytokine adjuvants. BALB/c mice were immunized intranasally at 0 and 28 days with rHA alone, rHA plus CT (1 μg/mouse), or rHA plus an IL-1 family cytokine (1 μg/mouse). Fourteen days after the final immunization, mice were intranasally infected with 256 HAU of influenza virus A/PR/8/34. Mice were monitored for survival (A) and weight loss (B) for 14 days after infection. The results are expressed as percent survival (A) and percent initial body weight (B). Data are presented as means ± SEM (n = 4 to 7).
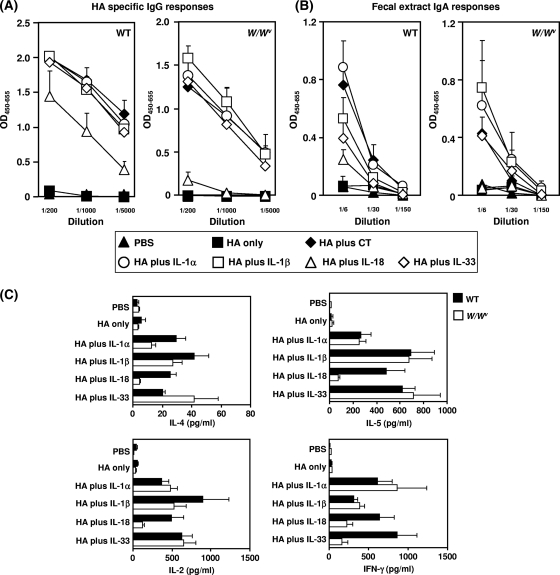
Role of MCs in rHA-specific immune responses induced by nasal immunization with rHA plus IL-1 family cytokines.
MCs are localized predominantly at the interface between the host and the environment (i.e., skin and mucosal surfaces). Recent reports have demonstrated the importance of IL-18-mediated MC activation for host defense, including innate sensing of pathogens (35) and recruitment of DCs and T lymphocytes to sites of inflammation. These findings prompted us to investigate whether MCs have a significant role in the immune response induced by IL-1 family cytokines as mucosal vaccine adjuvants. Hence, we examined MC-dependent rHA-specific systemic IgG and mucosal sIgA Ab responses induced by IL-1 family cytokine adjuvants. For this study, we compared the induction of specific Ab responses in MC-deficient (W/Wv) and WT mice immunized intranasally with rHA plus an IL-1 family cytokine (Fig. 7A and B). Both WT and W/Wv mice immunized with rHA had only minimal rHA-specific IgG Ab responses. However, rHA plus an IL-1 family cytokine induced significant rHA-specific IgG Ab responses in WT mice. W/Wv mice immunized with rHA plus IL-1α, IL-1β, or IL-33 also had significant rHA-specific IgG Ab responses (Fig. 7A), suggesting that IL-1α, IL-1β, and IL-33 act in an MC-independent manner. In contrast, the rHA-specific IgG Ab response induced in W/Wv mice by IL-18 was considerably lower than that in WT mice (Fig. 7A). Similar results were found for mucosal sIgA Ab responses: a significant response was seen with rHA plus IL-1α, IL-1β, or IL-33 in both WT and W/Wv mice, and a decreased response was seen with rHA plus IL-18 in W/Wv mice compared to WT mice (Fig. 7B). We then compared IL-4, IL-5, IL-2, and IFN-γ production in WT and W/Wv mice immunized with rHA plus IL-1 family cytokines (Fig. 7C). WT mice immunized with rHA plus IL-1 family cytokines showed significantly more rHA-specific IL-4, IL-5, IL-2, and IFN-γ production than did WT mice immunized with rHA alone. In contrast, the responses induced by rHA plus IL-18 were significantly reduced in W/Wv mice. In addition, although rHA-specific IL-2, IL-4, and IL-5 production in W/Wv mice immunized with rHA plus IL-33 was comparable to that in WT mice, the rHA-specific IFN-γ response was significantly reduced in W/Wv mice. Collectively, these results indicate that MCs have a crucial role in the rHA-specific immune response induced by nasal immunization with rHA plus IL-18. In particular, MCs appear to have an important role in regulating rHA-specific IFN-γ-mediated Th1-type immunity in mice immunized with rHA plus IL-33 as a mucosal vaccine adjuvant.
FIG. 7.
Role of MCs in induction of rHA-specific immune responses by nasal immunization with rHA plus IL-1 family cytokines. WBB6F1 W/Wv and WT mice were immunized intranasally at 0 and 28 days with rHA alone, rHA plus CT (1 μg/mouse), or rHA plus an IL-1 family cytokine (1 μg/mouse). Plasma and fecal extracts were collected 14 days after the final immunization and analyzed by ELISA for rHA-specific IgG in plasma (A) and rHA-specific sIgA in fecal extracts (B). (C) Also, 14 days after immunization, splenocytes from each group of WBB6F1 W/Wv and WT mice were cultured with 10 μg rHA/ml. Culture supernatants were harvested after a 3-day incubation, and rHA-specific cytokine production (IL-4, IL-5, IL-2, and IFN-γ) in the culture supernatants was analyzed using a Bio-Plex multiplex cytokine assay. Data are presented as means ± SEM (n = 5).
DISCUSSION
Of the 26 different IL cytokines studied here, intranasal immunization with rHA plus an IL-1 family cytokine (IL-1α, IL-1β, IL-18, and IL-33) induced the highest levels of rHA-specific systemic IgG. High levels of sIgA were also observed in the mucosa of IL-1 family cytokine-treated mice. However, IL-12 and IL-15 have been reported to promote systemic and mucosal immunity to intramucosally coadministered protein Ags (8, 45), although more frequent immunization was required to produce adjuvant activity. The apparent discrepancy concerning the adjuvant activity of IL-12 and IL-15 in this study and previous reports may be due to differences in immunization regimens and vaccine doses.
For IL-1 family cytokines, we showed that intranasal administration of rHA plus IL-1α, IL-18, or IL-33 induced higher levels of CD8+ CTLs than intranasal administration of rHA alone, whereas the level induced by rHA plus IL-1β was similar to that induced by rHA alone. In agreement with these results, IL-1β has been reported to have a pivotal role in development of Th2-type immune responses (20). A previous report by Shibuya et al. (36) showed that IL-1α is necessary for optimal Th1 development and IFN-γ secretion in BALB/c mice. In addition, Karupiah et al. (46) showed that IL-18 and IL-12p40 regulate cellular immune responses through CD8+ T-cell activation. Thus, our data are in agreement with previous reports that IL-1α and IL-18 play a pivotal role in inducing Th1-type immune responses. Furthermore, there have been a few reports on the potential of IL-33 to induce a Th1-type immune response (37). In the present study, we showed that of the IL-1 family cytokines, IL-33 induced the highest levels of CTL and IFN-γ+ cells. We are currently investigating the mechanism of IL-33 in Th1/CTL immunity.
We found that intranasal coadministration of influenza vaccine with IL-1 family cytokines provided protection against influenza viral infection, with IL-1β and IL-18 providing complete protection. It is known that nasal secretions containing locally produced sIgA and serum-derived IgG Abs contribute to forming a first line of defense for combating influenza viral infections (42, 44). Therefore, the prophylactic effects of IL-1 family cytokines may be due mainly to Ab-mediated immunity against influenza virus. Furthermore, previous studies have pointed out the importance of influenza-specific CD8+ CTLs for host recovery from lethal influenza virus infections and protection against further infection (7, 15). Although the mechanism by which IL-18 provided complete protection against influenza remains to be elucidated, high-avidity CD8+ CTLs induced by IL-1α, IL-18, or IL-33 probably confer protection against influenza viral infection. Recently, a requirement for NK cells or NKT cells for control of influenza virus infections was identified (10, 13). Since IL-18 is known to regulate NK and NKT cell activity (4, 38), it is possible that restimulation of these cells may have resulted in the reduction in virus replication and morbidity observed after viral challenge. We are currently investigating the involvement of these cell subsets in the induction of protection against influenza virus by IL-18.
Unfortunately, potent adjuvant action is often correlated with increased toxicity, as exemplified by CT adjuvant, which although it is potent is too toxic for human use. Therefore, one of the major challenges in adjuvant research is to gain potency while minimizing toxicity (17). Intranasal administration of 1 μg of an IL-1 family cytokine for four consecutive days has been shown to induce asthma-like symptoms, including airway hyperresponsiveness and goblet cell hyperplasia in the lungs (26). In contrast, in this study, we found that mice immunized intranasally with IL-1 family cytokines did not exhibit acute toxicity, i.e., there was no cytokine-induced mortality, no obvious weight loss, no abnormal behavior, and no histopathological changes. In addition, use of 0.1 μg of an IL-1 family cytokine as a nasal vaccine adjuvant was still effective at inducing systemic IgG and nasal sIgA Ab responses. Thus, although further safety evaluation is needed, our findings indicate a broad therapeutic utility for IL-1 family cytokines when used as adjuvants for mucosal vaccination.
To develop optimal vaccines for clinical applications, it is important to understand their mechanism of action on the immune system in terms of efficacy as well as safety (23). The present study demonstrates that the enhanced mucosal vaccine adjuvant effect of IL-18 operates via an MC-dependent mechanism. The rHA-specific immune response induced by intranasally administered rHA plus IL-18 in WT mice was significantly reduced in W/Wv mice. In addition, the level of the rHA-specific IFN-γ response in mice intranasally immunized with rHA plus IL-33 was minimal in W/Wv mice. Although studies are needed on the role of MCs in generation of Ag-specific immunity, the studies reported here show that MCs have a role in the effect of IL-18 as an adjuvant and in augmentation of the CTL response induced by IL-33 as a nasal vaccine adjuvant. MC activators (e.g., compound 48/80) have been reported to stimulate protective immune responses against infections (28, 32). In addition, these immune responses are correlated with DC trafficking and lymphocyte recruitment to draining lymph nodes (DLN). Nakae et al. (30) suggested that MC-derived tumor necrosis factor alpha (TNF-α) is required for enhanced recruitment of lymphocytes and DCs to DLN. MC-dependent induction of IL-18 mucosal vaccine adjuvant activity may involve these types of processes. In agreement with this possibility, the IL-18 receptor was highly expressed on the surfaces of MCs but not in nasal passage-associated lymphoid tissue CD11c+ DCs, and IL-18 induced robust TNF-α and IL-6 production from MCs in a concentration-dependent manner in vitro (unpublished data). Although further studies are required, IL-18 appeared to exhibit MC-dependent adjuvant activity that was not directly regulated by DC functions, such as DC migration and DC activation.
In summary, IL-1 family cytokines used as mucosal vaccine adjuvants induced two layers of protective immunity when administered intranasally with an influenza virus vaccine Ag, indicating that they may be suitable for use in antiviral nasal vaccines.
Acknowledgments
We have no financial conflicts of interest.
This study was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and from the Japan Society for the Promotion of Science (JSPS). This study was also supported in part by Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan and by Health Sciences Research Grants for Research on Publicly Essential Drugs and Medical Devices from the Japan Health Sciences Foundation.
Footnotes
Published ahead of print on 29 September 2010.
REFERENCES
- 1.Ada, G. 2001. Vaccines and vaccination. N. Engl. J. Med. 345:1042-1053. [DOI] [PubMed] [Google Scholar]
- 2.Ahlers, J. D., I. M. Belyakov, S. Matsui, and J. A. Berzofsky. 2001. Mechanisms of cytokine synergy essential for vaccine protection against viral challenge. Int. Immunol. 13:897-908. [DOI] [PubMed] [Google Scholar]
- 3.Arend, W. P., G. Palmer, and C. Gabay. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223:20-38. [DOI] [PubMed] [Google Scholar]
- 4.Baxevanis, C. N., A. D. Gritzapis, and M. Papamichail. 2003. In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J. Immunol. 171:2953-2959. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., and J. D. Ahlers. 2008. Functional CD8+ CTLs in mucosal sites and HIV infection: moving forward toward a mucosal AIDS vaccine. Trends Immunol. 29:574-585. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Invest. 102:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender, B. S., T. Croghan, L. Zhang, and P. A. Small, Jr. 1992. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J. Exp. Med. 175:1143-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122-128. [PubMed] [Google Scholar]
- 9.Boyaka, P. N., M. Ohmura, K. Fujihashi, T. Koga, M. Yamamoto, M. N. Kweon, Y. Takeda, R. J. Jackson, H. Kiyono, Y. Yuki, and J. R. McGhee. 2003. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J. Immunol. 170:454-462. [DOI] [PubMed] [Google Scholar]
- 10.Cerwenka, A., and L. L. Lanier. 2001. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 1:41-49. [DOI] [PubMed] [Google Scholar]
- 11.Croft, M. 2009. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9:271-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Gregorio, E., U. D'Oro, and A. Wack. 2009. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21:339-345. [DOI] [PubMed] [Google Scholar]
- 13.Diana, J., and A. Lehuen. 2009. NKT cells: friend or foe during viral infections? Eur. J. Immunol. 39:3283-3291. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello, C. A. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27:519-550. [DOI] [PubMed] [Google Scholar]
- 15.Doherty, P. C., J. M. Riberdy, and G. T. Belz. 2000. Quantitative analysis of the CD8+ T-cell response to readily eliminated and persistent viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli, S. J., S. Nakae, and M. Tsai. 2005. Mast cells in the development of adaptive immune responses. Nat. Immunol. 6:135-142. [DOI] [PubMed] [Google Scholar]
- 17.Griffin, M. R., M. M. Braun, and K. J. Bart. 2009. What should an ideal vaccine postlicensure safety system be? Am. J. Public Health 99(Suppl. 2):S345-S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes, B. F., and R. J. Shattock. 2008. Critical issues in mucosal immunity for HIV-1 vaccine development. J. Allergy Clin. Immunol. 122:3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heib, V., M. Becker, T. Warger, G. Rechtsteiner, C. Tertilt, M. Klein, T. Bopp, C. Taube, H. Schild, E. Schmitt, and M. Stassen. 2007. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood 110:946-953. [DOI] [PubMed] [Google Scholar]
- 20.Helmby, H., and R. K. Grencis. 2004. Interleukin 1 plays a major role in the development of Th2-mediated immunity. Eur. J. Immunol. 34:3674-3681. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45-S53. [DOI] [PubMed] [Google Scholar]
- 22.Hubbell, J. A., S. N. Thomas, and M. A. Swartz. 2009. Materials engineering for immunomodulation. Nature 462:449-460. [DOI] [PubMed] [Google Scholar]
- 23.Ishii, K. J., T. Kawagoe, S. Koyama, K. Matsui, H. Kumar, T. Kawai, S. Uematsu, O. Takeuchi, F. Takeshita, C. Coban, and S. Akira. 2008. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451:725-729. [DOI] [PubMed] [Google Scholar]
- 24.Kayamuro, H., Y. Abe, Y. Yoshioka, K. Katayama, T. Nomura, T. Yoshida, K. Yamashita, T. Yoshikawa, Y. Kawai, T. Mayumi, T. Hiroi, N. Itoh, K. Nagano, H. Kamada, S. Tsunoda, and Y. Tsutsumi. 2009. The use of a mutant TNF-alpha as a vaccine adjuvant for the induction of mucosal immune responses. Biomaterials 30:5869-5876. [DOI] [PubMed] [Google Scholar]
- 25.Kayamuro, H., Y. Yoshioka, Y. Abe, K. Katayama, T. Yoshida, K. Yamashita, T. Yoshikawa, T. Hiroi, N. Itoh, Y. Kawai, T. Mayumi, H. Kamada, S. Tsunoda, and Y. Tsutsumi. 2009. TNF superfamily member, TL1A, is a potential mucosal vaccine adjuvant. Biochem. Biophys. Res. Commun. 384:296-300. [DOI] [PubMed] [Google Scholar]
- 26.Kondo, Y., T. Yoshimoto, K. Yasuda, S. Futatsugi-Yumikura, M. Morimoto, N. Hayashi, T. Hoshino, J. Fujimoto, and K. Nakanishi. 2008. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 20:791-800. [DOI] [PubMed] [Google Scholar]
- 27.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, K. Fujihashi, and J. R. McGhee. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 28.McLachlan, J. B., C. P. Shelburne, J. P. Hart, S. V. Pizzo, R. Goyal, R. Brooking-Dixon, H. F. Staats, and S. N. Abraham. 2008. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat. Med. 14:536-541. [DOI] [PubMed] [Google Scholar]
- 29.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed] [Google Scholar]
- 30.Nakae, S., H. Suto, M. Kakurai, J. D. Sedgwick, M. Tsai, and S. J. Galli. 2005. Mast cells enhance T cell activation: importance of mast cell-derived TNF. Proc. Natl. Acad. Sci. U. S. A. 102:6467-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neutra, M. R., and P. A. Kozlowski. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148-158. [DOI] [PubMed] [Google Scholar]
- 32.Pulendran, B., and S. J. Ono. 2008. A shot in the arm for mast cells. Nat. Med. 14:489-490. [DOI] [PubMed] [Google Scholar]
- 33.Reddy, S. T., M. A. Swartz, and J. A. Hubbell. 2006. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27:573-579. [DOI] [PubMed] [Google Scholar]
- 34.Reed, S. G., S. Bertholet, R. N. Coler, and M. Friede. 2009. New horizons in adjuvants for vaccine development. Trends Immunol. 30:23-32. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki, Y., T. Yoshimoto, H. Maruyama, T. Tegoshi, N. Ohta, N. Arizono, and K. Nakanishi. 2005. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J. Exp. Med. 202:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibuya, K., D. Robinson, F. Zonin, S. B. Hartley, S. E. Macatonia, C. Somoza, C. A. Hunter, K. M. Murphy, and A. O'Garra. 1998. IL-1 alpha and TNF-alpha are required for IL-12-induced development of Th1 cells producing high levels of IFN-gamma in BALB/c but not C57BL/6 mice. J. Immunol. 160:1708-1716. [PubMed] [Google Scholar]
- 37.Smithgall, M. D., M. R. Comeau, B. R. Yoon, D. Kaufman, R. Armitage, and D. E. Smith. 2008. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 20:1019-1030. [DOI] [PubMed] [Google Scholar]
- 38.Son, Y. I., R. M. Dallal, R. B. Mailliard, S. Egawa, Z. L. Jonak, and M. T. Lotze. 2001. Interleukin-18 (IL-18) synergizes with IL-2 to enhance cytotoxicity, interferon-gamma production, and expansion of natural killer cells. Cancer Res. 61:884-888. [PubMed] [Google Scholar]
- 39.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stelekati, E., R. Bahri, O. D'Orlando, Z. Orinska, H. W. Mittrucker, R. Langenhaun, M. Glatzel, A. Bollinger, R. Paus, and S. Bulfone-Paus. 2009. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 31:665-676. [DOI] [PubMed] [Google Scholar]
- 41.Surh, C. D., and J. Sprent. 2008. Homeostasis of naive and memory T cells. Immunity 29:848-862. [DOI] [PubMed] [Google Scholar]
- 42.Tamura, S., Y. Ito, H. Asanuma, Y. Hirabayashi, Y. Suzuki, T. Nagamine, C. Aizawa, and T. Kurata. 1992. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J. Immunol. 149:981-988. [PubMed] [Google Scholar]
- 43.Toka, F. N., C. D. Pack, and B. T. Rouse. 2004. Molecular adjuvants for mucosal immunity. Immunol. Rev. 199:100-112. [DOI] [PubMed] [Google Scholar]
- 44.Tumpey, T. M., M. Renshaw, J. D. Clements, and J. M. Katz. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, X., X. Zhang, Y. Kang, H. Jin, X. Du, G. Zhao, Y. Yu, J. Li, B. Su, C. Huang, and B. Wang. 2008. Interleukin-15 enhance DNA vaccine elicited mucosal and systemic immunity against foot and mouth disease virus. Vaccine 26:5135-5144. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Y., G. Chaudhri, R. J. Jackson, and G. Karupiah. 2009. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 183:3324-3331. [DOI] [PubMed] [Google Scholar]