Abstract
After primary replication at the site of entry into the host, alphaherpesviruses infect and establish latency in neurons. To this end, they are transported within axons retrograde from the periphery to the cell body for replication and in an anterograde direction to synapses for infection of higher-order neurons or back to the periphery. Retrograde transport of incoming nucleocapsids is well documented. In contrast, there is still significant controversy on the mode of anterograde transport. By high-resolution transmission electron microscopy of primary neuronal cultures from embryonic rat superior cervical ganglia infected by pseudorabies virus (PrV), we observed the presence of enveloped virions in axons within vesicles supporting the “married model” of anterograde transport of complete virus particles within vesicles (C. Maresch, H. Granzow, A. Negatsch, B.G. Klupp, W. Fuchs, J.P. Teifke, and T.C. Mettenleiter, J. Virol. 84:5528-5539, 2010). We have now extended these analyses to the related human herpes simplex virus type 1 (HSV-1). We have demonstrated that in neurons infected by HSV-1 strains HFEM, 17+ or SC16, approximately 75% of virus particles observed intraaxonally or in growth cones late after infection constitute enveloped virions within vesicles, whereas approximately 25% present as naked capsids. In general, the number of HSV-1 particles in the axons was significantly less than that observed after PrV infection.
Herpesviruses are characterized by a distinct virion morphology and the property to establish latent infections with episodes of spontaneous reactivation. Herpesvirus virions contain a DNA genome enclosed in an icosahedral capsid shell, which is in turn embedded in tegument proteins and surrounded by a lipid envelope containing virally encoded, mostly glycosylated proteins. Within the Herpesviridae, three subfamilies, designated the Alpha-, Beta-, and Gammaherpesvirinae, have been recognized (9). The alphaherpesviruses contain pathogens of humans and animals with neuroinvasive properties resulting in infection of and latency in neurons. The genus Simplexvirus encompasses the ubiquitous human herpes simplex viruses, types 1 and 2 (HSV-1 and HSV-2), whereas varicella-zoster virus and several relevant animal pathogens, e.g., the porcine pseudorabies virus (PrV) (Suid herpesvirus 1 [30]), belong to the genus Varicellovirus.
Alphaherpesviruses are pantropic but neuroinvasive, i.e., they infect the nervous system after primary replication in mucosal membranes. Neuroinvasion entails two long-distance transport processes of different directionalities (3). There is general consent that retrograde intraaxonal transport of incoming alphaherpesvirus particles to the neuronal cell body for productive replication or establishment of latent infection is effected by dynein-mediated microtubule-associated transport of nucleocapsids coated with “inner” tegument proteins (1, 14, 26), as occurs during infection of nonpolarized cultured cells (reviewed in reference 39). After reactivation from latency, anterograde axonal transport to the periphery leads to the appearance of herpetic lesions (reviewed in references 8 and 12) and concomitant virus shedding. Anterograde transport to synapses connected with other neurons results in infection of higher-order neuronal sites and in viral encephalitis (reviewed in reference 11). The different directionalities are supposed to be influenced by differences in the transported viral cargo, i.e., nucleocapsids during entry and enveloped virions during egress (3).
Although this concept was attractive, it was not congruent with experimental findings. In HSV-1-infected neurons, viral structures observed in axons were identified as nucleocapsids lacking an envelope both in ultrastructural analyses (18, 31-33,36) and in fluorescence studies (37, 38). This was initially supported by reports of PrV-infected neurons (41, 42), prompting the hypothesis that viral subassemblies, i.e., nucleocapsids and associated proteins vs. envelopes and associated proteins, were transported separately, with virion formation occurring along the axon at varicosities (10) and/or at the synapse or growth cone (33), indicating that nucleocapsids represent viral cargo for retro- and anterograde transport (“separate model”).
A second model proposes virion assembly in the cytosol and intraaxonal transport of enveloped virions within secretory vesicles (“married model”), as occurs during egress of virions from the cell body of neurons (24, 28) and from nonpolarized cells (15). For PrV, it has now been largely accepted that enveloped virions within vesicles constitute the most abundant, if not exclusive, cargo for anterograde intraaxonal transport following high-resolution ultrastructural electron microscopical studies (5, 13, 28), as well as live-cell analysis by video microscopy of fluorescently labeled virions (2, 3, 25) and reinterpretation of earlier results (5, 8, 13, 41).
In contrast, the situation for HSV-1 is still unclear. Besides evidence for separate transport of viral nucleocapsids and envelope components (23, 34, 37, 38), enveloped capsids in vesicles (7, 23, 27) and sometimes both enveloped and naked nucleocapsids were detected (17, 20, 36). Recent live-cell imaging studies on transport of fluorescently labeled HSV-1 components provided evidence for cotransport of capsid and envelope components in congruence with the married model (2). Although the idea of a similar mechanism for this basic biological feature relevant for neuroinvasive alphaherpesviruses is intriguing, there is still the possibility of alternative solutions to the problem of how to transport viral cargo to peripheral sites in neurons in the different viruses.
Our studies of PrV made use of an assay system based on infection of explanted primary neurons from rat superior cervical ganglia followed by high-resolution electron microscopy (28). We now used the same system to analyze infection of primary rat neurons by the HSV-1 strains HFEM, SC16, and 17+. Strain HFEM harbors a mutation within the long terminal repeat region which eliminates one copy of the latency-associated genes (40). It is avirulent after intraperitoneal, subcutaneous, or intravenous infection but can establish a latent infection in mice after peripheral inoculation, demonstrating that it is neuroinvasive. Strain SC16 has been isolated from a human encephalitic brain (16). Strain 17+ (4) is also derived from a primary isolate. It has a nonsyncytial plaque morphology. Viruses were grown on African green monkey kidney (Vero) cells at 37°C in minimum essential medium (MEM) supplemented with 5% fetal calf serum (Invitrogen). Dissection and culture of primary neuronal cells were done exactly as described previously (6, 28). Neuronal cultures were infected after 7 days at 37°C with 1× 105 PFU of each virus strain diluted in neuronal culture medium. The inoculum was removed after 1 h and replaced with neuronal culture medium. Infected explants on microscope slides were fixed between 16 and 24 h postinfection and processed and analyzed by electron microscopy as described previously (28).
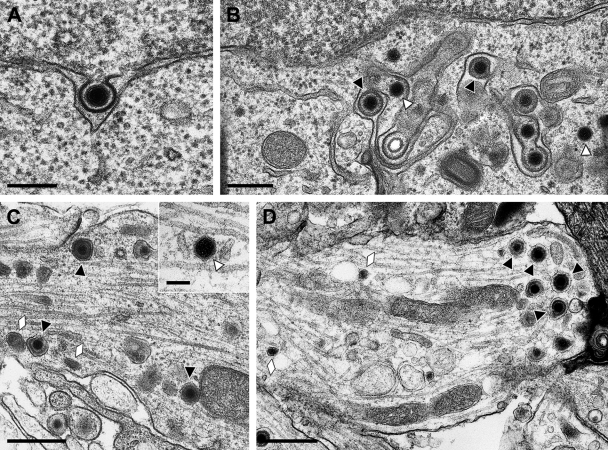
After infection with HSV-1 HFEM, primary envelopment at the inner nuclear membrane (Fig. 1A ) and virion formation by secondary envelopment in the cytosol (Fig. 1B) were readily observed, as in nonpolarized cells (reviewed in reference 29), confirming productive replication of this strain in the explanted neurons. When we analyzed virus particles in axons (Fig. 1C) or growth cones (Fig. 1D), mostly enveloped virions within vesicles were detected, as has been demonstrated for PrV (28), but naked capsids were also observed (Fig. 1C, inset). Quantitation of 48 different thin sections of three different assays resulted in the unambiguous identification of 140 virus particles in sections of axons or growth cones, of which 101 (72%) represented complete virions within vesicles and 39 (28%) represented naked capsids. Thus, during the late stage of infection with HSV-1 strain HFEM, most virus particles observed in axons or growth cones are enveloped virions within vesicles.
FIG. 1.
Ultrastructural analysis of primary rat neurons infected by HSV-1 HFEM. Micrographs show primary envelopment at the inner nuclear membrane (A), secondary envelopment in the cytosol (B), enveloped virions and neurovesicles (C) as well as a naked nucleocapsid (C, inset) in the axon, or enveloped virions and neurovesicles in the growth cone (D). Black triangles indicate enveloped virions; white triangles denote naked nucleocapsids. Neurovesicles are marked by lozenges. Bars: 200 nm in panel A, 300 nm in panel B, 500 nm in panels C and D, and 100 nm in panel C, inset.
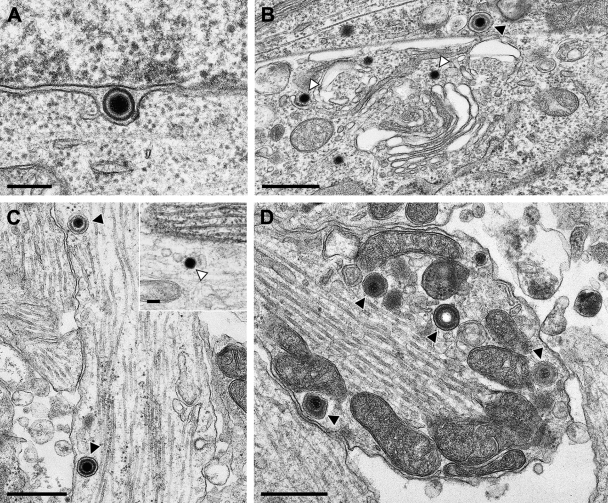
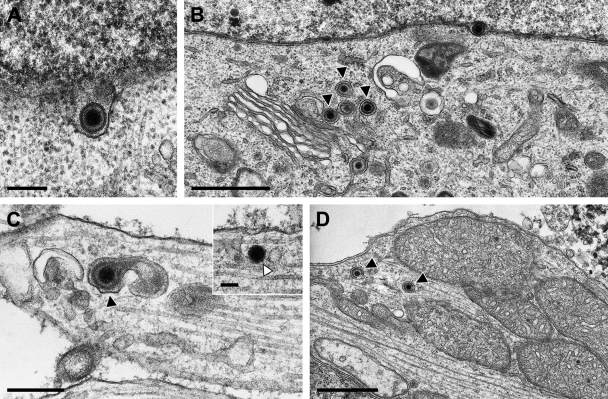
To assess whether this result was specific for strain HFEM or applicable also to other HSV-1 strains, we infected primary rat neurons in an identical fashion with 1× 105 PFU of strain SC16 or 17+. The results are shown in Fig. 2 and 3. Primary enveloped virions in the perinuclear cleft (Fig. 2A and 3A) and intracytoplasmic virion formation by secondary envelopment (Fig. 2B and 3B) demonstrated productive infection of these neurons by the two HSV-1 strains. As has also been observed after infection with HSV-1 HFEM, in axons (Fig. 2C) and growth cones (Fig. 2D) of HSV-1 SC16-infected cells, enveloped virions within vesicles were detected, including a rare case of an enveloped A-capsid lacking DNA (Fig. 2D). However, naked capsids were also present (Fig. 2C, inset). Similar observations were made after infection with strain 17+. Only a few virus particles were observed in axons and growth cones, which mostly presented as enveloped virions within vesicles, as shown in an axon (Fig. 3C) and a growth cone (Fig. 3D), besides occasional naked nucleocapsids, as demonstrated in an axon in Fig. 3C, inset.
FIG. 2.
Ultrastructural analysis of primary rat neurons infected by HSV-1 SC16. Micrographs show a primary enveloped virion in the perinuclear cleft (A), secondary envelopment in the cytosol (B), enveloped virions within vesicles (C), as well as a naked nucleocapsid (C, inset) within the axon, or enveloped virions including an enveloped empty capsid in the growth cone (D). Black triangles indicate enveloped virions; white triangles denote naked nucleocapsids. Bars: 200 nm in panel A, 500 nm in panels B to D, and 100 nm in panel C, inset.
FIG. 3.
Ultrastructural analysis of primary rat neurons infected by HSV-1 17+. Micrographs show a primary enveloped virion in the perinuclear cleft (A), secondary envelopment in the cytosol (B), an enveloped virion within a vesicle (C) and a naked nucleocapsid (C, inset) in the axon, or two enveloped virions in a growth cone (D). Black triangles indicate enveloped virions, white triangles denote naked nucleocapsids. Bars: 200 nm in panel A, 1 μm in panels B and D, 300 nm in panel D, and 100 nm in panel C, inset.
Quantitation was more difficult in HSV-1 SC16- or 17+-infected neurons, since significantly fewer virus particles could be observed beyond the cell body in axons or growth cones. However, analysis of 20 thin sections of two different assays after infection with HSV-1 SC16 showed 36 virus particles present in axons or growth cones, of which 27 (75%) were enveloped virions within vesicles and 9 (25%) represented nucleocapsids. A similar survey of 19 sections of two different assays after infection with HSV-1 17+ showed 29 virus particles present in axons or growth cones, of which 22 (75%) were enveloped virions within vesicles and 7 (25%) were naked nucleocapsids.
In comparing the three HSV-1 strains tested, the highest frequency of virus particles in axons and growth cones was observed in neurons infected by strain HFEM, which, however, was still significantly less than that found after PrV infection. We estimate that ca. 3- to 5-fold fewer virus particles were present in axons and growth cones of neurons infected by HSV-1 HFEM than was the case with PrV infection. This correlates with a more rapid and efficient neuroinvasion of PrV in murine infection models (19, 35). Nevertheless, a substantial number of virus particles could be identified in axons and growth cones of neurons infected by HSV-1 HFEM, SC16, and 17+. Interestingly, in all cases, enveloped virions within vesicles, as well as naked capsids, were observed at similar ratios of approximately 75% enveloped virions and approximately 25% naked nucleocapsids. These figures match well with results in a study using live-cell microscopy of fluorescently labeled viral components. Here, 65 to 70% of anterograde-transported HSV-1 capsids were associated with envelope glycoprotein B, whereas 30 to 35% were not (2). Thus, the majority of virus particles present in axons and growth cones are enveloped virions within vesicles, but naked capsids also represent a significant fraction. In contrast, in PrV infection, naked capsids were only occasionally observed in axons and growth cones, and more than 90% of virus particles were found to be intravesicular enveloped virions (28).
Unlike other imaging techniques, such as fluorescence microscopy, electron microscopy allows unambiguous identification of viral structures but provides no direct information on motion. Thus, there is uncertainty regarding the direction of transport of the observed viral particles. However, contrary to findings in previous studies (21-23, 31-34), we observed enveloped HSV-1 virions within vesicles in axons and growth cones, which could not be derived from entry events as could naked capsids during retrograde transport. Thus, the observation itself is of sufficient validity to support the notion that intraaxonal anterograde transport of both PrV and HSV-1 involves enveloped virions within vesicles. However, we cannot exclude anterograde transport of subviral components as well, since naked capsids have been observed previously (23, 31-34) and in our studies.
To clearly differentiate between retrograde and anterograde transport, compartmentalized chamber systems, e.g., Campenot chambers, had been used (6). They are suitable for following transport of fluorescently labeled viral structures in real time, but high-resolution electron micrographs of infected neurons from these chamber systems are difficult to obtain (13). We are currently working on establishing a procedure which allows combining unambiguous assessment of directionality with our high-resolution electron microscopy.
Our results differ from those of previous studies on HSV-1 infection in neurons using either transmission electron microscopy (18, 21-23, 31-34) or fluorescently labeled virion components (37, 38). In these studies, separate transport of capsids and enveloped components was observed. In contrast, a recent report using live-cell imaging of fluorescently labeled HSV-1 is congruent with our results in showing transport primarily of enveloped virions (2). Currently this discrepancy remains unexplained, but it may be due to the use of different viral strains, neuronal cultures, and readout systems. Thus, it is important to assay in standardized systems in parallel the different virus species, virus strains, and virus mutants to exclude confounding external influences as much as possible.
ADDENDUM IN PROOF
While this manuscript was in press, a study appeared online as an in press manuscript (J. Huang, H. M. Lazear, H. M. Friedman, Virology, 30 October 2010, doi: 10.1016/j.virol.2010.10.009). By electron microscopy of primary neurons grown in culture chambers, the authors also observed enveloped HSV-1 and PrV particles within vesicles in axons.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG Me 854/9).
We thank Petra Meyer for expert technical assistance and Mandy Jörn for photographic help. We highly appreciate the assistance of Lynn Enquist, Princeton University, in the establishment of the neuronal culture system. Strains HFEM and SC16 were kindly provided by Tony Minson, and strain 17+ by Valerie Preston.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Antinone, S., and G. A. Smith. 2010. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J. Virol. 84:1504-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinone, S. E., S. V. Zaichick, and G. A. Smith. 2010. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J. Virol. 84:13019-13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antinone, S. E., and G. A. Smith. 2006. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 80:11235-11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, S. M., D. A. Ritchie, and J. H. Subak-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. [DOI] [PubMed] [Google Scholar]
- 5.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 7.Cook, M. L., and J. G. Stevens. 1973. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7:272-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curanovic, D., and L. W. Enquist. 2009. Directional transneuronal spread of α-herpesvirus infection. Future Virol. 4:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison, A. J., R. Eberle, B. Ehlers, G. S. Hayward, D. J. McGeoch, A. C. Minson, P. E. Pellett, B. Roizman, M. J. Studdert, and E. Thiry. 2009. The order Herpesvirales. Arch. Virol. 154:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Regge, N., H. J. Nauwynck, K. Geenen, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, and H. W. Favoreel. 2006. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diefenbach, R. J., M. Miranda-Saksena, M. W. Douglas, and A. L. Cunningham. 2008. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 18:35-51. [DOI] [PubMed] [Google Scholar]
- 12.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 13.Feierbach, B., M. Bisher, J. Goodhouse, and L. W. Enquist. 2007. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J. Virol. 81:6846-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granzow, H., B. G. Klupp, and T. C. Mettenleiter. 2005. Entry of pseudorabies virus: an immunogold labelling study. J. Virol. 79:3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill, T. J., H. J. Field, and W. A. Blyth. 1975. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J. Gen. Virol. 28:341-353. [DOI] [PubMed] [Google Scholar]
- 17.Hill, T. J., H. J. Field, and A. P. Roome. 1972. Intra-axonal location of herpes simplex virus particles. J. Gen. Virol. 15:233-235. [DOI] [PubMed] [Google Scholar]
- 18.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klopfleisch, R., J. P. Teifke, W. Fuchs, M. Kopp, B.G. Klupp, and T. C. Mettenleiter. 2006. Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J. Virol. 80:5571-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensson, K., B. Ghetti, and H. M. Wisniewski. 1974. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 69:189-201. [DOI] [PubMed] [Google Scholar]
- 21.LaVail, J. H., A. N. Tauscher, A. Sucher, O. Harrabi, and R. Brandimarti. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience 146:974-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaVail, J. H., A. N. Tauscher, J. W. Hicks, O. Harrabi, G. T. Melroe, and D. M. Knipe. 2005. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J. Virol. 79:11142-11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaVail, J. H., K. S. Topp, P. A. Giblin, and J. A. Garner. 1997. Factors that contribute to the transneuronal spread of herpes simplex virus. J. Neurosci. Res. 49:485-496. [PubMed] [Google Scholar]
- 24.Leestma, J. E., M. B. Bornstein, R. D. Sheppard, and L. A. Feldman. 1969. Ultrastructural aspects of herpes simplex virus infection in organized cultures of mammalian nervous tissue. Lab. Invest. 20:70-78. [PubMed] [Google Scholar]
- 25.Liu, W. W., J. Goodhouse, N. L. Jeon, and L. W. Enquist. 2008. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS One 3:e2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. U. S. A. 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lycke, E., K. Kristensson, B. Svennerholm, A. Vahlne, and R. Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 65:55-64. [DOI] [PubMed] [Google Scholar]
- 28.Maresch, C., H. Granzow, A. Negatsch, B. G. Klupp, W. Fuchs, J. P. Teifke, and T. C. Mettenleiter. 2010. Ultrastructural analysis of virion formation and anterograde intraaxonal transport of the alphaherpesvirus pseudorabies virus in primary neurons. J. Virol. 84:5528-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mettenleiter, T. C. 2008. Pseudorabies virus, p. 341-351. In B. W. J. Mahy and M. Van Regenmortel (ed.), Encyclopedia of virology, 3rd ed., vol. 5. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 31.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda-Saksena, M., R. A. Boadle, A. Aggarwal, B. Tijono, F. J. Rixon, R. J. Diefenbach, and A. L. Cunningham. 2009. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J. Virol. 83:3187-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. U. S. A. 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomeranz, L. E., A. E. Reynolds, and C. J. Hengartner. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69:462-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 80:3592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder, A., B. Bruun, H. M. Browne, and D. C. Johnson. 2007. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J. Virol. 81:8337-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder, A., T. W. Wisner, and D. C. Johnson. 2006. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 80:11165-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 40.Spivack, J. G., and N. W. Fraser. 1988. Expression of herpes simplex virus type 1 (HSV-1) latency-associated transcripts and transcripts affected by the deletion in avirulent mutant HFEM: evidence for a new class of HSV-1 genes. J. Virol. 62:3281-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]