Abstract
Tetherin (BST2/CD317) potently restricts the particle release of human immunodeficiency virus type 1 (HIV-1) mutants defective in the accessory gene vpu. Vpu antagonizes tetherin activity and induces its cell surface downregulation and degradation in a manner dependent on the transmembrane (TM) domains of both proteins. We have carried out extensive mutagenesis of the HIV-1 NL4.3 Vpu TM domain to identify three amino acid positions, A14, W22, and, to a lesser extent, A18, that are required for tetherin antagonism. Despite the mutants localizing indistinguishably from the wild-type (wt) protein and maintaining the ability to multimerize, mutation of these positions rendered Vpu incapable of coimmunoprecipitating tetherin or mediating its cell surface downregulation. Interestingly, these amino acid positions are predicted to form one face of the Vpu transmembrane alpha helix and therefore potentially contribute to an interacting surface with the transmembrane domain of tetherin either directly or by modulating the conformation of Vpu oligomers. While the equivalent of W22 is invariant in HIV-1/SIVcpz Vpu proteins, the positions of A14 and A18 are highly conserved among Vpu alleles from HIV-1 groups M and N, but not those from group O or SIVcpz that lack human tetherin (huTetherin)-antagonizing activity, suggesting that they may have contributed to the adaption of HIV-1 to human tetherin.
Tetherin (CD317/BST2) is an interferon-induced type II membrane glycoprotein of unusual topology (4, 25, 44) that potently restricts the release of diverse mammalian enveloped viral particles from infected cells (24, 33, 42, 45, 49, 52, 63). The protein consists of a short 21-amino-acid cytoplasmic tail, a transmembrane (TM) domain, a predominantly helical extracellular domain containing three cysteine residues that mediate tetherin dimerization (1, 44, 48) and an extended parallel coiled-coil (18), and a C-terminal glycophosphatidylinositol anchor that links it back to the cellular membrane (25). These structural features are key to the mode of tetherin activity (48). Tetherin is localized to the plasma membrane (PM) and constitutively recycles through intracellular compartments (25, 50). It is incorporated into budding virions and acts as a physical tether, cross-linking the virion and cellular membranes, thereby preventing virus particle release from the host cell (11, 15, 16, 48). Strong evidence suggests that the dual membrane anchor of tetherin allows it to form parallel dimers with one terminal in the virion membrane and the other in the cell (48). The result of this is that mature viral particles are retained on cell surfaces by protease-sensitive linkages that contain tetherin, and virions can then be endocytosed and accumulate in endosomal compartments (41, 42, 48). This relatively nonspecific inhibition of virus release does not require that tetherin interact directly with any virally encoded structural protein; thus, several mammalian viruses have evolved to encode proteins that specifically inactivate tetherin function (5), the prototype being the human immunodeficiency virus type 1 (HIV-1) accessory protein Vpu (42, 63).
Tetherin antagonism is a highly conserved attribute among primate immunodeficiency viruses (14, 23, 27, 53, 68), implying that modulating tetherin activity is essential for these viruses in vivo. While the Vpu protein overcomes tetherin-mediated restriction of HIV-1, simian immunodeficiency viruses (SIVs) and HIV-2 that lack a vpu gene counteract tetherin through their Nef protein (23, 68) or envelope glycoproteins (14, 23, 27). Primate tetherin antagonism by both Vpu and Nef is species specific (13, 23, 36, 68). HIV-1 Vpu can counteract only human, gorilla, and chimpanzee tetherins, whereas SIV Nef proteins can target multiple primate tetherins but not the human protein (23, 36, 53, 67, 68). This specificity maps to residues in tetherin's TM domain for Vpu (13, 36, 51) and a cytoplasmic tail motif for Nef (23, 28, 68). It has been suggested that both the cytoplasmic tail (28, 36) and the TM domain (13, 36) have been subjected to high positive selection during primate evolution. To add more complexity, SIVcpz, the direct precursor of HIV-1, is a recombinant virus derived from SIVs that encoded either a Vpu or a Nef with tetherin-antagonizing activity (54). The SIVcpz Nef targeting of chimpanzee tetherin was maintained, while the Vpu activity was apparently lost (53). Since the Nef target sequence in the cytoplasmic tail of human tetherin (huTetherin) has been deleted, the Vpu protein appears to have readapted to tetherin antagonism in HIV-1, leading to speculation that Vpu-mediated antagonism of tetherin may have been a prerequisite for efficient human-to-human transmission (28, 53, 67).
Vpu is a 16-kDa membrane protein consisting of an N-terminal TM domain and a C-terminal cytoplasmic tail made up of two amphipathic alpha helices separated by a conserved casein kinase II phosphorylation motif that mediates binding to βTRCP1 and -2 (reviewed in reference 61). Vpu forms multimers in infected cells (30), with the TM domains forming a cation-permeable channel (10, 57), although the functional relevance of this for counteracting tetherin is unclear at present. In addition to antagonizing tetherin, a conserved function of most HIV-1/SIV Vpu proteins is to induce βTRCP-dependent ER-associated degradation of CD4 (3, 35, 38, 55, 65) to facilitate HIV-1 envelope maturation (66). In contrast, Vpu antagonizes tetherin in a post-ER compartment (58). Vpu localizes predominantly to the trans-Golgi network (TGN) and recycling endosomes (8, 64). In response to Vpu, tetherin is downregulated from the cell surface (63). Several reports also demonstrate that Vpu induces tetherin degradation dependent on the recruitment of a βTRCP2-SCF-Cullin1 ubiquitin ligase complex (6, 12, 22, 32, 39). While tetherin degradation is ubiquitin dependent, it is unclear whether it occurs in lysosomes (6, 22, 39) or proteasomally following an ERAD-like process (12, 13, 32). Furthermore, evidence suggests that degradation and, to some extent, cell surface downregulation of tetherin are dispensable events in tetherin antagonism by Vpu (40). Vpu localization to the TGN correlates with its ability to overcome tetherin (8), and recent studies demonstrate that tetherin also accumulates in the TGN in response to both Vpu and the envelope glycoproteins of HIV-2 and SIVtan (7, 14, 17, 27, 45). The TM domain of Vpu is essential for its antitetherin activity, and early studies demonstrated that deletion of parts of the TM (47), scrambling (56), or multiple amino acid replacements (62) blocked Vpu-enhanced virus release. Vpu coimmunoprecipitates with human, but not monkey, tetherin, suggesting that direct interaction between the two proteins is essential for antagonism (7, 22). However, the determinants in the Vpu TM domain required for this interaction have not been identified fully. In this study, we carried out extensive mutagenesis of the HIV-1 NL4.3 Vpu TM domain to show that a conserved face of the HIV-1 group M Vpu transmembrane helix is required for tetherin interaction and antagonism.
MATERIALS AND METHODS
Cells and plasmids.
All cells were maintained at 37°C/5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, United Kingdom) supplemented with 10% fetal calf serum (FCS) and gentamicin. HEK293T cells and HeLa cells were obtained from the American Tissue Culture Collection (ATCC), and the HIV-1 reporter cell line HeLa-TZMbl was kindly provided by John Kappes through the NIH AIDS Reagents Repository Program (ARRP); HeLa/CD4 cells were provided by A. Akrigg through the National Institute of Biological Standards and Controls (NIBSC) Centre for AIDS Reagents (Potters Bar, United Kingdom). Human HT1080 cells stably expressing human tetherin tagged extracellularly with an HA epitope have been described previously (45). The HIV-1 molecular clone plasmid pNL4.3 was obtained from the NIH ARRP, and the Vpu-defective counterpart has been described previously (41). pCR3.1 encoding codon-optimized HIV-1 NL4.3 Vpu tagged at the C terminus with a hemagglutinin (HA) epitope was derived from pVphu (43) (kindly provided by K. Strebel through the NIH ARRP). All transmembrane domain mutants of Vpu (Fig. 1A) were generated by QuikChange site-directed mutagenesis PCR by standard methods using the Phusion-II polymerase (New England Biolabs), and sequences were confirmed. pCR3.1 expression vectors encoding tagged or untagged human tetherins have been described elsewhere (24, 36, 42).
FIG. 1.
Scanning mutagenesis of the HIV-1 NL4.3 Vpu TM domain and tetherin antagonism. (A) A schematic representation of point mutations made in the TM domain of a codon-optimized HIV-1 NL4.3 Vpu protein. (B) 293T cells were transiently transfected with 500 ng of HIV-1 delVpu provirus, 50 ng of tetherin plasmid, and 25 ng of the indicated Vpu expression vector. At 48 h after transfection, the resulting viral supernatants were assayed for infectivity on HeLa-TZM indicator cells by measuring beta-galactosidase activity (top). Error bars are ± standard error of the mean (SEM) for three independent experiments. 293T cell lysates from one replicate of the assay were subjected to SDS-PAGE, blotted for Vpu-HA, with the 90-kDa heat shock protein (Hsp90) serving as a loading control (bottom), and analyzed by a LiCor quantitative imager. Numbers below the lanes indicate the percentages of relative Vpu expression compared to that of the wild-type protein. (C) 293T cells transfected with 500 ng of HIV-1 wt or HIV-1 delVpu and 50 ng of tetherin expression vectors with increasing doses of the indicated Vpu-HA mutant. The resulting infectivity was determined as described in the legend to panel B, and error bars represent ± SEM for three independent experiments. (D) Cell lysates and pelleted virions from one replicate of that shown in panel C were subjected to SDS-PAGE and LiCor Western blotting was performed for HIV-1 p24-CA, Vpu-HA, and Hsp90 (top). The histogram (bottom) indicates supernatant virion yield (p24 band intensity) relative to the release of HIV-1 wt in the absence of tetherin. RLU, relative light unit; YFP, yellow fluorescent protein; THN, tetherin.
Virus release assays.
Subconfluent HEK293T cells were transfected with 500 ng of proviral plasmid in combination with 50 ng of pCR3.1-huTetherin and variable concentrations of pCR3.1-Vpu-HA or mutants thereof using polyethyleneimine (1 mg/ml; Polysciences). The medium was replaced 16 h after transfection, and viral supernatants and cell lysates were harvested 48 h posttransfection. The infectivity of viral supernatants was determined by infecting HeLa-TZMbl and analyzing chemiluminescent beta-galactosidase activity 48 h later using Tropix beta-galactosidase reagent (Applied Biosystems). To analyze physical particle release, filtered supernatants were pelleted through a 20% sucrose-PBS cushion in a bench-top microcentrifuge at 20,000 × g for 90 min at 4°C, and pellets were resuspended in SDS-PAGE loading buffer. Virion and cell lysates were then subjected to SDS-PAGE and Western blotting for HIV-1 p24-CA using the monoclonal antibody (MAb) 187 (kindly provided by B. Chesebro through the NIH ARRP), Vpu-HA using mouse anti-HA monoclonal Ab (Covance), Hsp90 (rabbit polyclonal; Santa Cruz Biotechnologies), or Vpu (rabbit polyclonal; kindly provided by K. Strebel through the NIH ARRP [30]) and visualized by LiCor apparatus using secondary antibodies conjugated to fluorophores (IRDye 800 goat anti-rabbit and IRDye 680 goat anti-mouse antibodies).
Flow cytometry.
Subconfluent HeLa cells in 6-well dishes were transfected with 400 ng pCR3.1-enhanced green fluorescent protein (eGFP) and 400 ng pCR3.1-Vpu-HA or TM mutant. At 48 h posttransfection, the cells were harvested and stained for surface tetherin using a specific anti-BST2 monoclonal IgG2a antibody (Abnova) and goat anti-mouse IgG2a Alexa633-conjugated secondary antibody (Molecular Probes, Invitrogen, United Kingdom). Tetherin expression on GFP+ cells was then analyzed using a FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software. Alternatively Jurkat cells were infected with vesicular stomatitis virus G (VSV-G)-pseudotyped HIV-1 at a multiplicity of infection (MOI) of 0.2. Forty-eight hours later, cells were stained for surface tetherin expression as described above, fixed and permeabilized for 20 min (Cytofix/Cytoperm fixation/permeabilization kit; BD Biosciences), and stained for intracellular HIV-1 Gag using the KC57 antibody conjugated to phycoerythrin (PE) (Beckman-Coulter).
Immunofluorescence microscopy.
HeLa cells plated on glass coverslips were transfected with 100 ng of Vpu-HA expression vector or TM domain mutants. At 24 h posttransfection, the cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and immunostained using a mouse anti-HA monoclonal antibody (Covance) and sheep anti-human TGN46 (Serotec) followed by the appropriate donkey secondary antibodies coupled to Alexa 488 and 594 fluorophores (Molecular Probes, Invitrogen, United Kingdom). The coverslips were then mounted on slides using ProLong Antifade containing DAPI (4′,6-diamidino-2-phenylindole) (Molecular Probes, Invitrogen, United Kingdom) and examined on a Leica DM-IRE2 confocal microscope. Similarly, HT1080-tetherin-HA cells were plated on coverslips and infected with VSV-G-pseudotyped HIV-1 NL4.3 or Vpu mutant at an MOI of 0.2. Forty-eight hours later, the cells were fixed and stained with Vpu-, HA-, and TGN46-specific antibodies with the appropriate donkey secondary antibodies linked to Alexa 488, 647, and 594 dyes, respectively.
Immunoprecipitation.
Subconfluent HEK293T cells were transfected with 500 ng of Vpu-HA, the appropriate TM mutant or pCR3.1-YFP, and 500 ng of pCR3.1-huTetherin or pCR3.1-Vpu-YFP. At 48 h posttransfection, the cells were lysed on ice for 30 min on ice in buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, complete protease inhibitors (Roche), and 1% digitonin (Calbiochem). After removal of the nuclei, the resulting supernatants were precleared for 30 min at 4°C with 60 μl of protein G-agarose (Invitrogen). The supernatants were then incubated with 5 μg/ml mouse anti-HA (Covance) for 2 h at 4°C before addition of 50 μl of fresh protein G-agarose for a further 3 h. The beads were then washed 4 times in lysis buffer containing 0.1% digitonin and resuspended in SDS-PAGE loading buffer. Cell lysates and immunoprecipitates were then Western blotted for Vpu using mouse anti-HA and for tetherin using rabbit anti-BST2 (40) (kindly provided by K. Strebel through the NIH ARRP).
RESULTS
Identification and characterization of point mutations in the Vpu transmembrane domain that inhibit tetherin antagonism.
The TM domain of Vpu is an absolute requirement for enhancing virus particle release (56). Recent studies have demonstrated that Vpu and tetherin coimmunoprecipitate with each other in a species-specific manner, strongly suggesting a direct interaction (7). To better characterize the determinants of tetherin antagonism in the Vpu TM domain, we conducted scanning mutagenesis through this region (residues 5 to 28) of a codon-optimized HIV-1 NL4.3 Vpu bearing a C-terminal HA tag (Fig. 1A). All nonalanine residues were mutated individually to alanines, while bulkier hydrophobic leucine residues replaced those that were alanines. These Vpu TM mutants were then screened for their ability to rescue Vpu-defective HIV-1 from tetherin-mediated restriction. Human 293T cells, which lack endogenous tetherin expression, were transfected with HIV-1 NL4.3 delVpu proviruses in the presence of a fixed dose of a human tetherin expression vector and either a plasmid encoding HA-tagged wild-type (wt) Vpu or the TM domain mutants. At 48 h posttransfection, cell lysates and viral supernatants were harvested and analyzed by Western blotting or titrated on HeLa-TZM indicator cells (Fig. 1B). As expected, in the presence of human tetherin, the release of infectious HIV-1 delVpu was reduced approximately 50-fold, as determined by the infectivity of harvested supernatants on HeLa-TZM indicator cells, and expression of wild-type Vpu in trans rescued the majority of this defect. Two mutants, the A14L and W22A mutants, were markedly defective in viral rescue compared to the control, with a third, the A18L mutant, displaying a minor defect. Furthermore, several mutants (the V9A, V25A, and I27A mutants) also displayed slight (approximately 2-fold) defects compared to the wild type, but this appeared due to expression levels of the proteins rather than a functional defect per se. Interestingly mutation of S23, which has been shown previously to completely abolish the ion channel activity of Vpu (37), or V13, I17, and V21, which would line the pore of a putative channel (46), conferred no defect in tetherin antagonism, suggesting that formation of an ion conducting pore may not be required to relieve the restriction.
To characterize the functional mutants further, the A14L, A18L, and W22A mutants or two combined mutants, the A14L/W22A and A10L/A14L/A18L/W22A mutants, were rescreened against a fixed dose of tetherin, but with various levels of Vpu expression and virus release characterized by both infectious titer release and physical particle yield by quantitative Western blotting (Fig. 1C and D). Despite equivalent expression levels of Vpu and HIV-1 Gag in producer cells, neither the A14L mutant nor the W22A mutant could fully rescue HIV-1 delVpu from tetherin, and both remained defective compared to the wild-type Vpu, even at the highest inputs. The A18L mutant was the least defective and regained most of its function at higher plasmid inputs. Combining the A14L and W22A mutants into a single mutant, either in the context of the A14L/W22A or A10L/A14L/A18L/W22A mutant, rendered Vpu effectively unable to counteract tetherin at any level of expression.
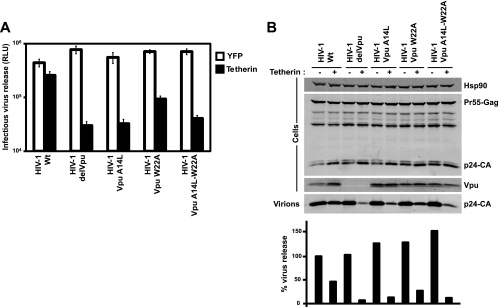
Finally we engineered A14L and W22A mutants back into the HIV-1 NL4.3 genome to place them under wild-type Vpu expression conditions and tested virus release from 293T cells transfected with tetherin (Fig. 2A and B). Consistent with expression of the mutants in trans, we found that the mutant carrying A14L alone and the combined mutant had a defect in release equivalent to a full Vpu deletion, whereas W22A retained a low level of tetherin antagonism. Thus, positions A14, W22, and, to a lesser extent, A18, appear to be key residues required for tetherin antagonism in the Vpu TM domain.
FIG. 2.
The effect of A14 and W22 mutations in the context of full-length proviral clones. HIV-1 wt, HIV-1 delVpu, HIV-1 Vpu A14L, HIV-1 Vpu W22A, and HIV-1 Vpu A14L/W22A proviral clones were transfected into 293T cells in the presence or absence of 50 ng of tetherin expression vector. Forty-eight hours later, the supernatants were assayed for infectivity on HeLa-TZM cells (A) and pelleted virions (B), and cell lysates were processed for Western blotting as described in the legend to Fig. 1.
The A14 and W22 mutants fail to downregulate tetherin from the cell surface.
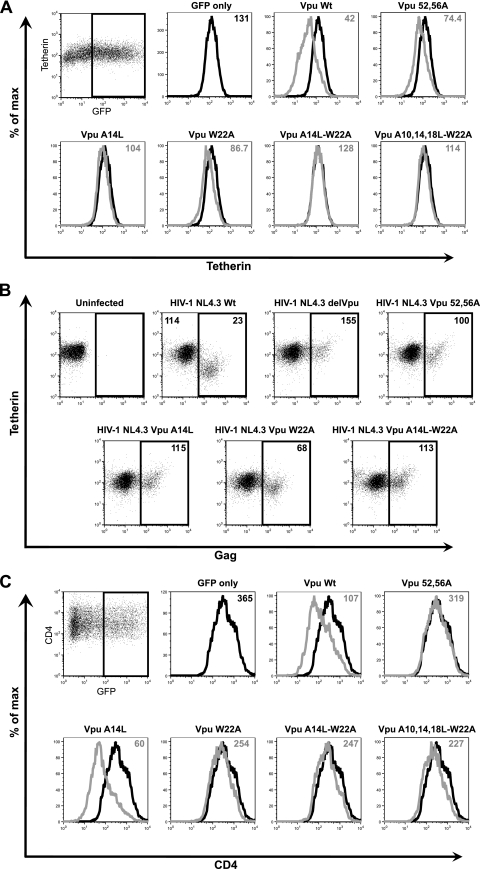
To further characterize the nature of the Vpu TM mutants, we first determined their effects on cell surface tetherin levels. Vpu expression leads to a downregulation of cell surface tetherin and subsequently a degradation step which itself may be dispensable for Vpu activity (12, 40, 63). Tetherin-positive HeLa cells were cotransfected with an empty vector control or Vpu expression vector in combination with a GFP marker plasmid. At 48 h posttransfection, the cells were harvested, stained for cell surface tetherin using a specific monoclonal antibody, and analyzed by flow cytometry (Fig. 3A). Tetherin was downregulated from the cell surface of GFP+ cells expressing Vpu, and as expected, downregulation was reduced in the presence of a Vpu mutant that does not interact with βTRCP1/2 and Vpu S52 56A (12, 39, 63). Both the A14L and W22A mutants failed to fully downregulate tetherin, and either multiple mutant containing both A14L and W22A mutations was completely defective for downregulation. To confirm this with relevant infected cells, CD4+ Jurkat T cells were infected with VSV-G-pseudotyped wild-type HIV-1, HIV-1 delVpu, HIV-1 Vpu2/6, HIV-1 Vpu A14L, HIV-1 Vpu W22A, or HIV-1 A14L/W22A, and 48 h later cells were stained for surface tetherin and intracellular Gag (Fig. 3B). As expected, Gag-positive cells in cultures infected with wild-type HIV-1, but not with HIV-1 delVpu or HIV-1 Vpu2/6, showed loss of tetherin expression at the cell surface. However, in line with our findings for transfected cells, Jurkat cells infected with viruses bearing an A14L mutation showed little cell surface tetherin reduction. The W22A mutant retained a residual ability to downregulate cell surface tetherin, again in line with our above observations. Thus, Vpu TM mutants that cannot antagonize tetherin function are concomitantly defective for cell surface tetherin downregulation and, by extension, degradation.
FIG. 3.
Effects of Vpu TM mutations on cell surface levels of tetherin and CD4. (A) HeLa cells were transfected with a wild-type Vpu (Vpu Wt)-encoding vector or the indicated mutant and a GFP-expressing construct. Cell surface staining for endogenous tetherin was analyzed by flow cytometry 48 h later. Histograms show the tetherin levels on GFP+ cells in empty vector control cells (black) or Vpu/Vpu mutant-expressing cells (gray). The median fluorescent intensities indicated in the top corner of each histogram are representative examples of 3 independent experiments. (B) Jurkat cells were infected with the indicated VSV-G-pseudotyped viral stocks at an MOI of 0.2. Forty-eight hours later, cells were stained for surface tetherin expression and intracellular Gag and analyzed by flow cytometry. Gag-positive infected cells were gated (black square), and surface tetherin levels were compared. Numbers indicate median fluorescence intensities of surface tetherin on the infected cells. (C) As shown in panel A, but the effects of Vpu-wt or Vpu-TM mutants on surface CD4 were examined in transfected HeLa/CD4 cells.
We also tested our Vpu mutants for their ability to downregulate CD4. Although CD4 and Vpu have been shown to interact through cytoplasmic tail interactions, a role for the TM domain has been implicated by some (62), but not others (56). HeLa/CD4 cells were transfected as described above, and cell surface CD4 levels were measured 48 h later (Fig. 3B). As expected, Vpu expression led to a reduction in surface CD4 that again was blocked in the presence of an S52 56A mutant. Vpu A14L retained the ability to downregulate CD4, indicating that this mutant is specifically defective for tetherin antagonism. In contrast, and consistent with the work of Tiganos et al. (62), the Vpu W22A mutant and multiple mutants bearing this lesion were also defective for CD4 downregulation. Thus, both tetherin and CD4 downmodulation require residues in the Vpu TM, but these determinants only partially overlap.
Defective Vpu TM mutants localize to the TGN.
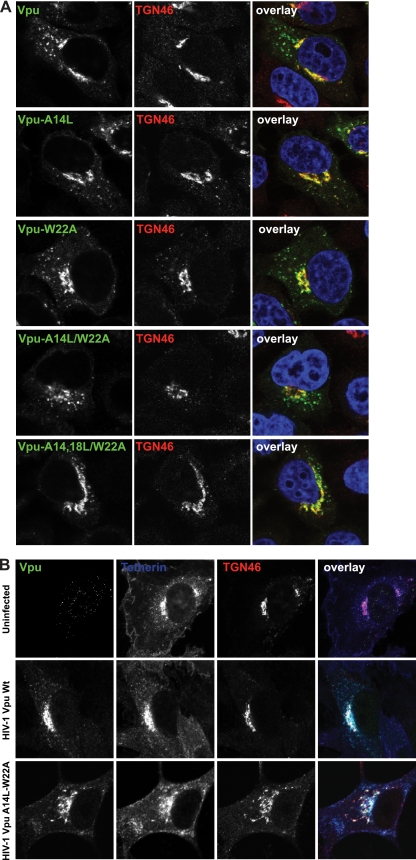
While CD4 degradation occurs in the endoplasmic reticulum (ER), Vpu-mediated virus release is sensitive to brefeldin A (58), suggesting that tetherin antagonism takes place outside the ER. Vpu localizes to the TGN and endosomal compartments, and the ability to localize to the TGN has been shown to be important for tetherin antagonism (8). Moreover, tetherin itself recycles via the TGN (25, 50), and recent data for both Vpu and HIV-2/SIV envelopes suggest that sequestration of tetherin in the TGN is associated with antagonism (7, 14, 17, 27). Since the TM domain(s) of integral membrane proteins may be involved in subcellular localization (59), we next sought to rule out whether our Vpu TM mutants were aberrantly localized. HeLa cells transfected with Vpu-HA or TM mutants were grown on coverslips, fixed 24 h later, immunostained for Vpu (anti-HA) and the TGN marker TGN46, and examined by confocal microscopy. As expected, wild-type Vpu-HA localizes predominantly to perinuclear and punctate structures, with much of the perinuclear signal overlapping with the TGN (Fig. 4A), consistent with previous studies. We found that all of our Vpu TM mutants localized similarly, with TGN accumulation visible in all cases. These data suggest that Vpu localization to the TGN is independent of the ability to counteract tetherin, and similarly, Vpu localizes to TGN46+ compartments in tetherin-negative cells (data not shown). We then examined Vpu and tetherin localization in infected HT1080 cells expressing tetherin bearing an extracellular HA tag (HT1080/tetherin-HA) (Fig. 4B). As expected, in these cells tetherin localizes to the plasma membrane (PM) as well as the TGN since both newly synthesized tetherin and that which recycles passes through the Golgi network en route to the PM (25, 50). Consistent with reports of TGN accumulation of tetherin in HIV-1-infected cells (7), we found that wild-type Vpu and tetherin colocalized in the TGN with a concomitant reduction in cell surface expression. In contrast, and consistent with the flow cytometry data presented in Fig. 3, cells infected with a virus bearing the A14L/W22A mutation showed no evidence of tetherin relocalization from the PM to the TGN. This indicates that the defect in tetherin antagonism for A14L and W22A mutants of Vpu is likely not due to gross subcellular mislocalization and that Vpu localization to the TGN and Vpu-induced accumulation of tetherin in TGN46-positive compartments are independent events.
FIG. 4.
Subcellular localization and multimerization of Vpu TM mutants. (A) HeLa cells were transfected by either 100 ng Vpu wt-HA or the indicated mutant. A total of 24 h later, the cells were fixed and stained for Vpu localization with anti-HA antibody (green) and a TGN marker (TGN46; red) and examined by confocal microscopy. (B) HT1080/THN-HA cells were infected with HIV-1 wt or HIV-1 Vpu A14L/W22A at an MOI of 0.2. Forty-eight hours later, the cells were fixed and stained for Vpu (green), TGN46 (red), and tetherin-HA (blue) and examined by confocal microscopy.
Tetherin poorly coimmunoprecipitates with Vpu TM mutants.
We then assessed the ability of Vpu TM mutants to coimmunoprecipitate tetherin as an indicator of direct interaction between the proteins. 293T cells were transfected with a human tetherin expression vector and wild-type Vpu-HA or the TM mutant. At 48 h after transfection, cell lysates were immunoprecipitated with an anti-HA antibody. As shown in Fig. 5A, tetherin (both precursor mannosylated and mature complex glycosylated forms) was efficiently coimmunoprecipitated with wild-type Vpu, despite total cellular tetherin levels being lower in cell lysates due to Vpu-induced degradation (Fig. 5A, low-exposure lysate panel). The A14L and W22A mutants displayed little or no tetherin degradation and only poorly coimmunoprecipitated the protein. Furthermore, combined mutants containing both A14L and W22A were further reduced in their ability to coimmunoprecipitate tetherin. Thus, the defect in tetherin antagonism in the A14L and W22A mutants correlates directly with their failure to interact with tetherin. Coupled with the observation that these mutants localize identically to wild-type Vpu, these data strongly suggest that these positions in the Vpu TM domain are critical for the interaction with tetherin. Interestingly, the fact that we coimmunoprecipitated the lower-molecular-weight, immature form of tetherin with Vpu may suggest that while Vpu exerts its effect on tetherin in a post-ER compartment, interaction may occur prior to further carbohydrate modification.
FIG. 5.
Vpu TM mutants are unable to coimmunoprecipitate tetherin but maintain the ability to multimerize with wild-type Vpu. (A) 293T cells were transfected with the indicated plasmid Vpu vectors or the indicated mutant and with tetherin. YFP only or tetherin only served as negative controls. Forty-eight hours later, Vpu was immunoprecipitated (IP) via the HA tag from cell lysates and subjected to SDS-PAGE. Total lysates and immunoprecipitates were then Western blotted for tetherin and Vpu-HA. Molecular mass markers are indicated, and blots are a representative example of three independent experiments. (B) 293T cells transfected with HA-tagged Vpu or Vpu A14L/W22A were lysed in 1% digitonin after 48 h, subjected to nonreducing SDS-PAGE without prior boiling, and Western blotted with an anti-HA monoclonal Ab. (C) 293T cells were transfected with Vpu-YFP and either Vpu-HA or Vpu A14L/W22A-HA. Forty-eight hours later, cell lysates were immunoprecipitated with anti-HA Abs as described in the legend to Fig. 5A, and whole lysates and IP fractions Western blotted for Vpu-HA and Vpu-YFP with anti-HA and anti-GFP antibodies, respectively. (D) 293T cells were transfected with wild-type HIV-1 NL4.3 proviral plasmid, 50 ng of tetherin, and the indicated amount of Vpu-HA or mutant, and infectious release was determined on HeLa-TZM cells 48 h later. Dotted lines represent wild-type and Vpu-defective viral titers in the absence of any expression of Vpu in trans. (E) Cell lysates of the 100-ng input described in the legend to panel D blotted with an anti-Vpu polyclonal Ab to allow simultaneous detection of the Vpu-HA expressed in trans and the wild-type Vpu expressed from the NL4.3 provirus.
Oligomers of Vpu can be detected in infected cells (21, 30), although their functional significance for tetherin antagonism is not known. We next asked whether we could detect Vpu mutants in multimeric forms in transfected cells. Western blots of postnuclear lysates of Vpu-HA-transfected 293T cells run under nonreducing conditions without prior boiling displayed a higher-molecular-mass form of Vpu (approximately 80 kDa) in addition to a monomer (Fig. 5B), consistent with previous observations (30). This species of Vpu was also detected for the Vpu A14L/W22A mutant, suggesting that our TM mutants did not abolish Vpu multimerization. In addition, this mutant also formed a larger (approximately 100- to 120-kDa) form. Furthermore, under the same immunoprecipitation conditions in which Vpu TM mutants failed to interact with tetherin, we could show that wild-type Vpu tagged with GFP could coimmunoprecipitate with both Vpu-HA and Vpu A14L/W22A-HA (Fig. 5C). Therefore, the defect in tetherin antagonism by our TM mutants was not due to an inability of the proteins to self-associate, although whether the nature or stability of these mutant oligomers is different or whether other cellular factors contribute to them is unknown. To characterize this further, we reasoned that if tetherin antagonism was mediated by functional Vpu multimers, Vpu A14L/W22A mutants might be dominant-negative inhibitors of tetherin antagonism. However, titration of Vpu TM mutants into HIV-1 wt viral release assays did not induce any enhanced sensitivity to tetherin, despite estimated mutant Vpu levels 10-fold over that expressed from the provirus (Fig. 5D and E). Thus, while not ruling out that Vpu acts as a multimer to target tetherin, these data show that the ability of Vpu-TM mutants to associate with wild-type Vpu does not compromise its function.
A14, A18, and W22 form a face of the TM helix that is conserved in group M and N, but not O, Vpu proteins.
We next looked at the conservation of the above TM positions in Vpu sequences deposited in the HIV Sequence Database (www.hiv.lanl.gov) (Fig. 6A). Aligning the TM domains of 1,197 group M Vpu sequences shows that the positions equivalent to A18 and W22 are highly conserved. The majority of sequences also have the equivalent of position 14 as A, but in many cases this can be V. Notably we did not find an L at this position in naturally occurring group M sequences. The same was observed with the small number of group N Vpu sequences available; the equivalents of positions 14, 18, and 22 were A, A, and W, respectively. Interestingly, this was not the case for HIV-1 group O Vpu proteins, which have recently been shown to be defective in tetherin antagonism (53). Here the position 14 equivalent is almost always L, and the majority of sequences have an N at position 18. Again the W is invariant, which, since group O Vpu proteins retain CD4 degradation activity (53), is consistent with W22A mutants of NL4.3 Vpu being defective for both tetherin and CD4 downregulation. Thus, the residues we have identified as important for tetherin antagonism in HIV-1 NL4.3 Vpu are conserved in viruses that can antagonize human tetherin, but not in those that cannot.
FIG. 6.
Residues A14, A18, and W22 form one face of the TM helix and are conserved in HIV-1 groups M and N, but not O. (A) Logo plots of the TM domains of Vpu sequences drawn from the HIV Sequence Database (www.hiv.lanl.gov). Group M comprises 500 clade B, 200 clade C, 200 clade A, 200 clade D, 42 clade F, 48 clade G, and 7 clade H TM sequences. Arrows indicate the equivalent positions of NL4.3 Vpu A14, A18, and W22 residues. (B) Positions of A14, A18, and W22 (red) in the solid-state NMR structure of the NL4.3 Vpu TM domain determined in membranes and the modeled tetrameric and pentameric assemblies based on it, as determined by Park et al. (46). Images were generated in PyMol from the RSCB protein database entries 1PI7 (pentamer) and 1PI8 (tetramer).
The solid-state nuclear magnetic resonance (NMR) structure of the NL4.3 Vpu TM domain in lipid membranes has been determined previously (34, 46). The core TM domain (residues 8 to 25) forms a slightly kinked alpha helix tilted at approximately 13 degrees to the vertical. Residues A10, A14, A18, and W22 form one diagonal face of the helix (Fig. 6B). Because Vpu can form ion-permeable channels, this structure was further modeled to account for this, and the most favorable conformations that would allow channel function were predicted to be a tetramer or a pentamer (46) (Fig. 6B). In this conformation, W22 is predicted to protrude outward from the pentamer and may give stability to the structure in the membrane. A14 and A18 are positioned at the interface with the adjacent monomers, and therefore, their replacement with bulkier leucine residues may impinge on this interaction and, although not sufficient to block Vpu multimerization, alter the conformation or stoichiometry of Vpu oligomers. Alternatively, or in addition to contributing to interhelical contacts or stability, A14, A18, and W22 might also contribute to a conserved binding surface for interaction with another protein, potentially tetherin.
DISCUSSION
The mechanism by which Vpu antagonizes tetherin activity is unclear at present. While Vpu expression leads to either endosomal or proteasomal degradation of tetherin (6, 12, 13, 22, 32, 39), the ultimate destruction of tetherin appears dispensable (7, 40). Moreover, tetherin accumulation in the TGN in response to Vpu suggests that removal of tetherin from the cell surface is a result of Vpu inhibiting tetherin recycling/transport to the plasma membrane, and Vpu localization to the TGN, mediated by determinants in its cytoplasmic tail, correlates with this activity (7, 8). Furthermore, in CD4+ T cells, removal of tetherin itself from the cell surface may not be strictly required to block its antiviral activity (40). In this study we have characterized transmembrane domain mutants of HIV-1 NL4.3 Vpu that cannot antagonize tetherin-mediated restriction of virion release, with major defects associated with changes at positions A14 and W22. Vpu TM mutants defective for tetherin antagonism are concomitantly defective for tetherin downregulation from the cell surface and, by extension, degradation. This directly correlates with the inability of these mutants to interact with tetherin in coimmunoprecipitations. Since these residues are conserved in group M and N Vpu proteins and their mutation does not grossly affect Vpu localization to the TGN or prevent Vpu from interacting with itself, we propose that A14, W22, and, to a lesser extent, A18 contribute to the interaction between tetherin and Vpu. In the absence of this interaction, antagonism of the antiviral activity of tetherin, its cell surface downregulation, and its ultimate degradation are all impaired.
While it cannot be ruled out that a cellular cofactor mediates Vpu-tetherin interactions, the genetic evidence for direct binding between their TM domains is strong. First, the species specificity of HIV-1 NL4.3 Vpu maps to positively selected residues in the tetherin TM domain, suggesting that viral countermeasures that target the tetherin TM may have acted as a selective pressure on tetherin evolution (13, 36). In particular, a GI deletion toward the N terminus (cytoplasmic) and a T45I change at the C terminus (extracellular) of the tetherin TM account for the sensitivity of human, but not macaque, tetherin to NL4.3 Vpu, although additional residues can contribute to Vpu sensitivity (36). Moreover, the Vpu proteins from other HIV-1 group M strains have a more expanded tropism for other primate tetherins (53). However, in the case of HIV-1 NL4.3, this species specificity correlates with the lack of coimmunoprecipitation with insensitive primate tetherin variants (7). Second, a recent study demonstrated fluorescence resonance energy transfer (FRET) between Vpu and human tetherin that was dependent on the Vpu TM domain (2). Finally, deletion mutations within the Vpu TM that encompass W22 or the complete scrambling of its sequence have long been known to inhibit Vpu-mediated virus release (47, 56, 62). Our data build on these observations, implicating specific Vpu TM residues in the interaction with tetherin that do not affect TGN localization or block self-association.
Modeling of the Vpu TM structure has historically been done on the basis that it forms a multimeric cation-permeable channel in cellular membranes (10, 57). Since scrambling the TM domain blocks both ion channel activity and efficient virus release, it was thought that these activities were related (10, 57). Indeed, amiloride-based drugs that block Vpu channel function have been shown to display antiviral activity against HIV-1 in (tetherin-positive) macrophages (9), and other studies report that chimeric Vpu proteins bearing the TM domain of the influenza A virus proton channel M2 were functional for virus release and sensitive to rimantadine (19, 20). However, since we found that mutation of S23, known to be required for channel activity (60) and the potential target for amilorides (26), had no effect on tetherin inactivation in our assays, there appears to be a discrepancy in directly correlating cation transport and virus release (tetherin antagonism). The role of ion transport in Vpu function is therefore unclear, but given the multifunctional nature of HIV-1 accessory proteins (31), it is possible that the ion channel activity is related to a yet-to-be-defined role of Vpu in HIV-1 replication.
We do not know the stoichiometry of the Vpu-tetherin interaction or indeed whether Vpu multimerization is required for its antagonism. Oligomers of Vpu can be detected in infected cells (30). NMR structures of the Vpu TM have been used to predict the conformation of Vpu multimers; however, as discussed above, these have revolved around its ion channel activity. More recent data suggest that there might be an equilibrium of Vpu monomers and different multimeric forms (29), while another FRET-based study provided evidence that Vpu-Vpu interactions could be detected in Golgi-related structures but not in the ER (21), suggesting that Vpu multimerization may occur differentially in subcellular compartments. Based on the NMR structural model of a Vpu pentamer shown in Fig. 6 (46), an A14L mutation might disrupt interhelical interactions in Vpu multimers and a W22A mutant might lose the stability imparted by the tryptophan side chain. However, neither mutation disrupts the ability of Vpu to interact with itself nor alters its subcellular distribution, although they may lead to additional multimeric forms at steady state (Fig. 5B). Furthermore, V13A and I16A mutants, which in the predicted multimers would be associated with A14 on the adjacent helix, lack significant defects in tetherin antagonism. A14 and W22 might also contribute to a direct tetherin-interacting surface formed upon Vpu multimerization. However, one piece of data from our study is not consistent with Vpu targeting tetherin as a functional multimer. Despite overexpressing Vpu mutants in excess, we were unable to observe any dominant-negative activity of A14L and W22A mutants against wild-type HIV-1 virion release in the presence of tetherin, as might be expected if A14L/W22A mutations were disrupting the integrity or conformation of functional Vpu multimers. While not itself demonstrating that Vpu acts on tetherin as a monomer, this observation shows that despite being unable to mediate tetherin interaction, the ability of the A14L/W22A mutant to multimerize with the wild-type protein does not block its inactivation of tetherin. Alternatively, if Vpu did act on tetherin as a monomer, the fact that A14, A18, and W22 form a face of the TM helix might be indicative of the residues forming a binding surface. If so, they could directly interact with residues in the tetherin TM domain known to be required for sensitivity to Vpu. Further in-depth structure/function analyses are required to distinguish between these possibilities. Such studies will be essential for the design of any potential Vpu inhibitors.
The ability to counteract tetherin is conserved among primate lentiviruses, indicating that this attribute is essential for replication/transmission of these viruses in vivo (14, 23, 27, 42, 53, 68). Recent studies have suggested that the ability of HIV-1 Vpu to counteract human tetherin is a “reacquisition” of an ancestral Vpu function in the SIVgsn/SIVmon/SIVmus lineage that was lost in SIVcpz presumably due to redundancy with the tetherin antagonism function of Nef (53). The selective pressures that lead to the specific deletion of the SIV Nef target sequence (G/DDIWK) in the cytoplasmic tail of human tetherin are unclear (23, 28, 68), although prehistoric human exposure to Nef-like proteins from pathogenic viruses is a possible explanation. Regardless, the spread of SIVcpz to humans to become HIV-1 presented the virus with a problem; the tetherin countermeasure was ineffective (54). Recent data from the Emerman group have demonstrated that swapping the majority of the TM domain of HIV-1 Vpu, including the residues identified herein, renders SIVcpz Vpu active against human tetherin (28). The differences between the HIV-1 Vpu and SIVcpz Vpu TM domains indicate that multiple changes occurred during the adaption of HIV-1 Vpu to humans. Interestingly, in terms of acquiring tetherin antagonism and maintaining CD4 downregulation, there is evidence that this was fully achieved only by HIV-1 group M, derived from the SIVcpz zoonosis that gave rise to the HIV/AIDS pandemic (53). Data from the small number of group N Vpu proteins available suggest that tetherin antagonism has developed to some extent here too, although these proteins now do not target CD4. HIV-1 group O Vpu proteins, by contrast, are devoid of tetherin antagonistic activity, while remaining capable of targeting CD4 (53). We note with interest that the conservation of an A and, to a lesser extent a V, at the equivalent to position 14 in the Vpu TM is found only in Vpu proteins from groups M and N, whereas in group O it is invariably L and is often acidic in SIVcpz. In our hands, an A14L mutation was selectively defective for tetherin and had no effect on CD4 downregulation. In contrast, the W residue is invariant in all HIV-1/SIVcpz Vpu TMs, consistent with its role in both tetherin antagonism and CD4 degradation. Position 18, which when mutated to a leucine has a lesser effect on tetherin antagonism in NL4.3 Vpu, is also highly conserved in groups M and N but in group O is most often a polar residue (N or S). Thus, the residues identified in our screen are associated with Vpu proteins that can antagonize tetherin, but not those that cannot. Whether these residues are sufficient to confer tetherin antagonism to group O Vpu or whether they are necessary only in the context of other differences in the TM domains, as suggested by the data of Lim et al. (28), remains to be determined. As shown in Fig. 6A, several other positions in the group M Vpu TMs are highly conserved, particularly surrounding A14 and W22, and when mutated individually, they had little or no effect on tetherin antagonism in our transfection assays but may have subtle context-dependent roles. Additional features of the cytoplasmic tail that modulate Vpu localization to the TGN also play a role in tetherin antagonism (8), and it is at present unknown whether these are functional in group O Vpu proteins.
In summary, we have identified conserved determinants in the HIV-1 Vpu TM domain that contribute to tetherin interaction and antagonism. Further studies will determine the structural implications of these mutations on tetherin binding and the role of Vpu multimerization in the process and potentially allow the design of antiviral candidates to disrupt Vpu-tetherin interactions.
Acknowledgments
We thank Paul Bieniasz, Greg Towers, Klaus Strebel, John Kappes, Bruce Chesebro, the NIH AIDS Reagents Repository, and the NIBSC Centre for AIDS Reagents for the kind gift of reagents. We are especially grateful to Michael Linden and Julian Bergeron for helpful discussions, Anna Le Tortorec for reagents, and other members of the Neil lab for support and encouragement.
This work was supported by a Wellcome Research Career Development Fellowship (WT082274MA) and an MRC project grant (G0801937) to S.J.D.N.
Author contributions: R.V. and S.J.D.N. designed the experiments; R.V. performed the experiments; R.V. and S.J.D.N. analyzed the data and wrote the paper.
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Andrew, A. J., E. Miyagi, S. Kao, and K. Strebel. 2009. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banning, C., J. Votteler, D. Hoffmann, H. Koppensteiner, M. Warmer, R. Reimer, F. Kirchhoff, U. Schubert, J. Hauber, and M. Schindler. 2010. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PLoS One 5:e9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binette, J., M. Dube, J. Mercier, D. Halawani, M. Latterich, and E. A. Cohen. 2007. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology 4:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasius, A. L., E. Giurisato, M. Cella, R. D. Schreiber, A. S. Shaw, and M. Colonna. 2006. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 177:3260-3265. [DOI] [PubMed] [Google Scholar]
- 5.Douglas, J. L., J. K. Gustin, K. Viswanathan, M. Mansouri, A. V. Moses, and K. Fruh. 2010. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 6:e1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas, J. L., K. Viswanathan, M. N. McCarroll, J. K. Gustin, K. Fruh, and A. V. Moses. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/tetherin via a βTrCP-dependent mechanism. J. Virol. 83:7931-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubé, M., B. B. Roy, P. Guiot-Guillain, J. Binette, J. Mercier, A. Chiasson, and E. A. Cohen. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé, M., B. B. Roy, P. Guiot-Guillain, J. Mercier, J. Binette, G. Leung, and E. A. Cohen. 2009. Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewart, G. D., K. Mills, G. B. Cox, and P. W. Gage. 2002. Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu. Eur. Biophys. J. 31:26-35. [DOI] [PubMed] [Google Scholar]
- 10.Ewart, G. D., T. Sutherland, P. W. Gage, and G. B. Cox. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 70:7108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick, K., M. Skasko, T. J. Deerinck, J. Crum, M. H. Ellisman, and J. Guatelli. 2010. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6:e1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffinet, C., I. Allespach, S. Homann, H. M. Tervo, A. Habermann, D. Rupp, L. Oberbremer, C. Kern, N. Tibroni, S. Welsch, J. Krijnse-Locker, G. Banting, H. G. Krausslich, O. T. Fackler, and O. T. Keppler. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285-297. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R. K., S. Hue, T. Schaller, E. Verschoor, D. Pillay, and G. J. Towers. 2009. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta, R. K., P. Mlcochova, A. Pelchen-Matthews, S. J. Petit, G. Mattiuzzo, D. Pillay, Y. Takeuchi, M. Marsh, and G. J. Towers. 30 October 2009. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U. S. A. [Epub ahead of print.] doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed]
- 15.Habermann, A., J. Krijnse Locker, H. Oberwinkler, M. Eckhardt, S. Homann, A. Andrew, K. Strebel, and H. G. Krausslich. 2010. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84:4646-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammonds, J., J. J. Wang, H. Yi, and P. Spearman. 2010. Immunoelectron microscopic evidence for tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6:e1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser, H., L. A. Lopez, S. J. Yang, J. E. Oldenburg, C. M. Exline, J. C. Guatelli, and P. M. Cannon. 2010. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology 7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz, A., N. Miguet, G. Natrajan, Y. Usami, H. Yamanaka, P. Renesto, B. Hartlieb, A. A. McCarthy, J. P. Simorre, H. Gottlinger, and W. Weissenhorn. 2010. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 7:314-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hout, D. R., L. M. Gomez, E. Pacyniak, J. M. Miller, M. S. Hill, and E. B. Stephens. 2006. A single amino acid substitution within the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein renders simian-human immunodeficiency virus (SHIV(KU-1bMC33)) susceptible to rimantadine. Virology 348:449-461. [DOI] [PubMed] [Google Scholar]
- 20.Hout, D. R., M. L. Gomez, E. Pacyniak, L. M. Gomez, B. Fegley, E. R. Mulcahy, M. S. Hill, N. Culley, D. M. Pinson, W. Nothnick, M. F. Powers, S. W. Wong, and E. B. Stephens. 2006. Substitution of the transmem- brane domain of Vpu in simian-human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig-tailed macaques. Virology 344:541-559. [DOI] [PubMed] [Google Scholar]
- 21.Hussain, A., S. R. Das, C. Tanwar, and S. Jameel. 2007. Oligomerization of the human immunodeficiency virus type 1 (HIV-1) Vpu protein-a genetic, biochemical and biophysical analysis. Virol. J. 4:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwabu, Y., H. Fujita, M. Kinomoto, K. Kaneko, Y. Ishizaka, Y. Tanaka, T. Sata, and K. Tokunaga. 2009. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284:35060-35072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia, B., R. Serra-Moreno, W. Neidermyer, A. Rahmberg, J. Mackey, I. B. Fofana, W. E. Johnson, S. Westmoreland, and D. T. Evans. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouvenet, N., S. J. Neil, M. Zhadina, T. Zang, Z. Kratovac, Y. Lee, M. McNatt, T. Hatziioannou, and P. D. Bieniasz. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kupzig, S., V. Korolchuk, R. Rollason, A. Sugden, A. Wilde, and G. Banting. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694-709. [DOI] [PubMed] [Google Scholar]
- 26.Lemaitre, V., R. Ali, C. G. Kim, A. Watts, and W. B. Fischer. 2004. Interaction of amiloride and one of its derivatives with Vpu from HIV-1: a molecular dynamics simulation. FEBS Lett. 563:75-81. [DOI] [PubMed] [Google Scholar]
- 27.Le Tortorec, A., and S. J. Neil. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966-11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim, E. S., H. S. Malik, and M. Emerman. 2010. Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J. Virol. 84:7124-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, J. X., S. Sharpe, R. Ghirlando, W. M. Yau, and R. Tycko. 2010. Oligomerization state and supramolecular structure of the HIV-1 Vpu protein transmembrane segment in phospholipid bilayers. Protein Sci. 19:1877-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldarelli, F., M. Y. Chen, R. L. Willey, and K. Strebel. 1993. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J. Virol. 67:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malim, M. H., and M. Emerman. 2008. HIV-1 accessory proteins-ensuring viral survival in a hostile environment. Cell Host Microbe 3:388-398. [DOI] [PubMed] [Google Scholar]
- 32.Mangeat, B., G. Gers-Huber, M. Lehmann, M. Zufferey, J. Luban, and V. Piguet. 2009. HIV-1 Vpu neutralizes the antiviral factor tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5:e1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansouri, M., K. Viswanathan, J. L. Douglas, J. Hines, J. Gustin, A. V. Moses, and K. Fruh. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marassi, F. M., C. Ma, H. Gratkowski, S. K. Straus, K. Strebel, M. Oblatt-Montal, M. Montal, and S. J. Opella. 1999. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc. Natl. Acad. Sci. U. S. A. 96:14336-14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 36.McNatt, M. W., T. Zang, T. Hatziioannou, M. Bartlett, I. B. Fofana, W. E. Johnson, S. J. Neil, and P. D. Bieniasz. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehnert, T., A. Routh, P. J. Judge, Y. H. Lam, D. Fischer, A. Watts, and W. B. Fischer. 2008. Biophysical characterization of Vpu from HIV-1 suggests a channel-pore dualism. Proteins 70:1488-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meusser, B., and T. Sommer. 2004. Vpu-mediated degradation of CD4 reconstituted in yeast reveals mechanistic differences to cellular ER-associated protein degradation. Mol. Cell 14:247-258. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell, R. S., C. Katsura, M. A. Skasko, K. Fitzpatrick, D. Lau, A. Ruiz, E. B. Stephens, F. Margottin-Goguet, R. Benarous, and J. C. Guatelli. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyagi, E., A. J. Andrew, S. Kao, and K. Strebel. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neil, S. J., S. W. Eastman, N. Jouvenet, and P. D. Bieniasz. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen, K. L., M. llano, H. Akari, E. Miyagi, E. M. Poeschla, K. Strebel, and S. Bour. 2004. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology 319:163-175. [DOI] [PubMed] [Google Scholar]
- 44.Ohtomo, T., Y. Sugamata, Y. Ozaki, K. Ono, Y. Yoshimura, S. Kawai, Y. Koishihara, S. Ozaki, M. Kosaka, T. Hirano, and M. Tsuchiya. 1999. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem. Biophys. Res. Commun. 258:583-591. [DOI] [PubMed] [Google Scholar]
- 45.Pardieu, C., R. Vigan, S. J. Wilson, A. Calvi, T. Zang, P. Bieniasz, P. Kellam, G. J. Towers, and S. J. Neil. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, S. H., A. A. Mrse, A. A. Nevzorov, M. F. Mesleh, M. Oblatt-Montal, M. Montal, and S. J. Opella. 2003. Three-dimensional structure of the channel-forming trans-membrane domain of virus protein “u” (Vpu) from HIV-1. J. Mol. Biol. 333:409-424. [DOI] [PubMed] [Google Scholar]
- 47.Paul, M., S. Mazumder, N. Raja, and M. A. Jabbar. 1998. Mutational analysis of the human immunodeficiency virus type 1 Vpu transmembrane domain that promotes the enhanced release of virus-like particles from the plasma membrane of mammalian cells. J. Virol. 72:1270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Caballero, D., T. Zang, A. Ebrahimi, M. W. McNatt, D. A. Gregory, M. C. Johnson, and P. D. Bieniasz. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radoshitzky, S. R., L. Dong, X. Chi, J. C. Clester, C. Retterer, K. Spurgers, J. H. Kuhn, S. Sandwick, G. Ruthel, K. Kota, D. Boltz, T. Warren, P. J. Kranzusch, S. P. Whelan, and S. Bavari. 2010. Infectious Lassa virus, but not filoviruses, is restricted by Bst-2/tetherin. J. Virol. 84:10569-10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rollason, R., V. Korolchuk, C. Hamilton, P. Schu, and G. Banting. 2007. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120:3850-3858. [DOI] [PubMed] [Google Scholar]
- 51.Rong, L., J. Zhang, J. Lu, Q. Pan, R. P. Lorgeoux, C. Aloysius, F. Guo, S. L. Liu, M. A. Wainberg, and C. Liang. 2009. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83:7536-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakuma, T., T. Noda, S. Urata, Y. Kawaoka, and J. Yasuda. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauter, D., M. Schindler, A. Specht, W. N. Landford, J. Munch, K. A. Kim, J. Votteler, U. Schubert, F. Bibollet-Ruche, B. F. Keele, J. Takehisa, Y. Ogando, C. Ochsenbauer, J. C. Kappes, A. Ayouba, M. Peeters, G. H. Learn, G. Shaw, P. M. Sharp, P. Bieniasz, B. H. Hahn, T. Hatziioannou, and F. Kirchhoff. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauter, D., A. Specht, and F. Kirchhoff. 2010. Tetherin: holding on and letting go. Cell 141:392-398. [DOI] [PubMed] [Google Scholar]
- 55.Schubert, U., L. C. Anton, I. Bacik, J. H. Cox, S. Bour, J. R. Bennink, M. Orlowski, K. Strebel, and J. W. Yewdell. 1998. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol. 72:2280-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schubert, U., S. Bour, A. V. Ferrer-Montiel, M. Montal, F. Maldarell, and K. Strebel. 1996. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J. Virol. 70:809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schubert, U., A. V. Ferrer-Montiel, M. Oblatt-Montal, P. Henklein, K. Strebel, and M. Montal. 1996. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 398:12-18. [DOI] [PubMed] [Google Scholar]
- 58.Schubert, U., and K. Strebel. 1994. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 68:2260-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharpe, H. J., T. J. Stevens, and S. Munro. 2010. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142:158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpe, S., W. M. Yau, and R. Tycko. 2006. Structure and dynamics of the HIV-1 Vpu transmembrane domain revealed by solid-state NMR with magic-angle spinning. Biochemistry 45:918-933. [DOI] [PubMed] [Google Scholar]
- 61.Strebel, K. 2007. HIV accessory genes Vif and Vpu. Adv. Pharmacol. 55:199-232. [DOI] [PubMed] [Google Scholar]
- 62.Tiganos, E., J. Friborg, B. Allain, N. G. Daniel, X. J. Yao, and E. A. Cohen. 1998. Structural and functional analysis of the membrane-spanning domain of the human immunodeficiency virus type 1 Vpu protein. Virology 251:96-107. [DOI] [PubMed] [Google Scholar]
- 63.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varthakavi, V., R. M. Smith, K. L. Martin, A. Derdowski, L. A. Lapierre, J. R. Goldenring, and P. Spearman. 2006. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic 7:298-307. [DOI] [PubMed] [Google Scholar]
- 65.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J. Virol. 66:226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, S. J., L. A. Lopez, H. Hauser, C. M. Exline, K. G. Haworth, and P. M. Cannon. 2010. Anti-tetherin activities in Vpu-expressing primate lentiviruses. Retrovirology 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, F., S. J. Wilson, W. C. Landford, B. Virgen, D. Gregory, M. C. Johnson, J. Munch, F. Kirchhoff, P. D. Bieniasz, and T. Hatziioannou. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]