Abstract
Outbreaks of smallpox (i.e., caused by variola virus) resulted in up to 30% mortality, but those who survived smallpox infection were regarded as immune for life. Early studies described the levels of neutralizing antibodies induced after infection, but smallpox was eradicated before contemporary methods for quantifying T-cell memory were developed. To better understand the levels and duration of immunity after smallpox infection, we performed a case-control study comparing antiviral CD4+ and CD8+ T-cell responses and neutralizing antibody levels of 24 smallpox survivors with the antiviral immunity observed in 60 smallpox-vaccinated (i.e., vaccinia virus-immune) control subjects. We found that the duration of immunity following smallpox infection was remarkably similar to that observed after smallpox vaccination, with antiviral T-cell responses that declined slowly over time and antiviral antibody responses that remained stable for decades after recovery from infection. These results indicate that severe, potentially life-threatening disease is not required for the development of sustainable long-term immunity. This study shows that the levels of immunity induced following smallpox vaccination are comparable in magnitude to that achieved through natural variola virus infection, and this may explain the notable success of vaccination in eradicating smallpox, one of the world's most lethal diseases.
Variola virus (VAR) is the causative agent of smallpox, an extinct human disease that had a mortality rate ranging from 1% (Variola minor) to as high as 30% (Variola major). Humans represent the only known host or reservoir for VAR and, following massive global eradication efforts, smallpox was declared officially eradicated in 1980 (12). Despite being extinct in nature, the threat of bioterrorism has led to a resurgence of interest in smallpox and smallpox vaccination since it is possible that undisclosed stocks of virus exist or that VAR or VAR-like orthopoxviruses could be developed through genetic engineering and used as biological weapons (13, 34).
The last case of natural smallpox was reported in 1977 in Somalia and the last laboratory-associated case of smallpox occurred in Great Britain in 1978 (12). The last documented outbreak of smallpox in the United States occurred in Texas in 1949 (20). In the United States, routine smallpox vaccination ceased by 1972, and routine smallpox vaccination ceased worldwide by 1980 following eradication of the naturally occurring disease. Smallpox vaccination is performed by infecting a patient with a closely related orthopoxvirus, vaccinia virus (VAC), leading to a localized infection at the site of inoculation and cross-protective immunity against smallpox. Since smallpox is no longer circulating in nature and smallpox vaccination is limited mainly to individuals with occupational risk (e.g., military personnel, laboratory workers, etc.), we have an opportunity to measure the persistence of antiviral immunity to these two divergent orthopoxvirus infections in the relative absence of re-exposure/reinfection over a prolonged period of time. Although several studies have examined the duration of immunity following smallpox vaccination (2, 4, 9, 17, 31, 35), little is known about the magnitude or duration of immunity following natural smallpox infection itself (32, 35). This is an important question because smallpox infection is believed to confer lifelong protective immunity (5), whereas protective immunity following smallpox vaccination represents a topic of considerable debate; protective immunity is either long-lived (2, 9, 10, 15, 17, 31, 33, 35) or may persist for only 3 to 5 years (19, 21, 26, 28).
Since the last documented cases of smallpox occurred more than 30 years ago, it is becoming increasingly more difficult to find subjects who have survived smallpox infection. Prior studies have examined antibody levels (35) and/or T-cell responses (32) in a small number of smallpox-immune subjects with varying results. However, a formal case-control analysis of immunity following natural smallpox infection versus smallpox vaccination has not been previously performed, and this represents the objective of the present study. We describe here the results of a case-control study involving 24 smallpox survivors, including 8 subjects with a history of smallpox infection and 16 subjects with a history of smallpox infection, in addition to a history of one or more smallpox vaccinations. The levels of antiviral immunity in these two groups of smallpox survivors were compared to 60 control subjects with one or more smallpox vaccinations but with no history of smallpox infection. Antiviral CD4+ and CD8+ T-cell responses could be identified for up to 83 years after smallpox infection, but T-cell memory declined slowly over time with a pattern similar to that observed following smallpox vaccination. In contrast to T-cell memory, antiviral antibody responses remained elevated and showed no evidence of decline following smallpox vaccination or smallpox infection. These results demonstrate that the relative magnitude and duration of antiviral immunity following smallpox infection and smallpox vaccination (i.e., VAC infection) are strikingly similar despite the considerable differences in virulence between VAR and VAC infections.
MATERIALS AND METHODS
Study population.
A cohort of subjects with self-reported cases of smallpox infection (n = 24, Table 1) were compared to matched case-controls (n = 60, i.e., 2.5 controls for each subject, Table 2) in terms of their antiviral antibody and T-cell responses to orthopoxvirus antigens. Two potential smallpox-immune subjects were excluded due to an unreliable infection history (based on an extensive medical history questionnaire) and had not been diagnosed by a healthcare professional at the time of illness. Case-control samples were obtained from subjects who participated in a previous cross-sectional study (17) and were matched for years post-orthopoxvirus infection, number of orthopoxvirus infections, gender, and age. Other additional subjects included 10 unvaccinated subjects who served as negative controls and one recently vaccinated subject (1 year post-smallpox vaccination) who served as an internal positive control for virus-specific T-cell and antibody analyses. Subjects were recruited between 2002 and 2004. All subjects provided informed written consent before signing research authorization forms that complied with the U.S. Health Insurance Portability and Accountability Act, completing medical history questionnaires and providing a 50-ml blood sample used to prepare serum and peripheral blood mononuclear cells (PBMC) that were cryopreserved prior to analysis. Subjects were not financially compensated for their participation in the study. All clinical studies were conducted according to the Declaration of Helsinki principles and approved by the Institutional Review Board of Oregon Health and Science University.
TABLE 1.
Smallpox infection historya
| Subject | Yr of VAR infection | Subject age (yr) | Location of VAR infection | Yr of last vaccination | Quarantine | Physician diagnosed | Measles | Varicella | Period (yr) postinfection | CD4/106 CD4 | CD8/106 CD8 | ELISA titer | NT50 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 185 | 1935-1936 | 16-17 | Pasco, WA | 1955 (6) | Yes | Yes | Yes | Unsure | 48 | 4 | 43 | 1,055 | 246 |
| 186 | 1935-1936 | 7-8 | Pasco, WA | 1970 (5) | Yes | Yes | Yes | No | 33 | 212 | <1 | 2,114 | 300 |
| 344 | 1927 | <1 | Juneau, Al | 1950 (1) | Yes | Yes | Yes | Yes | 53 | <1 | 13 | 24,283 | 481 |
| 348 | 1929 | 6-7 | Portland, OR | NAb | Yes | 74 | 44 | 25 | 1,182 | 64 | |||
| 378 | 1935-1939 | 7-11 | Colorado | 1940 (1) | Yes | Unsure | 63 | <1 | <1 | 2,291 | 166 | ||
| 379 | 1952-1953 | 2-3 | Germany | NA | Unsure | Yes | No | No | 50.5 | 28 | <1 | 4,841 | 59 |
| 381 | 1925 | 8 | Portland, OR | NA | Yes | Yes | Yes | Yes | 78 | 4 | 4 | 32,803 | 14 |
| 382 | 1930 | 12 | Vancouver, WA | NA | Yes | Yes | Yes | Yes | 73 | 54 | 88 | 10,213 | 218 |
| 391 | 1931 | 7 | Troutdale, OR | 1931 (1) | Yes | Nurse diagnosed | Yes | Yes | 72 | 7 | 7 | 548 | 44 |
| 392 | 1924-1925 | 3 | Byesville, OH | 1970 (2) | Yes | Yes | Yes | Yes | 33 | 40 | 38 | 4,199 | 239 |
| 393 | 1928-1929 | 9-10 | Portland, OR | 1928 (2) | Yes | Yes | Yes | Yes | 75 | 71 | 69 | 5,434 | 64 |
| 394 | 1933-1934 | 7-8 | Seattle, WA | NA | Yes | Yes | Yes | Yes | 69.5 | 7 | <1 | 3,278 | 17 |
| 395 | 1935-1936 | 1-2 | Portland, OR | 1970 (7) | Unsure | Unsure | Yes | Yes | 33 | 50 | <1 | 3,340 | 184 |
| 396 | 1927 | 5 | Hillsboro, OR | 1968 (5) | Yes | Yes | Yes | Yes | 35 | 658 | 311 | 3,578 | 63 |
| 399 | 1928-1929 | 11-12 | Kadoca, SD | 1974 (1) | Yes | Yes | Yes | Yes | 29 | <1 | <1 | 5,270 | 199 |
| 400 | 1926 | 5 | Nebraska | 1926 (1) | Yes | Yes | Yes | Yes | 77 | 10 | 4 | 887 | 162 |
| 401 | 1936 | 8 | Riverton, WY | 1949 (2) | Yes | Yes | Yes | Yes | 54 | 32 | <1 | 1,592 | 15 |
| 402 | 1929-1930 | 3-4 | Kansas | NA | Yes | Yes | Yes | Yes | 73.5 | 10 | 11 | 424 | 7 |
| 403 | 1920 | 4 | Sellwood, OR | NA | Yes | Yes | No | Yes | 83 | 22 | 38 | 2,237 | 37 |
| 429 | 1935 | 2-3 | Tacoma, WA | NA | Yes | No | Yes | Yes | 68 | <1 | <1 | 601 | 16 |
| 431 | 1938 | 10-11 | Shahalis, WA | 1976 (2) | Yes | Yes | Yes | Yes | 27 | 6 | <1 | 20,779 | 528 |
| 471 | 1923 | 7 | Seattle, WA | 1952 (8) | Yes | Yes | Yes | Yes | 51 | 10 | 41 | 1,106 | 40 |
| 490 | 1958 | 3 | Lahore, Pakistan | 1963 (2) | Yes | Yes | Yes | Yes | 40 | 21 | 20 | 1,671 | 104 |
| 515 | 1930 | 3 | Portland, OR | 1957 (3) | Yes | Yes | Yes | Yes | 47 | 4 | 171 | 3,188 | 30 |
Subjects filled out a medical history questionnaire describing their age, the date and location at the time of smallpox (VAR) infection, and verifying information such as whether they were diagnosed with smallpox by a physician/medical professional and/or whether they were quarantined. Information on other rash illnesses, including measles and varicella-zoster virus infection (i.e., chickenpox), was also provided. If the subject provided a range of dates for VAR infection or smallpox vaccination (i.e., VAC infection), then an average was used for calculating the years after infection or vaccination. The total number of vaccinations is shown in parentheses following the year of the last vaccination. Six subjects were vaccinated prior to contracting clinically apparent (albeit mild) smallpox, including two individuals (subjects 185 and 391) who were vaccinated less than a week prior to the eruption of smallpox, one individual (subject 490) who was vaccinated 1 month prior to smallpox, two individuals (subjects 400 and 471) who described being vaccinated “during the time of the outbreak,” and one individual (subject 393) who was vaccinated twice with both vaccinations described as “no-takes” at undetermined times prior to contracting smallpox.
NA, not applicable.
TABLE 2.
Study group characteristicsa
| Characteristic | Group |
|||
|---|---|---|---|---|
| 1 (VAR; n = 8) | 2 (VAC; n = 20) | 3 (VAR-VAC; n = 16) | 4 (VAC-VAC; n = 40) | |
| Mean age in yr (range) | 76 (52-86) | 70 (63-80) | 77 (48-87) | 64 (43-76) |
| Mean no. of infections or vaccinations (range) | 1 | 1 | 4 (2-9) | 3 (2-7) |
| Mean period (yr) after last infection/vaccination (range) | 71 (51-83) | 66 (61-75) | 48 (27-77) | 45 (33-75) |
| % Male subjects | 25 | 50 | 50 | 43 |
Subjects were divided into four groups based on their infection history. Group 1 consisted of cases of smallpox (VAR) with no history of smallpox vaccination. Group 2 consisted of case-controls for group 1 and included subjects with 1 smallpox vaccination (VAC) but no history of smallpox infection. Group 3 (VAR-VAC) consisted of cases of smallpox with a history of one or more smallpox vaccinations, and group 4 (VAC-VAC) consisted of case-controls for group 3 with a history of multiple smallpox vaccinations but no history of smallpox infection.
ELISA and neutralization assays.
Orthopoxvirus-specific enzyme-linked immunosorbent assay (ELISA) was performed as previously described (17) using whole-VAC lysate (inactivated by pretreatment with 3% H2O2 for 2 h). An internal positive control was included on all plates to normalize between plates and between assays performed on different days. Serial 3-fold dilutions of sera were incubated for 1 h on preblocked plates. H2O2 was added to the plates at a final concentration of 3%, and the plates were incubated for an additional 30 min. After a washing step, the plates were incubated with horseradish peroxidase-conjugated antibodies specific for human IgG (clone G18-145; Pharmingen) for 1 h. After another washing step, detection reagents were added, and the plates were analyzed on a VersaMax ELISA plate reader (Molecular Devices). Antibody titers were determined by log-log transformation of the linear portion of the curve, using 0.1 optical density units as the endpoint and performing conversion on final values.
Neutralization assays were performed as previously described (17). Briefly, serial 2-fold dilutions of heat-inactivated serum were incubated with VAC (∼75 PFU) for 2 h at 37°C before plating the mixture on Vero cell monolayers in six-well plates. After 1 h, the cells were overlaid with 0.5% agarose and incubated for 4 days to allow for plaque formation. Monolayers were fixed with 75% methanol-25% acetic acid, the agarose was removed, and the monolayer was stained with 0.1% crystal violet in phosphate-buffered saline containing 0.2% formaldehyde. The 50% neutralization titer (NT50) was defined as the serum dilution resulting in 50% reduction of plaques and was calculated by log-log transformation of the linear portion of the curve. Logarithmic transformation of the data was used to calculate the titer, and conversion was performed on final values.
ICCS.
Intracellular cytokine staining (ICCS) was performed as previously described (17). Briefly, PBMC were cultured at 37°C with 6% CO2 in RPMI containing 5% heat-inactivated fetal bovine serum (HyClone), 20 mM HEPES, 2 mM l-glutamine, and antibiotics with or without VAC (sucrose gradient-purified intracellular mature virus, strain Western Reserve) at an MOI of 0.3. After 12 h of culture, brefeldin A was added for an additional 6 h and cells were stained overnight at 4°C with antibodies specific for CD4 (clone L200; Pharmingen) and CD8b (clone 2ST8.5H7; Beckman Coulter). Cells were fixed, permeabilized, and stained intracellularly with antibodies specific for gamma interferon (IFN-γ; clone 4S.B3; Pharmingen) and tumor necrosis factor alpha (TNF-α; clone Mab11; eBioscience). Samples were acquired on an LSRII flow cytometer (Becton Dickinson) using FACS-Diva software (Becton Dickinson), typically obtaining 2 to 3 million events per sample (range, 0.6 to 7.8 million events). Samples were analyzed by using FlowJo software (Tree Star). Live cell gating was performed using forward- and side-scatter characteristics. Quantitation of cytokine-positive cells was determined after first gating on CD4+ CD8− or CD4− CD8+ T cells and subtracting the number of events from uninfected cultures.
Statistical analysis.
Demographic variables were compared across the groups to assess group likeness. Demographic differences between groups were assessed by the Fisher exact and chi-square tests for categorical variables and by the Wilcoxon rank sum test for continuous variables. Group comparisons of patterns of change in immunity measures over time were performed using regression analysis. Log-transformed ELISA and neutralizing antibody data were analyzed using linear regression models for normally distributed data; however, CD4+ and CD8+ ICCS results contained many truncated values falling below the limits of instrument detection and requiring statistical models accommodating of interval censored data with a Weibull distribution. Analyses were performed by using SAS software, version 9.2.
RESULTS
Subjects and case-controls.
Subjects with a history of smallpox infection (Table 1) provided informed written consent, medical history (including specific information about their exposure to smallpox), and a blood sample that was used to prepare serum for serological analysis of antiviral antibody levels and PBMC for analysis of orthopoxvirus-specific CD4+ and CD8+ T-cell memory. Of the 24 subjects, 22 (92%) reported being infected with smallpox in the United States during outbreaks that occurred in the 1920s and 1930s. Most of the subjects reported their smallpox infections in the Northwest United States, and the timeframe is consistent with records of recurrent smallpox outbreaks in this region (29) and with the last documented cases of smallpox occurring in 1949 (20). Two subjects contracted smallpox in other countries, Germany (1952 to 1953) or Pakistan (1958), and these represent the most recent samples from VAR-infected individuals within the cohort.
VAR-immune subjects and their controls were split into four groups (Table 2). Group 1 (VAR) includes eight individuals with a self-reported history of smallpox infection but no history of smallpox vaccination. Serving as case-controls for group 1, group 2 (VAC) includes 20 subjects who received a single smallpox vaccination (i.e., one VAC infection). Group 3 (VAR-VAC) includes 16 subjects who reported smallpox infection in addition to receiving one or more smallpox vaccinations. Smallpox vaccination occurred prior to smallpox infection in 6 of the 16 subjects in this group. Despite contracting smallpox, the other 10 subjects in this group still received one or more smallpox vaccinations after recovering from the initial disease. These vaccinations were mainly performed due to requirements for school enrollment, international travel, or enlistment in the military. One subject (subject 395) received six smallpox vaccinations at monthly intervals as “a treatment for canker sores” in 1970. Serving as case-controls for the VAR-VAC group, group 4 (VAC-VAC) includes 40 subjects who experienced a similar number of orthopoxvirus infections (i.e., received two or more smallpox vaccinations) but never developed smallpox infection.
There were no significant differences in gender between the VAR and VAC groups or between the VAR-VAC and VAC-VAC groups (P = 0.40 and P = 0.77, respectively [Fisher exact test]). Likewise, there were no significant differences in the number of total orthopoxvirus infections experienced by the VAR-VAC and VAC-VAC groups (P = 0.32 [Fisher exact test]). The average number of years since smallpox infection in the VAR group (mean, 71 years) was significantly longer than the average number of years since smallpox vaccination in the VAC group (mean, 66 years, P = 0.02 [Wilcoxon rank sum test]), whereas there was no significant difference in the number of years since the last orthopoxvirus infection between the VAR-VAC (mean, 48 years) and the VAC-VAC (mean, 45 years) groups (P = 0.96 [Wilcoxon rank sum test]).
Duration of antiviral CD4+ T-cell memory.
To measure orthopoxvirus-specific T-cell memory, we infected PBMC with an optimized concentration of purified VAC as previously described (14, 16, 17). VAC preferentially infects human monocytes (1, 14), and these cells present viral peptides to both virus-specific CD4+ T cells and CD8+ T cells. Quantitation of virus-specific T cells is determined directly ex vivo based on the induction of IFN-γ and TNF-α expression that is measured by ICCS analysis (Fig. 1a). VAC represents the most effective orthopoxvirus to use for stimulating virus-specific T cells in vitro because its large genome contains peptide epitopes that are cross-reactive to other closely related orthopoxviruses, including monkeypox virus (14, 15, 32), cowpox virus (1), and VAR (Fig. 1a). VAR was not used in these T-cell assays due to feasibility and biosafety constraints, as well as the likelihood that it will be ineffective at stimulating T-cell responses if it contains the same immune evasive properties as other orthopoxviruses such as monkeypox virus or cowpox virus that, similar to VAR, spread via monocytic cell-associated viremia and which are essentially undetectable by virus-specific T-cell immunosurveillance (1, 6, 14).
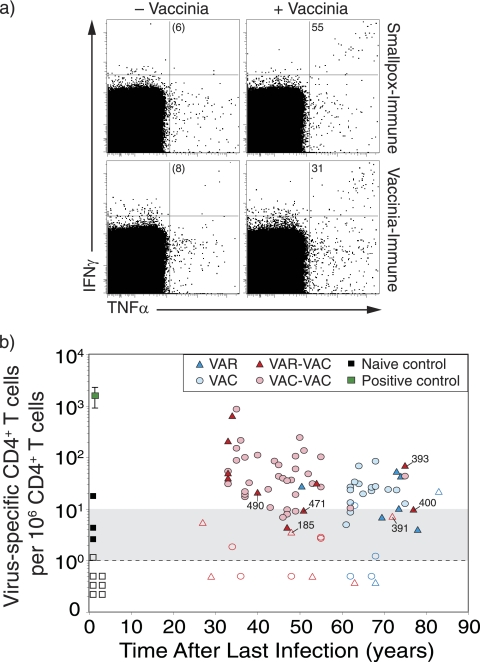
FIG. 1.
Virus-specific CD4+ T-cell memory after smallpox infection or vaccination. (a) Representative flow cytometry dot plots from a smallpox-immune subject (73 years after infection) and a VAC-immune subject (64 years after vaccination). PBMC were incubated in medium (− Vaccinia) or with purified virus (+ Vaccinia) and virus-specific T cells were identified by intracellular cytokine staining. The dot plots are pregated on CD4+CD8− T cells and show the number of IFN-γ+ TNF-α+ events per 106 CD4+ T cells after background subtraction (− Vaccinia). (b) Analysis of orthopoxvirus-specific CD4+ T-cell memory as a function of time after smallpox infection or vaccination. Filled symbols represent samples scoring more than 2-fold over background, and open symbols represent samples that were not 2-fold over background. Samples that scored between 1 and 9 events/106 T cells are considered equivocal (as noted by the gray shading). Samples scoring below the limits of detection were given values of <1 per 106. Group 1 (VAR) includes subjects with one smallpox infection, group 2 (VAC) includes subjects with one smallpox vaccination, group 3 (VAR-VAC) includes subjects with a history of smallpox infection in addition to one to eight smallpox vaccinations, and group 4 (VAC-VAC) includes subjects who received between two to seven smallpox vaccinations. Symbols in the VAR-VAC group that are marked with subject identification (ID) number represent subjects who were vaccinated prior to contracting smallpox to distinguish them from subjects within that group who were only vaccinated after recovery from smallpox. Naive controls (n = 10) represent unvaccinated subjects born after 1972. A positive control (1 year postvaccination) was included in each experiment.
Following background subtraction, the frequency of IFN-γ+ TNF-α+ CD4+ T cells per million peripheral CD4+ T cells was determined and plotted as a function of time postinfection (Fig. 1b). A positive control sample (1 year after smallpox vaccination) was included in each experiment to confirm the antigenicity of the virus preparation and verify the staining conditions. Ten unvaccinated negative controls (born after 1972 with no history of smallpox vaccination) were also included in the analysis to confirm the specificity of the assay. Similar to previous studies (17), 9/10 (90%) of naive subjects scored < 10 IFN-γ+ TNF-α+ CD4+ T cells per 106 CD4+ T cells. Based on these results, a score of 1 to 9 IFN-γ+ TNF-α+ CD4+ T cells per 106 CD4+ T cells was considered equivocal (as illustrated by gray-shaded region), and a score of <1 IFN-γ+ TNF-α+ CD4+ T-cell per 106 CD4+ T cells was below our limit of detection (dashed line). Low, but detectable CD4+ T-cell memory was identified for up to 83 years after natural smallpox infection (Fig. 1b). As a group, 5/8 (62%) of the VAR-immune subjects maintained unequivocal CD4+ T-cell memory (range, 51 to 83 years). These results are similar to the VAC control group that demonstrated similar levels of durable immunity, with 15/20 (75%) of the subjects maintaining antiviral CD4+ T-cell memory for up to 75 years (range, 61 to 75 years). VAR-immune subjects who also received smallpox vaccination (VAR-VAC) are an important group because the repertoire of smallpox-specific T cells that are cross-reactive to VAC antigens has been preferentially selected/expanded in vivo by vaccination. In this case, VAC represents a homologous antigen for measuring the levels and duration of antiviral T-cell memory. Similar to the VAR group that only experienced natural smallpox infection, 9/16 (56%) of the VAR-VAC group maintained unequivocal orthopoxvirus-specific CD4+ T-cell memory. The case-control VAC-VAC group showed somewhat higher memory T-cell frequencies with 32/40 (80%) of the subjects maintaining antiviral CD4+ T-cell responses during a similar span of time after infection. There were no significant differences in the magnitude or the slope of decay in CD4+ T-cell memory when comparing VAR to the VAC control group (P = 0.69, Wald chi-square) or comparing VAR-VAC to the VAC-VAC control group (P = 0.93, Wald chi-square). Together, these results indicate that CD4+ T-cell memory is long-lived after smallpox infection but declines slowly over time with a pattern that is similar to that observed after smallpox vaccination (10, 17).
Analysis of antiviral CD8+ T-cell memory.
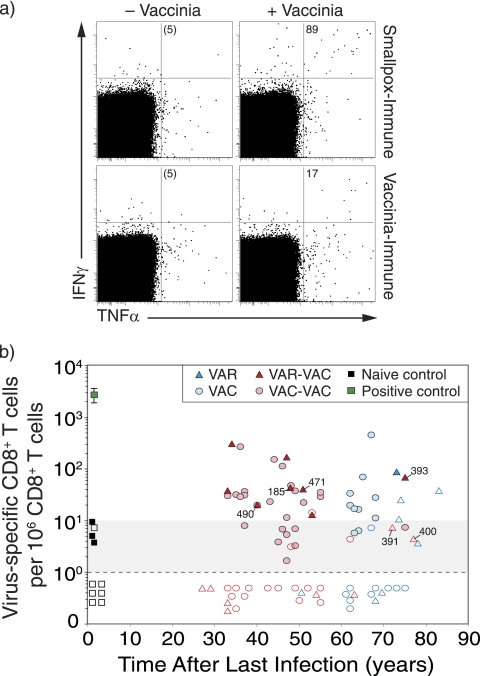
Quantitation of CD8+ T-cell memory was performed in parallel to the analysis of antiviral CD4+ T-cell responses as described above. Similar to orthopoxvirus-specific CD4+ T cells, virus-specific CD8+ T cells from smallpox-immune or VAC-immune subjects expressed both IFN-γ and TNF-α after antigenic stimulation (Fig. 2a), and the frequency of IFN-γ+ TNF-α+ CD8+ T cells per 106 CD8+ T cells was determined as a function of time postinfection (Fig. 2b). Antiviral CD8+ T-cell memory was demonstrated for up to 83 years after smallpox infection. However, as a group, only 4/8 (50%) of VAR-immune subjects maintained an unequivocal CD8+ T-cell memory. This is similar to the VAC control group in which 8/20 (40%) subjects maintained orthopoxvirus-specific CD8+ T-cell responses. Smallpox-immune subjects who received smallpox vaccination (VAR-VAC) also showed demonstrable CD8+ T-cell immunity in 8/16 (50%) of the cases, and this was similar to their control group (VAC-VAC) in which 17/40 (43%) of subjects maintained antiviral CD8+ T-cell memory. There were no significant differences observed in the frequency or the slope of decay in CD8+ T-cell memory when comparing VAR to VAC (P = 0.17, Wald chi-square) or when comparing VAR-VAC to the VAC-VAC control groups (P = 0.67, Wald chi-square). This indicates that orthopoxvirus-specific CD8+ T-cell memory is maintained in about half of the subjects, regardless of whether they contracted natural smallpox, were vaccinated against smallpox, or experienced a combination of smallpox infection and smallpox vaccination(s).
FIG. 2.
Virus-specific CD8+ T-cell memory after smallpox infection or vaccination. (a) Representative flow cytometry dot plots from a smallpox-immune subject (73 years after infection) and a VAC-immune subject (64 years after vaccination). The dot plots are gated on CD4− CD8+ T cells and show the number of IFN-γ+ TNF-α+ cells per 106 CD8+ T cells after background subtraction (− Vaccinia). (b) Orthopoxvirus-specific CD8+ T cells were measured as a function of time after infection or vaccination. The samples were analyzed in parallel to the antiviral CD4+ T-cell responses examined in Fig. 1, and the same symbols and group definitions are as described in the legend to Fig. 1.
Quantitation of antiviral antibody responses.
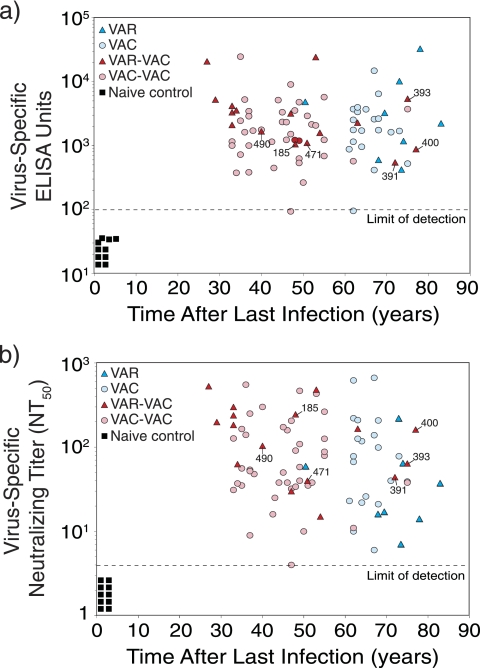
Orthopoxvirus-specific antibody responses were measured by two independent approaches; ELISA and plaque reduction neutralizing assays (Fig. 3). The ELISA approach (Fig. 3a) provides information on the overall levels of orthopoxvirus-specific antibody responses regardless of neutralizing activity. All of the smallpox-immune subjects and VAC-immune subjects maintained detectable levels of orthopoxvirus-specific serum IgG, regardless of the time since the last infection or vaccination. Unlike CD4+ T-cell memory (Fig. 1) or CD8+ T-cell memory (Fig. 2), which declined over time, antiviral IgG responses measured by ELISA remained essentially unchanged. Measurement of humoral immunity by neutralizing assays (Fig. 3b) provides information on biologically active antibody levels (e.g., antibody that can neutralize infectious VAC) and is independent of the immunoglobulin isotype or subclass. Similar to the ELISA titers, all of the smallpox-immune and VAC-immune subjects maintained detectable neutralizing antibody titers with no statistically significant differences in titer changes over time for the VAR to VAC (P = 0.88 [F-test]) and the VAR-VAC to VAC-VAC case-control groups (P = 0.63 [F-test]). Together, this indicates that humoral immunity is long-lived regardless of which orthopoxvirus infection is encountered.
FIG. 3.
Durable antiviral antibody responses are induced by both smallpox infection and vaccination. (a) Quantitation of antiviral IgG responses by ELISA. In these experiments, ELISA plates were coated with VAC lysate and the titers of virus-specific serum IgG were determined and expressed as ELISA units (EU). Samples that scored <100 EU are considered seronegative, and samples from naive, unvaccinated subjects (n = 10) score below this cutoff value (designated by the dashed line). (b) Quantitation of orthopoxvirus-specific neutralizing antibody responses. In these studies, 2-fold dilutions of serum were incubated with VAC, and plaque reduction neutralization tests were performed. The NT50 is defined as the reciprocal of the serum dilution required to reduce infectious virus (i.e., VAC plaques) by 50%. Symbols and group definitions are as described in the legend to Fig. 1.
DISCUSSION
In this study, we determined the levels of antiviral T-cell memory and antibody responses following smallpox infection (i.e., VAR) and compared this to the levels of immunity induced by smallpox vaccination (i.e., VAC). Of the 24 smallpox survivors in the study, 8 reported a history of smallpox infection and 16 reported a history of smallpox infection in addition to one or more smallpox vaccinations. These two groups of smallpox-immune individuals were compared to case-control groups who received either one smallpox vaccination or multiple smallpox vaccinations, respectively. Based on these comparisons, there were no significant differences in the relative level or duration of immunity induced by smallpox infection versus smallpox vaccination. Although the sample size was too small to reliably measure T-cell or antibody half-life, in each case, antiviral T-cell memory declined slowly over time, whereas humoral immunity was maintained essentially for life. Based on these results, it appears that smallpox vaccination effectively recapitulates the levels and types of immunity induced by smallpox infection itself, and this may be the key to the protective immunity afforded by this successful vaccine and its role in the eradication of smallpox.
Analysis of cellular immunity against orthopoxviruses can be performed by a variety of assays, including IFN-γ ELISPOT, IFN-γ ELISA, and ICCS, or by measuring other functions after extended in vitro stimulation such as cytolytic activity or proliferative capacity (3, 33). Combinations of phenotypic markers such as CD38/HLA-DR or Ki67/BcL-2 can also be used, at least during the acute phase of infection, to estimate the total number of virus-specific CD8+ T cells (27). These cellular techniques often require more complex experimental manipulations than analysis of serum antibody responses and may be more prone to experimental variation and interpretation. Prior studies on T-cell memory following smallpox infection or vaccination have indicated that T-cell memory is either short-lived or long-lived (33), and this may be due, at least in part, to the types of assays conducted and how the analysis is performed. A further complication with analysis of T-cell memory following orthopoxvirus infection is that the relatively low frequency of virus-specific T cells induced by infection/vaccination can be difficult to measure. In one study (10), IFN-γ ELISPOT analysis showed that VAC-specific T cells could be detected for up to 50 years after smallpox vaccination. In this example, the negative controls (i.e., unvaccinated subjects) scored less than three spot-forming cells per million PBMC (10), and this allowed for low-frequency VAC-specific T cells to be detected in the range of 4 to 50 spot-forming cells per million PBMC for 20 to 50 years after smallpox vaccination. In another study (7), the IFN-γ ELISPOT background was set at 50 spot-forming cells per million PBMC and, with this cutoff value, <20% of the subjects maintained T-cell memory after smallpox vaccination. This suggests that the sensitivity of a given assay can impact the interpretation of T-cell memory levels and the duration of immunity.
In our studies, we used intracellular cytokine staining analysis to identify virus-specific CD4+ and CD8+ T cells that expressed both IFN-γ and TNF-α following direct ex vivo stimulation with VAC (Fig. 1 and 2). Although this approach detects only the subset of virus-specific T cells that produce both of these cytokines, we found that this combination was the most specific and allows detection of very low-frequency events (14, 15, 17). In this present study, we measured the levels of T-cell memory following smallpox infection or smallpox vaccination and expected to see higher levels of T-cell memory after smallpox infection since recovery from smallpox infection is believed to confer lifelong immunity against reinfection. Instead, we found that smallpox infection induced T-cell responses of similar magnitude and duration as that observed after smallpox vaccination and likewise declined slowly over time (Fig. 1 and 2). The low but detectable level of T-cell memory observed in these studies contrasts with a previous study that found only 1/8 VAR-immune subjects retained long-term T-cell memory as measured by IFN-γ ELISPOT analysis (32). The reasons for these differences are unclear. Historical studies indicate that immunity against smallpox wanes over time with the severity of disease increasing steadily as a function of time since vaccination (18). However, immunity against lethal smallpox infection does not change appreciably over time (18, 24, 33) with survival rates of 98, 94, and 93% during intervals of 0 to 10 years, 11 to 20 years, or >20 years postvaccination, respectively (24, 33). These historical findings, in combination with the results of the T-cell analysis described here, suggests that T-cell memory may play a role in reducing disease severity after exposure to smallpox but that T cells are unlikely to be the sole component of protective immunity since antiviral T-cell responses decline markedly over time (Fig. 1 and 2), whereas protection against lethal smallpox infection does not.
Analysis of humoral immunity following smallpox vaccination has provided new insight into a fundamental difference between the humoral and cell-mediated arms of the adaptive immune response to orthopoxvirus infection. Unlike virus-specific T-cell responses that decline over time (4, 10, 17), virus-specific memory B-cell responses appear stable (2, 10), and antiviral antibody responses following smallpox or smallpox vaccination are likely maintained for a lifetime (Fig. 3). Although smallpox infection is expected to confer lifelong protection, we did not find significantly higher antibody titers in the VAR or VAR-VAC groups compared to their vaccinated case controls (Fig. 3). This is consistent with a previous study that found no differences in ELISA or neutralizing antibody titers between subjects who received smallpox vaccination and those who recovered from smallpox infection (35).
One potential caveat to this study is that we did not use VAR in our in vitro assays, and this could lead to an underestimate of the total antiviral immune response in subjects who contracted smallpox. We believe that this is unlikely to be an issue since we have two groups of smallpox survivors: those who contracted smallpox but were never vaccinated (the VAR group) and those who were vaccinated either before or after smallpox infection (the VAR-VAC group). This latter group is important because they represent an immunological “rosetta stone” in the sense that their antiviral T-cell and antibody responses should be focused mainly on cross-reactive epitopes that are shared between VAC and VAR. When this group was compared to their appropriate case-controls (the VAC-VAC group), we found no significant differences in the magnitude or duration of T-cell memory or humoral immune responses.
The correlate(s) of protective immunity against smallpox have not been formally proven (33). Although T cells are likely to play a role in protection, studies in nonhuman primates challenged with the closely related orthopoxvirus, monkeypox, have shown that virus-specific antibody is both necessary and sufficient for protection against lethal infection (11). These results are further supported by clinical experience during smallpox outbreaks in which administration of neutralizing antibodies can aid in preventing smallpox or reducing symptoms if administered prior to exposure (8, 22, 23, 25, 30) or can provide therapeutic benefit and protect against lethal smallpox infection if given shortly after symptom onset (8, 33). In the studies described here, VAR was not used to measure T-cell or antibody responses but, if similar to monkeypox virus (14), VAR might evade T-cell recognition and fail to stimulate T cells in vitro. This is an important question, and it will be interesting to learn whether or not VAR evades T-cell responses, since this may be one of the reasons why neutralizing antibodies play such an important role in protection against this group of viruses. Likewise, we did not use VAR antigens in our analysis of antibody responses by ELISA or neutralizing assays. Fortunately, we have a group of individuals (the VAR-VAC group) who provide a bridge between VAR-immune and VAC-immune subjects because this group of individuals either were vaccinated prior to contracting smallpox (n = 6) or were boosted with VAC after resolving smallpox infection (n = 10) and therefore would be expected to have developed cross-reactive VAC-specific antibodies that would bind VAC antigens in ELISA and neutralizing assays. Since the VAR-VAC group demonstrated antibody titers that were not significantly different from that observed in their vaccinated case-controls (the VAC-VAC group), this suggests that smallpox vaccination induces levels of immunity similar to that achieved through natural smallpox infection.
The main goal of the present study was to provide a bridging study to compare the differences in the levels and duration of humoral and cell-mediated immunity induced by smallpox infection versus smallpox vaccination. Our results were somewhat surprising because we found similar antiviral immune responses regardless of whether the subjects had been vaccinated or had contracted natural smallpox infection. The robust and long-lived immune response initiated by smallpox vaccination may be the main factor of its success in combating smallpox outbreaks and serves as an excellent model for determining the underlying mechanisms involved with maintaining long-term immunological memory to acute viral infection.
Acknowledgments
We thank each of the study subjects for the generous gift of their time and participation in this research project.
This study has been funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services contract number HHSN266200400029 (to J.M.H. and M.K.S.), R41 AI063675 (to M.K.S.), PHS grant 5 M01 RR00334, and ONPRC grant RR00163 (to M.K.S.). This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research.
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Alzhanova, D., D. M. Edwards, E. Hammarlund, I. G. Scholz, D. Horst, M. J. Wagner, C. Upton, E. J. Wiertz, M. K. Slifka, and K. Fruh. 2009. Cowpox virus inhibits the transporter associated with antigen processing to evade T-cell recognition. Cell Host Microbe 6:433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amanna, I. J., N. E. Carlson, and M. K. Slifka. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903-1915. [DOI] [PubMed] [Google Scholar]
- 3.Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320-337. [DOI] [PubMed] [Google Scholar]
- 4.Amara, R. R., P. Nigam, S. Sharma, J. Liu, and V. Bostik. 2004. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J. Virol. 78:3811-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 346:1300-1308. [DOI] [PubMed] [Google Scholar]
- 6.Byun, M., M. C. Verweij, D. J. Pickup, E. J. Wiertz, T. H. Hansen, and W. M. Yokoyama. 2009. Two mechanistically distinct immune evasion proteins of cowpox virus combine to avoid antiviral CD8 T cells. Cell Host Microbe 6:422-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combadiere, B., A. Boissonnas, G. Carcelain, E. Lefranc, A. Samri, F. Bricaire, P. Debre, and B. Autran. 2004. Distinct time effects of vaccination on long-term proliferative and IFN-γ-producing T-cell memory to smallpox in humans. J. Exp. Med. 199:1585-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzi, G., and J. P. Kircher. 1941. Immunotherapie de la variole. Bull. Inst. Hyg. Maroc 1:59-68. [Google Scholar]
- 9.Crotty, S., and R. Ahmed. 2004. Immunological memory in humans. Semin. Immunol. 16:197-203. [DOI] [PubMed] [Google Scholar]
- 10.Crotty, S., P. Felgner, H. Davies, J. Glidewell, L. Villarreal, and R. Ahmed. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969-4973. [DOI] [PubMed] [Google Scholar]
- 11.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740-747. [DOI] [PubMed] [Google Scholar]
- 12.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication, p. 1469. The pathogenesis, immunology, and pathology of smallpox and vaccinia. World Health Organization, Geneva, Switzerland.
- 13.Finkel, E. 2001. Australia: engineered mouse virus spurs bioweapon fears. Science 291:585. [DOI] [PubMed] [Google Scholar]
- 14.Hammarlund, E., A. Dasgupta, C. Pinilla, P. Norori, K. Fruh, and M. K. Slifka. 2008. Monkeypox virus evades antiviral CD4+ and CD8+ T-cell responses by suppressing cognate T-cell activation. Proc. Natl. Acad. Sci. U. S. A. 105:14567-14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarlund, E., M. W. Lewis, S. V. Carter, I. Amanna, S. G. Hansen, L. I. Strelow, S. W. Wong, P. Yoshihara, J. M. Hanifin, and M. K. Slifka. 2005. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 11:1005-1011. [DOI] [PubMed] [Google Scholar]
- 16.Hammarlund, E., M. W. Lewis, J. M. Hanifin, E. L. Simpson, N. E. Carlson, and M. K. Slifka. 2008. Traditional smallpox vaccination with reduced risk of inadvertent contact spread by administration of povidone iodine ointment. Vaccine 26:430-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 18.Hanna, W. 1913. Studies in smallpox and vaccination. William Wood & Company, New York, NY.
- 19.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283:1279-1282. [DOI] [PubMed] [Google Scholar]
- 20.Irons, J. V., T. D. Sullivan, E. B. M. Cook, G. W. Cox, and R. A. Hale. 1953. Outbreak of smallpox in the lower Rio Grande valley of Texas in 1949. Am. J. Public Health 43:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karem, K. L., M. Reynolds, C. Hughes, Z. Braden, P. Nigam, S. Crotty, J. Glidewell, R. Ahmed, R. Amara, and I. K. Damon. 2007. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 14:1318-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempe, C. H., T. O. Berge, and B. England. 1956. Hyperimmune vaccinial gamma globulin. Pediatrics 18:177-188. [PubMed] [Google Scholar]
- 23.Kempe, C. H., C. Bowles, G. Meiklejohn, T. O. Berge, L. St. Vincent, B. V. S. Babu, S. Govindarajan, N. R. Ratnakannan, A. W. Downie, and V. R. Murthy. 1961. The use of vaccinia hyperimmune gammaglobulin in the prophylaxis of smallpox. Bull. World Health Organ. 25:41-48. [PMC free article] [PubMed] [Google Scholar]
- 24.Mack, T. M. 1972. Smallpox in Europe, 1950-1971. J. Infect. Dis. 125:161-169. [DOI] [PubMed] [Google Scholar]
- 25.Marennikova, S. S. 1962. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull. World Health Organ. 27:325-330. [PMC free article] [PubMed] [Google Scholar]
- 26.Meltzer, M. I., I. Damon, J. W. LeDuc, and J. D. Millar. 2001. Modeling potential responses to smallpox as a bioterrorist weapon. Emerg. Infect. Dis. 7:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, J. D., R. G. van der Most, R. S. Akondy, J. T. Glidewell, S. Albott, D. Masopust, K. Murali-Krishna, P. L. Mahar, S. Edupuganti, S. Lalor, S. Germon, C. Del Rio, M. J. Mulligan, S. I. Staprans, J. D. Altman, M. B. Feinberg, and R. Ahmed. 2008. Human effector and memory CD8+ T-cell responses to smallpox and yellow fever vaccines. Immunity 28:710-722. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole, T., M. Mair, and T. V. Inglesby. 2002. Shining light on “dark winter”. Clin. Infect. Dis. 34:972-983. [DOI] [PubMed] [Google Scholar]
- 29.Palmquist, E. E. 1947. The 1946 smallpox experience in Seattle. Can. J. Public Health 38:213-219. [PubMed] [Google Scholar]
- 30.Peirce, E. R., F. S. Melville, A. W. Downie, and M. J. Duckworth. 1958. Antivaccinial gamma-globulin in smallpox prophylaxis. Lancet 2:635-638. [DOI] [PubMed] [Google Scholar]
- 31.Putz, M. M., I. Alberini, C. M. Midgley, I. Manini, E. Montomoli, and G. L. Smith. 2005. Prevalence of antibodies to vaccinia virus after smallpox vaccination in Italy. J. Gen. Virol. 86:2955-2960. [DOI] [PubMed] [Google Scholar]
- 32.Sivapalasingam, S., J. S. Kennedy, W. Borkowsky, F. Valentine, M. X. Zhan, P. Pazoles, A. Paolino, F. A. Ennis, and N. H. Steigbigel. 2007. Immunological memory after exposure to variola virus, monkeypox virus, and vaccinia virus. J. Infect. Dis. 195:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slifka, M. K. 2004. Immunological memory to viral infection. Curr. Opin. Immunol. 16:443-450. [DOI] [PubMed] [Google Scholar]
- 34.Smith, G. L., and G. McFadden. 2002. Smallpox: anything to declare? Nat. Rev. Immunol. 2:521-527. [DOI] [PubMed] [Google Scholar]
- 35.Taub, D. D., W. B. Ershler, M. Janowski, A. Artz, M. L. Key, J. McKelvey, D. Muller, B. Moss, L. Ferrucci, P. L. Duffey, and D. L. Longo. 2008. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am. J. Med. 121:1058-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]