Abstract
Respiratory syncytial virus (RSV) is a major cause of morbidity and mortality. Previous studies have suggested that T-cell responses may contribute to RSV immunopathology, which could be driven by dendritic cells (DCs). DCs are productively infected by RSV, and during RSV infections, there is an increase of DCs in the lungs with a decrease in the blood. Pediatric populations are particularly susceptible to severe RSV infections; however, DC responses to RSV from pediatric populations have not been examined. In this study, primary isolated DCs from cord blood and adult peripheral blood were compared after RSV infection. Transcriptional profiling and biological network analysis identified transforming growth factor beta (TGF-β) and associated signaling molecules as differentially regulated in the two age groups. TGF-β1 was decreased in RSV-infected adult-blood DCs but increased in RSV-infected cord blood DCs. Coculture of adult RSV-infected DCs with autologous T cells induced secretion of gamma interferon (IFN-γ), interleukin 12p70 (IL-12p70), IL-2, and tumor necrosis factor alpha (TNF-α). Conversely, coculture of cord RSV-infected DCs and autologous T cells induced secretion of IL-4, IL-6, IL-1β, and IL-17. Addition of purified TGF-β1 to adult DC-T-cell cocultures reduced secretion of IFN-γ, IL-12p70, IL-2, and TNF-α, while addition of a TGF-β chemical inhibitor to cord DC-T-cell cocultures increased secretion of IL-12p70. These data suggest that TGF-β acts as a major regulator of RSV DC-T-cell responses, which could contribute to immunopathology during infancy.
Human respiratory syncytial virus (RSV), an enveloped negative-strand RNA virus in the family Paramyxoviridae, is the most common viral cause of severe bronchiolitis and pneumonia in infants and young children (10). RSV mainly infects cells of the nasopharynx and lung but can also be detected in circulating blood mononuclear cells (16). Both adaptive and innate responses play a role in protecting hosts from RSV disease. Antibodies do not appear to play a major role in controlling primary infection (25). However, studies using depletion of B lymphocytes indicate that antibodies are necessary for preventing reinfection, and the level of passively acquired anti-RSV maternal antibodies appears to correlate with decreased risk of severe RSV disease (25). Additionally, T helper 2 (Th2)-biased cytokine immune responses may correlate with increased risk of severe disease (3, 21). In the BALB/c mouse model, NK cells and CD8+ T lymphocytes produce gamma interferon (IFN-γ) in response to RSV infection (33, 34). CD8+ and CD4+ T lymphocytes are essential for RSV clearance following primary infection (26). While mild disease does not require medical intervention, severe disease may require mechanical removal of secretions, humidified-oxygen treatment, or mechanical ventilation (10). Available therapies for severe RSV illness include aerosolized treatment with the nucleoside analog ribavirin and passive immunoprophylaxis with RSV neutralizing antibodies; however, by the time of presentation to the hospital with clinical illness, antiviral therapy typically is ineffective. A licensed RSV vaccine is not available (10).
Dendritic cells (DCs) are the most potent of the antigen-presenting cells (APCs) that serve as the first line of defense against invading pathogens. Human monocyte-derived (MD) and mouse bone marrow-derived (BMD) DCs can be infected productively with RSV (6, 27, 50, 53). Infection of DCs with RSV induces upregulation of the costimulatory molecules CD40, CD80, and CD86 and secretion of interleukin 6 (IL-6), IL-10, tumor necrosis factor alpha (TNF-α), IL-1β, and IL-12p70 (6, 27). Infants experiencing severe RSV respiratory disease have increased numbers of DCs in nasal washes and decreased numbers of blood DCs (20).
Neonates exhibit different adaptive immune responses than adults, which are highlighted by known functional immaturities in early immune responses to pathogens. At birth, humans exhibit immature splenic marginal zones, decreased expression of complement receptor 2 on neonatal B cells, low serum levels of the complement component C3, and absence of lymphoid follicles, follicular-dendritic cell networks, and germinal centers (1, 13, 56, 59, 60). Furthermore, neonatal naïve B cells express reduced levels of the IL-4 receptor alpha chain and exhibit reduced IL-4 signaling and elevated susceptibility to apoptosis in culture (58). Neonates also mount immature innate immune responses with cytokine production biased against Th1 responses, show low expression of major histocompatibility complex (MHC) II on APCs, and mount only modest IL-12 responses to stimuli (42). Neonatal monocytes exhibit reduced production of IFN-α and TNF-α in response to Toll-like receptor (TLR) agonist stimulation (2, 14, 15).
DCs have the potential to drive pathogenic T-cell responses during RSV infection. Because DCs from neonates exhibit functional immaturity, they may not respond to RSV infection in the same manner as adult DCs, but these differences have not been studied extensively. In the current study, we compared the responses of RSV-infected adult and cord blood DCs in detail. We found that adult and cord blood DCs differentially regulate transforming growth factor beta (TGF-β) signaling. TGF-β is a family of secreted proteins that affect both differentiation and effector functions of T lymphocytes. Coculture of RSV-infected DCs with autologous T cells identified TGF-β1 as a major regulator for RSV-induced T-cell responses. These findings shed light on the molecular and cellular basis for the differing immune responses to RSV infection between infants and adults.
MATERIALS AND METHODS
Cells and viruses.
HEp-2 cells were maintained in Opti-Mem I medium (Invitrogen) supplemented with 2% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The RSV wild-type strain A2 was expanded in HEp-2 monolayer cultures. Virus was isolated from infected cell monolayers after scraping and pelleting, three successive freeze-thaw cycles, and resuspension in fresh medium. RSV was purified further with two rounds of centrifugation at 2,500 × g for 10 min at 4°C and then filtration through a 45-μm filter. For mock infections, uninfected HEp-2 cell culture monolayers were harvested and treated as described above.
Isolation, infection, and flow cytometry of dendritic cells.
Human peripheral blood was collected with the approval of the Vanderbilt University Institutional Review Board. Sixty milliliters of peripheral blood was collected from healthy adult donors, or 10 to 25 ml blood was collected from umbilical cords. Peripheral blood mononuclear cells (PBMCs) and cord blood mononuclear cells (CBMCs) were isolated using Ficoll (Sigma; Histopaque 1077). Mixed suspensions of primary plasmacytoid and conventional DCs were enriched using Miltenyi blood dendritic cell isolation kit II (catalog no. 130-091-379) and a VarioMACS magnet, as directed by the manufacturer. Cells from each donor were divided in half and were either infected at a multiplicity of infection (MOI) of 3.0 with RSV wild-type strain A2 grown in HEp-2 cells or mock infected with the supernatant from a parallel uninfected HEp-2 cell culture monolayer. Enrichment of DCs was confirmed by flow cytometry using Lin (CD3, CD14, CD16, CD19, CD20, and CD56)-fluorescein isothiocyanate (FITC), CD11c-allophycocyanin, and CD123-phycoerythrin (PE) (BD) on a custom Becton-Dickinson LSRII cytometer. Compensation was calculated using CompBeads (BD). Flow cytometry data were analyzed using FlowJo software v7.5 (TreeStar).
Transcriptional-profile analysis.
Mock- or RSV-infected DCs were pelleted 24 h postinfection and washed once with phosphate-buffered saline (PBS), and then RNA was extracted organically with Trizol and chloroform and further purified with RNeasy (Qiagen), as directed by the manufacturer. The RNA was DNase treated (Qiagen) as directed by the manufacturer. The RNA concentration was measured with a Nanodrop spectrophotometer and was sent to the Vanderbilt Microarray Shared Resources (VMSR) core facility. The RNA integrity was analyzed by the VMSR core on an Agilent 2100 bioanalyzer. RNAs with RNA integrity numbers equal to or greater than 7.0 were used for further analysis, amplified with a Nugen amplification kit as directed by the manufacturer, and hybridized to an Affymetrix human U1.33 plus 2.0 3′ untranslated region (UTR) transcriptional-profile array. The arrays were scanned with a GeneChip Scanner 3000 7G with AutoLoader. Data from all 12 arrays were batch normalized with the robust multichip average (RMA) quartile algorithm using Expression Console software (Affymetrix). The fold change of the expression level for each gene was calculated, comparing RSV-infected to donor-matched mock-infected DCs. Lists of genes with 2-fold or more increased or decreased expression levels were compiled. Lists of genes that were commonly upregulated or downregulated within healthy adult donor DCs or cord blood DCs were compiled. The data were analyzed using Ingenuity Pathway Analysis (Ingenuity Systems).
Real-time reverse transcription-PCR.
Real-time PCR was performed on RNA prepared as described above. Copy DNA was synthesized from 50 ng of RNA using a High Capacity Reverse Transcription cDNA kit (Applied Biosystems), as directed by the manufacturer. Individual gene products were detected by real-time PCR performed on a SmartCycler II (Cepheid) using TaqMan gene expression assays and TaqMan universal PCR major mix (Applied Biosystems), as directed by the manufacturer. The expression assays that were used included GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (4352934E) and TGF-β1 (Hs00998130_m1, Hs00171257_m1, and Hs00998133_m1) (Applied Biosystems). Custom TaqMan assays were also designed for fusion (F) and attachment (G) RSV genes (Applied Biosystems Assays on Demand). Statistics were calculated using the Wilcoxon rank sum test.
DC-T-cell cocultures and cytokine analysis.
DCs were enriched from blood as described above. DCs (1 × 104 or 1 × 103 total cells) were mock infected or inoculated with RSV strain A2 at an MOI of 3 for 2 h at 37°C. Flowthrough from the DC isolation was collected, and untouched pan-T cells were isolated as directed by the manufacturer (Miltenyi; 130-091-156). T cells were resuspended at a concentration of 1 × 106 cells/ml in RPMI (Invitrogen) supplemented with 10% human AB serum (Sigma), 1% nonessential amino acids (Invitrogen), 2 mM l-glutamine (Mediatech), penicillin-streptomycin (Mediatech), and 0.1% β-mercaptoethanol (Sigma). Two hours after inoculation, the DCs were washed with PBS and overlaid with 1 × 105 autologous T cells. Human recombinant TGF-β1 was added to the culture daily where indicated at a dose of 10 ng/ml (R&D Systems). The TGF-β inhibitor SB431542 was added daily to cultures where indicated at a dose of 100 μg/ml (Sigma).
Supernatants from cocultures were collected on day 0, 1, 3, or 5 postinfection and used for 10-plex cytokine analysis with a custom Multi-Spot assay (Meso Scale Discovery). Analytes included human IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-13, IL-17, and TNF-α. The lower limits of detection for the cytokine were as follows (pg/ml): IFN-γ, 0.4; IL-1β, 0.2; IL-2, 0.7; IL-4, 0.3; IL-6, 0.9; IL-10, 0.4; IL-12p70, 2.3; IL-13, 1.8; IL-17, 0.4; and TNF-α, 0.5. Supernatants were also assayed for secretion of latent TGF-β1 with a singleplex cytokine assay (Meso Scale Discovery). Assays were performed as directed by the manufacturer and read using a Sector Imager 2400 (Meso Scale Discovery).
RESULTS
RSV-infected adult and cord blood DCs regulate TGF-β differently.
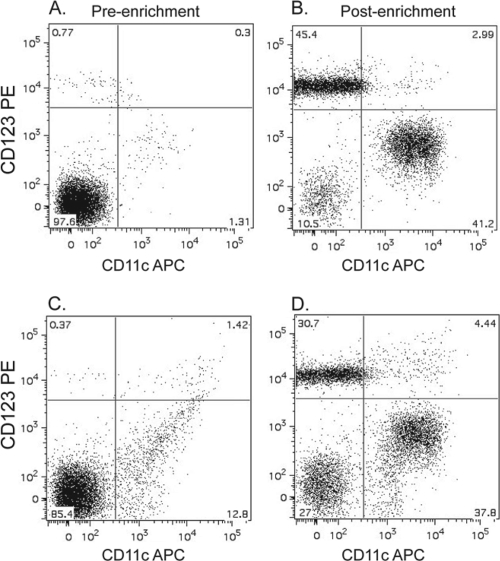
DC responses to RSV infection were examined using cells from adult PBMCs and cord blood mononuclear cells. DCs used for experiments were enriched by magnetic isolation. Enrichment was confirmed by flow cytometry. Prior to enrichment, approximately 1% of lineage-negative cells are plasmacytoid (pDCs) or conventional (cDCs) DCs (Fig. 1). After enrichment, approximately 90% of lineage-negative cells or 85% of ungated cells are DCs (Fig. 1), which confirms the effectiveness of magnetic isolation. It has previously been reported that ratios of pDCs to cDCs are approximately equivalent in adult peripheral and cord blood (61).
FIG. 1.
Plasmacytoid and conventional DCs are enriched through magnetic isolation. Mixed pools of plasmacytoid and conventional DCs were isolated by a two-step magnetic enrichment from adult PBMCs. First, B cells and monocytes were depleted with CD19 and CD14 antibody cocktail, followed by enrichment of DCs with CD304, CD141, and CD1c antibody cocktails. DC purity was confirmed by flow cytometry using FITC-conjugated lineage cocktail (CD3, CD14, CD16, CD19, CD20, and CD56), allophycocyanin-conjugated CD11c, and PE-conjugated CD123 antibodies. Shown is a representative flow cytometric data plot of FITC-negative populations of DCs prior to (A) and after (B) enrichment, as well as total populations of DCs prior to (C) and after (D) enrichment.
In order to examine if DCs from adult PBMCs and cord blood mononuclear cells respond differently to RSV, we examined transcriptional profiles using Affymetrix gene expression arrays in primary DCs after inoculation with RSV. RNA from adult and cord blood DCs that were either mock or RSV infected was hybridized to a human U1.33 plus 2.0 3′ UTR transcriptional-profile array. After data normalization, lists of genes that were increased or decreased in expression by 2-fold or more after RSV infection were compiled and categorized in four ways: (i) genes that were increased in adult DCs but not in cord DCs, (ii) genes that were decreased in adult DCs but not cord DCs, (iii) genes that were increased in cord DCs but not adult DCs, and (iv) genes that were decreased in cord DCs but not adult DCs. We analyzed these gene sets using Ingenuity Pathway Analysis software to identify biological networks that were differentially regulated in adult and cord DCs. The networks were numerically ranked by the software according to the percentage of genes present from a list within a known network. Of the top 3 networks examined from each of the four gene lists, 5 of 12 networks contained TGF-β family members and signaling partners within the TGF-β pathway. Two representative networks are shown in Fig. S1 in the supplemental material. The top-ranked network of genes that was decreased in adult DCs but not cord DCs had TGF-β1 at a major node point (see Fig. S1A in the supplemental material). The top-ranked network of genes increased in cord blood DCs but not adult DCs contained TGF-β1, the TGF-β transcription factors SMAD3 and SMAD5, and TGF-β-induced factor homeobox 1 (see Fig. S1B in the supplemental material).
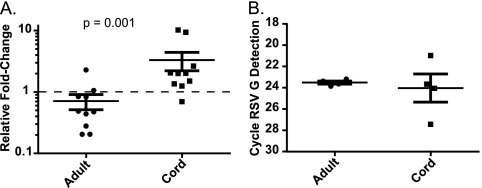
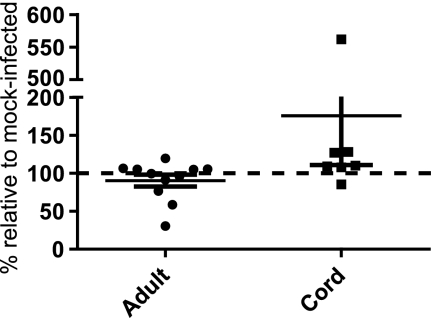
Differential regulation of the tgf-β1 gene in cord blood versus adult cells in response to RSV infection was confirmed by real-time reverse transcription-PCR. The relative fold change of TGF-β1 after RSV infection was calculated after GAPDH normalization via the 2−ΔΔCT method (43) within donor-matched pairs. TGF-β1 decreased to 0.71 relative fold change in response to RSV infection in adult DCs and increased to 3.3 relative fold-change in response to RSV infection in cord DCs (Fig. 2A). We confirmed that both cord and adult DCs were infected by detection of the RSV G gene product using RT-PCR (Fig. 2B). After GAPDH normalization, RSV G was detected at similar cycle thresholds in both adult (23.5) and cord (24.0) DCs (Fig. 2B). Changes in secretion of TGF-β1 protein were assayed by immunoassay at 24 h postinoculation. Absolute amounts of secreted TGF-β1 differed significantly from donor to donor; therefore, the relative changes in TGF-β1 were calculated as percentages of cytokine in RSV-infected DCs in comparison to mock-infected DCs within donor-matched pairs. In adult DCs after RSV inoculation, secreted TGF-β1 levels were 90% that of mock-infected DCs, (Fig. 3). In cord DCs, TGF-β1 levels were 175% that of mock-infected DCs after RSV infection (Fig. 3). On a per donor basis, we noted that when cord blood DCs responded to RSV infection, they secreted increased amounts of TGF-β. However, responses were not detected in all samples, and the responses were not statistically significant with the sample numbers that we studied. Taken together, these mRNA array, RT-PCR, and protein data all suggested that TGF-β1 is differentially regulated in response to RSV infection in cord versus adult DCs.
FIG. 2.
TGF-β1 transcript levels decreased in adult DCs after RSV infection. Adult or cord DCs were inoculated with RSV. TGF-β1, GAPDH, and RSV G transcripts were quantitated by reverse transcription and real-time PCR. (A) The relative fold change of TGF-β1 in RSV- versus mock-infected DCs in donor-matched pairs was calculated after GAPDH normalization by the 2−ΔΔCT method. The dashed line indicates a 1-fold change (i.e., no relative change). Points below the line indicate a decrease in TGF-β1, and points above the line indicate increased TGF-β1. Data points from 10 adult and 10 cord blood samples are shown. The data are plotted as the mean, and the error bars represent the standard errors of the mean. P values were calculated with a Wilcoxon rank sum test. (B) Cycle thresholds of GAPDH-normalized RSV G in RSV-infected adult or cord DCs. Data points from four adult and four cord blood samples are shown. The data are plotted as the mean, and the error bars represent the standard errors of the mean.
FIG. 3.
RSV infection of cord DCs increased secretion of TGF-β1 protein. Adult or cord blood DCs were inoculated with RSV. The supernatant concentration of TGF-β1 was determined by immunoassay. Relative amounts of TGF-β1 were calculated in donor-matched RSV-infected DCs compared to mock-infected cells. The dashed line indicates no change. Data points from 11 sets of adult DCs and 7 sets of cord blood DCs are shown. The data are plotted as the mean, and the error bars represent the standard errors of the mean.
T cells from adult and cord blood cocultured with RSV-infected DCs secrete different cytokines.
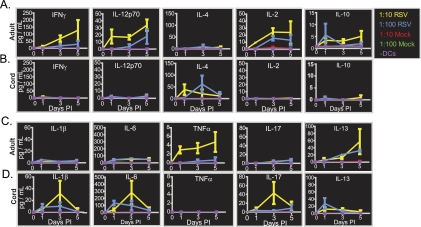
A major characteristic of TGF-β1 is the ability to affect both T-cell differentiation and T-cell effector functions. In order to test the potential biological consequences of differential TGF-β1 secretion by cord and adult DCs after RSV infection, we established a DC-T-cell coculture model. TGF-β1 could block differentiation and effector functions of Th1 and Th2 CD4+ cells and promote inducible regulatory T cells (iTregs) or Th17 cells. The ability of RSV-infected DCs to induce secretion of cytokines in donor-matched T cells was tested in a coculture model. CD3+ T cells were collected from adult or cord blood mononuclear cells and cultured with autologous mock- or RSV-infected DCs. The supernatants were tested for the presence of IFN-γ, IL-12p70, IL-4, IL-1β, IL-6, TNF-α, IL-17, IL-10, and IL-13 by immunoassay. Neither adult nor cord blood cocultures secreted the cytokines tested when cultured in the absence of DCs (Fig. 4A to D, purple line). DCs without T cells did not secrete large amounts of cytokines, suggesting cytokine secretion was primarily from DC-stimulated T cells. Specifically, DCs contributed 1.3% IFN-γ, 0.5% IL-1β, 5.0% IL-2, 4% IL-4, 8% IL-6, 30% IL-10, 2.5% IL-12p70, 3% IL-13, 3.7% IL-17, and 30% TNF-α (data not shown). Adult cocultures secreted IFN-γ, IL-12p70, IL-2, and TNF-α when cultured with RSV-infected DCs, but not with mock-infected DCs (Fig. 4A and C). Additionally, adult cocultures secreted more IFN-γ, IL-12p70, IL-2, and TNF-α when DCs and T cells were cultured at a ratio of 1:10 (Fig. 4, yellow) than when they were cultured at a ratio of 1:100 (Fig. 4, blue), indicating that cytokine secretion depended on the number of RSV-infected DCs (Fig. 4A and C). In marked contrast, cocultures from cord blood did not secrete IFN-γ, IL-12p70, IL-2, or TNF-α, but rather secreted IL-4, IL-1β, IL-6, and IL-17 (Fig. 4B and D). Secretion of IL-1β, IL-6, and IL-17 depended on the number of RSV-infected DCs, while equal levels of IL-4 were secreted when cultures were performed at ratios of 1:10 or 1:100 (Fig. 4B and D). IL-4 secretion was delayed approximately 2 days when DCs and T cells were cultured at ratios of 1:100, indicating that RSV-infected DCs may control the kinetics of IL-4 secretion in cord blood T cells. By day 5, cytokine secretion in adult cocultures increased, whereas secretion in cord cocultures decreased. This phenomenon may reflect the absence of IL-2 secretion in cord cocultures, and by day 5, cord T cells may not have been proliferating. Some (5 pg/ml) IL-10 was secreted in adult DC-T-cell cocultures, but this finding did not depend on the number of RSV-infected DCs (Fig. 4A). In cord blood cocultures, IL-10 was not detected (Fig. 4B). Up to 50 pg/ml of IL-13 was detected in both adult and cord DC-T-cell cocultures; however, similar levels were secreted in both sets of cultures, and detection did not depend on the number of DCs (Fig. 4C and D). It should be noted that cord blood DCs were cocultured with cord T cells and adult DCs with adult T cells, introducing a potential experimental difference in T cells that was independent of the DC response. Although studying cord versus adult DCs on a common panel of T cells would be desirable, such a setup is not feasible. Alloreactivity in the MHC locus would lead to high T-cell activation in this setting, confounding the study of the effect of the DC phenotype on the response to RSV.
FIG. 4.
RSV-infected DCs induced cytokine secretion in adult and cord DCs. Cytokines secreted in DC-T-cell cocultures were assayed in a 5-day time course. Constant numbers of T cells were cocultured with various numbers of donor-matched DCs at ratios of 1:10 DCs to T cells (red and yellow) or 1:100 DCs to T cells (green and blue) or without DCs (purple). Prior to the addition of T cells to the culture, DCs were mock infected (red and green) or infected with RSV at an MOI of 3.0 (yellow and blue). IFN-γ, IL-12p70, IL-4, IL-2, and IL-10 were assayed in adult (A) or cord (B) blood cocultures. IL-1β, IL-6, TNF-α, IL-17, and IL-13 were also assayed in adult (C) or cord (D) blood cocultures. The data shown are from a representative experiment with three different sets of donor-matched pairs from adult peripheral blood and three different sets of donor-matched pairs from cord blood. The data are plotted as the mean, and the error bars represent the standard errors of the mean. PI, postinfection.
In summary, RSV infection induced mutually exclusive T-cell responses in adult versus cord blood DCs. During RSV infection, adult DCs, but not cord blood DCs, support secretion of the prototypical Th1 cytokines IFN-γ, IL-12p70, and IL-2, which are necessary for efficient T-cell proliferation, and proinflammatory TNF-α. In contrast, RSV-infected cord blood DCs support secretion of the proinflammatory IL-6 and IL-1β cytokines, the Th2 cytokine IL-4, and the Th17 cytokine IL-17. The T cells used for these assays included CD4+ and CD8+ T cells. Previously published data have indicated that ratios of CD4+ to CD8+ T cells are approximately equal in cord and adult blood (1.2 to 1.7) (12, 17, 28). Therefore, cytokine secretion differences are not likely due to differences in CD4+/CD8+ ratios. However, approximately 85% of CD4+ cells in cord blood are naïve compared to 40 to 50% in adult blood (12, 17, 28). It is possible that both secretion of TGF-β1 and the differences in naïve-T-cell percentages contribute to the cytokine responses.
Manipulation of TGF-β1 in DC-T-cell cocultures changes the T-cell cytokine secretion profile.
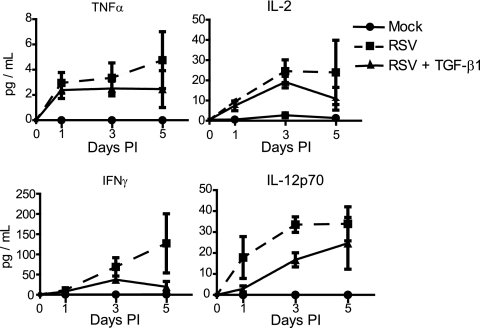
In order to determine if differential regulation of TGF-β1 contributed to the mutually exclusive cytokine secretion profiles of adult and cord blood DC-T-cell cocultures, adult DCs and CD3+ T cells were cultured in the presence of human recombinant TGF-β1. Addition of TGF-β1 reduced secretion of IFN-γ, TNF-α, and IL-2 (Fig. 5). Secretion of IL-12p70 was also decreased transiently by the addition of purified TGF-β1 on day 3 postinoculation; however, by day 5 postinoculation, IL-12p70 was equivalent to that in T cells cocultured with RSV-infected DCs in the absence of exogenous TGF-β1 (Fig. 5). These data are consistent with the role of TGF-β1 in control of DC-induced T-cell secretion of cytokines.
FIG. 5.
Secretion of TNF-α, IL-2, IFN-γ, and IL-12p70 is reduced by addition of purified TGF-β1 to adult DC-T-cell cocultures. DCs and T cells from cord blood were cocultured at a ratio of 1:10 in the presence or absence of purified TGF-β1. Prior to addition of T cells, the DCs were either mock infected or infected with RSV. Secretion of TNF-α (top left), IL-2 (top right), IFN-γ (bottom left), or IL-12p70 (bottom right) was quantitated by immunoassay in a 5-day time course. The data shown are from a representative experiment with three different sets of donor-matched pairs from adult peripheral blood. The data are plotted as the mean, and the error bars represent the standard errors of the mean.
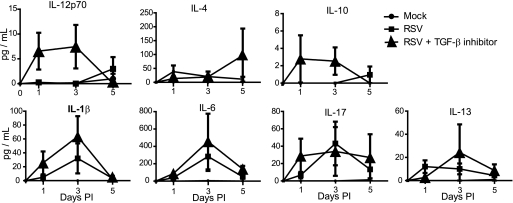
In cord cell cocultures, IL-12p70 increased in response to SB431542, a TGF-β inhibitor (Fig. 6). SB431542 blocks activation of the TGF-β signal cascade through type I receptors by specifically blocking the kinase activity of activin receptor-like kinases without blocking other kinases, such as jun-N-terminal kinase (JNK), extracellular signal-related kinase (ERK), or mitogen-activated protein kinases (MAPK) (35). SB431542 would act on any cells that have TGF-β receptors, which includes both CD4+ and CD8+ T cells. In response to inhibitor, IL-10, IL-1β, and IL-6 modestly increased, though the error bars overlap (Fig. 6). IL-4 and IL-13 also increased in response to inhibitor, though at only one time point (Fig. 6). IL-17 did not change in response to inhibitor (Fig. 6). These data suggest that a block in TGF-β1 controls IL-12 secretion, though not necessarily secretion of IL-4, IL-1β, IL-6, or IL-17 directly.
FIG. 6.
Secretion of IL-12p70 is increased by addition of a TGF-β inhibitor to cord blood DC-T-cell cocultures. DCs and T cells from cord blood were cocultured at a ratio of 1:10 in the presence or absence of the TGF-β inhibitor SB431542. Prior to the addition of T cells, the DCs were either mock infected or infected with RSV. Secretion of IL-12p70, IL-4, IL-10, IL-1β, IL-6, IL-17, and IL-13 was quantitated by enzyme-linked immunosorbent assay (ELISA) in a 5-day time course. The data shown are from a representative experiment with three different sets of donor-matched pairs from cord blood. The data are plotted as the mean, and the error bars represent the standard errors of the mean.
Manipulation of TGF-β1, either through addition of recombinant protein in adult cocultures or through inhibition of TGF-β in cord cocultures, changed the cytokine secretion profiles of both adult and cord blood cocultures after RSV infection. These data confirmed that TGF-β1 is a regulator of the observed developmentally controlled differences in responses of adult and neonatal DCs and specifically appears to affect skewing of the Th1-type response. Notably, inhibition of TGF-β signaling through use of a chemical inhibitor increased IL-12, but not IL-1β or IL-6, in cord cocultures, suggesting that IL-1β and IL-6 may be regulated by an independent mechanism. A possible contributor to differential IL-1β and IL-6 secretion may be the abundance of naïve T cells in cord blood. The observation that we could manipulate the adult DC-T-cell responses away from a cord-like response suggests that the TGF-β pathway could serve as a major therapeutic target for prevention or treatment of neonatal RSV disease.
DISCUSSION
The molecular and cellular basis for enhanced RSV disease severity in infants is incompletely understood. The current study suggests that altered control of TGF-β expression in neonatal DCs may be a critical regulatory event that affects the T-cell response and disease expression. Prior to these studies, the dominant RSV pathogenesis model had been that infants may exhibit a Th2-biased response that is associated with more severe disease. Studies in mouse models suggest that Th2-biased responses promote enhanced disease severity (3). Vaccine studies in infants and children in the 1960s using formalin-inactivated RSV led to enhanced disease upon subsequent natural infection. Infection of mice with formalin-inactivated RSV induces Th2-biased CD4+ responses (26). In humans, genetic studies have identified small nucleotide gene polymorphisms (SNPs) that correlate with susceptibility to severe infection, including IL-4 and the IL-4 receptor, which could affect the Th1/Th2 balance (9, 32, 47). In our studies, cord blood DCs stimulated secretion of IL-4 in cocultures, suggesting that they may support a Th2-biased response.
Infants are more susceptible to severe RSV infection, and neonatal DCs mount immature innate immune responses with cytokine production biased against Th1 responses and associated with modest levels of IL-12 (15). Furthermore, neonatal monocytes exhibit reduced production of IFN-α and TNF-α in response to TLR agonist stimulation (2, 14, 15). In the present study, we hypothesized that DCs from adult or cord blood would respond differently to RSV infection and drive different T helper responses that could affect the severity of RSV disease. Using biological-pathway analysis, we identified TGF-β as the most important factor that is regulated differently in DCs from adults versus those from infants in response to RSV infection.
A hallmark of the function of TGF-β is the ability to affect both differentiation and effector functions of T lymphocytes. TGF-β blocks T-cell proliferation in vitro (38). TGF-β also blocks differentiation of naïve CD4+ into Th1 and Th2 cells through downregulation of the essential transcription factors T-bet and GATA-3 (22, 23, 31, 46). Mice with attenuated TGF-β signaling have CD4+ T cells that spontaneously differentiate into Th1 and Th2 cells (24). The downregulation of TGF-β1 in adult DCs that we observed in response to RSV infection may allow the differentiation of Th1 and Th2 cells to mount the efficient adaptive immune response that is characteristic of adults.
In contrast to the effect on Th1 and Th2 cells, secretion of TGF-β promotes differentiation of iTregs and Th17 cells. Secreted alone, TGF-β promotes differentiation of iTregs, which are suppressive. However, in the presence of IL-6, TGF-β promotes differentiation of immunopathological Th17 cells, which secrete IL-17A, IL-17F, and IL-23 (5, 11). Alone, IL-6 and TGF-β induce a Th17 subtype that produces IL-10 and suppresses Th17-associated immunopathology (45). However, IL-10-producing Th17 cells can be driven to become pathogenic Th17 cells when IL-6 is secreted in combination with either IL-23 or IL-1β (41, 45). Notably, in our studies, RSV-infected cord blood cocultures secreted IL-17, IL-6, and IL-1β but did not secrete IL-10. Our data suggest that cord DCs, but not adult DCs, may support differentiation of pathogenic Th17 cells. In humans, TGF-β appears to promote differentiation of Th17 cells through a blockade in the Th1 differentiation process (52). Our data are consistent with this notion in that use of a TGF-β inhibitor in cord cocultures did not affect IL-1β or IL-6 but did increase secretion of IL-12, which is necessary for Th1 differentiation. Such a Th17-biased response could contribute to the severity of RSV disease through T-cell-mediated immunopathology.
Th17 cells and TGF-β have been implicated in airway inflammation. IL-17A and TGF-β are expressed in the airways of patients with asthma (8, 48). Furthermore, in a mouse model, secretion of IL-17 correlates with neutrophil influx in the lung and bronchial hyperreactivity (30). Immunohistochemical examination of bronchiolar and alveolar tissues from infants with severe RSV disease showed large numbers of neutrophils, suggesting that neutrophilic infiltrate occurs in the lungs of ill infants (64). Notably, both TGF-β and IL-17A function as neutrophil chemoattractants (7, 49). These data suggest that overexpression of TGF-β or IL-17 may promote infiltration of neutrophils, which may be involved in the development of severe RSV airway disease. Furthermore, an SNP in the receptor for TGF-β was found to be protective against severe RSV infection in preterm infants (55).
The data presented in this paper are consistent with those in a previous study that determined that neonatal monocytes are deficient in the ability to produce IL-12p70 and to induce IFN-γ (39). Additionally, in our study, cord blood DCs failed to support secretion of IL-2 and proinflammatory TNF-α in DC-T-cell cocultures. TNF-α can promote viral clearance through enhanced internalization of complement-coated viral particles by neutrophils (62). The reduced ability of infants to secrete TNF-α in response to RSV infection may result in decreased viral clearance.
TGF-β is translated as an inactive proprotein that is embedded in the plasma membrane. The latent cytokine can be activated by numerous stimuli, including heat or a change in pH, or directly by integrin stimulation. Once latent TGF-β is activated, it induces phosphorylation, activation, and nuclear translocation of SMAD transcription factors, which induce transcription of more TGF-β, as well as TGF-β-induced genes (36). Other viruses induce activation and secretion of TGF-β. The influenza virus protein neuraminidase directly activates latent TGF-β (54), and reovirus and hepatitis C activate TGF-β signaling (4, 29). It has recently been reported that RSV infection of epithelial cells induces secretion of TGF-β1 and that treatment of RSV-infected cultured cells with TGF-β enhances viral replication (19, 44). Additionally, TGF-β treatment of cells also enhances rhinovirus replication (57). Therefore, enhanced secretion of TGF-β may contribute to higher levels of viral replication in neonates during infection. However, this mechanism would be most likely operating in trans, as our data indicate that within DCs, RSV produces equivalent gene products in cord and adult DCs.
Considering these data as a whole, RSV infection of neonatal human DCs appears to activate TGF-β signaling pathways. Differential regulation of TGF-β in cord and adult DCs may involve a mechanism of activation of latent TGF-β, with secondary inhibition of TGF-β signaling in adult DCs but not cord DCs. Activation of JNK blocks transcription of TGF-β (63). RSV F protein directly stimulates TLR4, which can activate JNK upon ligand binding (40). Expression of TLR4, the TLR adapter molecule MyD88, and several members of the tumor necrosis factor receptor (TNFR) family of proteins is decreased in preterm neonates and increases with gestational age (18, 37, 51). If cord blood DCs express lower levels of TLR4, they may fail to activate JNK and induce a secondary negative signal to turn off activation of TGF-β. What may result is direct activation of TGF-β by RSV in both adult and cord DCs and then a secondary inhibitory signal in adult DCs but not cord DCs.
In conclusion, we have identified TGF-β1 as a major regulator of the altered nature of neonatal DC-induced T-cell responses during RSV infection. Due to its potent effects on Th1/Th2 differentiation, induction of inflammatory Th17 cells, chemoattraction of neutrophils, and enhancement of viral replication, TGF-β secretion has the potential to determine the outcome of infection and may be a major mediator of disease severity in infants and young children. The pharmacologic reversion of this skewing in neonatal cells to an adult phenotype by blocking activation of the TGF-β signaling cascade suggests the potential for development of new therapeutic strategies for RSV disease. Furthermore, researchers developing experimental RSV vaccine strategies for use in neonates should consider the effects of novel antigens and immunomodulators, such as adjuvants, on the induction of TGF-β pathways in neonatal antigen-presenting cells.
Supplementary Material
Acknowledgments
We gratefully acknowledge Mark Hicar and Bernardo Mainou for critical reviews of the manuscript.
Flow cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The VMC Flow Cytometry Core Laboratory is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). All microarray experiments were performed in the Vanderbilt Microarray Shared Resource. The Vanderbilt Microarray Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Digestive Disease Center (P30 DK58404), and the Vanderbilt Vision Center (P30 EY08126). N.J.T. was supprted by T32 HL69765 and F32 AI080117. J.E.C. was supprted by a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
Footnotes
Published ahead of print on 6 October 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 2.Angelone, D. F., M. R. Wessels, M. Coughlin, E. E. Suter, P. Valentini, L. A. Kalish, and O. Levy. 2006. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 60:205-209. [DOI] [PubMed] [Google Scholar]
- 3.Aung, S., Y. W. Tang, and B. S. Graham. 1999. Interleukin-4 diminishes CD8(+) respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J. Virol. 73:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckham, J. D., K. Tuttle, and K. L. Tyler. 2009. Reovirus activates transforming growth factor beta and bone morphogenetic protein signaling pathways in the central nervous system that contribute to neuronal survival following infection. J. Virol. 83:5035-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 6.Boogaard, I., M. van Oosten, L. S. van Rijt, F. Muskens, T. G. Kimman, B. N. Lambrecht, and A. M. Buisman. 2007. Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells. Immunology 122:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes, M. E., J. B. Allen, Y. Ogawa, and S. M. Wahl. 1991. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J. Clin. Invest. 87:1108-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakir, J., J. Shannon, S. Molet, M. Fukakusa, J. Elias, M. Laviolette, L. P. Boulet, and Q. Hamid. 2003. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 111:1293-1298. [DOI] [PubMed] [Google Scholar]
- 9.Choi, E. H., H. J. Lee, T. Yoo, and S. J. Chanock. 2002. A common haplotype of interleukin-4 gene IL4 is associated with severe respiratory syncytial virus disease in Korean children. J. Infect. Dis. 186:1207-1211. [DOI] [PubMed] [Google Scholar]
- 10.Collins, P. L., and J. E. Crowe. 2007. Respiratory syncytial virus and metapneumovirus, p. 1601-1646. In D. M. Knipe (ed.), Fields virology, 5th ed., vol. II. Lippincott Williams & Wilkins, Philadelphia PA. [Google Scholar]
- 11.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 12.D'Arena, G., P. Musto, N. Cascavilla, G. Di Giorgio, S. Fusilli, F. Zendoli, and M. Carotenuto. 1998. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 83:197-203. [PubMed] [Google Scholar]
- 13.Davis, C. A., E. H. Vallota, and J. Forristal. 1979. Serum complement levels in infancy: age related changes. Pediatr. Res. 13:1043-1046. [DOI] [PubMed] [Google Scholar]
- 14.De Wit, D., V. Olislagers, S. Goriely, F. Vermeulen, H. Wagner, M. Goldman, and F. Willems. 2004. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103:1030-1032. [DOI] [PubMed] [Google Scholar]
- 15.De Wit, D., S. Tonon, V. Olislagers, S. Goriely, M. Boutriaux, M. Goldman, and F. Willems. 2003. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 21:277-281. [DOI] [PubMed] [Google Scholar]
- 16.Domurat, F., N. J. Roberts, Jr., E. E. Walsh, and R. Dagan. 1985. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J. Infect. Dis. 152:895-902. [DOI] [PubMed] [Google Scholar]
- 17.Erkeller-Yuksel, F. M., V. Deneys, B. Yuksel, I. Hannet, F. Hulstaert, C. Hamilton, H. Mackinnon, L. T. Stokes, V. Munhyeshuli, F. Vanlangendonck, et al. 1992. Age-related changes in human blood lymphocyte subpopulations. J. Pediatr. 120:216-222. [DOI] [PubMed] [Google Scholar]
- 18.Förster-Waldl, E., K. Sadeghi, D. Tamandl, B. Gerhold, U. Hallwirth, K. Rohrmeister, M. Hayde, A. R. Prusa, K. Herkner, G. Boltz-Nitulescu, A. Pollak, and A. Spittler. 2005. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr. Res. 58:121-124. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs, J. D., D. M. Ornoff, H. A. Igo, J. Y. Zeng, and F. Imani. 2009. Cell cycle arrest by transforming growth factor {beta}1 enhances replication of respiratory syncytial virus in lung epithelial cells. J. Virol. 83:12424-12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill, M. A., A. K. Palucka, T. Barton, F. Ghaffar, H. Jafri, J. Banchereau, and O. Ramilo. 2005. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 191:1105-1115. [DOI] [PubMed] [Google Scholar]
- 21.Glezen, W. P., A. Paredes, J. E. Allison, L. H. Taber, and A. L. Frank. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98:708-715. [DOI] [PubMed] [Google Scholar]
- 22.Gorelik, L., S. Constant, and R. A. Flavell. 2002. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 195:1499-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorelik, L., P. E. Fields, and R. A. Flavell. 2000. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165:4773-4777. [DOI] [PubMed] [Google Scholar]
- 24.Gorelik, L., and R. A. Flavell. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171-181. [DOI] [PubMed] [Google Scholar]
- 25.Graham, B. S., L. A. Bunton, J. Rowland, P. F. Wright, and D. T. Karzon. 1991. Respiratory syncytial virus infection in anti-mu-treated mice. J. Virol. 65:4936-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero-Plata, A., A. Casola, G. Suarez, X. Yu, L. Spetch, M. E. Peeples, and R. P. Garofalo. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, D. T., M. J. Schumacher, J. Locascio, F. J. Besencon, G. B. Olson, D. DeLuca, L. Shenker, J. Bard, and E. A. Boyse. 1992. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 89:10006-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan, M., D. Selimovic, H. Ghozlan, and O. Abdel-kader. 2009. Hepatitis C virus core protein triggers hepatic angiogenesis by a mechanism including multiple pathways. Hepatology 49:1469-1482. [DOI] [PubMed] [Google Scholar]
- 30.He, R., M. K. Oyoshi, H. Jin, and R. S. Geha. 2007. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc. Natl. Acad. Sci. U. S. A. 104:15817-15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath, V. L., E. E. Murphy, C. Crain, M. G. Tomlinson, and A. O'Garra. 2000. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 30:2639-2649. [DOI] [PubMed] [Google Scholar]
- 32.Hoebee, B., E. Rietveld, L. Bont, M. Oosten, H. M. Hodemaekers, N. J. Nagelkerke, H. J. Neijens, J. L. Kimpen, and T. G. Kimman. 2003. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J. Infect. Dis. 187:2-11. [DOI] [PubMed] [Google Scholar]
- 33.Hussell, T., C. J. Baldwin, A. O'Garra, and P. J. Openshaw. 1997. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur. J. Immunol. 27:3341-3349. [DOI] [PubMed] [Google Scholar]
- 34.Hussell, T., and P. J. Openshaw. 1998. Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 79:2593-2601. [DOI] [PubMed] [Google Scholar]
- 35.Inman, G. J., F. J. Nicolas, J. F. Callahan, J. D. Harling, L. M. Gaster, A. D. Reith, N. J. Laping, and C. S. Hill. 2002. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62:65-74. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins, G. 2008. The role of proteases in transforming growth factor-beta activation. Int. J. Biochem. Cell Biol. 40:1068-1078. [DOI] [PubMed] [Google Scholar]
- 37.Kaur, K., S. Chowdhury, N. S. Greenspan, and J. R. Schreiber. 2007. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood 110:2948-2954. [DOI] [PubMed] [Google Scholar]
- 38.Kehrl, J. H., L. M. Wakefield, A. B. Roberts, S. Jakowlew, M. Alvarez-Mon, R. Derynck, M. B. Sporn, and A. S. Fauci. 1986. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J. Exp. Med. 163:1037-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan, S., M. Craven, R. C. Welliver, N. Ahmad, and M. Halonen. 2003. Differences in participation of innate and adaptive immunity to respiratory syncytial virus in adults and neonates. J. Infect. Dis. 188:433-439. [DOI] [PubMed] [Google Scholar]
- 40.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 41.Laurence, A., and J. J. O'Shea. 2007. T(H)-17 differentiation: of mice and men. Nat. Immunol. 8:903-905. [DOI] [PubMed] [Google Scholar]
- 42.Levy, O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379-390. [DOI] [PubMed] [Google Scholar]
- 43.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 44.McCann, K. L., and F. Imani. 2007. Transforming growth factor beta enhances respiratory syncytial virus replication and tumor necrosis factor alpha induction in human epithelial cells. J. Virol. 81:2880-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGeachy, M. J., K. S. Bak-Jensen, Y. Chen, C. M. Tato, W. Blumenschein, T. McClanahan, and D. J. Cua. 2007. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8:1390-1397. [DOI] [PubMed] [Google Scholar]
- 46.Neurath, M. F., B. Weigmann, S. Finotto, J. Glickman, E. Nieuwenhuis, H. Iijima, A. Mizoguchi, E. Mizoguchi, J. Mudter, P. R. Galle, A. Bhan, F. Autschbach, B. M. Sullivan, S. J. Szabo, L. H. Glimcher, and R. S. Blumberg. 2002. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 195:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puthothu, B., M. Krueger, J. Forster, and A. Heinzmann. 2006. Association between severe respiratory syncytial virus infection and IL13/IL4 haplotypes. J. Infect. Dis. 193:438-441. [DOI] [PubMed] [Google Scholar]
- 48.Redington, A. E., J. Madden, A. J. Frew, R. Djukanovic, W. R. Roche, S. T. Holgate, and P. H. Howarth. 1997. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am. J. Respir. Crit. Care Med. 156:642-647. [DOI] [PubMed] [Google Scholar]
- 49.Reibman, J., S. Meixler, T. C. Lee, L. I. Gold, B. N. Cronstein, K. A. Haines, S. L. Kolasinski, and G. Weissmann. 1991. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc. Natl. Acad. Sci. U. S. A. 88:6805-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudd, B. D., M. A. Schaller, J. J. Smit, S. L. Kunkel, R. Neupane, L. Kelley, A. A. Berlin, and N. W. Lukacs. 2007. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 178:5820-5827. [DOI] [PubMed] [Google Scholar]
- 51.Sadeghi, K., A. Berger, M. Langgartner, A. R. Prusa, M. Hayde, K. Herkner, A. Pollak, A. Spittler, and E. Forster-Waldl. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195:296-302. [DOI] [PubMed] [Google Scholar]
- 52.Santarlasci, V., L. Maggi, M. Capone, F. Frosali, V. Querci, R. De Palma, F. Liotta, L. Cosmi, E. Maggi, S. Romagnani, and F. Annunziato. 2009. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur. J. Immunol. 39:207-215. [DOI] [PubMed] [Google Scholar]
- 53.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz-Cherry, S., and V. S. Hinshaw. 1996. Influenza virus neuraminidase activates latent transforming growth factor beta. J. Virol. 70:8624-8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siezen, C. L., L. Bont, H. M. Hodemaekers, M. J. Ermers, G. Doornbos, R. Van't Slot, C. Wijmenga, H. C. Houwelingen, J. L. Kimpen, T. G. Kimman, B. Hoebee, and R. Janssen. 2009. Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes. Pediatr. Infect. Dis. J. 28:333-335. [DOI] [PubMed] [Google Scholar]
- 56.Tasker, L., and S. Marshall-Clarke. 2003. Functional responses of human neonatal B lymphocytes to antigen receptor cross-linking and CpG DNA. Clin. Exp. Immunol. 134:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas, B. J., M. Lindsay, H. Dagher, N. J. Freezer, D. Li, R. Ghildyal, and P. G. Bardin. 2009. Transforming growth factor-beta enhances rhinovirus infection by diminishing early innate responses. Am. J. Respir. Cell Mol. Biol. 41:339-347. [DOI] [PubMed] [Google Scholar]
- 58.Tian, C., G. K. Kron, K. M. Dischert, J. N. Higginbotham, and J. E. Crowe, Jr. 2006. Low expression of the interleukin (IL)-4 receptor alpha chain and reduced signalling via the IL-4 receptor complex in human neonatal B cells. Immunology 119:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Timens, W., A. Boes, T. Rozeboom-Uiterwijk, and S. Poppema. 1989. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J. Immunol. 143:3200-3206. [PubMed] [Google Scholar]
- 60.Timens, W., T. Rozeboom, and S. Poppema. 1987. Fetal and neonatal development of human spleen: an immunohistological study. Immunology 60:603-609. [PMC free article] [PubMed] [Google Scholar]
- 61.Ueda, Y., M. Hagihara, A. Okamoto, A. Higuchi, A. Tanabe, K. Hirabayashi, S. Izumi, T. Makino, S. Kato, and T. Hotta. 2003. Frequencies of dendritic cells (myeloid DC and plasmacytoid DC) and their ratio reduced in pregnant women: comparison with umbilical cord blood and normal healthy adults. Hum. Immunol. 64:1144-1151. [DOI] [PubMed] [Google Scholar]
- 62.van Strijp, J. A., M. E. van der Tol, L. A. Miltenburg, K. P. van Kessel, and J. Verhoef. 1991. Tumour necrosis factor triggers granulocytes to internalize complement-coated virus particles. Immunology 73:77-82. [PMC free article] [PubMed] [Google Scholar]
- 63.Ventura, J. J., N. J. Kennedy, R. A. Flavell, and R. J. Davis. 2004. JNK regulates autocrine expression of TGF-beta1. Mol. Cell 15:269-278. [DOI] [PubMed] [Google Scholar]
- 64.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.