Abstract
Wnt signaling activates at least three different pathways involved in development and disease. Interactions of secreted ligands and inhibitors with cell-surface receptors result in the activation or regulation of particular downstream intracellular cascades. During the developmental stages of otic vesicle closure and beginning morphogenesis, the forming inner ear transcribes a plethora of Wnt-related genes. We report expression of 23 genes out of 25 tested in situ hybridization probes on tissue serial sections. Sensory primordia and Frizzled gene expression share domains, with Fzd1 being a continuous marker. Prospective nonsensory domains express Wnts, whose transcripts mainly flank prosensory regions. Finally, Wnt inhibitor domains are superimposed over both prosensory and nonsensory otic regions. Three Wnt antagonists, Dkk1, SFRP2, and Frzb are prominent. Their gene expression patterns partly overlap and change over time, which adds to the diversity of molecular micro-environments. Strikingly, prosensory domains express Wnts transiently. This includes (1) the prosensory otic region of high proliferation, neuroblast delamination, and programmed cell death at stage 20/21 (Wnt3, -5b, -7b, -8b, -9a, -11), and (2) sensory primordia at stage 25 (Wnt7a, Wnt9a). In summary, robust Wnt-related gene expression shows both spatial and temporal tuning during inner ear development as the otic vesicle initiates morphogenesis and prosensory cell fate determination.
Keywords: inner ear, otic vesicle, hearing, vestibular, sensory primordia, Wnts, Fzd, Wnt-antagonists
Introduction
Wnt proteins are a large family of secreted factors that have been linked with various developmental processes such as boundary formation, convergence-extension during gastrulation, neural tube closure, cell proliferation, axial polarity of the whole embryo as well as specific organs, cell fate specification, planar cell polarity and axon guidance. Wnts can transmit a signal through at least three intracellular signaling pathways. The canonical pathway exercises control over the stabilization versus degradation of β-catenin, which can act as a transcriptional regulator when present in the nucleus (Dale, 1998). The second pathway influences the release of intracellular calcium (Slusarski et al., 1997; Kohn and Moon, 2005), while the third involves activation of RhoA and has been linked to planar cell polarity (PCP) (Mlodzik, 2002).
The choice of intracellular pathway depends, in part, on the developmental context in which the Wnts act, including receptor availability, relative levels of Wnt inhibitors, and availability of intracellular signaling components (Moon et al., 1997; Cadigan and Nusse, 1997; Miller et al., 1999; Jones and Jomary, 2002; Hendrickx and Leyns, 2008). For example, it was shown that Wnt5a can signal either through the canonical pathway via Fzd4 and its LRP5 co-receptor or through a calcium-independent non-canonical pathway via Ror2 receptor tyrosine kinase; furthermore these two signaling pathways act antagonistically on the readout of a downstream β-catenin-responsive reporter gene (Mikels and Nusse, 2006).
Wnts and Frizzleds are known to regulate several aspects of inner ear development at both early and late stages. At the onset of ear formation, Wnts are implicated in otic induction in chicken (Ladher et al., 2000) and Xenopus (Park and Saint-Jeannet, 2008). However, in zebrafish, interference with canonical Wnt signaling does not prevent otic formation (Phillips et al., 2004). Canonical Wnt signal acts instructively to determine the size of the otic placode (Ohyama et al., 2006; Jayasena et al., 2008) and this may explain the size reduction of zebrafish otic vesicles observed after overexpression of the Wnt antagonist, Dkk1 (Phillips et al., 2004). Based on Dkk1-mediated Wnt inhibition in chickens, Freter et al. (2008) interpret Wnt signaling as permissive and important during otic commitment rather than for initial otic induction. Misexpression of Wnt4, which is normally restricted to the ventral otocyst in frogs, leads to brain defects and lateral displacement of the otocyst in zebrafish and Xenopus (Ungar et al., 1995). Interference with β-catenin mRNA levels by antisense oligos reduces cell proliferation and the number of otic ganglion neurons in the chicken inner ear (Matsuda and Keino, 2000). Data from our lab show that Wnt/β-catenin signaling can influence the decision between auditory and vestibular sensory fate (Stevens et al., 2003), although it is still unknown whether there is an endogenous Wnt ligand with a comparable function. Finally, Frizzleds are implicated in the establishment of cochlear hair cell polarity (Wang et al., 2006). Knockout of Fzd4 causes degeneration of the organ of Corti and hearing loss in mice (Wang et al., 2001; Xu et al., 2004).
Gene expression studies further implicate Wnt signaling at several time points of otic development, although a full spatial and temporal survey has yet to be reported for the stages under consideration here (from otic vesicle closure through early morphogenesis). Anecdotal findings include asymmetric expression of Wnt2b, Wnt3a and Wnt6 in the otic vesicle of different vertebrate embryos (Parr et al., 1993; Zakin et al., 1998; Grove et al., 1998; Hollyday et al., 1995; Lillevali et al., 2006). Chicken Frizzled genes are expressed non-uniformly in the otic cup (Stark et al., 2000) and show partial overlap with a sensory marker, Serrate-1 (Lewis and Davies, 2002; Stevens et al., 2003). A Wnt and Frizzled gene expression survey in mice using Optical Projection Tomography shows several genes in the otic vesicle (Summerhurst et al., 2008). Finally, we reported expression of 22 genes in the Wnt pathway in the developing ventral ear and cochlear duct of the chicken (Sienknecht and Fekete, 2008), with a time course that begins approximately when the period discussed in this paper leaves off.
Several key morphogenetic events take place between otic cup closure and overt cochlear duct differentiation; some of these are accompanied by programmed cell death. Outgrowth of the endolymphatic duct from the dorsal otocyst begins almost immediately after the ventrolateral edge of the otic cup moves upward to meet the dorsal rim and fuses with it to separate the otic tissue from the surface ectoderm (Brigande et al., 2000). Eventually the endolymphatic duct enlarges at its most dorsal tip to form the endolymphatic sac. Formation of the endolymphatic duct is extraordinarily robust; in some cases it is virtually the only structure remaining in severely dysmorphic phenotypes resulting from gene knockouts such as Six1 (Ozaki et al., 2004). The specification of the mouse dorsal otocyst, including the endolymphatic duct, has been shown to be controlled by Wnt/β-catenin signaling via Wnt1 and Wnt3a expressed and presumably released from the dorsal hindbrain (Riccomagno et al., 2005).
Ventrally, the pars inferior will extend to create the cochlear duct. Outgrowth and/or specification of the cochlear duct can be regulated by Sonic hedgehog signaling emanating from the ventral midline (Bok et al., 2005; Riccomagno et al., 2002). Furthermore, the width and length of the cochlear duct are altered in mice carrying mutations in genes traditionally associated with PCP (Montcouquiol et al., 2003; Wang et al., 2005a), leading to the suggestion that cochlear duct elongation may occur through a convergence-extension process similar to that associated with axis elongation in gastrulating vertebrate embryos. Wnt4, Wnt5 and Wnt11, that preferentially signal through non-canonical pathways, have been linked to axial convergence-extension in zebrafish and Xenopus (Heisenberg et al., 2000; Kilian et al., 2003; Du et al., 1995; Wallingford et al., 2001). Intriguingly, earlier we reported distinct expressions of a number of Wnts, with Wnt4, -5a, and -11 in particular, at the ventral tip of the elongating cochlear duct (Sienknecht and Fekete, 2008).
These morphogenetic changes take place concurrently with cell fate specification. As early as the otic cup stage, neuroblasts delaminate from the floor of the cup and migrate medially to generate the statoacoustic ganglion. Within the otic epithelium, patches of cells assume a sensory fate and will differentiate into either auditory or vestibular sensory organs. The process of specifying and segregating the sensory anlage is not fully understood, although it depends on the transcription factor, Sox2 (Kiernan et al., 2005). A number of marker genes allow for prosensory identification well before overt differentiation, including molecules associated with Notch, BMP, Neurotrophin, FGF and, as shown here, Wnt signaling as well as a few transcription factors (Wu and Oh, 1996; Morrison et al., 1999; Cole et al., 2000; Qiu et al., 2004; Schimmang, 2007; for reviews see Fekete and Wu, 2002; Fekete and Sienknecht, 2007).
Here we present an extensive survey of gene expression for a total of 25 probes for Wnts, Frizzleds or Wnt inhibitors over the course of the chicken’s early ear morphogenesis using tissue section in situ hybridization methods. Shown are exemplary data of all 23 genes that have otic expression. We begin at the tear-drop shaped otic vesicle stage (Hamburger-Hamilton stage 16) and proceed through stages that include endolymphatic duct formation, the establishment of the statoacoustic ganglion, the appearance of distinct sensory primordia and the beginning of cochlear duct outgrowth (s25/26).
Material and Methods
Tissue preparation
White Leghorn chicken embryos ranging from stage (s)16 to s26 were harvested and staged according to established criteria (Hamburger and Hamilton, 1951). RNase-free treated embryos were processed as described previously (Sienknecht and Fekete, 2008). Briefly, immersion fixation overnight at 4ºC in 4% paraformaldehyde in phosphate buffered saline was followed by cryo-protection in 15% sucrose in phosphate buffered saline and embedding in TFM (Tissue Freezing Medium, TBS, Triangle Biomedical Sciences). Frozen serial sections of 15μm were collected onto a set of 5–10 slides in a consecutive alternating manner. Depending on the developmental stage every second to 5th section was probed for the same gene, which allowed for a comparison of two to maximal five genes per specimen, including prosensory markers. Experiments were replicated and the order of slide series per tested probe was changed routinely to ensure the representation of different section levels for three dimensional expression pattern information. Transverse and coronal sections were obtained by cutting angles parallel or perpendicular, respectively, to the dorso-ventral axis of the otic anlage.
in situ hybridization on tissue sections
Riboprobes for chicken Wnts and Wnt-related genes (Chapman et al., 2004) were made from plasmids provided by the laboratories of G. Schoenwolf and C. Tabin. The 25 genes studied are listed as follows with gene accession number, sequence and the number of experiments for stages 16–26 in parentheses:
Wnt1 (AY753286,1 – 373, 4, no otic expression), Wnt2b (NM_204336, 687 – 1067, 7), Wnt3 (NM_001081696, 621 – 1034, 10), Wnt3a (NM_204675, 697 – 1088, 7), Wnt4 (NM_204783, 798 – 1191, 10), Wnt5a (NM_204887, 994 – 1364, 16), Wnt5b (NM_001037269, 656 – 1017, 9), Wnt6 (NM_001007594, 557 – 930, 14), Wnt7a (NM_204292, 555 – 1244 + 3’UTR, 5), Wnt7b (NM_001037274, 679 – 1067, 10), Wnt8b (AY753292, 440 – 834, 7), Wnt9a (NM_204981, 72 – 1132, 10), Wnt11 (NM_204784, 1 – 1827, 10), Fzd1 (NM_001030337, 1148 – (1839), 12), Fzd2 (NM_204222, 288 – 1430, 11), Fzd4 (NM_204099, 818 – 3757, 9), Fzd7 (NM_204221, 1034 – 3090, 17), Fzd8 (XM_418566, 728 – 1452, 13), Fzd9 (XM_425392, 219 – 1746, 18), Fzd10 (NM_204098, 1 – 2244, 8), Dkk1 (XM_421563, 478 – 1064, 9), Cer (NM_204823, 44 – 877, 4, no otic expression), SFRP1 (NM_204553, 250 – 468, 9), SFRP2 (NM_204773, 1 – 1594, 13), Frzb (NM_204772, 655 – 1822, 20).
In each experiment the hybridization was replicated on 2 to 5 slides, with few exceptions. In addition, different molecular markers such as Ser1, Lfng (Adam et al., 1998), and BMP4 (Wu and Oh, 1996) were probed on selected adjacent sections to identify prosensory domains. In situ hybridizations were performed as described previously (Sienknecht and Fekete, 2008; Sanchez-Calderon et al., 2005) with riboprobe concentrations of ~1μg/ml. Hybridization was detected with Anti-Digoxigenin-AP Fab fragments (Roche, 1:3500) followed by alkaline phosphatase (AP) precipitation with BM Purple AP Substrate (Roche Applied Science). Throughout the remainder of the manuscript, chicken genes and transcripts are capitalized and presented in italics to distinguish them from proteins.
Photomicrographic documentation
Photomicrographs of tissue sections of all in situ hybridization results were captured and stored in a large image database (Aperture 2.1, Apple Inc., Cupertino, CA) to allow filename- and keyword-based searches and data comparisons of section series. The figure panels in this study provide exemplary data to support the reported results. Cropping of photomicrographs and a minimal amount of contrast and brightness adjustment was carried out with Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA).
Axon immunhistochemistry
Identification of prosensory patches in the developing otic vesicle was aided by double labeling of in situ hybridized tissue sections with 3A10 antibody (1:50, Developmental Studies Hybridoma Bank, DSHB, University of Iowa, USA). 3A10 is a monoclonal against chicken ventral spinal cord that recognizes an uncharacterized antigen that is found in the auditory nerve, and is used to label the axons as previously described (Sienknecht and Fekete, 2008; Sanchez-Calderon et al., 2005).
Results
Otic vesicle expression patterns at s16-s19
The timeframe s16 to s19 includes the phase when neuroblasts delaminate from the neurogenic otic anlage (s13–21, Adam et al., 1998) and the anterior-posterior inner ear axis is specified (s17, Wu et al., 1998). The combined expression of both Lfng and Ser1, which are partially overlapping along the A-P axis, is used to indicate the sensory-competent region of the otic vesicle (Cole et al., 2000). Neuroblasts originate from a subdomain of this neurogenic region (Alsina et al. 2004). By s19 finally, the first two prosensory patches associated with presumptive anterior (superior) and posterior cristae are evident (Wu and Oh, 1996).
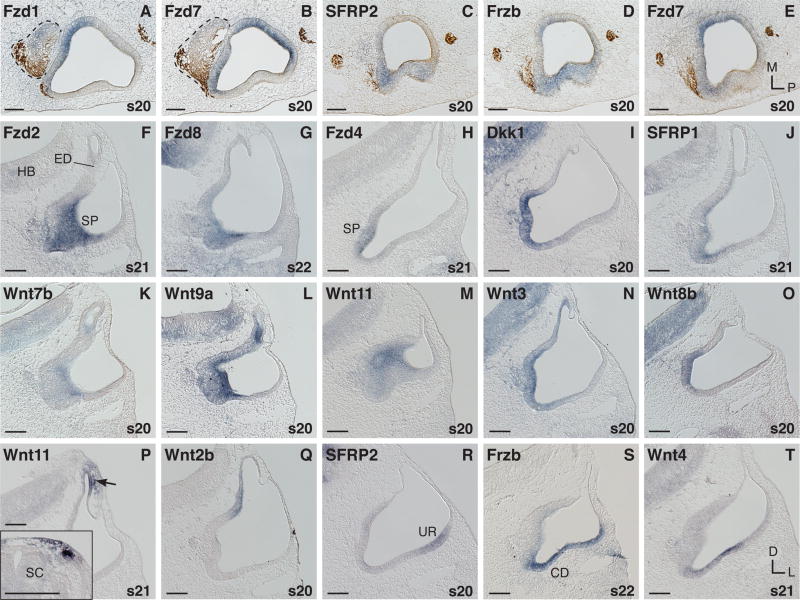
As the otic cup is closing (s16/17), this epithelium expresses several Frizzled receptor genes (Fzd1, -2, -7, -9) and the Wnt antagonists Dkk1, SFRP2, and Frzb (Fig. 1). Frizzled mRNA is mainly confined to the neurosensory-competent ventral half of the otic vesicle up to and including the equatorial zone as confirmed by marker gene expression (Fig. 1A, H, K, N). Fzd1 is strong in the anterior-medial half of the otocyst and includes neuroblasts in the ganglion (Fig. 1B). Concurrently, mRNA expression of the Wnt antagonist SFRP2 is more restricted and concentrated rostrally in the corresponding otic region, excluding the ganglion (Fig. 1C). Fzd9 extends from the anterior otocyst and statoacoustic ganglion into the lateral wall of the otic vesicle (Fig. 1D). Dkk1 resembles this pattern, including some delaminating ganglion neuroblasts (Fig. 1E). Likewise, Fzd7 is present in the anterior and lateral otocyst but only weakly in the adjacent ganglion (Fig. 1F). Partially overlapping with the Fzd7 domain, in the lateral wall is Frzb (compare Fig. 1F and G, and 1I and L). By stage 18, the Fzd7 and Frzb domains are more similar (Fig. 1J and M). We compared these two transcripts to the prosensory markers, Ser1 and Lfng, and conclude that both Fzd7 and Frzb encroach into prosensory territory. Fzd7 partially overlaps with both Lfng (Fig. 1A and F) and Ser1 (Fig. 1I and K). In contrast Frzb overlaps with Ser1 (Fig. 1K and L) but not with Lfng (compare Fig. 1A with G, and 1H with L, and 1N with O). Fzd2 transcripts are present in the medial-ventral otocyst (Fig. 1P), co-expressed with Fzd7 (Fig. 1Q). Continuing this domain, the medial otocyst transcribes Fzd1 (Fig. 1R).
Fig. 1. mRNA expression in the otic vesicle (s16–18).
A-G and N,O: Horizontal sections through otic vesicles (horizontal level in schema). Anterior is to the left and hindbrain rhombomeres are at the top of the image. H-M and P-T: Transversal sections (vertical level in schema). A: Lfng, prosensory marker expression in the anterior and medial otic vesicle at the equatorial level of the vesicle, and in the adjacent SAG. B-G: Comparison of overlapping mRNA expression of three Frizzleds (B,D,F) with three Wnt inhibitors (C,E,G), respectively, on adjacent section levels. H-O: Comparison of Frizzled receptor transcripts, Fzd7, with the Wnt inhibitor gene, Frzb, and prosensory markers (Lfng and Ser1). Consecutive sections of the same embryo are: H,I and L; J and M; and N,O, respectively. Horizontal sections in N,O are through the ventral otic vesicle. P-R: Pro-neurosensory domain expressing Frizzleds (consecutive sections of the same embryo). S,T: Otic Wnt gene expression at s18. Abbreviations: ED, endolymphatic duct; D, dorsal; HB, hindbrain; L, lateral; M, medial; P, posterior; s, embryonic stage. SAG, statoacoustic ganglion (dashed line). In situ hybridization signals (purple) are counterstained by axon labeling with 3A10 antibody (brown) in B, Q, and R. Scale bar =100μm.
None of the ligands could be detected in the inner ear as early as s16. By s18, transcripts for Wnts associated with canonical Wnt signaling, such as Wnt2b and -3a, are confined to the dorsal-medial otic vesicle in the anlage of the endolymphatic duct and sac (Fig. 1S, Wnt2b). While Wnt3a expression later also expands in the dorsal ear anlage to include the base of the vertical canal pouch (summarized in Fig. 5), Wnt2b transcription remains exclusively dorsomedial in the endolymphatic system (latest test s41, embryonic day 15). Although weakly, Wnt5a is also present in the prospective endolymphatic sac at s18 (Fig. 1T). In addition, the otic epithelium of the middle and posterior vesicle expresses Wnt5a with the signal concentrated towards the otocyst lumen (Fig. 1T).
Otocyst expression patterns at s20-s23
Axis specification in the dorsal-ventral dimension of the forming inner ear takes place at s21 to 22 (Wu et al., 1998). The first presumptive sensory macula is evident in the saccule at s20 as an extended patch of BMP4-expressing prosensory cells in the medial otocyst (Wu and Oh, 1996). Of the three cristae ampullares, the last to arise is the lateral crista primordium at s22/23. By s23, the prosensory field of the presumptive auditory epithelium (basilar papilla) and the vestibular lagena macula can be detected by molecular markers (Wu and Oh, 1996).
Continuing earlier stages, Fzd1 and Fzd7 domains complement each other in the prosensory domain with partial overlap (compare Fig. 2A, and B). Fzd1 is transcribed in the medial-anterior otic vesicle and in the statoacoustic ganglion (Fig. 2A). Fzd7 is more prominent in the lateral-anterior vesicle, but extends medially, and in addition it is strong in the medial-posterior epithelium (Fig. 2B and E). Overlapping with Fzd7 (Fig. 2E) in its lateral-anterior domain is the expression of SFRP2 and Frzb (Fig. 2C, D). Both inhibitor genes are transcribed to a different extent in the lateral otic vesicle (Fig. 2C, D, and R, S). New otic Frizzled expression is seen for Fzd8 that is barely detectable until s21 and rises at s22 (Fig. 2G), and Fzd4 of which transcription begins at s21 (Fig. 2H).
Fig. 2. mRNA expression in the otocyst (s20–22).
A-E: Horizontal sections through the broad middle otocyst; anterior is to the left, statoacoustic ganglion (SAG) dashed line. A,B: Comparison of Fzd1 and Fzd7 on adjacent sections. C,D: Expression of two Wnt inhibitors, SFRP2 and Frzb, compared with E: Fzd7 on adjacent sections of the same ear. F-T: Transverse sections. F-O: Transcripts in the anterior and medial otocyst. F-H: Fzds. I,J: Wnt antagonists. K-O: Wnts. F,G and K-M: Anterior-middle section level through the otocyst. H-J and N,O: Middle-posterior otocyst section level. P: Neural crest derived mesenchyme cells expressing Wnt11 adjacent to the endolymphatic sac primordium (arrow). P inset: Wnt11 expression in neural crest cells and the dorsal somite at trunk levels (same embryo as in P). Q: Dorsal-medial expression of Wnt2b. R-T: Lateral otocyst expressors. Abbreviations: CD, cochlear duct; D, dorsal; ED, endolymphatic duct/sac; HB, hindbrain; L, lateral; M, medial; P, posterior; s, embryonic stage; SC, spinal cord; SP, sensory primordium; UR, utricular recess. In situ hybridization signals (purple) are counterstained by axon labeling with 3A10 antibody (brown) in A-E. Scale bar =100μm.
The anterior-medial otocyst includes a thickened epithelial domain located near the origin of the endolymphatic duct that corresponds to the prosensory patch of the saccular macula primordium. Extending into the ventral-medial part of the otocyst, this region is described as the area of highest cell proliferation between s16 and s21 (Alvarez et al., 1989; Hemond and Morest, 1991). In addition, Lang et al. (2000) find that it contains foci of increased programmed cell death beginning at s19. This domain expresses all Frizzled genes with the exception of Fzd10. Fig. 2F-H show examples: anteriorly Fzd2 (F), and Fzd8 (G) and more posteriorly Fzd4 (H). Wnt inhibitor genes such as Dkk1 (Fig. 2I), less strongly SFRP1 (Fig. 2J) and SFRP2 (see Fig. 3C for s24) are present as well. Transiently, limited to s20/21, the anterior-medial and medial-ventral otocyst express numerous ligand genes (Wnt3, -5b, -7b, -8b, -9a, and -11). Fig. 2K-O show examples of a weak (Wnt7b, K) and several more prominent Wnt-expressors in this prosensory domain: Wnt9a (L), -11 (M), -3 (N), and -8b (O). Wnts that are transcribed transiently by this prosensory region are otherwise characteristic of nonsensory areas at later stages, with the exception of Wnt7b that is transcribed extensively in the lagena macula and basilar papilla later by s34 (see Sienknecht and Fekete, 2008, Fig. 4B). Following this transient anterior-medial otic expression of Wnt11 at s20 (Fig. 2M), this transcript disappears from the otocyst until s25 (Fig. 3I). However, we find Wnt11 expression adjacent to the dorsal otocyst in cells that appear to be neural crest derived (Fig. 2P) between s21 and s23. As a positive control, migrating neural crest cells in the trunk region are labeled by Wnt11 at this time point (Fig. 2P inset).
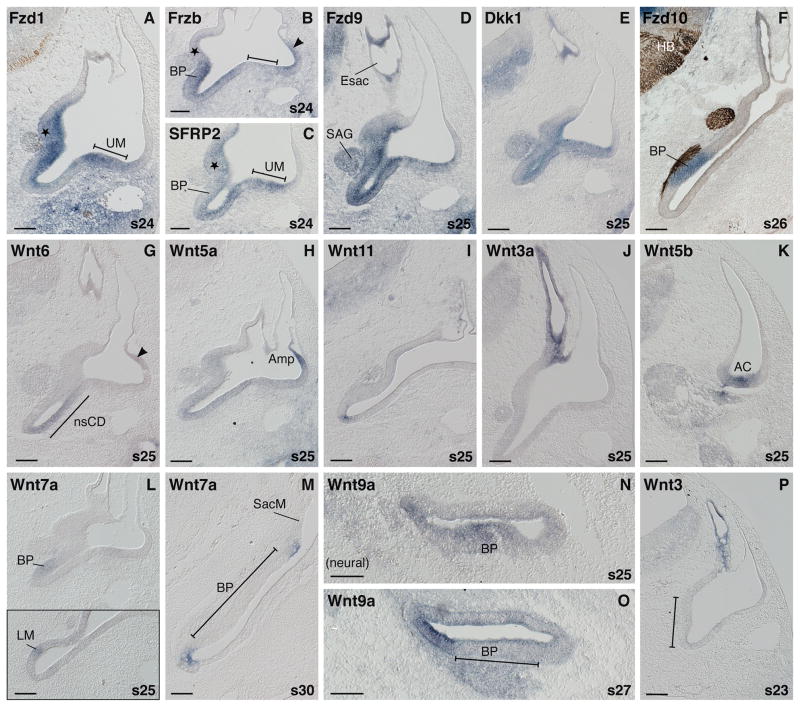
Fig. 3. mRNA expression during otic morphogenesis (s23–30).
A: Prosensory domains are labeled by Fzd1 expression including the prosensory saccule (asterisk)/basilar papilla patch on the medial side and utricular macula (UM) laterally. B,C: Complementary expression patterns of two Wnt inhibitors, Frzb and SFRP2 (asterisk, prosensory saccule; bar, prosensory utricle; BP, prosensory basilar papilla). D,E: Broad otic expression of Fzd9 and Dkk1. F: Exclusive expression of Fzd10 in the distal-abneural BP primordium. G: Nonsensory lateral wall of the cochlear duct (nsCD, demarcated by black line) transcribing Wnt6. H: Lateral ampulla expresses Wnt5a. (Arrow head in B and G indicate weak transcription of Frzb and Wnt6, respectively in the same region). I: Ventral tip of the cochlear duct labeled by locally restricted expression of Wnt11. J: Endolymphatic sac and duct express Wnt3a. K: Wnt5b expression in the anterior crista (AC) region at s25. L,L inset: Weak Wnt7a transcription in the primordia of the auditory BP and vestibular lagena macula (LM) at s25. M: Nonsensory expression of Wnt7a at s30. Transcripts border the saccular macula (SacM) and flank the BP but are not present in the prosensory domains. N: At s25 the BP primordium is expressing Wnt9a, shown in cross section (neural is to the left). The neural side of the forming cochlear duct also shows some Wnt9a transcription. O: At s27 the neural side of the cochlear duct, the prospective homogene cell domain, expresses Wnt9a, flanking the BP domain. P: Wnt3 transcripts label the endolymphatic sac and duct but are not present in the ventral medial domain (bar) at s23. Abbreviations: AC, anterior crista; Amp, semicircular canal ampulla; BP, basilar papilla; D, dorsal; Esac, endolymphatic sac; HB, hindbrain; L, lateral; LM, lagenar macula; M, medial; nsCD, nonsensory cochlear duct; P, posterior; s, embryonic stage; SAG, statoacoustic ganglion; SacM, saccular macula, UM, utricular macula. In situ hybridization signals (purple) are counterstained by axon labeling with 3A10 antibody (brown) in F. Scale bar =100μm.
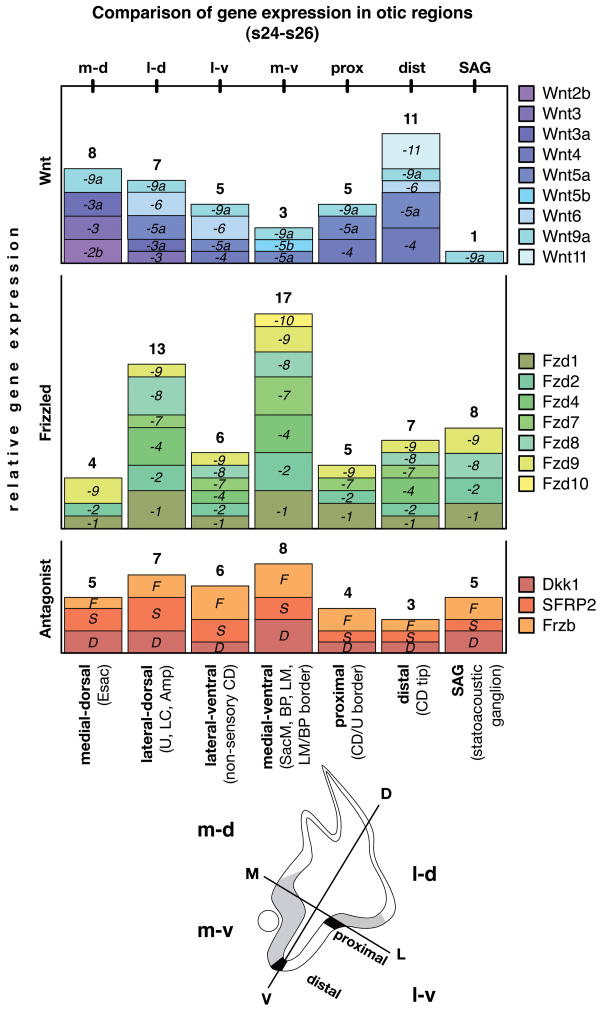
Fig. 4. Relative comparison of gene expression in otic regions (s24-s26).
Qualitatively scored expression of Wnts (blue), Frizzleds (green), and Wnt antagonists (red) allow a comparison of different otic regions and the diversity of genes contributing to each expression domain. The box height refers to a qualitative score from 1–3 that indicates either a) relative expression strength/distinctiveness (signal vs. background and compared to positive control tissue of the same embryo); b) expression duration; or c) local restriction within the domain. That means, a gene with the score 1 (small box) is either expressed weakly in that domain compared to its expression elsewhere in the embryo, or a score 1 refers to a limited duration within the time window discussed, or finally a score 1 indicates a localized expression e.g. only in parts of the lateral dorsal otic anlage. The number above each diagram block is the sum of expression units (scores), representing relative gene expression per otic region. Schematic provides orientation of otocyst quadrants (m-d, m-v, l-d, l-v), illustrates locations of prosensory domains (gray), and indicates proximal and distal border domains (black). Abbreviations: Amp, semicircular canal ampulla; BP, basilar papilla; CD, cochlear duct; CD/U border, border between CD and U; D, dorsal; Esac, endolymphatic sac; L, lateral; LC, lateral crista; LM, lagena macula; LM/BP border, inter-sensory border between LM and BP (not indicated in schema); M, medial, s, embryonic stage; SacM, saccular macula, U, utricle, V, ventral.
Three major nonsensory domains of the developing inner ear are: (1) the outgrowing endolymphatic sac and duct located medial-dorsal; (2) the presumptive utricular recess joint with the forming lateral ampulla located lateral-dorsal; and (3) the lateral wall of the pars inferior, the presumptive cochlear duct located ventral-lateral. Each of these 3 domains expresses transcripts for Wnt ligands, receptors and inhibitors. Specifically, the presumptive endolymphatic domain expresses Wnt2b (Fig. 2Q), -3 (Fig. 2N), -3a (see later Fig. 3J), -9a (Fig. 2L), and several Frizzleds (Fzd1, -2, and -9, see Fig. 3D for Fzd9 at s25), along with Dkk1 (see Fig. 3E for s25) and SFRP2 (not shown). Cells in the presumptive utricular recess express SFRP2 (Fig. 2R), Frzb (Fig. 2S), and the forming semicircular canal ampulla expresses Wnt5a (see Fig. 3H for s25), as well as weakly Wnt3a and -6 (both not shown). The prospective nonsensory cochlear duct transcribes Wnt4 (Fig. 2T). Congruent with this, Frzb is present at s20 and extends its expression by s22 to the entire ventral-lateral wall (Fig. 2S), excluding the medial prosensory domain. Weak expression of Wnt3, Dkk1 and SFRP1 is also detectable in the ventral-lateral otocyst (cf. Fig. 2N, I, and J). In addition Fzd2, and -9, which are broadly expressed (see Fig. 3D, Fzd9), are present in the lateral wall of the pars inferior and Fzd7 that extends from its prosensory domains into adjacent nonsensory areas (not shown).
Inner ear primordium expression patterns at s24-s26
According to (Wu and Oh, 1996) the last large prosensory domain, the utricular macula, is recognizable by s24. At this stage, Frizzleds demarcate all the prosensory patches of the developing inner ear (Fig. 3A, Fzd1). Not restricted to prosensory domains, but including the endolymphatic sac and the nonsensory cochlear duct, are the expressions of Fzd2 and Fzd9 (Fig. 3D, Fzd9) as well as Dkk1 (Fig. 3E). The last Frizzled receptor gene, Fzd10, is uniquely localized in the basilar papilla by s26 (Fig. 3F), as described in more detail previously (Sienknecht and Fekete, 2008).
Expressed differentially in both prosensory and nonsensory domains are the Wnt inhibitor genes, Frzb and SFRP2, apart from the aforementioned Dkk1. In detail, Frzb is transcribed most strongly in the prospective auditory organ (the basilar papilla), less so in vestibular prosensory patches at s24 (Fig. 3B). Furthermore, the nonsensory cochlear duct (Fig. 3B) and the forming lateral ampulla region of the dorsal ear transcribe Frzb (Fig. 3B, arrow head). In contrast, SFRP2’s highest expression levels are in vestibular prosensory patches (Fig. 3C, SacM, star; UM, bar) and in the nonsensory cochlear duct, while less strong in the presumptive basilar papilla at this time (Fig. 3C).
Wnts, in contrast, are mainly confined to nonsensory domains (Fig. 3G-J), with some transient exceptions (see below). The nonsensory cochlear duct expresses Wnt6 (Fig. 3G), plus SFRP2 as described before, and weakly both SFRP1 and Wnt5a (Fig. 3H, Wnt5a). In the posterior cochlear duct, however, the latter is more apparent at the distal tip of the ventral ear (not shown, see Sienknecht and Fekete, 2008, Fig. 1L), sharing this domain with Wnt4 and Wnt11 (Fig. 3I, Wnt11). Another prominent nonsensory expression domain is the forming ampulla of the lateral crista in the dorsal ear. These cells express both Wnt5a (Fig. 3H) and Frzb in particular (the latter boldly by s25; see Fig. 3B arrow head for weak labeling at s24). Also faintly detectable in the lateral ampulla at this stage is Wnt6 (Fig. 3G, arrow head). Medial-dorsal, the endolymphatic apparatus is distinctly labeled by Wnt3a (Fig. 3J), -3 (Fig. 3P) and -2b (see Fig. 2Q, for a younger stage). Although not as restricted to this domain, the expression of Wnt9a is most intense in the forming endolymphatic anlage (see Fig. 2L). SFRP2, Frzb, and the aforementioned Dkk1 (Fig. 3E), are present in this domain, too.
In addition to the prosensory domains, Frizzled receptor genes are prominent in the statoacoustic ganglion (namely these are Fzd1, -2, -8, and -9) (Fig. 3D, Fzd9). The only ligand gene expressed in the ganglion is Wnt9a (see Sienknecht and Fekete, 2008, Fig. 5F). All three inhibitors (e.g. Fig. 3E, Dkk1) are present with SFRP2 being the weakest. Wnt11 is expressed in glia or Schwann cells as cranial nerves enter the hindbrain and is first apparent at s23 (see s36 in Sienknecht and Fekete, 2008, Fig. 5G). Cranial nerve glia or Schwann cells also express Wnt5a and Fzd7 at s24–26 and beyond (not shown).
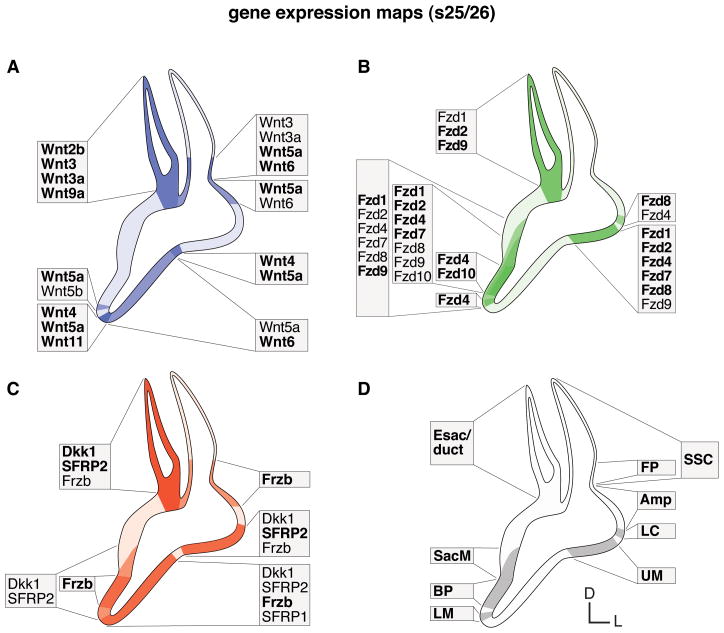
Fig. 5. Summary of gene expression domains of the morphogenetic inner ear (s25/26).
Schematic illustration summarizes maps of characteristic expression domains established in the regionalized otic epithelium. A: Wnts (blue). B: Fzds (green). C: Wnt inhibitors (red). D: prosensory domains (gray). Color intensities refer to co-expression of multiple genes and expression strength. Faint background color indicates broad and indistinct gene expression. Text boxes group and list expressors within the region. Comparison of A: Wnt ligands and B: Fzd receptors show mostly complementary expression domains. Prosensory areas express Fzds, whereas nonsensory and border domains express Wnts. The exception is the endolymphatic apparatus that co-expresses both Wnts and Fzds. C: Wnt inhibitor maps combine to differentially cover both the Wnt and Fzd domains. Abbreviations: Amp, semicircular canal ampulla; BP, basilar papilla; D, dorsal; Esac/duct, endolymphatic sac/duct; FP, semicircular canal fusion plate; L, lateral; LC, lateral crista; LM, lagenar macula; s, embryonic stage; SacM, saccular macula; SSC, semicircular canal; UM, utricular macula.
In Figure 4, we provide a block diagram that compares regions of qualitatively scored Wnt-related gene expression in the morphogenetic inner ear from s24 to s26. This reveals several centers of high transcriptional activity and their composition of Wnt pathway genes. Wnt expression peaks at the distal tip of the pars inferior (dist.), the outgrowing cochlear duct. Wnt transcription in the endolymphatic sac (medial-dorsal) is relatively high, followed by the lateral-dorsal inner ear in the region of the forming lateral ampulla and the superior semicircular canal base (fusion plate). Peak areas for Frizzleds and Wnt inhibitors are the regions that give rise to the prosensory patches in the medial-ventral (m-v) and the lateral-dorsal (l-d) inner ear anlage.
At s25, when the prosensory patches become distinct, several Wnts are expressed transiently in these domains. For example, the dorsal ear expresses Wnt5b in the domain of the anterior crista (Fig. 3K). Wnt7a shows a weak expression primarily in the ventral ear (basilar papilla and lagena macula; Fig. 3L and inset). Yet, prosensory domains are negative again for Wnt7a soon thereafter. Fig. 3M reports this down-regulation for the time point two days later (s30). The window of s25/26 is also when Wnt9a transcripts appear transiently in the auditory primordium as shown in cross section through the cochlear duct (Fig. 3N). Shortly thereafter, at s27, Wnt9a has mostly vanished from the prosensory domain and remains as a flanking border along the non-innervated neural side (Fig. 3O). Another example for a transient expression is Wnt3 that appears in the ventromedial prosensory patch at s20 (see Fig. 2N) and then disappears from the entire ventral ear by s23, maintaining only its endolymphatic sac and duct expression (Fig. 3P).
Excluding those transient expression events, Figure 5 summarizes domain maps of (1) Wnts (ligand genes, in blue), (2) Frizzleds (receptor genes, in green) and (3) Wnt antagonists (inhibitor genes, in red) during morphogenesis from s25/s26 and later. Wnts establish mainly nonsensory and border domains, while Frizzleds demarcate areas of sensory primordia, and all these domains are superimposed by differential Wnt inhibitor gene expressions. The endolymphatic apparatus is the only otic region where all three Wnt signaling entities regularly overlap.
Discussion
Correlation of Frizzleds with neurosensory development
Our results refine and extend a previous study by (Stark et al., 2000) regarding Frizzled gene expression in the avian otic anlage as well as other cranial placodes. They observed Fzd1, -2, and -7 from s11/12 (otic cup) to s15/16 (closing otic vesicle), with expression diminished by s16–18. In contrast, we find the expression of these genes persists to later stages. Furthermore, we show here distinct otic expression of three Frizzled genes (Fzd4, -8, and -9) that they described as not prominent in cranial placodes at similar stages. These discrepancies may be due to less reliable mRNA detection when performed as whole mount in situ hybridization, particularly after closure of the superficial otic cup.
Frizzled family members vary in the onset of their inner ear expression. The closing otic vesicle expresses Fzd1, -2, -7, and -9 at s16, while Fzd4 (s21), -8 (s22), and -10 (s25/26) show distinct otic transcription at progressively later stages of development. The early expression overlaps with the neurogenic region that gives rise to delaminating neuroblasts and expression of Fzd1 persists in the newly-released neuroblasts at s16 (Fig. 1B). Concurrently, there is focal expression of SFRP2 within the otic vesicle at the site of delamination (Fig. 1C) that continues in the lateral vestibule at s20 (Fig. 2R) and persists within and adjacent to the presumptive utricular macula at s25/26 (summarized in Fig. 5C). The macular region remains neurogenic through s27 (Stone et al., 2003) and shows persistent expression of one or more Frizzled genes during the interval from s16-s27 (summarized for s25/26 in Fig. 5B). This raises the interesting question of whether the binding of SFRP2 to Frizzled receptors might be involved either in the specification or repulsion of neuroblasts.
The Frizzled genes are also associated with prosensory domains, including both ventromedial and lateral (summarized in Fig. 5B). This suggests a potential function for Wnt signaling in the development of sensory identity, which is also under the control of the proneural transcription factor, Sox2 (Uchikawa et al., 1999; Neves et al., 2007; Kiernan et al., 2005). Such a role for Frizzleds in the formation and maintenance of neural-competent precursors has been reported by Van Raay et al. (2005) for the developing Xenopus retina. There, blocking of either Fzd5 or canonical Wnt signaling inhibits Sox2 expression and promotes non-neural fate. A Frizzled requirement was also reported during neurogenesis in Drosophila (Bhat, 1998).
Finally, expression of Frizzleds in the statoacoustic ganglion neurons raises the question of whether they might regulate axon guidance as has been shown elsewhere (Drescher, 2005; Bovolenta et al., 2006). It seems there are at least three possibilities for Frizzled-mediated responsiveness: (1) attraction via Wnt inhibitors that are abundant in the prosensory domains; (2) attraction via Wnt ligands that appear transiently in the prosensory domains (see below); or (3) repulsion via Wnt ligands that flank the sensory domains at most stages.
In some systems, such as Drosophila, Frizzleds appear to function redundantly (Bhanot et al., 1999), and indeed we show several examples where there are multiple overlapping Frizzled genes. Yet our results also show spatial and temporal differences in Frizzled gene expression that suggest distinct non-redundant functions during inner ear development.
Correlation of Wnts with non-sensory domains
Typically, nonsensory borders expressing Wnts flank sensory primordia presenting Frizzled receptors. This arrangement is consistent with the possibility of paracrine signaling from nonsensory to sensory territories as we noted in a previous study (Sienknecht and Fekete, 2008) and that we can now extend to earlier time points. Interactions between sensory and nonsensory domains seems crucial for inner ear development, as shown by the requirement of FGF activity in the sensory cristae to promote adjacent semicircular canal formation in chickens (Chang et al., 2004) and conversely the nonsensory expression of Foxg1 for proper patterning of the mouse organ of Corti (Pauley et al., 2006). Transcription of several Wnt inhibitors in sensory and nonsensory domains further suggests that paracrine Wnt signaling may be tightly regulated.
Spatial expression of Wnts that commonly signal through a non-canonical pathway, such as Wnt4, -5a and -11, are found in association with the lateral otocyst and ventral tip of the outgrowing cochlear duct. A role for these proteins in convergent extension of the cochlear duct is conceivable based on the expression data reported here and the finding that Wnt5a and Wnt11 are involved in convergent extension movements during gastrulation of birds and zebrafish (Hardy et al., 2008; Kilian et al., 2003; Zhu et al., 2006). Furthermore, Wnt5a mutant mice have a shortened cochlear duct, a fact that has been connected to convergent extension failure (Qian et al., 2007). In addition, in vitro data from Wnt5a mutant mice indicate involvement of this ligand in mesenchymal condensation during otic capsule chondrogenesis (Liu et al., 2008).
Finally, the ventral expression of preferentially non-canonical Wnts, such as Wnt5a and Wnt11, could suggest an antagonistic relationship with dorsal Wnts, such as the canonical Wnt3a. Given that both Wnt11 and Wnt5a can effectively antagonize Wnt3a-activated β-catenin signaling (Maye et al., 2004), an otic-autonomous Wnt-Wnt interplay either establishing or refining non-overlapping canonical vs. non-canonical Wnt territories in the otocyst becomes an attractive idea.
The large number of Wnt-related genes expressed in the endolymphatic duct raises the possibility that it may be an important signaling center acting on nearby cells in or around the dorsal otocyst, including the hindbrain and migrating neural crest cells. Yet, it should also be remembered that Wnt ligands, as well as the Wnt antagonists, are secreted molecules which means the location of their transcription does not necessarily correlate directly with the proteins’ functions. One possibility is that a region of high transcriptional turnover, such as the endolymphatic sac appears to be, is particularly in need of control by Wnt inhibitors to prevent Wnt signaling in loco.
Taken together, regionalized expression of several Wnts, Wnt receptors and Wnt inhibitor genes in the otic anlage indicates that the system is carefully regulating Wnt responsiveness.
Transient Wnt signaling in prosensory domains
Unexpected was the observation of Wnt transcription within prosensory domains, although temporally restricted and often short term (cf. s20/21 and s25). Because this overlaps both spatially and temporally with receptor gene expression, it suggests a probable role of autocrine Wnt signaling as well.
In the mouse otocyst, canonical Wnt activity is restricted dorsally by counteractive Shh signaling originating ventrally (Riccomagno et al., 2002; 2005). For the chicken otocyst, we confirm the dorsal-medial predominance of Wnt genes that activate β-catenin dependent signaling such as Wnt2b and -3a. In mice, the dorsal restriction of Wnt1 and Wnt3a expression, coupled with ventral, cochlear defects in Wnt1 and Wnt3a knockouts, was initially puzzling. However, Cre-lox-mediated lineage tracing of Wnt-responding cells provided a plausible explanation: dorsally-derived cells received a canonical Wnt signal before migrating to the medial-ventral otocyst (Riccomagno et al., 2005). While we do not dispute this idea, in chickens there may be a more direct opportunity for Wnt signaling to influence cells of the medial-ventral otocyst. This region shows transient expression of numerous Wnt genes (Wnt3, -5b, -7b, -8b, -9a and -11 at s20/21). Timing and location of this expression corresponds to the specification of a Sox2-expressing prosensory domain (Neves et al., 2007) that is mitotically active (Lang et al., 2000). Interestingly, canonical Wnt signaling is thought to maintain an undifferentiated, proliferating progenitor population, whereas non-canonical Wnts facilitate differentiation, as evident during osteogenesis in vitro in human mesenchymal stem cells (Boland et al., 2004). However, the set of genes we find co-expressed at s20/21 is per se not further instructive in this respect, although, considering recent reports, it does not contradict canonical signaling (cf. Kim et al., 2008; Lewis et al., 2008, Wnt3; Kuorelahti et al., 2007, Wnt5b, Wnt7b; Wang et al., 2005b, Wnt7b; Cadigan and Liu, 2006, Wnt8b; Guo et al., 2004; Person et al., 2005, Wnt9a; Tao et al., 2005, Wnt11).
Forced expression of activated β-catenin in nonsensory territories of the chicken inner ear induces the formation of ectopic sensory patches of vestibular nature during a sensitive period that ends by s23 (Stevens et al., 2003). This time frame precedes the second time point, s25, when a few Wnts (e.g. Wnt7a and Wnt9a) occur transiently in prosensory domains. Shortly thereafter Wnts disappear again from these domains. Both the observed temporal restriction of Wnt expression in prosensory domains and the functional data lead to the conclusion that a tight temporal control system is necessary for prosensory specification in the developing inner ear.
Furthermore, Wnt7b, one of the expressors in the prosensory domain at s20/21, disappears from prosensory territory for nearly 5 days, reappearing at embryonic day 8 (s34) in the auditory basilar papilla and the vestibular lagena macula. By that time, both sensory organ primordia show a robust and non-overlapping expression of different Wnt inhibitor genes, i.e., SFRP2 in the basilar papilla and Frzb in the lagena macula (Sienknecht and Fekete, 2008). Thus, (a) autocrine canonical Wnt signaling influences are likely to be prevented in the sensory primordia for an extended time and (b) the gene expression data are consistent with a role of Wnts in conjunction with Wnt inhibitor activity in the proper formation of distinct auditory and vestibular sensory organs.
Shortly after the medial prosensory domain is established, a focus of apoptosis appears in the medial wall of the otocyst (Fekete et al., 1997; Lang et al., 2000). This leads us to consider whether Wnt signaling might influence programmed cell death during inner ear development. He et al. (2004) provided evidence that blocking Wnt1 leads to apoptosis in human cancer cell lines, although others found the opposite in the chicken hindbrain in vivo, where Wnt1 overexpression causes ectopic programmed cell death (Ellies et al., 2000). Comparison of our Wnt-related gene expression patterns to maps of cell proliferation and cell death during chicken inner ear development by Lang et al. (2000) revealed some regions of interest. Wnt9a, for example, shows resemblance to the distribution of proliferating cells that incorporated BrdU over development. In the region called the ventral-medial ‘hot spot’ of cell death (s19–23), where proliferating and apoptotic cells are intermingled, Wnt9a overlaps with Dkk1 at s20 (Fig. 2L, Wnt9a; 2I, Dkk1). Interestingly, Wnt9a promotes cell proliferation during chicken heart development, an effect that is antagonized by Frzb (Person et al., 2005). Furthermore, the authors show that inhibition of Wnt9a by overexpression of dominant-negative Wnt9a results in an increase in apoptotic (TUNEL-positive) cells in cardiac atrioventricular canal cultures. A role of Dkk1 in cell proliferation and cell death was suggested by Mukhopadhyay et al. (2001). Grotewold and Rüther (2002) provided evidence that Dkk1 enhances apoptosis during limb development. Finally, another Wnt antagonist, SFRP2, modulates programmed cell death in chicken hindbrain rhombomeres via a Wnt-BMP4 feedback-loop as demonstrated by Ellies et al. (2000).
Inner ear domains under control of Wnt antagonists
Three Wnt inhibitors, Dkk1, Frzb, and SFRP2 are notable for their robust transcription. Present from early otic stages throughout inner ear development, Wnt inhibitor genes show partial overlap in their expression patterns. Figure 6 maps six differently composed domains established by Wnt signaling modulators at two exemplary time points.
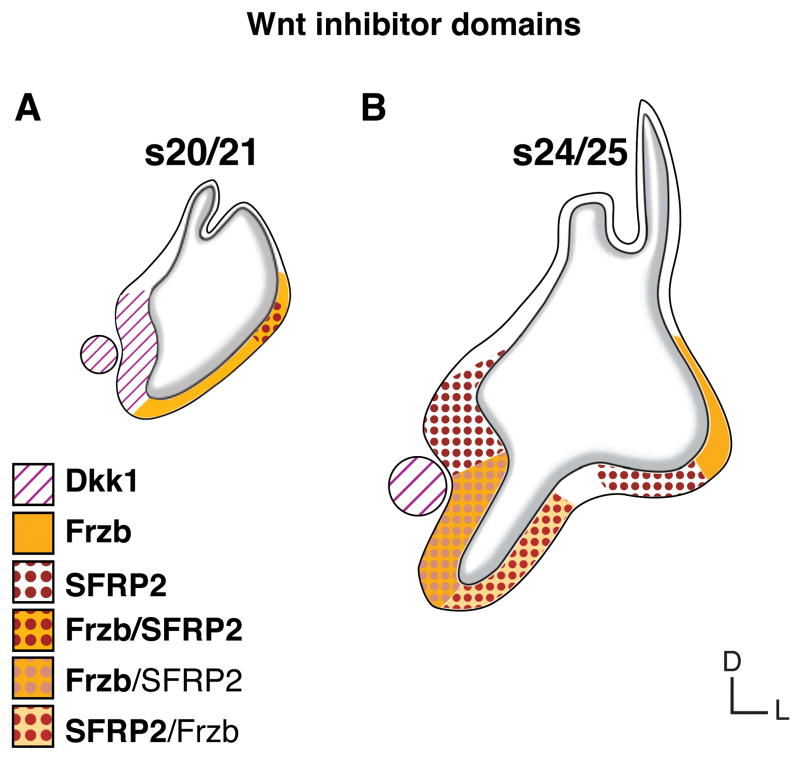
Fig. 6. Wnt inhibitor domains.
Comparison of two time points, A: otocyst stage 20/21 and B: inner ear primordium stage 24/25, reveals six differently composed Wnt inhibitor domains with distinct or overlapping expression of three Wnt inhibitor genes: (1) Dkk1 at s20 in the medial otocyst and SAG (circle); (2) Frzb at s20 in the lateral otocyst, and at s24/25 lateral-dorsal (in the vestibule); (3) co-expression of Frzb and SFRP2 at s20 in the lateral-dorsal otocyst; (4) SFRP2 at s24/25 in prosensory vestibular primordia; (5) co-expression with strong SFRP2 and mild Frzb at s24/25 lateral-ventral (in the nonsensory pars inferior); (6) co-expression with strong Frzb and mild SFRP2 at s24/25 medial-ventral (in the prosensory BP primordium). Not shown are the sporadic and weak expression of SFRP1 (see Fig. 2J) and an underlying broad expression of Dkk1 by s24/25 (Fig. 3E). Bold and plain font face in the legend refers to relative expression strength. Abbreviations: BP, basilar papilla; D, dorsal; L, lateral; s, embryonic stage; (SAG), statoacoustic ganglion.
Nonetheless, dynamic changes over time are drastic. For example, SFRP2 shifts from vestibular sensory domains to the auditory primordium. During the divergence of vestibular and auditory sensory cell fates, SFRP2 is expressed mainly in prospective vestibular prosensory patches and the nonsensory pars inferior, thus flanking the prospective auditory epithelium (Fig. 6, s24/25). Later, (s27-s36), SFRP2 is prominently expressed in the auditory epithelium (Sienknecht and Fekete, 2008) but not in any vestibular sensory organ primordium (at least until s39, embryonic day 13, by s40 this changes, unpublished data). Frzb, in contrast, is mainly present in prospective nonsensory territories at s20–22, but then becomes transcribed in the auditory primordium by s24/25 (Fig. 6). Later, during differentiation of the basilar papilla, when SFRP2 is strongly expressed in this tissue, the transcription of Frzb restricts to the neural-basal basilar papilla by s30 before diminishing from the auditory organ as reported previously; Frzb remains in the vestibular lagena macula (Sienknecht and Fekete, 2008).
Based on the particular set of genes expressed in the medial-ventral (neurosensory) otic vesicle at s20/21, we suggested that the neurosensory domain may be responsive to β-catenin pathway signaling factors for a small time window, with canonical Wnt activity serving to promote a prosensory fate. However, such a function seems, at first glance, to be inconsistent with the high level of Dkk1 expression we observed in this same domain. Dkk1 is a potent inhibitor of canonical Wnt signaling (reviewed in Niehrs, 2006). One plausible explanation is that the antagonistic effect of Dkk1 may be overruled by the accumulated presence of numerous presumed canonical-pathway Wnt ligands (e.g. Wnt7b, -8b, -9a). Moreover, Dkk1 might actually not interfere with canonical signaling after all. In fact, there is evidence for Dkk1 as a direct inhibitor of the non-canonical PCP pathway acting independently of β-catenin (Caneparo et al., 2007). Furthermore, Cha et al. (2008) show that during dorsal-ventral axis formation in Xenopus, Dkk1 limits the site and amount of activation of Wnt target genes by modulating JNK activation, a key component of non-canonical signaling. In the light of this knowledge, Dkk1 could very well be permissive for canonical Wnt signaling in the otic neurosensory domain.
Modulation of Wnt signaling by co-expression and therefore cumulative gene activity seems likely and could provide vast plasticity. For example, activity of both Dkk1 and SFRP2 in the anterior otic vesicle at s16 could be crucial to pass the threshold for inhibition of a given signaling event. Conversely, ligands with regionally overlapping expression and the tendency to form functional complexes could together reach an expression threshold to reduce the efficacy of inhibitors. A situation like this has been described by Cha et al. (2008) for Xenopus embryos. There, Dkk1 antagonizes signaling of the broadly expressed Wnt5a, except in the dorsal blastula where Wnt11 mRNA is enriched. Co-expression of both ligands, which tend to form a functional complex of two homodimers, passes a threshold for activity and the Dkk1 antagonism becomes ineffective, allowing transduction of the Wnt signal and leading to expression of Wnt target genes.
The striking prominence of secreted Wnt signaling antagonists during otic development, their differential expression and dynamic changes over time that we report here, suggests that Wnt signaling is likely to be under tight control spatially and temporally.
Summary
Expression of a plethora of Wnt-related genes is concomitant with inner ear development as the otic vesicle initiates morphogenesis and prosensory cell fate determination. Co-expression and spatiotemporal tuning create specific developmental contexts for responding cells. In addition, topological patterns and temporal dynamics of Wnt inhibitor gene expression lead to a catalog of molecular micro-environments that likely specify and regulate Wnt activities. Detailed knowledge of overlapping expression of several Wnts, Frizzleds, and/or Wnt inhibitors, respectively, may assist in the design of experiments for functional analyses by suggesting a need to target functional units rather than single genes. This may also help to overcome the common problem of analyzing functional redundancies.
Acknowledgments
We acknowledge the important technical assistance of Deb Biesemeier and Katie Holmes. We thank Susan Chapman, Gary Schoenwolf, Cliff Tabin, Doris Wu, Julian Lewis and Bruce Morgan for probes. Our research is funded through NIH RO1DC002756.
References
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267(1):119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Alvarez IS, Martín-Partido G, Rodríguez-Gallardo L, González-Ramos C, Navascués J. Cell proliferation during early development of the chick embryo otic anlage: quantitative comparison of migratory and nonmigratory regions of the otic epithelium. J Comp Neurol. 1989;290:278–288. doi: 10.1002/cne.902900208. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126(18):4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95(7):1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132(9):2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Rodriguez J, Esteve P. Frizzled/RYK mediated signalling in axon guidance. Development. 2006;133(22):4399–4408. doi: 10.1242/dev.02592. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Kiernan AE, Gao X, Iten LE, Fekete DM. Molecular genetics of pattern formation in the inner ear: Do compartment boundaries play a role? Proc Natl Acad Sci U S A. 2000;97(22):11700–11706. doi: 10.1073/pnas.97.22.11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11(24):3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Caneparo L, Huang YL, Staudt N, Tada M, Ahrendt R, Kazanskaya O, Niehrs C, Houart C. Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev. 2007;21(4):465–480. doi: 10.1101/gad.406007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135(22):3719–3729. doi: 10.1242/dev.029025. [DOI] [PubMed] [Google Scholar]
- Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development. 2004;131(17):4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229(3):668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424(3):509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329(Pt 2):209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher U. A no-Wnt situation: SFRPs as axon guidance molecules. Nat Neurosci. 2005;8(10):1281–1282. doi: 10.1038/nn1005-1281. [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol. 1995;15(5):2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies DL, Church V, Francis-West P, Lumsden A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development. 2000;127(24):5285–5295. doi: 10.1242/dev.127.24.5285. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Sienknecht UJ. The Inner Ear. In: Moody Sally A., editor. Principles of Developmental Genetics. Academic Press, Elsevier; 2007. pp. 631–655. [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12(1):35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Homburger SA, Waring MT, Riedl AE, Garcia LF. Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development. 1997;124:2451–2461. doi: 10.1242/dev.124.12.2451. [DOI] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135(20):3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21(5):966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125(12):2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18(19):2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–91. [PubMed] [Google Scholar]
- Hardy KM, Garriock RJ, Yatskievych TA, D’Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320(2):391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6(1):7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405(6782):76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Morest DK. Ganglion formation from the otic placode and the otic crest in the chick embryo: mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991;184(1):1–13. doi: 10.1007/BF01744256. [DOI] [PubMed] [Google Scholar]
- Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008;50(4):229–243. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52(1):9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135(13):2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. BioEssays. 2002;24(9):811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120(4):467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48(5):780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kuorelahti A, Rulli S, Huhtaniemi I, Poutanen M. Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology. 2007;148(8):3694–3703. doi: 10.1210/en.2007-0249. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290(5498):1965–1968. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- Lang H, Bever MM, Fekete DM. Cell proliferation and cell death in the developing chick inner ear: spatial and temporal patterns. J Comp Neurol. 2000;417(2):205–220. doi: 10.1002/(sici)1096-9861(20000207)417:2<205::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lewis J, Davies A. Planar cell polarity in the inner ear: How do hair cells acquire their oriented structure? J Neurobiol. 2002;53(2):190–201. doi: 10.1002/neu.10124. [DOI] [PubMed] [Google Scholar]
- Lewis SL, Khoo PL, De Young RA, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson RV, Robb L, Tam PP. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135(10):1791–1801. doi: 10.1242/dev.018853. [DOI] [PubMed] [Google Scholar]
- Lillevali K, Haugas M, Matilainen T, Pussinen C, Karis A, Salminen M. Gata3 is required for early morphogenesis and Fgf10 expression during otic development. Mech Dev. 2006;123(6):415–429. doi: 10.1016/j.mod.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Liu W, Li L, Li G, Garritano F, Shanske A, Frenz DA. Coordinated molecular control of otic capsule differentiation: functional role of Wnt5a signaling and opposition by sfrp3 activity. Growth Factors. 2008;26(6):343–354. doi: 10.1080/08977190802442013. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Keino H. Roles of beta-catenin in inner ear development in rat embryos. Anat Embryol (Berl) 2000;202(1):39–48. doi: 10.1007/pl00008243. [DOI] [PubMed] [Google Scholar]
- Maye P, Zheng J, Li L, Wu D. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem. 2004;279(23):24659–24665. doi: 10.1074/jbc.M311724200. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18(55):7860–7872. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18(11):564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423(6936):173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Moon R, Brown J, Yang-Snyder J, Miller J. Structurally related receptors and antagonists compete for secreted wnt ligands. Cell. 1997;88:725–728. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84(1–2):169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1(3):423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503(4):487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133(5):865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131(3):551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet JP. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev Biol. 2008;324(1):108–121. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr B, Shea M, Vassileva G, McMahon A. Mouse wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235(9):2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol. 2005;278(1):35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Storch EM, Lekven AC, Riley BB. A direct role for Fgf but not Wnt in otic placode induction. Development. 2004;131(4):923–931. doi: 10.1242/dev.00978. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306(1):121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Xu H, Haddon C, Lewis J, Jiang YJ. Sequence and embryonic expression of three zebrafish fringe genes: lunatic fringe, radical fringe, and manic fringe. Dev Dyn. 2004;231(3):621–630. doi: 10.1002/dvdy.20155. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16(18):2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19(13):1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Martin-Partido G, Hidalgo-Sanchez M. Pax2 expression patterns in the developing chick inner ear. Gene Expr Patterns. 2005;5(6):763–773. doi: 10.1016/j.modgep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Schimmang T. Expression and functions of FGF ligands during early otic development. Int J Dev Biol. 2007;51(6–7):473–481. doi: 10.1387/ijdb.072334ts. [DOI] [PubMed] [Google Scholar]
- Sienknecht UJ, Fekete DM. Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J Comp Neurol. 2008;510(4):378–395. doi: 10.1002/cne.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390(6658):410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93(1–2):195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol. 2003;261(1):149–164. doi: 10.1016/s0012-1606(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. Expression of Prox1 defines regions of the avian otocyst that give rise to sensory or neural cells. J Comp Neurol. 2003;460(4):487–502. doi: 10.1002/cne.10662. [DOI] [PubMed] [Google Scholar]
- Summerhurst K, Stark M, Sharpe J, Davidson D, Murphy P. 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19) Gene Expr Patterns. 2008;8(5):331–348. doi: 10.1016/j.gep.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120(6):857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev. 1999;84(1–2):103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Ungar AR, Kelly GM, Moon RT. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech Dev. 1995;52(2–3):153–164. doi: 10.1016/0925-4773(95)00386-f. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46(1):23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45(1):225–227. [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005a;37(9):980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26(8):2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21(13):4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005b;25(12):5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16(20):6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Nunes FD, Choo D. Axial specification for sensory organs versus non-sensory structures of the chicken inner ear. Development. 1998;125:11–20. doi: 10.1242/dev.125.1.11. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Zakin LD, Mazan S, Maury M, Martin N, Guenet JL, Brulet P. Structure and expression of Wnt13, a novel mouse Wnt2 related gene. Mech Dev. 1998;73(1):107–116. doi: 10.1016/s0925-4773(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Zhu S, Liu L, Korzh V, Gong Z, Low BC. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal. 2006;18(3):359–372. doi: 10.1016/j.cellsig.2005.05.019. [DOI] [PubMed] [Google Scholar]