Abstract
Before I begin, I want to add my own personal reminiscence. I knew Dave Hume for almost 14 years, slightly for the first 4 and well for the last 10. I first talked to him at an elevator entrance at the Greenbrier Hotel in West Virginia, in April, 1959, and for the last time in April, 1973, in the lower lobby of the Century Plaza Hotel in Los Angeles. In May, 1973, I was in the railroad station in Albuquerque, New Mexico, when I learned from my grief-stricken youngest son that Dave was dead. It is strange how the exact details of these and some other memories in between, of the time I spent with Dave Hume, stand out with the same clarity as what I was doing when I learned of the bombing of Pearl Harbor, the assassination of John Kennedy, but very few other things. The most eloquent tribute to Dave Hume I have heard was the briefest, coming from a non-medical friend who told me sadly, “He really was a dynamite guy!”
There is almost no aspect of clinical organ transplantation into which Dave Hume did not breathe life. Liver transplantation was no exception. It will surprise no one that his contributions were important and concisely stated, although never published. He gave his observations to one of us (T.E.S.) as personal communications throughout the years and granted permission for their use in a book published 5 years ago.1 They should be listed briefly.
Hume performed one of the earliest auxiliary hepatic transplantations, to the splenic fossa of a recipient whose abdomen could not accommodate the extra organ plus the host liver. Undaunted, he proceeded to remove the total native liver. In another trial, this time with orthotopic transplantation, Hume described hyperacute rejection, which if it was a valid diagnosis was the first and only documented example of this complication destroying a liver homograft. Finally, one of Hume’s recipients who had a hepatoma plus cirrhosis lived for about a year postoperatively after liver replacement, eventually dying with widespread metastases similar to those we have recorded after hepatic transplantation for the indication of malignancy.1
There is no point in saying more about these experiences of Hume, since, important as they were, they were really peripheral to his main interests. Instead, I would like to discuss three aspects of orthotopic liver transplantation that might introduce either new data or new ideas. These concern our survival statistics, hyperacute rejection in livers, and the problem of biliary duct reconstruction.
SURVIVAL STATISTICS
According to the April 1974 report on liver transplantation being prepared by Dr. Carl G. Groth for the American College of Surgeons Registry, about 200 patients have had liver replacement.2 Since 1963 we have contributed 82 to this total, at a rate since 1967 ranging from 6 to 13 per year (Table 1). We have had 18 and 9 recipients, respectively, who have lived for more than 1 and 2 years. Thirteen recipients are still alive from 2 weeks to almost 5 years postoperatively. The 4 longest survivors are 4 years, 10 months; 4 years, 4 months; 3 years, 10 months; and 3 years, 2 months.
Table 1.
Cases of Orthotopic Liver Transplantation Treated in Denver
| Lived | ||||
|---|---|---|---|---|
| Years | Number | 1 year | 2 years | Alive Now |
| 1963–1966 | 6 | 0 | 0 | 0 |
| 1967 | 6 | 1 | 0 | 0 |
| 1968 | 12 | 5 | 2 | 0 |
| 1969 | 6 | 2 | 1 | 1 |
| 1970 | 10 | 2 | 1 | 1 |
| 1971 | 11 | 2 | 2 | 2 |
| 1972 | 11 | 5 | 3 | 3 |
| 1973 | 13 | 1 | 0 | 3 |
| 1974 (to April 1) | 7 | 0 | 0 | 3 |
| 82 | 18 | 9 | 13 | |
There have been 10 late deaths, from 12 to 41 months postoperatively, and for the reasons listed in Table 2. The latest mortality was at 3 years, 5 months, following about of Hemophilus septicemia (OT 19). The homograft arteries contained the same kind of occlusive lesions that have been seen in renal transplants.3
Table 2.
Present Status of 18 One-Year Survivors after Orthotopic Liver Transplantation. Eight Are Still Alive from 14 to 58 Months. The Other 10 Eventually Died from the Causes Listed Below
| OT Number |
Time of Death (months) |
Cause of Death |
|---|---|---|
| 15 | 12 | Recurrent cancer |
| 29 | 12 | Serum hepatitus and liver failure |
| 8 | 13 | Recurrent cancer |
| 58 | 13½ | ?Chronic rejection |
| ?Recurrent hepatitus | ||
| 16 | 13½ | Rejection and liver failure |
| 14 | 14 | Recurrent cancer |
| 54 | 19 | Multiple liver abscesses necessitating retransplantation |
| 36 | 20 | Systemic Nocardia infection and chronic aggressive hepatitis |
| 13 | 30 | Rejection and liver failure following retransplantation |
| 19 | 41 | Hemophilus septicemia and secondary liver and renal failure |
The causes for the high acute failure rate have been discussed elsewhere.1 The single most important factor has been a multiplicity of technical misadventures of which complications of biliary duct reconstruction lead the list (see next section). Poor control of rejection and systemic infection are the next leading causes of death.
THE STRATEGY OF BILE DUCT RECONSTRUCTION
As was just mentioned, the Achilles’ heel of liver transplantation has been biliary duct reconstruction. The different techniques we have used to restore bile drainage include choledochocholedochostomy with or without a T tube (not applicable with biliary atresia), cholecystoduodenostomy after ligation of the graft common duct, and choledochoduodenostomy. Because of continuing dissatisfaction with all of the aforementioned techniques of duct reconstruction, we have recently embarked on a trial of Roux-en-Y cholecystojejunostomy (see later under Possible Solutions). The lethal complications with most or all of these procedures were of two general kinds, one obvious and proved and the other subtle and still speculative.
Statement of The Problem
Mechanical problems
The obvious biliary duct problems have been obstruction and biliary fistula from anastomotic leaks. In our 82 cases of orthotopic liver transplantation the initial biliary reconstruction was eventually shown to be unsatisfactory, leading either to death or early reoperation in 25 cases (Table 3), for the staggering incidence of 30%; the true frequency was undoubtedly even higher, since many patients died so early postoperatively that an incipient duct problem would not yet have been manifest. In 13 of the 25 recipients an effort was made at secondary repair. Even in these 13 reoperated cases the biliary duct problem was an important contributory or the main cause of death in at least 9.
Table 3.
Kind of Primary Bile Duct Reconstruction Used in 82 Consecutive Cases of Orthotopic Liver Transplantation
| Cholecystoduo- denostomy |
Choledochocho- ledochostomy |
Roux-en-Y Cholecystoje- junostomy |
Choledochoduo- denostomy |
Cholecysto- Loop Jejunostomy |
Totol | |
|---|---|---|---|---|---|---|
| Number | 59 | 9 | 8 | 4 | 2 | 82 |
| Obstruction | 15 | 0 | 2 | 0 | 0 | 17* |
| Fistula | 2 | 5 | 0 | 1 | 0 | 8* |
In these 25 cases, reoperations were performed in 13 patients with attempt at duct reconstruction. A satisfactory recovery followed in only 4 of the recipients. Two later died after 6 and 13½ months post-transplantation survival. The other 2 are alive after 3 months and 2 years, respectively. Both survivors now have the final biliary duct reconstruction shown in Fig. 2C.
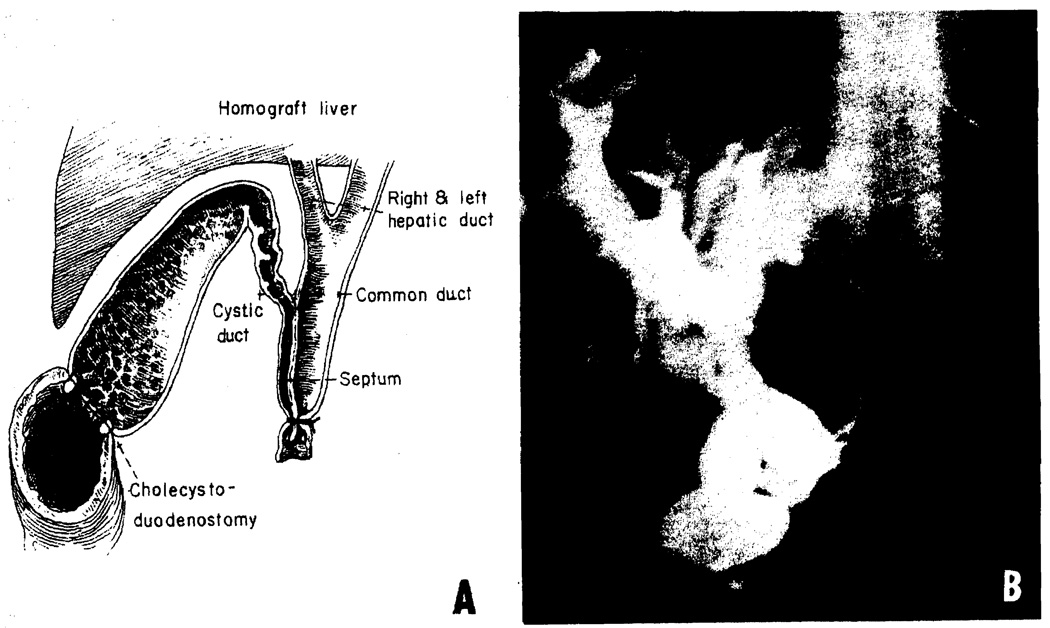
None of the commonly used methods of biliary duct reconstruction was trouble-free (Table 3). With cholecystoduodenostomy, fistulae were uncommon, but obstruction occurred in 25% of cases. The obstructions ranged from accidental acute ligation of the cystic duct before performing cholecystoduodenostomy (Fig. 1A) to delayed obstruction (Fig. 1B) of the cystic duct in some cases, apparently due to cytomegalovirus (CMV) infection weeks or months postoperatively.4 Most commonly, no obvious etiologic cause was evident, accounting for the partial cystic duct obstruction. With choledochocholedochostomy or choledochoduodenostomy the leading complication was biliary fistula formation.
Fig. 1.
Two kinds of biliary duct obstruction after cholecystoduodenostomy. (A) The anatomic basis for a technical error that cost the life of 3 patients. Distal ligation of the double-barreled extrahepatic duct system resulted in total biliary obstruction. This recurrent accident has caused us to perform cholangiography on all liver homografts before transplantation. (B) The kind of biliary obstruction caused by stenosis of the cystic duct. Martineau reported that cytomegalovirus infection of the duct could be responsible for this development.4
There were two obstructions with Roux-en-Y cholecystojejunostomy. In one, the kind of cystic duct ligation shown in Fig. 1A had not been recognized and was not diagnosed until autopsy. In the other case, there was partial obstruction (Fig. 3B) of the cystic duct necessitating conversion of the ultimate hookup shown in Fig. 2C.
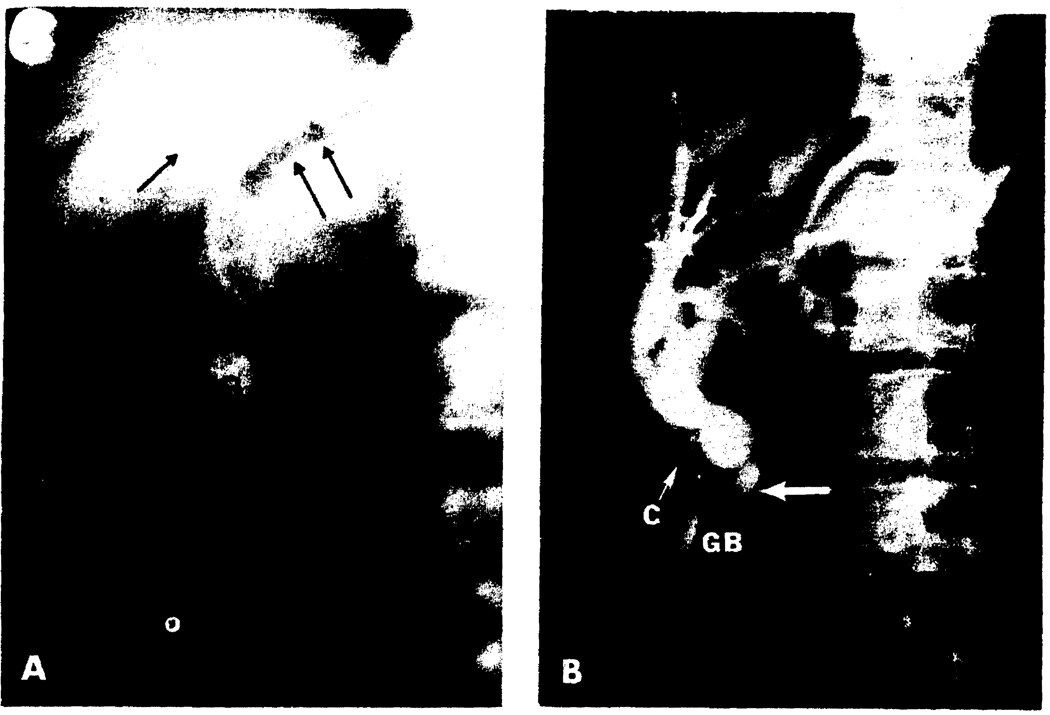
Fig. 3.
Post-transplantation cholangiographic studies. (A) Intravenous cholangiogram in a 47-year-old recipient of a hepatic homograft, the biliary drainage for which was with Roux-en-Y cholecystojejunostomy (Fig. 2B). The patient’s liver function studies were normal at the time of the examination. However, the findings of a very slightly dilated common duct and air in the biliary system (arrows) are suspicious for low-grade obstruction. (B) A percutaneous transhepatic cholangiogram performed 4 weeks post-transplantation because of persistent elevations of the serum bilirubin (8–10 mg/100 ml). At the time of transplantation, biliary drainage had been established with a Roux-en-Y cholecystojejunostomy (Fig. 2B). After obtaining this study, the patient was re-explored, the gallbladder removed, and the Roux limb anastomosed to the dilated common duct (large arrow), as shown in Fig. 2C. The patient’s jaundice rapidly cleared, and he now has normal liver function 3 months post-transplantation. GB, gallbladder; CD, common bile duct; C, cystic duct.
Fig. 2.
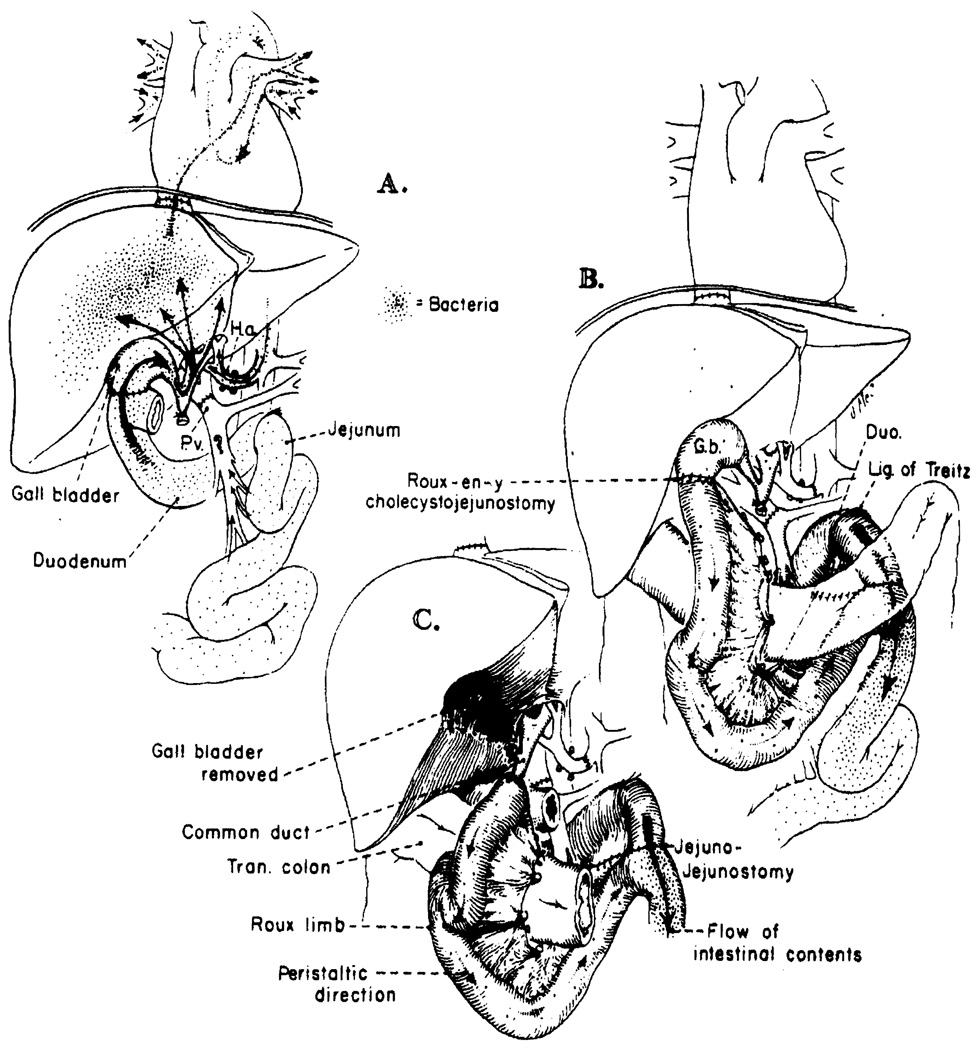
Schematic representation of the bacterial contamination or lack thereof in three different kinds of biliary reconstruction. (A) cholecystoduodenostomy. This extremely simple operation probably carries the greatest risk of graft infection. (B) Roux-en-Y cholecystojejunostomy. This operation protects from hepatic sepsis by placing the new liver outside the main gastrointestinal stream. The isoperistaltic limb is made at least 18 in. long. (C) Roux-en-Y choledochojejunostomy. The end-to-end duct-to-bowel anastomosis is simple if the duct is dilated, as would be the case if a conversion became necessary from B to C.
Special bacteriologic complications
With the well-defined technical complications cited above, clinical evidence of cholangitis (including bacteremia) is easily understandable and is often accompanied by histopathologic findings of cholangitis. In addition, a subtle and as yet hypothetical complication may occur in spite of an apparently satisfactory biliary duct reconstruction. It has been reported by us that systemic infection and even asymptomatic bacteremia are common problems in liver recipients.1 For years there has been strong justification to believe that the transplanted liver itself was the portal of entry by which microorganisms of all kinds gained access to the bloodstream. The variety of bacteria that were cultured from peripheral veins of patients, both early and many months after operation, was strikingly similar to that found in dogs and pigs subjected to liver injury or hepatic transplantation.5 In the human liver recipients with bacteremia the failure to find any other focus of infection necessitated indictment of the homograft (as a site of entry) by the process of exclusion. The two routes of entry could be the portal vein or the duct system, but the former possibility seems less and less important.
The exposed relation of the duct system of the orthotopic liver to gastrointestinal flora is probably the first step in bacterial “leak” through the homograft, which may well be bacteriologically porous without the presence of histopathologically significant cholangitis. This situation after cholecystoduodenostomy is depicted in Fig. 2A. If bacteria enter the circulation through the duct system of hepatic homografts, the logical solution would be to carry out liver transplantation as far removed from the mainstream of the gastrointestinal tract as is possible, as has been illustrated in Figs. 2B and 2C.
Practical Solutions
The five guiding principles we are now attempting to follow are: (1) avoidance of stents or drains; (2) preservation of maximum extrahepatic biliary duct tissue; (3) intensification of diagnostic efforts to differentiate between duct obstruction and rejection, including performance of cholangiography i n all homografts prior to transplantation; (4) early reoperation for suspicion of obstruction; (5) placement of the liver in a relatively bacteria-free relation to the mainstream gastrointestinal continuity. None of the presently available operations completely meets all of these objectives, so that considerable individualization of care is necessary.
A Roux-en-Y cholecystojejunostomy (Fig. 2B), our present procedure of choice, permits all the above listed objectives to be partly met. If postoperative biliary obstruction later develops, the Roux limb can be detached, the gallbladder removed, and an anastomosis performed to the now dilated common duct (Fig. 2C).
The most important objection to this approach is that a Roux-en-Y cholecystojejunostomy can be an extremely difficult added procedure at the end of a long and arduous liver transplantation. The typical adult liver recipient is dying of hepatic failure and has massive collaterals in the small-bowel mesentery. In addition, the mesentery is usually thickened and waterlogged with edema fluid. Construction of a Roux-en-Y isolated limb under these conditions may require 3 to 6 additional hours of operating time in a patient who has already sustained thousands of milliliters of blood loss. Under these adverse conditions, it may be the better part of valor to perform a simple cholecystoduodenostomy with the objective of returning later.
If at the time of transplantation the gallbladder were found to be defective, we would them make a selection between choledochocholedochostomy with T-tube stenting, and a Roux-en-Y choledochojejunostorny.
No matter what the initial procedure, an intense suspicion about the cause for postoperative jaundice is a necessary condition of postoperative management. The simplest precaution is to perform routine intravenous cholangiography in the early postoperative period (Fig. 3A). In almost all of our patients who develop jaundice, transhepatic cholangiography (Fig. 3B) and percutaneous needle biopsy are now performed. Cholangiography has been greatly expedited by our use of the Chiba needle introduced in Japan6,7 and now being used in several American centers. These thin-walled small-caliber needles have great flexibility that permits the diagnostic studies to be done with an improvement in safety (Fig. 3B).
It is not yet established that these changes in policy will improve the results after liver transplantation. Our approach is fundamentally different from that proposed by Calne, who believes that duct-to-duct reconstruction over a T-tube and preservation of the sphincter of Oddi will be the better solution.8 The fact that different methods are being tried to solve a generally recognized set of problems should be of advantage in evolving solutions that can eventually be agreed upon.
HYPERACUTE REJECTION
The pathophysiology of hyperacute rejection has been well worked out in recent years. Fixation of antibody to the transplant is apparently the initiating event, as was first noted in kidney homografts after breaches of red-blood-type barriers.9 In later years the predominant cause of hyperacute rejection has been the presence in the recipient of antigraft cytotoxic antibodies, as was first described by Terasaki10 and confirmed by Kissmeyer-Nielsen11 and others.12,14
In experimental animals of widely disparate relationship, an experiment of nature with hyperacute rejection may be constructed, as for example in transplanting organs from pigs to dogs.15 The serum of dogs contains heterospecific antiporcine cytotoxic antibodies.
With either homografts or heterografts transplanted to recipients that possess preformed antigraft antibodies, the actual destruction of the homograft or heterograft is a complex process in which formed blood elements and clotting factors are entrapped by the graft.13–15 The resulting occlusion of the major vessels causes ischemic necrosis and a characteristic purple or mottled appearance.
It is probable that the kidneys, because of the special filtering properties of the renal microvasculature, are unusually prone to the irreversible consequences of hyperacute rejection. In contrast, the liver may be unusually resistant, as the ability of pig livers to perform rudimentary functions for a number of hours while being perfused with human blood might have predicted.16 Even in the difficult pig-to-dog heterograft model in which kidneys are grossly rejected in a few seconds the liver often does not suffer this fate for more than an hour.15
The resistance of the liver to hyperacute rejection may prove to be sufficiently great to permit transplantation under conditions that would be categorically unacceptable for kidneys. If so, an important stricture on the practicality of the procedure will be eased. Patients dying of liver disease usually cannot wait for an ideal homograft. If transplantation could be conducted in spite of preformed antibody states, patients deprived in the past of a trial at treatment would no longer be arbitrarily excluded. In this connection, our previously unreported experience is of potential interest.
ABO incompatibility
In 1972, 3 patients with ABO-mismatched livers were transplanted to recipients whose conditions were considered sufficiently grave that they could not wait (Table 4). Hyperacute rejection did not occur, and no obvious adverse consequences were seen. The titers of antigraft isoagglutinins were highly variable, and at least in one case reached prodigious levels (Table 5). Eventually the three patients all died, but the pathologic findings were remarkably minor. The homograft of 1 of the patients (OT 60) became partially obstructed by the mechanism of cystic duct stenosis shown in Fig. 2B; following biliary reconstruction, the recipient died of pulmonary sepsis. The other 2 patients had almost no abnormalities in their livers when they died of infectious complications.
Table 4.
Three Cases of Orthotopic Transplantation of ABO-incompatible Livers
| OT Number |
Age (years) |
Diagnosis | ABO Types | Preoperative Isoagglutinin Titer |
Survival (days) |
Cause of Death | Pathologic Changes in Liver |
|---|---|---|---|---|---|---|---|
| Donor Recipient | |||||||
| 59 | 11/12 | Biliary atresia | AB → A | 1:4 (anti-B) | 173 | Septicemia (from liver?) | (Arterial and arteriolar narrowing (post rejection) |
| 60 | 46 | Primary biliary cirrhosis | AB → A | 1:32 (anti-B) | 61 | Septicemia (from liver?) | Mild cytomegaloviral infection |
| Pulmonary emboli | No rejection | ||||||
| 61 | 42 | Postnecrotic cirrhosis | A → O | 1:512 (anti-A) | 41 | Disseminated herpes and cytomegalovirus | Cytomegalovirus infection |
| No rejection | |||||||
| Pulmonary emboli | |||||||
| Brain infarction |
Table 5.
Serial Antigraft Isoagglutinin Titers in the Three Recipients of ABO-incompatible Livers Described in Table 4
| Post-transplantation Day | OT 59 (Anti-B) |
OT 60 (Anti-B) |
OT 61 (Anti-A) |
|---|---|---|---|
| 0 | 1:4 | 1:32 | 1:512 |
| 1 | 1:4 | 1:16 | |
| 3 | 1:1 | 1:4 | 1:64 |
| 5 | 1:1 | 1:2 | 1:64 |
| 7 | 1:1 | 1:8 | 1:2048 |
| 9 | 1:4 | 1:64 | 1:8192 |
| 11 | 1:4 | 1:64 | 1:8192 |
| 13 | 1:4 | 1:32 | 1:4096 |
| 15 | 1:4 | 1:16 | 1:2048 |
| 17 | — | 1:8 | 1:1024 |
| 19 | 1:2 | 1:4 | 1:1024 |
| 21 | 1:1 | 1:4 | 1:512 |
| 28 | 1:1 | 1:2 | 1:256 |
| 35 | 1:2 | 1:2 | 1:128 |
| 42 | 1:2 | 1:2 | |
| 49 | 1:2 | 1:1 | |
| 56 | 1:2 | 1:8 | |
| 63 | 1:2 | ||
| 70 | 1:2 | ||
| 77 | 1:8 | ||
| 84 | 1:4 |
Cytotoxic antibodies
This ability of the liver to remain healthy under conditions that would be predictably harmful to most kidneys is a noteworthy feature that has been seen in other preformed antibody situations. During the last 2 years, 3 patients with antidonor cytotoxic antibodies have been given livers. In all 3 cases cytotoxins were also present against most of the donors of an indifferent lymphocyte screening panel. Thus the prospects of finding a liver without a positive cytotoxic antibody cross-match were considered nil. As a consequence, a decision was made to proceed despite the potentially adverse prognostic implications.
None of the 3 patients developed hyperacute rejection, although they all eventually died from weeks to months later (Table 6), in 2 cases with relatively good livers. In OT 71, the homograft seemed to have been severely damaged by ischemia, as well as cellular rejection, although its poor initial function could have been a manifestation of acute antibody-mediated injury. After 10 days the organ was removed and replaced by a chimpanzee heterograft, against which the recipient cytotoxins also reacted. The chimpanzee liver functioned for most of the 14 subsequent days of the patient’s life. Upon pathologic examination the initial homograft had many focal areas of necrosis compatible with the diagnosis of ischemic injury. In contrast, the heterograft was well preserved. Centrilobular cholestasis was a prominent feature. Otherwise, there was little evidence of rejection. This was our third trial of chimpanzee-to-man heterotransplantation, the other two having been previously reported.1,17
Table 6.
Three Cases of Orthotopic Hepatic Transplantation in which the Recipients Had Antidonor Cytotoxic Antibodies
| OT Number | Age (years) |
Diagnosis | Preoperative Cytotoxicity Titer |
Survival | Cause of Death | Pathologic Changes in Liver |
|---|---|---|---|---|---|---|
| 58 | 34 | Chronic aggressive hepatitis | 1:2 | 407 days | Stopped immunosuppression | Resolution of previous obstructive changes at 8½-month biopsy (no autopsy) |
| Hepatic insufficiency | ||||||
| 63 | 49 | Primary biliary cirrhosis | 1:64 | 26 days | Gastrointestinal hemorrhage | Normal liver |
| 71 | 1 11/12 | Biliary atresia | 1:16 (homograft) | (Removal of the graft at 10 days) | Acute rejection, cellular and humoral | |
| 1:16 (heterograft) | 14 days after retransplantation | Pulmonary edema, bronchial hemorrhage | No evidence of cellular rejection. | |||
| Centrilobular cholestasis. |
It goes without saying that preformed antibody states should be avoided if at all possible. However, the experience cited both with the ABO red cell and cytotoxic antibodies makes it possible that this kind of positive cross-match is not an absolute but only a relative contraindication for liver transplantation.
SUMMARY
An account of 82 consecutive orthotopic liver transplantations carried out in Colorado is given. Eighteen patients have lived for 1 year postoperatively, and the longest survival is almost 5 years.
Much of the high failure rate is attributable to the technical difficulty of the operation and especially to complications of the biliary duct reconstruction. A strategy for biliary duct reconstruction is advanced that is designed to place the liver as far outside the mainstream gastrointestinal tract as possible, to avoid unnecessary sacrifice of biliary duct tissue and to facilitate reoperation at the slightest sign of a technical complication.
An experience is cited in 6 patients who received 6 homografts and 1 chimpanzee heterograft in which livers were transplanted against preferred anti-red-cell isoagglutinins or leukocyte cytotoxins. Hyperacute rejection did not occur, nor was there convincing evidence of antibody-mediated rejection in any case. The conclusion is that the liver may be more resistant to hyperacute rejection than is the kidney.
Supplementary Material
Acknowledgments
This work was supported by research grants from the Veterans Administration, by grants AI-AM-08898 and AM-07772 from the National Institutes of Health, and by grants RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources. National Institutes of Health.
REFERENCES
- 1.Starzl TE, Putnam CW. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders; 1969. (For those interested in Hume’s life and work, his personal reports of these cases are on pages 279 and 502.) [Google Scholar]
- 2.Groth CG. personal communication. [Google Scholar]
- 3.Starzl TE, Porter KA, Schroter G, et al. N Engl J Med. 1973;289:82. doi: 10.1056/NEJM197307122890207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martineau G, Porter KA, Corman J, et al. Surgery. 1972;72:604. [PMC free article] [PubMed] [Google Scholar]
- 5.Brettschneider L, Tong JL, Boose DS, et al. Arch Surg. 1968;97:313. doi: 10.1001/archsurg.1968.01340020177021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchiya Y. Jap J Gastroenterol. 1969;66:438. [Google Scholar]
- 7.Okuda K, Tanikawa K, Emura T, et al. Dig Dis. 1974;19:21. doi: 10.1007/BF01073350. [DOI] [PubMed] [Google Scholar]
- 8.Calne RY, Williams R. Br Med J. 1968;4:535. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE. Experience in Renal Transplantation. Philadelphia: W. B. Saunders; 1964. [Google Scholar]
- 10.Terasaki PI, Marchioro TL, Starzl TE. In: Histocompatibility Testing. van Rood JJ, Amos DB, editors. Washington, D.C: National Academy of Sciences, National Research Council; 1965. p. 83. [Google Scholar]
- 11.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Lancet. 1966;2:662. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 12.Williams GM, Hume DM, Hudson RP, Jr, et al. N Engl J Med. 1968;279:611. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 13.Simpson KM, Bunch DL, Amemiya H, et al. Surgery. 1970;68:77. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Boehmig HJ, Amemiya H, et al. N Engl J Med. 1970;283:383. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giles GR, Boehmig HJ, Lilly J, et al. Transplant Proc. 1971;2:522. [PMC free article] [PubMed] [Google Scholar]
- 16.Eiseman B, Liem DS, Raffucci F. Ann Surg. 1965;162:329. doi: 10.1097/00000658-196509000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giles GR, Boehmig HJ, Amemiya H, et al. Transplant Proc. 1970;2:506. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.