Abstract
Double-strand breaks in chromosomal DNA are repaired efficiently in eukaryotic cells through pathways that involve direct religation of broken ends, or through pathways that utilize an unbroken, homologous DNA molecule as a template for replication. Pathways of repair that require homology initiate with the resection of the 5' strand at the break site, to uncover the 3' single-stranded DNA that becomes a critical intermediate in single-strand annealing and in homologous strand exchange. Resection of the 5' strand is regulated to occur most efficiently in S and G2 phases of the cell cycle when sister chromatids are present as recombination templates. The mechanisms governing resection in eukaryotes have been elusive for many years, but recent work has identified the major players in short-range processing of DNA ends as well as the extensive resection of breaks that has been observed in vivo. This review focuses on the Mre11/Rad50/Xrs2(Nbs1) complex and the Sae2(CtIP) protein and their roles in initiating both short-range and long-range resection, the effects of topoisomerase-DNA conjugates on resection in vivo, and the relationship between these factors and NHEJ proteins in regulating 5' strand resection in eukaryotic cells.
1. Introduction
The repair of DNA double-strand breaks (DSBs) through mechanisms of homologous recombination initiates with the processing of the broken DNA strands, primarily removal of the 5' strand [1,2]. This process of 5' strand resection is essential for the eventual establishment of a Rad51 filament on the 3' strand, which performs the homology search for a target to use as a template for replication [3]. In eukaryotes, the 3' single-stranded DNA (ssDNA) generated during resection is first coated by the RPA complex before exchange for Rad51 by mediator proteins [4], and plays an important biological role in recruitment and activation of Mec1(ATR) in the replication checkpoint [5]. In budding yeast, both resection and checkpoint activation occur much more efficiently in S and G2 phases of the cell cycle when sister chromatids are present [6,7]. 5' strand resection is thus a critical event in the initiation of homologous recombination as well as in S phase cell cycle checkpoint control in eukaryotic cells. Resection enzymes and their roles in vivo have recently been reviewed in DNA Repair [8], thus this review focuses primarily on the mechanisms by which the Mre11/Rad50/Xrs2(Nbs1) (MRX(N)) complex and Sae2(CtIP) facilitate DNA end processing.
2. DSB resection in budding yeast
DSB resection was observed decades ago [1] but only in the last few years have the molecular details of this process become more apparent [8-10]. Most of these details have been elucidated in S. cerevisiae, where resection of DSBs has primarily been measured during meiotic recombination, or at sites of inducible endonuclease-generated breaks in vegetatively growing cells. The extent of resection can vary depending on the presence of functional strand invasion machinery, as well as the availibility and location of a homologous template, which also determines the kinetics of repair [11,12]. Under circumstances where strand invasion is blocked by the absence of recombination factors or a homologous template, tens of thousands of nucleotides can be removed from the 5' strand at the break site [11,13]. When a homologous template is available and strand invasion can occur, the extent of resection is significantly less (~2 to 4 kb in the case of allelic recombination and ~3 to 6 kb in the case of ectopic recombination), which correlates well with the faster kinetics of gene conversion between homologs relative to recombination between different chromosomes [11]. The rate of resection has been reported to be approximately 4 kb/hr in vivo at different chromosomal locations [11-14], although the initiation of resection is not necessarily concerted [2].
3. Mechanisms of MRX/Sae2 stimulation of resection
3.1. MRX and Sae2 introduction
Mre11/Rad50 complexes are found in all organisms and are important for efficient DSB repair, as well as for signaling of DSBs that occurs through the Tel1(ATM) protein kinase in eukaryotes [15,16]. The Mre11 protein is in the lambda phosphatase family of phosphoesterases, and recombinant Mre11 proteins from several divergent species exhibit exo- and endonuclease activity in vitro [17-24]. Exonuclease activity in manganese occurs in the 3' to 5' direction, while endonuclease activity is observed in manganese on single-strand/double-strand transitions and on hairpin loops. In magnesium, Mre11 does not act as an exonuclease but rather shows weak endonucleolytic activity on the 5' strand of linear DNA ends [25-27]. Mre11 binds to the Rad50 protein, which is in the Structural Maintenance of Chromosomes (SMC) family of large ATPases containing long coiled-coils that separate Walker A and Walker B ATP-binding domains. Rad50 proteins exhibit ATPase and adenylate kinase activities in vitro [28-30], and the human MRN complex was also shown to catalyze opening of duplex DNA ends in a manner that is stimulated by ATP [31]. In eukaryotic cells, Mre11 and Rad50 also associate with a third protein, Nbs1(NBN, Nibrin, Xrs2 in budding yeast), which regulates the activities of the other components and is essential for the functional interactions between the MRX(N) complex and Tel1(ATM)[31-35].
In organisms that undergo sexual reproduction, MRX(N) complexes are essential for the processing of DSBs that are created by Spo11, a toposiomerase-like enzyme related to TOP6A from archaea [36] that forms a covalent attachment with DNA at a catalytic tyrosine residue [37,38]. In many organisms, MRN also regulates telomere maintenance through telomerase activity and also through telomerase-independent recombination [8], and in some organisms MRN is important for efficient nonhomologous end joining (NHEJ)[39]. All of these scenarios involve DNA ends, which are bound by MRN and in some cases also processed through the activity of Mre11 [25-27]. MRX complexes are required for the association of DSB ends in vivo in budding yeast [40,41], and have been observed tethering DNA ends together in vitro [42]. Mre11 dimers from P. furiosus were crystallized with two molecules of DNA associating end to end [43], suggesting that Mre11 may hold the ends of DNA molecules together in the context of Mre11/Rad50 complexes.
The Sae2 protein in budding yeast does not associate physically with MRX but deletion strains share many phenotypes with strains expressing hypomorphic alleles of the MRX complex, most notably Mre11 nuclease-deficient alleles and rad50S alleles that block meiotic DSB processing [44-46]. Sae2-deficient yeast strains were also found to exhibit defects in mitotic DSB repair, specifically those involving hairpin structures as intermediates [47-49]. Rattray et al observed that large palindromic duplications resulted from misrepair of a DSB within an inverted repeat in sae2Δ cells, which was postulated to result from fold-back and replication of 3' single-stranded DNA intermediates [47,48]. Lobachev et al also found that the processing of spontaneous DNA breaks at sites of inverted repeats was altered in sae2Δ, MRX-deficient, Mre11 nuclease-deficient, and rad50S strains, which was also suggested to arise because of a failure to process hairpin DNA structures [49]. Recombinant Sae2 binds linear DNA independently of MRX and exhibits endonuclease activity on 5' flap structures as well as on hairpin DNA in vitro [50]. Hairpin structures were cleaved within single-stranded regions adjacent to the hairpin rather than at the hairpin tip, thus it was suggested that Mre11 nuclease activity might help to create single-stranded DNA which could then be cleaved by Sae2.
The MRX complex and the Sae2/Com1 protein have been associated with DSB resection for many years because mutations in the genes encoding these factors generate a complete block to 5' strand removal in meiosis. rad50S and mre11S hypomorphic mutations, Mre11 nuclease-deficient alleles, and null mutations in Sae2 yield covalent complexes of Spo11 on the 5' strand of DNA at the break site [24,44-46,51,52]. The strict requirement for Mre11 nuclease activity and for the Sae2 protein is not as pronounced in vegetatively growing yeast cells, however, where resection of endonuclease-induced breaks is only delayed in the absence of MRX [53], and nearly unaffected by mutations that inactivate Mre11 nuclease activity [52]. Deletion of Sae2 also causes a delay of resection, similar to that seen with rad50S strains [54].The primary difference between meiotic and mitotic DSBs is the presence of covalent Spo11-DNA intermediates in meiosis, which presumably are removed by a combination of MRX nuclease activity and the activity of Sae2.
3.2. Long-range resection of DSBs in yeast
Long-range resection of endonuclease-induced breaks in budding yeast was shown to be dependent on two redundant pathways: the 5' to 3' exo- and endonuclease Exo1, or an alternative pathway consisting of the 5' flap endonuclease Dna2 in combination with the helicase Sgs1 and its partners RmiI and Top3 [13,55-57]. Although inactivation of either of these pathways alone has little effect on DSB resection, removal of both blocks all long-range (0.5 to 30 kb) resection. In cells lacking Exo1 and either Sgs1 or Dna2, the broken DNA ends were processed very slowly on the 5' strand but only 50 to 300 nt was removed [13,55]. This short-range processing was found to be dependent on the MRX complex and the Sae2 protein.
MRX deficient yeast cells with functional Exo1 and Dna2/Sgs1/Rmi1/Top3 pathways show deficiencies in resection, primarily at early time points and at sites close to the site of the break [13,55]. The study by Zhu et al examined the kinetics of resection in wild-type vs. rad50Δ cells, showing that 5' strand degradation very close to the break site is significantly delayed in cells lacking MRX [13]. The rate of resection far from the break (28 kb) was not as significantly altered in the absence of MRX, but ~20% of the breaks were never resected in rad50Δ cells. These observations clearly point to a role for MRX in the initiation of resection; not that MRX is essential for the start of resection at every break site, but that it accelerates the rate of initiation. This also suggests that initiation of resection is a rate-limiting step, consistent with a recent study showing that initiation of resection likely occurs with a stochastic, slower rate compared to the rate measured for processive resection far from the break site, based on quantitative measurement of ssDNA intermediates in vivo [2]. The observation that overexpression of Exo1 can at least partially suppress the sensitivity of MRX-deficient strains to DNA damaging agents [58-62] is also consistent with the idea that one of the primary functions of MRX in DSB resection is to help load Exo1 (or Dna2/Sgs1/Rmi1/Top3) onto DNA ends.
It is also clear that the stimulatory effect of MRX on the extensive resection of 5' strands can occur independently through both redundant pathways: Exo1 and Dna2/Sgs1/RmiI/Top3 [13]. Evidence for MRX stimulation of Exo1 comes from the comparison of sgs1Δ and sgs1Δrad50Δ cells [13], where it is clear that the sgs1Δrad50Δ cells show a marked decrease in resection at sites close to the break point. Similarly, MRX also appears to be important for the Sgs1 pathway since an exo1Δrad50Δ strain exhibits a population of unresected DSBs, which differs dramatically from exo1Δ cells that are essentially wild-type in resection close to the DSB [13].
3.3. Recruitment of Exo1 and Dna2
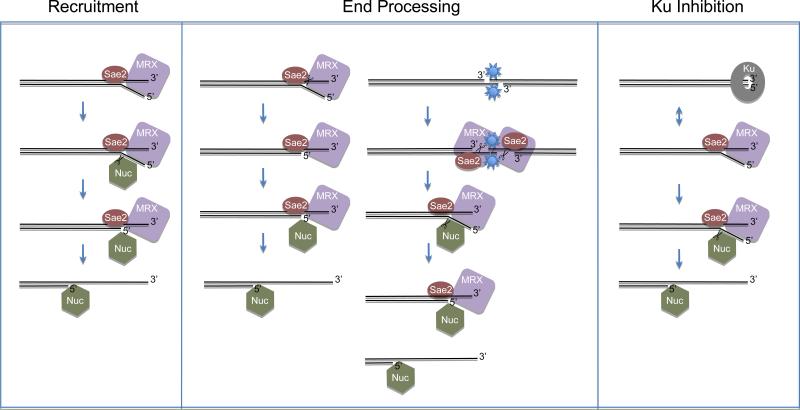
Recombinant yeast Exo1 was used to reconstitute resection in vitro and to determine the effects of MRX and Sae2 on its activity [25]. These experiments showed that there are at least 3 distinct mechanisms that contribute to the effects of MRX and Sae2 on yeast Exo1 (Fig. 1). The first of these occurs through recruitment of the Exo1 protein, which is a relatively inefficient, distributive 5' to 3' exonuclease as well as 5' flap endonuclease in vitro [63]. Exo1 showed little activity on linear DNA when present at a molar excess over ends of 3-5:1, yet under these conditions MRX and Sae2 dramatically stimulated Exo1 nuclease activity [25]. At higher ratios of Exo1 to DNA, Exo1 activity was observed in the absence of MRX and Sae2, but MRX and Sae2 still markedly increased the extent of resection. Most of this effect was attributed to an increase in Exo1 binding to DSB ends in the presence of MRX and Sae2. This effect was not dependent on the nuclease activity of Mre11, as a nuclease-deficient MRX complex exhibited ~80% of the activity of wild-type MRX. Protein-protein interactions were not observed between Exo1 and either MRX or Sae2, so it is possible that a change in DNA structure, created by MRX and Sae2, may increase the affinity of Exo1 for DNA. Previous experiments with the human MRN complex suggest that this is likely a branched structure [31,35], consistent with the observation that Exo1 preferentially binds to branched DNA in comparison to fully complementary ends [25]. In addition, MRX and Sae2 stimulated the endonucleolytic products generated by Exo1 on the 5' strand of DNA, also consistent with the presence of an opened structure at the DNA end.
Figure 1.
Schematic diagram of proposed mechanisms by which the MRX complex and Sae2 facilitate the activity the downstream enzymes, shown as “Nuc”, to represent either Exo1 or Dna2(Sgs1/Rmi1/Top3) nucleases. See text for details. Recruitment (left panel): MRX and Sae2 promote nuclease binding to DNA by creating a higher-affinity binding site for the nuclease (here shown as a branched DNA structure). Nuclease bound to the MRX/Sae2-bound DNA makes an endonucleolytic cut (scissors) on the 5' strand. Further resection occurs by nucleases independently of MRX/Sae2. The event that triggers MRX and Sae2 dissociation is not known. End processing on unmodified ends (center panel, left side): MRX and Sae2 cleave the 5' strand of the DNA end through endonucleolytic activity (scissors); in the case of Exo1, this short 3' overhang facilitates nuclease binding and digestion. End processing on modified ends (center panel, right side): MRX and Sae2 cleave an adduct from the DNA (shown here as the 5' strand but in theory could be the 3' strand), allowing access of nucleases to the ends. Alternatively, Dna2 may also perform adduct cleavage [56]. Ku inhibition (right panel): the Ku heterodimer binds avidly to DNA ends; this occurs in competition with MRX binding. Some of the time MRX wins this competition and blocks Ku from binding; this allows loading of Exo1 and resection. Dna2 binding to DNA ends in vivo is relatively unaffected by Ku, while Exo1 binding is strongly affected [64], thus the nuclease in this case is likely to be Exo1. The exact configuration of the proteins on the DNA is not known, although Exo1 has been shown to crosslink to a DNA end on the 5' strand, while Sae2 crosslinks to the DNA on the 3' strand in strand-specific crosslinking experiments [25].
The recruitment of Exo1 by MRX has also been observed in vivo, where loading of Exo1 at an HO-induced break was observed to be significantly decreased in mre11Δ or rad50Δ strains [64]. A similar effect was also observed with Dna2 in MRX-deficient strains [64], also suggesting that the stimulatory effect of MRX is substantially attributable to recruitment of the enzymes responsible for long-range DNA end processing.
3.4. DNA end processing by MRX/Sae2
In addition to Exo1 recruitment in the presence of MRX/Sae2, a less efficient mechanism of Exo1 stimulation was also observed which acts at the step of DSB processing [25]. In previous studies, Mre11/Rad50 complexes from P. furiosus, D. radiodurans, E. coli, S. cerevisiae, and humans were all found to exhibit 3' to 5' exonuclease activity and endonuclease activity in manganese in vitro [17-24]. However, in more physiological conditions with magnesium, the P. furiosus, S. cerevisiae, and humans complexes showed very weak, if any, 3' to 5' exonuclease activity, but did show endonuclease activity on the 5' strand of linear DNA ends [25-27]. The predominant cleavage sites for the 5' endonuclease activity were between 10 and 50 nt from the DSB. In resection assays with either P. furiosus MR or yeast MRX, incubation of the linear DNA substrate with MR was found to increase subsequent resection by downstream enzymes (the helicase/nuclease complex HerA/NurA in the case of P. furiosus and Exo1 with the S. cerevisiae proteins), even when the MR(X) was removed prior to this incubation [25,27]. Limited 5' strand resection by an unrelated 5' to 3' exonuclease also promoted Exo1 activity [25]. Thus, removal of the 5' strand to generate a short 3' overhang has a stimulatory effect on the downstream enzymes that act together with Mre11/Rad50 complexes, which also affects binding of the downstream enzymes to the processed (3' overhang) DNA ends [27]. In vivo, MRX/Sae2-dependent processing at endonuclease-induced break sites can only be observed directly when Exo1 and either Sgs1 or Dna2 is inactivated [13,55]. Under these conditions, about 100 nt [55] (up to 200 or 300 nt in a minority of cells [13]) were found to be removed from the 5' strand. The 5' endonucleolytic cleavage of DSBs observed in vitro is likely the source of this inefficient processing seen in exo1Δsgs1Δ cells in vivo. One question that is difficult to answer in vivo is whether MRX and Sae2 actually cleave the 5' strand at a break under circumstances where the Exo1 and Dna2 pathways are functional. In vitro experiments with MRX and Exo1 suggest that the Exo1 endonuclease activity on the 5' strands at a break is much more robust than MRX/Sae2 activity [25], thus it is likely that in a wild-type cell, Exo1 (or Dna2/Sgs1/Rmi1/Top3) is recruited to DNA ends and performs the majority of the processing of the 5' strand.
While Mre11 nuclease activity appears to play a relatively minor role in the resection of DSBs induced by endonucleases in budding yeast, nuclease-deficient MRX complexes show only partial complementation of mre11Δ strains when assayed for survival of ionizing radiation (IR) [52]. Since MRX is recruited to DSB sites generated by either an endonuclease or by IR [65], it is possible that the requirement for MRX in the repair of IR-induced damage is due to the fact that IR can generate base damage, protein-DNA adducts, or other altered structures at the end of DSBs that require Mre11 nuclease activity for removal. These complicated structures would likely not be present on all the ends, thus one might expect a hypomorphic phenotype with the nuclease-deficient mutant. MRX-dependent generation of oligonucleotides from linear DNA was also observed in Xenopus egg extracts, where the oligonucleotide products of 5' strand resection were shown to regulate ATM protein kinase activity [66]. These oligonucleotides may thus constitute an additional link between DNA end processing and checkpoint activation in eukaryotic cells.
Interestingly, budding yeast cells expressing an Mre11 nuclease-deficient allele are extremely sensitive to IR when also deleted for Dna2 and the Pif1 helicase, despite the presence of wild-type Exo1 in these cells [56]. The Dna2 nuclease function, rather than the helicase function, was found to be responsible for this effect. Thus the function of Dna2 is partially redundant with MRX with respect to processing of IR-induced damage, and this function is distinct from that of Exo1. Expression of a helicase-proficient, nuclease-deficient form of Dna2 was also shown to be lethal in cells expressing an Mre11 nuclease-deficient allele, suggesting that structures generated by Dna2 (presumably at replication forks) specifically require Mre11-dependent processing in the presence of a helicase+/nuclease- form of Dna2 [56].
3.5. Rad50S mutations and DSB processing
rad50S mutations in MRX completely block meiotic DSB resection [46] but only delay resection in vegetatively growing yeast cells, similar to strains lacking Sae2 [25,54]. The rad50S mutations were identified by mutagenesis of the N-terminal ATPase domain and genetic screening for alleles that abolish Rad50 function in meiosis but show nearly wild-type function in vegetatively growing cells [46]. Despite the location of these mutations in the ATPase domain, rad50S mutations do not block the ATPase activity of MRX [67]. Functionally equivalent mutations were also identified in Mre11 [24,51,52]. Overexpression of Sae2 can suppress the delay of resection observed in rad50S strains [54], suggesting that these mutations may alter the relationship between MRX and Sae2 on DNA ends. The MRX complex remains at DSB sites longer in rad50S strains [68], also similar to that seen in sae2Δ strains, thus the unknown event(s) that precipitate loss of MRX from DNA ends involve both MRX and Sae2 interactions and are abrogated or delayed when Rad50 proteins contain the rad50S mutation. Sae2 still localizes to DSBs in rad50s cells, however, consistent with the ability of Sae2 to bind to DNA independently of MRX, thus the defect is not simply in Sae2 binding [68]. The molecular basis of the rad50S defect is not understood, but reconstitution of Exo1-mediated resection in vitro also showed a defect in Exo1 activity in the presence of Rad50S MRX in comparison to the wild-type complex [25], thus it may be possible to dissect the properties of this mutant with purified components.
rad50S forms of the mammalian MRN complex have also been studied extensively in the mouse, where it is clear that Rad50S MRN generates constitutive checkpoint signaling by ATM [69-71]. rad50S mice exhibit increased apoptosis in hematopoietic cells and spermatogonia, leading to early death due to anemia, and these outcomes can be suppressed by loss of even one allele of ATM, Chk2, or p53 [69,72]. Increased signaling in rad50S yeast cells is also observed in a mec1 background (in which DNA damage-induced checkpoint signaling is Tel1-dependent), leading to constitutive formation of activated Rad53/Rad9 complexes [73]. Rad50S MRX(N) complexes behave genetically as hypermorphic alleles relative to the function of MRN in ATM signaling, in that their phenotypes can be abrogated by mutations that disrupt the MRN complex or ATM [69,73].
Usui et al demonstrated that rad50S or a sae2Δ deletion in yeast can rescue MMS sensitivity and checkpoint functions in a mec1Δ deletion strain [73]. This result suggests a relationship between 5' strand processing and Tel1(ATM) activation, such that stabilization of the end of the 5' strand promotes Tel1(ATM) kinase activity, while removal of the 5' strand inhibits activity. Evidence in support of this model was also obtained in human cell extracts, where ATM activation was found to decrease proportionally with 5' strand processing, opposite to that of ATR activation which depends on extensive, RPA-coated ssDNA [74]. ATM activation in vitro through the human MRN complex was shown to be dependent on opening of linear duplexes to expose ssDNA ends [35], also consistent with the idea that the single strands, particularly the 5' strand, are an essential component of the ATM activation mechanism. It is possible that Rad50S MRX(N) complexes may hold the 5' strand of DNA at a break in a configuration that is not as suitable for endonucleolytic cleavage by Mre11 or Sae2, and in this way may activate Tel1(ATM) longer than would be the case in wild-type cells. This would conceivably yield novel phenotypes associated with ATM hyperactivation that would be very different from cells lacking MRX(N).
The defect in Rad50S MRX(N) complexes may be related to processing of topoisomerase lesions, since cells from rad50S mice are hypersensitive to camptothecin, a toposiomerase I poison, yet show little sensitivity or chromosomal aberrations in the context of IR or UV irradiation, or other DNA damaging agents [70]. There is not necessarily a perfect correspondence between camptothecin sensitivity and hypersignaling through Tel1, however, since Mre11 nuclease-deficient mutants are camptothecin sensitive yet do not rescue signaling in mec1 strains [73].
3.6. MRX/Sae2 and processing of topoisomerase covalent conjugates
In contrast to vegetatively growing cells, budding yeast strains expressing nuclease-deficient Mre11 alleles show a complete block in meiotic DSB resection [52], because the putative type II topoisomerase Spo11 is covalently attached to the 5' strands at every break [37]. Spo11 was subsequently found to be removed from chromosomes with a short oligonucleotide (~12 nt or 21-37 nt) covalently attached [75], showing that DSB processing during meiosis initiates with an endonucleolytic incision of the DNA rather than direct reversal of the tyrosyl-DNA bond. Removal of Spo11 by this mechanism was also shown to be dependent on MRX and Sae2 [75], consistent with their activity as 5' endonucleases in vitro [25,50]. Rec12(Spo11) removal from DSBs in fission yeast is similarly dependent on MRN and Ctp1 and requires Mre11 nuclease activity, although in this case only one size of oligonucleotide (13 to 29 nt) was observed attached to Rec12 [76-78].
The strict requirement for Mre11 nuclease activity in Spo11 removal also suggests the possibility that MRX may be involved in removing topoisomerase II covalent complexes in vegetatively growing cells. This hypothesis was tested in budding yeast using the same immunoprecipitation/Tdt labeling technique used to assay Spo11-DNA processing, but generation of the topoisomerase II-oligonucleotide product was not altered in rad50S or sae2Δ cells [75]. The idea that MRX(N) complexes may cleave covalent topoisomerase conjugates is still of interest, however, because other studies have found that MRX and Sae2 do contribute to the survival of budding yeast cells during exposure to camptothecin, a topoisomerase I poison, in a parallel pathway to that of Tdp1, a 3' tyrosyl-DNA phosphodiesterase [79-81]. A study in fission yeast also found that rad50S and nuclease-deficient forms of the MRN complex exhibit hypersensitivity to both topoisomerase I and II poisons (camptothecin and TOP-53, respectively) relative to methylmethanesulfonate and IR sensitivity [82]. In addition, TOP-53-induced topoisomerase II covalent complexes were observed at elevated levels in rad50Δ, Mre11 nuclease-deficient, and ctp1Δ (functional equivalent of Sae2 in fission yeast) strains. Studies with the E. coli Mre11/Rad50 complex, SbcCD, also showed that the complex can cleave DNA on the 5' strand with a biotin-avidin complex attached to that strand, suggesting that removal of large protein complexes from DNA ends through endonucleolytic cleavage of the DNA is a conserved activity of this complex [83].
In contrast to the situation in yeast, Mre11 nuclease activity appears to be critically important in mammalian cells, as a nuclease-deficient mutation in Mre11 causes early embryonic lethality in the mouse and prevents proliferation of mouse embryonic fibroblasts [84], similar to genetic knockout of the Mre11 gene [85]. The reasons for this striking phenotype are not clear, although it was shown using a conditional allele that a failure in DNA repair (as measured by IR sensitivity, genomic instability, RPA and Rad51 foci after IR, and ISceI-induced homology-directed repair), rather than a defect in ATM activation, are responsible [84]. These findings show that Mre11 nuclease activity plays an important role in DNA repair at non-meiotic DSBs, to the extent that Mre11 null cells and cells expressing the Mre11 nuclease-deficient allele appeared identical for all of the repair phenotypes. It is not clear if removal of topoisomerase II covalent complexes constitutes a large part or any part of the role of Mre11 nuclease activity in human cells, as a novel phosphodiesterase (Tdp2/TTRAP) has been identified in human cells that directly removes 5' tyrosyl linkages [86], similar to the 3' tyrosyl removal catalyzed by Tdp1 [87], and this was shown to be the major source of this activity in human cells. It is certainly possible that MRN could provide an alternative pathway of endonucleolytic removal of topoisomerase II, however, in parallel to the direct removal by Tdp2. It will be important to determine what proportion of topoisomerase I and II covalent complexes are removed via endonucleolytic cleavage of the DNA adjacent to the attachment site, and if the MRN complex has a role in these cleavage events in mammalian cells.
3.7. MRX/Sae2 and competition with Ku
In addition to recruitment of downstream resection enzymes and processing of DNA ends to facilitate 5' strand resection, there is at least one other mechanism by which MRX promotes DSB resection in eukaryotic cells which involves factors important for NHEJ. NHEJ is often considered to be a pathway that competes with homologous recombination, particularly in the G1 phase of the cell cycle. In mammalian cells, NHEJ operates throughout the cell cycle [88], however, and it is still not clear what controls pathway choice at DSBs that occur during S and G2. A number of studies have shown that DSB resection is increased in efficiency in the absence of Ku, and to a lesser extent in the absence of other core NHEJ factors Dnl4 (ligase IV) and Lif1 (Xrcc4) [2,89-91]. This effect is primarily observed in G1 and is dependent on the MRX complex and Exo1, although resection is limited to the region proximal to the break site [2,64,90]. Resection is not increased by deletion of Ku in G2 cells, but can be delayed by overexpression of Ku [90]. These results suggest that Ku inhibits MRX(N) binding to DNA, which has also been observed in vitro with recombinant human Ku and MRN complexes [31].
While Ku inhibits MRX-dependent resection (at least in G1), it appears that the converse is also true: MRX inhibits Ku activity at DNA ends. MRX deletion strains show a dramatic increase in the binding of Ku to DSBs [64,89]. The mre11-3 nuclease deficient allele of Mre11 can suppress this effect [89], showing that the presence of MRX, rather than its DNA processing activity, is important in inhibiting Ku binding. Inhibiting Ku is not the only role of MRX in the processing of DNA breaks, however, as deletion of Ku in a strain lacking MRX does not restore resection rates to wild-type levels and only partially restores radiation resistance [64,90,92]. In vitro experiments have demonstrated that Ku strongly inhibits Exo1-mediated resection and that MRX/Sae2 can at least partially recover Exo1 activity under these conditions [64], consistent with the idea that MRX and Ku compete for DNA ends.
The role of NHEJ factors in regulating DSB resection is intimately linked to cell cycle control of resection, since NHEJ is used as an alternative to homologous recombination in G1, but not in G2 [6,7,90,93]. Cyclin-dependent kinase (CDK) activity is essential for long-range resection, which favors homologous recombination during S and G2, a period of the cell cycle when sister chromatids are present. Limited DSB resection can occur in G1 [65] which increases in the absence of Ku, but even this is limited to DNA regions close to the break site [2]. The difference between G1 and G2 cells may be explained by differential binding of Ku, because Ku was reported to be associated with DSBs more efficiently in G2 relative to G1, in a manner that is dependent on CDK activity [93]. However, another study reported that levels of Ku at break sites were similar in G1 vs G2, thus it is unclear at this point how and if Ku loading onto DNA ends is regulated during the cell cycle phase [90]. Ku does not appear to be a direct target of CDK [93], so the mechanism by which CDK inhibits NHEJ during G2 is unknown.
3.8. Sae2/CtIP and regulation of resection by CDK
Identification of CDK targets that regulate resection has been a major interest in the field. One of the only CDK phosphorylation events that has been shown to be important for resection in vivo is the single CDK site on the Sae2 protein, the endonuclease that cooperates with MRX in recuitment of Exo1 and Dna2/Sgs1 and DNA end processing. Mutation of S267 in Sae2 to alanine blocks DSB resection in vivo [94], although we do not yet know what the phosphorylation does to activate the protein. Monomeric Sae2 (both wild-type and S267A) produced in E. coli is active in DNA binding and endonuclease activity while dimeric Sae2 is relatively inactive [50,95], thus it is possible that the CDK phosphorylation in yeast helps to activate the inactive dimer, perhaps in conjunction with Tel1/Mec1 (ATM/ATR) phosphorylation events that occur in S and G2 [96]. Sae2 and MRX can bind independently to DNA breaks, shown in vivo [68] and in vitro [50], and Sae2 was found to form foci at time points when MRX foci were dissociating, almost always before the appearance of Rad52 foci [68]. It is attractive to speculate that post-translational modification of Sae2 may regulate the kinetics of these events, but whether this is the case is not known. Mutation of five Tel1/Mec1 sites in Sae2 to alanine generated MMS sensitivity that approached the sensitivity of a sae2Δ strain [96], suggesting that these sites are important for Sae2 function in vivo. However, mutation of the same sites in vitro caused a deficiency in nuclease activity, even though the E. coli-expressed protein is not phosphorylated [50]. Thus these mutations in SQ/TQ sites cause functional problems independently of phosphorylation and make the investigation of the roles of these modifications difficult in vivo.
The functional ortholog of Sae2 in other eukaryotes is the CtIP/Ctp1 protein, which shows almost no sequence similarity to Sae2 apart from a short stretch of amino acids in the C-terminus of the proteins, yet has been shown to be remarkably similar in its functions in DSB resection [97-102]. Ctp1 in fission yeast was shown to be required for efficient resection of DSBs and is regulated at the level of transcription to be present at high levels in S and G2 phases of the cell cycle [103]. In chicken DT40 cells and in human cells in culture, CtIP also is important for resection in S and G2 and is phosphorylated by CDK at two sites that control the efficiency of resection [101,102]. One of these sites (S327) also regulates association of CtIP with the Brca1 tumor suppressor [104], a factor which has been known to be critical for homologous recombination for some time [105] but the exact function of which remains unknown. CtIP in DT40 cells was also shown to act in G1 phase, in addition to its known roles in S and G2, although its function in G1 cells appears to be in the microhomology-mediated end-joining (MMEJ) pathway [101]. A function for CtIP in MMEJ was not unexpected because Sae2 also is an important component of Ku-independent MMEJ in budding yeast [106].
There are a few differences between CtIP and Sae2, most notably the fact that Ctp1 in S. pombe and CtIP in mammalian cells require MRN for localization to DSBs [103] whereas Sae2 localizes independently [68,99]. Mammalian CtIP binds directly to the MRN complex in a manner that involves both the N-terminus and the C-terminus of CtIP, with the N-terminus being particularly important for DNA damage localization [99,107]. CtIP in mammals is significantly larger than yeast Sae2 (897 a.a. vs. 345 a.a.) and also is lacking in recognizable domains apart from a coiled-coil region identified in the N-terminus that mediates homodimerization [108,109].
CtIP was also shown to be required for DSB resection in Xenopus egg extracts, where the MRN complex and ATM were found to be essential for CtIP loading onto DNA ends [100]. The kinase activity of ATM was required for CtIP localization on damaged DNA, but the identity of the target of ATM is currently unknown. You et al. also identified a DNA-binding motif within the central region of CtIP that is necessary and sufficient for localization to DNA damage sites, although binding of a small fragment containing this motif was independent of ATM activity [100]. The authors suggest that modification of full-length CtIP (by ATM or other enzymes) after DNA damage might uncover this DNA-binding motif to promote damage localization.
4. Summary and future questions
We now understand much more about the mechanisms of DSB resection than we did a few years ago. The enzymes responsible for long-range resection in S. cerevisiae have been identified as Exo1 and Dna2/Sgs1/Rmi1/Top3 [13,55-57], and it appears that at least Exo1 function in resection has been conserved in human cells [110]. The RecQ helicase BLM also has been shown to stimulate the activity of human Exo1 in vitro [111], and to affect DSB resection in human cells [57], suggesting that it may be an important component of the system in higher organisms. The WRN helicase and Dna2 have also been implicated in DSB resection in Xenopus egg extracts [112], so it is possible that both the Exo1 and Dna2 pathways may operate in higher eukaryotes.
It is now clear that the MRX complex does not catalyze long-range resection directly, but rather is involved in a number of activities that together promote long-range resection: recruitment of downstream enzymes, processing of DNA ends to promote recuitment and remove covalent adducts, and blocking the binding of NHEJ factors to DSB ends (summarized in Fig. 1). We also know that CDK phosphorylation regulates Sae2/CtIP activity at DNA breaks [94], but relatively little detail has emerged about how this affects the activities of these proteins, or how CDK activity might block the inhibitory effects of Ku on DSB resection. Finally, there is evidence that ATM activity is important for CtIP loading on DNA but the target and mechanism of this effect is still under investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Sun H, Treco D, Szostak JW. Extensive 3'-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- 2.Zierhut C, Diffley JF. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008;27:1875–1885. doi: 10.1038/emboj.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovejoy CA, Cortez D. Common mechanisms of PIKK regulation. DNA Repair (Amst) 2009;8:1004–1008. doi: 10.1016/j.dnarep.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, Haber JE, Foiani M. DNA endresection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mimitou EP, Symington LS. DNA end resection: many nucleases make light work. DNA Repair (Amst) 2009;8:983–995. doi: 10.1016/j.dnarep.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimitou EP, Symington LS. Nucleases and helicases take center stage in homologous recombination. Trends Biochem Sci. 2009;34:264–272. doi: 10.1016/j.tibs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Huertas P. DNA resection in eukaryotes: deciding how to fix the break. Nat Struct Mol Biol. 17:11–16. doi: 10.1038/nsmb.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung WH, Zhu Z, Papusha A, Malkova A, Ira G. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet. 6:e1000948. doi: 10.1371/journal.pgen.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaze MB, Pellicioli A, Lee SE, Ira G, Liberi G, Arbel-Eden A, Foiani M, Haber JE. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol Cell. 2002;10:373–385. doi: 10.1016/s1097-2765(02)00593-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Amours D, Jackson SP. The Mre11 complex: at the crossroads of dna repair and checkpoint signalling. Nat. Rev. Mol. Cell. Biol. 2002;3:317–327. doi: 10.1038/nrm805. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 17.Paull TT, Gellert M. The 3' to 5' exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol. Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- 18.Trujillo KM, Sung P. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50/Mre11 complex. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.M105482200. [DOI] [PubMed] [Google Scholar]
- 19.Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- 20.Connelly JC, de Leau ES, Okely EA, Leach DR. Overexpression, purification, and characterization of the SbcCD protein from Escherichia coli. J. Biol. Chem. 1997;272:19819–19826. doi: 10.1074/jbc.272.32.19819. [DOI] [PubMed] [Google Scholar]
- 21.Hopfner KP, Karcher A, Shin D, Fairley C, Tainer JA, Carney JP. Mre11 and rad50 from pyrococcus furiosus: cloning and biochemical characterization reveal an evolutionarily conserved multiprotein machine. J Bacteriol. 2000;182:6036–6041. doi: 10.1128/jb.182.21.6036-6041.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamble VA, Misra HS. The SbcCD complex of Deinococcus radiodurans contributes to radioresistance and DNA strand break repair in vivo and exhibits Mre11-Rad50 type activity in vitro. DNA Repair (Amst) 9:488–494. doi: 10.1016/j.dnarep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 1998;17:6412–6425. doi: 10.1093/emboj/17.21.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 25.Nicolette ML, Lee K, Guo Z, Rani M, Chow JM, Lee SE, Paull TT. A direct role for Mre11/Rad50/Xrs2 and Sae2 in 5’ strand resection of DNA double-strand breaks. 2010. submitted. [DOI] [PMC free article] [PubMed]
- 26.Hopkins B, Paull TT. The P. furiosus Mre11/Rad50 complex promotes 5' strand resection at a DNA double-strand break. Cell. 2008;135:250–260. doi: 10.1016/j.cell.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopkins BB, Paull TT. THE P. FURIOSUS MRE11/RAD50 COMPLEX PROMOTES 5' STRAND RESECTION THROUGH END PROCESSING AND NURA RECRUITMENT TO DNA BREAKS. 2010. submitted.
- 28.Bhaskara V, Dupre A, Lengsfeld B, Hopkins BB, Chan A, Lee JH, Zhang X, Gautier J, Zakian VA, Paull TT. Rad50 Adenylate Kinase Activity Regulates DNA Tethering by Mre11/Rad50 complexes. Molecular Cell. 2007;25:647–661. doi: 10.1016/j.molcel.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Trujillo KM, Van Komen S, Roh DH, Krejci L, Lewis LK, Resnick MA, Sung P, Tomkinson AE. Effect of amino acid substitutions in the rad50 ATP binding domain on DNA double strand break repair in yeast. J Biol Chem. 2005;280:2620–2627. doi: 10.1074/jbc.M410192200. [DOI] [PubMed] [Google Scholar]
- 30.Hopfner KP, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP, Tainer JA. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 31.Paull TT, Gellert M. Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes & Dev. 1999;13:1276–1288. doi: 10.1101/gad.13.10.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakada D, Matsumoto K, Sugimoto K. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 2003;17:1957–1962. doi: 10.1101/gad.1099003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 36.Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 37.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 38.Ehmsen KT, Heyer WD. Biochemistry of Meiotic Recombination: Formation, Processing, and Resolution of Recombination Intermediates. Genome Dyn Stab. 2008;3:91–164. doi: 10.1007/7050_2008_039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18:134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 40.Kaye JA, Melo JA, Cheung SK, Vaze MB, Haber JE, Toczyski DP. DNA breaks promote genomic instability by impeding proper chromosome segregation. Curr Biol. 2004;14:2096–2106. doi: 10.1016/j.cub.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Lobachev K, Vitriol E, Stemple J, Resnick MA, Bloom K. Chromosome fragmentation after induction of a double-strand break is an active process prevented by the RMX repair complex. Curr Biol. 2004;14:2107–2112. doi: 10.1016/j.cub.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 42.Trujillo KM, Roh DH, Chen L, Van Komen S, Tomkinson A, Sung P. Yeast xrs2 binds DNA and helps target rad50 and mre11 to DNA ends. J Biol Chem. 2003;278:48957–48964. doi: 10.1074/jbc.M309877200. [DOI] [PubMed] [Google Scholar]
- 43.Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, Moiani D, Carney JP, Russell P, Tainer JA. Mre11 dimers coordinate DNA end bridging and nucleaseprocessing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prinz S, Amon A, Klein F. Isolation of COM1, a new gene required to complete meiotic double-strand break-induced recombination in Saccharomyces cerevisiae. Genetics. 1997;146:781–795. doi: 10.1093/genetics/146.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keeney S, Kleckner N. Covalent protein-DNA complexes at the 5' strand termini of meiosis- specific double-strand breaks in yeast. Proc. Natl. Acad. Sci. 1995;92:11274–11278. doi: 10.1073/pnas.92.24.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. doi: 10.1016/0092-8674(90)90524-i. [DOI] [PubMed] [Google Scholar]
- 47.Rattray AJ, McGill CB, Shafer BK, Strathern JN. Fidelity of mitotic double-strand-break repair in Saccharomyces cerevisiae: a role for SAE2/COM1. Genetics. 2001;158:109–122. doi: 10.1093/genetics/158.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rattray AJ, Shafer BK, Neelam B, Strathern JN. A mechanism of palindromic gene amplification in Saccharomyces cerevisiae. Genes Dev. 2005;19:1390–1399. doi: 10.1101/gad.1315805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobachev KS, Gordenin DA, Resnick MA. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell. 2002;108:183–193. doi: 10.1016/s0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 50.Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 Is an Endonuclease that Processes Hairpin DNA Cooperatively with the Mre11/Rad50/Xrs2 Complex. Mol Cell. 2007;28:638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nairz K, Klein F. mre11S--a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes & Dev. 1997;11:2272–2290. doi: 10.1101/gad.11.17.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreau S, Ferguson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivanov EL, Sugawara N, White CI, Fabre F, Haber JE. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clerici M, Mantiero D, Lucchini G, Longhese MP. The Saccharomyces cerevisiae Sae2 protein promotes resection and bridging of double strand break ends. J Biol Chem. 2005;280:38631–38638. doi: 10.1074/jbc.M508339200. [DOI] [PubMed] [Google Scholar]
- 55.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budd ME, Campbell JL. Interplay of Mre11 nuclease with Dna2 plus Sgs1 in Rad51-dependent recombinational repair. PLoS ONE. 2009;4:e4267. doi: 10.1371/journal.pone.0004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsubouchi H, Ogawa H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol. Cell. Biol. 1998;18:260–268. doi: 10.1128/mcb.18.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamankhah M, Fontanie T, Xiao W. The Saccharomyces cerevisiae mre11(ts) allele confers a separation of DNA repair and telomere maintenance functions. Genetics. 2000;155:569–576. doi: 10.1093/genetics/155.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreau S, Morgan EA, Symington LS. Overlapping functions of the Saccharomyces cerevisiae Mre11, Exo1 and Rad27 nucleases in DNA metabolism. Genetics. 2001;159:1423–1433. doi: 10.1093/genetics/159.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis LK, Karthikeyan G, Westmoreland JW, Resnick MA. Differential suppression of DNA repair deficiencies of Yeast rad50, mre11 and xrs2 mutants by EXO1 and TLC1 (the RNA component of telomerase) Genetics. 2002;160:49–62. doi: 10.1093/genetics/160.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SE, Bressan DA, Petrini JH, Haber JE. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair (Amst) 2002;1:27–40. doi: 10.1016/s1568-7864(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 63.Tran PT, Erdeniz N, Dudley S, Liskay RM. Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst) 2002;1:895–912. doi: 10.1016/s1568-7864(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 64.Shim EY, Chung W-H, Nicolette ML, Yu Z, Davis M, Zhu Z, Paull TT, Ira G, Lee SE. Saccharomyces cerevisiae Mre11/Rad50/Xrs2 and Ku proteins regulate association of Exo1 and Dna2 with DNA breaks. EMBO J. 2010 doi: 10.1038/emboj.2010.219. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barlow JH, Lisby M, Rothstein R. Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell. 2008;30:73–85. doi: 10.1016/j.molcel.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jazayeri A, Balestrini A, Garner E, Haber JE, Costanzo V. Mre11-Rad50-Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008;27:1953–1962. doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paull TT, Nicolette ML. (unpublished observations)
- 68.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Morales M, Theunissen JW, Kim CF, Kitagawa R, Kastan MB, Petrini JH. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as aDNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morales M, Liu Y, Laiakis EC, Morgan WF, Nimer SD, Petrini JH. DNA damage signaling in hematopoietic cells: a role for Mre11 complex repair of topoisomerase lesions. Cancer Res. 2008;68:2186–2193. doi: 10.1158/0008-5472.CAN-07-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usui T, Petrini JH, Morales M. Rad50S alleles of the Mre11 complex: questions answered and questions raised. Exp Cell Res. 2006;312:2694–2699. doi: 10.1016/j.yexcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JH. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Usui T, Ogawa H, Petrini JH. A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol Cell. 2001;7:1255–1266. doi: 10.1016/s1097-2765(01)00270-2. [DOI] [PubMed] [Google Scholar]
- 74.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33:547–558. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neale MJ, Pan J, Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartsuiker E, Mizuno K, Molnar M, Kohli J, Ohta K, Carr AM. Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, butRec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol Cell Biol. 2009;29:1671–1681. doi: 10.1128/MCB.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milman N, Higuchi E, Smith GR. Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-oligonucleotide complexes. Mol Cell Biol. 2009;29:5998–6005. doi: 10.1128/MCB.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rothenberg M, Kohli J, Ludin K. Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS Genet. 2009;5:e1000722. doi: 10.1371/journal.pgen.1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C, Pouliot JJ, Nash HA. Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proc Natl Acad Sci U S A. 2002;99:14970–14975. doi: 10.1073/pnas.182557199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng C, Brown JA, You D, Brown JM. Multiple endonucleases function to repair covalent topoisomerase I complexes in Saccharomyces cerevisiae. Genetics. 2005;170:591–600. doi: 10.1534/genetics.104.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simon JA, Szankasi P, Nguyen DK, Ludlow C, Dunstan HM, Roberts CJ, Jensen EL, Hartwell LH, Friend SH. Differential toxicities of anticancer agents among DNA repair and checkpoint mutants of Saccharomyces cerevisiae. Cancer Res. 2000;60:328–333. [PubMed] [Google Scholar]
- 82.Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol Cell. 2009;33:117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Connelly JC, de Leau ES, Leach DR. Nucleolytic processing of a protein-bound DNA end by the E. coli SbcCD (MR) complex. DNA Repair (Amst) 2003;2:795–807. doi: 10.1016/s1568-7864(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 84.Buis J, Wu Y, Deng Y, Leddon J, Westfield G, Eckersdorff M, Sekiguchi JM, Chang S, Ferguson DO. Mre11 nuclease activity has essential roles in DNA repair and genomic stability distinct from ATM activation. Cell. 2008;135:85–96. doi: 10.1016/j.cell.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Y, Weaver DT. Conditional gene targeted deletion by Cre recombinase demonstrates the requirement for the double-strand break repair Mre11 protein in murine embryonic stem cells. Nucleic Acids Res. 1997;25:2985–2991. doi: 10.1093/nar/25.15.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5'-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 87.Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286:552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- 88.Beucher A, Birraux J, Tchouandong L, Barton O, Shibata A, Conrad S, Goodarzi AA, Krempler A, Jeggo PA, Lobrich M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Hefferin ML, Chen L, Shim EY, Tseng HM, Kwon Y, Sung P, Lee SE, Tomkinson AE. Role of Dnl4-Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol. 2007;14:639–646. doi: 10.1038/nsmb1261. [DOI] [PubMed] [Google Scholar]
- 90.Clerici M, Mantiero D, Guerini I, Lucchini G, Longhese MP. The Yku70-Yku80 complex contributes to regulate double-strand break processing and checkpoint activation during the cell cycle. EMBO Rep. 2008;9:810–818. doi: 10.1038/embor.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishna S, Wagener BM, Liu HP, Lo YC, Sterk R, Petrini JH, Nickoloff JA. Mre11 and Ku regulation of double-strand break repair by gene conversion and break-induced replication. DNA Repair (Amst) 2007;6:797–808. doi: 10.1016/j.dnarep.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bressan DA, Baxter BK, Petrini JH. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Shim EY, Davis M, Lee SE. Regulation of repair choice: Cdk1 suppresses recruitment of end joining factors at DNA breaks. DNA Repair (Amst) 2009;8:1235–1241. doi: 10.1016/j.dnarep.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature. 2008;455:689–692. doi: 10.1038/nature07215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paull TT, Lengsfeld B. (unpublished observations)
- 96.Baroni E, Viscardi V, Cartagena-Lirola H, Lucchini G, Longhese MP. The functions of budding yeast Sae2 in the DNA damage response require Mec1- and Tel1-dependent phosphorylation. Mol Cell Biol. 2004;24:4151–4165. doi: 10.1128/MCB.24.10.4151-4165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Penkner A, Portik-Dobos Z, Tang L, Schnabel R, Novatchkova M, Jantsch V, Loidl J. A conserved function for a Caenorhabditis elegans Com1/Sae2/CtIP protein homolog in meiotic recombination. EMBO J. 2007;26:5071–5082. doi: 10.1038/sj.emboj.7601916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uanschou C, Siwiec T, Pedrosa-Harand A, Kerzendorfer C, Sanchez-Moran E, Novatchkova M, Akimcheva S, Woglar A, Klein F, Schlogelhofer P. A novel plant gene essential for meiosis is related to the human CtIP and the yeast COM1/SAE2 gene. EMBO J. 2007;26:5061–5070. doi: 10.1038/sj.emboj.7601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.You Z, Shi LZ, Zhu Q, Wu P, Zhang YW, Basilio A, Tonnu N, Verma IM, Berns MW, Hunter T. CtIP links DNA double-strand break sensing to resection. Mol Cell. 2009;36:954–969. doi: 10.1016/j.molcel.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem. 2009;284:9558–9565. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Limbo O, Chahwan C, Yamada Y, de Bruin RA, Wittenberg C, Russell P. Ctp1 is a cell-cycle-regulated protein that functions with Mre11 complex to control double-strand break repair by homologous recombination. Mol Cell. 2007;28:134–146. doi: 10.1016/j.molcel.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–7720. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 105.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 106.Lee K, Lee SE. Saccharomyces cerevisiae Sae2-and Tel1-dependent single-strand DNA formation at DNA break promotes microhomology-mediated end joining. Genetics. 2007;176:2003–2014. doi: 10.1534/genetics.107.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan J, Chen J. N terminus of CtIP is critical for homologous recombination-mediated double-strand break repair. J Biol Chem. 2009;284:31746–31752. doi: 10.1074/jbc.M109.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubin MJ, Stokes PH, Sum EY, Williams RS, Valova VA, Robinson PJ, Lindeman GJ, Glover JN, Visvader JE, Matthews JM. Dimerization of CtIP, a BRCA1- and CtBP-interacting protein, is mediated by an N-terminal coiled-coil motif. J Biol Chem. 2004;279:26932–26938. doi: 10.1074/jbc.M313974200. [DOI] [PubMed] [Google Scholar]
- 109.Kim H, Huang J, Chen J. CCDC98 is a BRCA1-BRCT domain-binding protein involved in the DNA damage response. Nat Struct Mol Biol. 2007;14:710–715. doi: 10.1038/nsmb1277. [DOI] [PubMed] [Google Scholar]
- 110.Bolderson E, Tomimatsu N, Richard DJ, Boucher D, Kumar R, Pandita TK, Burma S, Khanna KK. Phosphorylation of Exo1 modulates homologous recombination repair of DNA double-strand breaks. Nucleic Acids Res. 38:1821–1831. doi: 10.1093/nar/gkp1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liao S, Toczylowski T, Yan H. Identification of the Xenopus DNA2 protein as a major nuclease for the 5'->3' strand-specific processing of DNA ends. Nucleic Acids Res. 2008;36:6091–6100. doi: 10.1093/nar/gkn616. [DOI] [PMC free article] [PubMed] [Google Scholar]