Abstract
This study aimed to determine the prevalence of metabolic syndrome (MeS) and its individual components in Jordanian children and adolescents aged 7–18 years and determine the factors that are associated with clustering of metabolic abnormalities. MeS was defined using the International Diabetes Federation (IDF) definition. The prevalence of MeS was estimated from 512 subjects who had complete information on all MeS components. The prevalence of MeS according to IDF criteria was 1.4% in subjects aged between 10 and 15.9 years and 3.6% in subjects aged between 16 and 18 years. When categorized according to body mass index (BMI), the prevalence of the MeS was 15.1% in obese subjects, compared to 0.3% in subjects with normal BMI, and 3.0% in overweight subjects. In conclusion, our results indicate that although the prevalence of MeS is low in Jordanian children and adolescents, a large proportion of them had one or two metabolic abnormalities.
1. Introduction
Previous studies revealed that cardiovascular diseases (CVD) begin in childhood [1, 2], and endothelial damage may occur early in the life of children with lipid abnormalities [3, 4]. The Cardiovascular Risk in Young Finns Study [5] showed a positive correlation between the number of risk factors at age12–18 years and carotid artery wall thickness at age 33–39 years. Furthermore, Morrison et al. [6] showed that the metabolic syndrome (MeS) in childhood predicts MeS and type 2 diabetes mellitus in adulthood.
While earlier studies in childhood had focused on the clustering of risk factors for cardiovascular diseases [7, 8], recent studies had reported the prevalence of MeS during childhood [9–11]. Several definitions have been proposed for MeS in children and some of these definitions followed the ATP III guidelines [9–14]. The International Diabetes Federation (IDF) consensus worldwide definition of the MeS is divided according to age-groups because of developmental challenges presented by age-related differences in children and adolescents: 6 –<10 years, 10 –<16 years, and ≥16 years [15, 16].
The prevalence of the MeS varied by the definition used, the weight status of the children, sex, and ethnicity. Cook et al. [9] reported a prevalence of 4.2% among children and adolescents of 12–19 years of age using the third National Health and Nutrition Examination Survey (NHANES III) data. A prevalence of 3.6% in youth 8–17 years of age was reported by researchers from the Bogalusa Heart Study [17]. Much higher prevalence rates were reported in children who were overweight or obese [9, 13], where the prevalence reached 50% in severely obese youngsters [13].
Data pertaining to MeS in children are scarce and most studies were conducted in developed countries with very little is known about this syndrome in children in the middle east and Arab countries including Jordan. Therefore, this study aimed to determine the prevalence of MeS and its individual components in Jordanian children and adolescents aged 7–18 years and determine the factors that are associated with clustering of metabolic abnormalities.
2. Methods
2.1. Sampling
A national population-based household sample was selected from the 12 governorates of Jordan. These 12 governorates belong to the three regions of the country, namely, the north, middle, and south. A complex multistage sampling technique was used to select the households, taking into consideration the geographic distribution of the population as well as the urban-rural residence. As the population is covered by an extensive network of health centers and because the study procedures have to take place in a medical setting, the selection of households was health center-oriented. The health director in each governorate was contacted and asked to identify at least two health centers in which to conduct the study procedures. He was asked to select the health centers so that urban and rural areas in each governorate are represented and the selected centers have enough space to host the study team, participants, and equipments. A total of 31 health centers were identified and people served by these centers were targeted. A systematic sample of households was selected from the population served by the selected health centers. The number of selected households was approximately proportional to the population in each region.
In each selected area, one day before data collection, 2-membered teams (a male and a female each) visited the selected households, explained the purpose of the study, and invited all members aged ≥7 years, who were available at the time of visit, to attend the health centre in the next day after an overnight fast. Subjects on regular medications were asked not to take their medications early at that day and to bring all their medications with them to the survey site. To encourage participation, the study team worked in all week days including official holidays except Fridays during the entire study period. Of the 1600 families invited to participate, 1282 (80.1%) families (5640 subjects aged between 7 and 90 years) took part in this study. Of the total 1290 children and adolescents (aged 18 years or less) who were invited to participate, 1046 (81.1%) subjects responded and participated in the study.
2.2. Data Collection
All field work was carried out between 1st of July and 30th of November, 2009. Participants attended the health centers in the morning (8–11 am) with a minimum fasting time of 10 hours. A pilot-tested structured questionnaire was prepared and administered by trained interviewers to collect relevant information that is necessary to answer the current research question and other selected research questions that will be addressed in separate publications. The questionnaire sought information on demographics, medical history and medication use, level of education, average monthly family income, and amount of exercise per week. Information on other variables necessary for other research questions was collected. Two questions related to physical activity were modified from the Leisure Time Exercise Questionnaire to assess the physical activity of participants [18]. The two questions individually assessed moderate and vigorous activity asking, “In the past week, how many hours did you spend doing moderate activity" and “In the past week, how many hours did you spend doing vigorous activity". More than 10 examples of common specific activities were given after each question.
2.3. Measurements and Laboratory Analysis
Anthropometric measurements including weight, height, waist, and hip circumferences were measured with the subjects wearing light clothing and no shoes. Height and weight were determined to the nearest 0.1 cm. WC was measured to the nearest centimeter using nonstretchable tailors measuring tape at the midpoint between the bottom of the rib cage and above the top of the iliac crest during minimal respiration. Hip circumference was measured at the widest part of the body below the waist. Readings of systolic (SBP) and diastolic blood pressure (DBP) were taken in duplicates with the subject seated and the arm at heart level, after at least 5 minutes of rest, using standardized mercury sphygmomanometer with appropriate arm cuff length. The mean of these two determinations was used to express the individual's systolic and diastolic blood pressures.
For laboratory analysis and all biochemical measurements, two sets of fasting blood samples were drawn from a cannula inserted into the antecubital vein into sodium fluoride potassium oxalate tubes for glucose and lithium heparin vacuum tubes for lipids. Samples were centrifuged at 3000 rpm for 10 min within 1 hour at the survey site, and plasma was transferred to separate labeled tubes and transferred immediately in cold boxes filled with ice to the central laboratory of the National Center for diabetes and endocrinology. All biochemical measurements were carried out by the same team of laboratory technicians and the same method throughout the study period.
Triglycerides values were obtained on COBAS INTEGRA 700 with the cassette COBAS INTEGRA Triglycerides using enzymatic, colorimetric method (GPO/PAP) with glycerol phosphate oxidase and 4-aminophenazone. Total cholesterol was analyzed using enzymatic, colorimetric method with COBAS INTEGRA Cholesterol Gen.2. HDL cholesterol and LDL cholesterol values were obtained on COBAS INTEGRA 700 using homogeneous enzymatic colorimetric assay. Other laboratory analysis was also performed for several blood constituents, but it is not described here because they are irrelevant to the current report.
2.4. Definition of Variables
To define MeS according to IDF definition, age was divided into three groups: 7 to <10 years, 10 to <16, and 16 years and older. The MeS was not diagnosed in children younger than 10 years old as stated in the IDF definition [15, 16]. For children aged between 10 and 15 years, a diagnosis of the MeS was made as the presence of abdominal obesity (WC ≥90th percentile or adult cut-off if lower) and the presence of two or more of the other components: Elevated TG (≥1.7 mmol/L (≥150 mg/dL)), low HDL cholesterol (<1.03 mmol/L (<40 mg/dL)), high blood pressure (Systolic ≥130 mm Hg or diastolic ≥85 mm Hg), and elevated blood glucose (≥5.6 mmol/L (100 mg/dL)). IDF criteria for adults [19] were used to define MeS in those aged 16 years or more. Although that the MeS was not diagnosed for children younger than 10 years, other components were defined, for all subjects who had complete information on that component, as those for children aged between 10 and 15 years. Of children and adolescents selected, 10 subjects had missing information on at least one of the anthropometric measures, blood pressure measurements, and lipid measurements. However, only 671 subjects had blood glucose measurements. MeS was defined and reported for subjects aged 10 years or more who had complete information on all components of MeS (n = 512).
Body mass index (BMI) was calculated as the ratio of weight in kilograms to the square of height in meters, and then standardized for sex and age using data from the Center for Disease Control and Prevention (USA) [20]. Overweight was defined as a BMI between the 85th and 95th percentiles, and obesity as a BMI greater than or equal to the 95th percentile [21].
2.5. Data Management and Statistical Analysis
Data were entered into computer using the Statistical Package for Social Sciences software, SPSS (SPSS Inc., Chicago, IL, USA) version 15. Participants' characteristics were described using means, standard deviations, and percentages wherever appropriate. The differences between percentages were analyzed using χ2 test, and the differences between two means were analyzed using independent sample t-test. Multivariable logistic regression models were fit to determine factors associated with various clustering of CVD risk factors (outcome variables): at least one metabolic abnormality, at least two metabolic abnormalities, and at least three metabolic abnormalities. Logistic regression was conducted using backward stepwise elimination method to reach the “best fitting model" that includes the significant variables only. Similar analysis was repeated for each outcome variable. A P-value of less than .05 was considered statistically significant.
3. Results
3.1. Participants' Characteristics
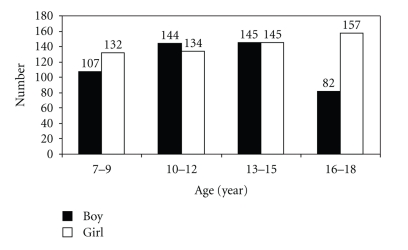
This study included a total of 1046 subjects (478 boys and 568 girls) aged between 7 and 18 years with a mean of 12.5 (3.2) year. Figure 1 shows the age distribution for boys and girls. About 46.7% of subjects were living in the north, 43.5% in the middle, and 9.8% in the south of Jordan. Table 1 shows their anthropometric, lipid profile, and clinical characteristics according to age and gender.
Figure 1.
The age distribution of Jordanian 478 boys and 568 girls aged between 7 and 18 years.
Table 1.
Anthropometric, lipid profile, and clinical characteristics for boys and girls according to age.
| Boys | Girls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤12 | >12 | ≤12 | >12 | |||||||
| N | Mean (SD) | N | Mean (SD) | P-value | N | Mean (SD) | N | Mean (SD) | P-value | |
| Weight (Kg) | 190 | 28.3 (8.3) | 283 | 52.1 (15.5) | <.005 | 208 | 29.8 (10.0) | 357 | 52.0 (13.2) | <.005 |
| Weight Z score | 190 | −0.81 (0.48) | 283 | 0.54 (0.88) | <.005 | 208 | −0.87 (0.62) | 357 | 0.50 (0.82) | <.005 |
| Height (cm) | 190 | 130.1 (9.3) | 238 | 157.0 (13.0) | <.005 | 208 | 131.3 (10.3) | 357 | 154.7 (7.3) | <.005 |
| Height Z score | 190 | −0.91 (0.53) | 283 | 0.61 (0.74) | <.005 | 208 | −1.04 (0.73) | 357 | 0.61 (0.52) | <.005 |
| Body mass index (BMI) Kg/m2 | 190 | 16.5 (3.3) | 283 | 20.8 (4.56) | <.005 | 208 | 16.9 (3.5) | 357 | 21.6 (4.5) | <.005 |
| BMI Z score | 190 | −0.56 (0.73) | 283 | 0.38 (0.98) | <.005 | 208 | −0.62 (0.73) | 357 | 0.36 (0.96) | <.005 |
| Waist circumference (cm) | 193 | 55.7 (7.9) | 283 | 68.9 (10.8) | <.005 | 209 | 56.5 (8.3) | 356 | 67.3 (8.7) | <.005 |
| Hip circumference (cm) | 193 | 68.1 (7.8) | 282 | 86.4 (11.0) | <.005 | 210 | 71.4 (9.5) | 356 | 90.9 (11.0) | <.005 |
| Systolic blood pressure | 193 | 101.4 (9.6) | 283 | 110.0 (12.2) | <.005 | 210 | 101.1 (12.1) | 355 | 108.5 (9.8) | <.005 |
| Diastolic blood pressure | 193 | 66.4 (8.1) | 282 | 70.7 (9.3) | <.005 | 210 | 66.4 (9.3) | 355 | 71.5 (8.1) | <.005 |
| Total cholesterol (mg/dL) | 195 | 164.1 (31.7) | 283 | 159.0 (34.1) | .099 | 210 | 166.5 (32.9) | 358 | 163.8 (31.6) | .323 |
| HDL cholesterol (mg/dL) | 195 | 52.1 (13.9) | 283 | 45.3 (13.0) | <.005 | 210 | 49.9 (11.8) | 358 | 48.4 (12.1) | .167 |
| LDL cholesterol (mg/dL) | 195 | 90.1 (26.7) | 283 | 86.6 (27.8) | .170 | 210 | 92.7 (27.7) | 358 | 89.7 (25.7) | .189 |
| Triglyceride (mg/dL) | 195 | 96.8 (58.9) | 283 | 113.8 (62.0) | .003 | 210 | 111.8 (61.6) | 358 | 109.4 (52.7) | .618 |
| Fasting blood glucose (mg/dL) | 123 | 85.5 (10.4) | 175 | 87.3 (18.8) | .320 | 134 | 81.1 (10.2) | 239 | 83.8 (15.2) | .062 |
3.2. The Prevalence of MeS
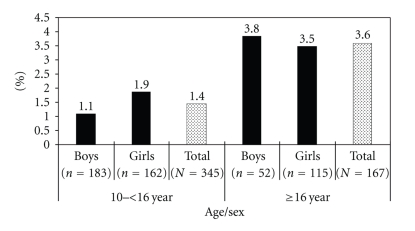
The prevalence of MeS according to IDF criteria was estimated from 512 subjects (235 boys, 277 girls, 345 aged 10–15.9 years and 167 aged 16–18 years) who had complete information on all MeS components. The prevalence of MeS according to IDF criteria was 1.4% in subjects aged between 10 and 15.9 years (1.1% for boys and 1.9% for girls, P-value =.556) and 3.6% in subjects aged between 16 and 18 years (3.8% for boys and 3.5% for girls, P-value =.906) (Figure 2). There was no significant difference in the prevalence of MeS between the two age groups (P-value =.117) and between boys and girls in both age groups.
Figure 2.
The prevalence of metabolic syndrome among boys and girls using the International Diabetes Federation (IDF) consensus worldwide definition according to the age group (10–<16 year and 16–18 year).
When categorized according to BMI category, the prevalence of the MeS was 15.1% in obese subjects, compared to 0.3% in subjects with normal BMI (BMI < 85th percentile) and 3.0% in overweight subjects.
3.3. The Prevalence of Individual Metabolic Abnormalities
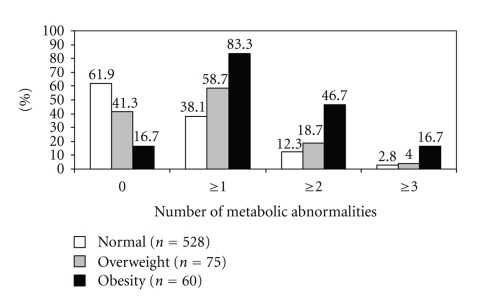
In the whole sample, the most commonly found abnormality was low HDL cholesterol (26.1%) followed by high TG (17.2%). The prevalence rates were 7.2% for high fasting glucose, 6.2% for high blood pressure, and 5.8% for increased waist circumference. About 44.4% had at least one metabolic abnormality, 16.1% had at least two metabolic abnormalities, and 4.2% had at least three metabolic abnormalities. The prevalence of clustering of metabolic abnormalities according to BMI is shown in Figure 3.
Figure 3.
The prevalence of clustering of metabolic abnormalities among Jordanian children and adolescent aged between 7 and 18 years according to body mass index.
Table 2 shows the prevalence of individual metabolic abnormalities for boys and girls according to age. The prevalence for children aged ≤12 and >12 years, respectively, was 3.2% and 8.0% for high blood pressure, 14.6% and 18.9% for high TG levels, 20.5% and 29.6% for low HDL levels, and 3.7% and 7.1% for increased waist circumference. The prevalence of at least one CVD risk factors was 36.5% and 49.3%, respectively. The prevalence of obesity (waist circumference), low HDL cholesterol, high blood pressure, at least one metabolic abnormality, and at least three metabolic abnormalities were significantly higher among subjects aged > 12 years compared to those among subjects aged ≤12 years.
Table 2.
The prevalence of individual metabolic abnormalities for boys and girls according to age.
| Age (year) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | ≤12 year | >12 year | |||||
| Boys n (%) | Girls n (%) | Total n (%) | Boys n (%) | Girls n (%) | Total n (%) | P-value (age groups) | |
| Abdominal obesity | .025 | ||||||
| Yes | 4 (2.1) | 11 (5.3) | 15 (3.7) | 16 (5.7) | 29 (8.2) | 45 (7.1) | |
| No | 189 (97.9) | 197 (94.7) | 386 (96.3) | 266 (94.3) | 325 (91.8) | 591 (92.9) | |
| Triglycerides level | .072 | ||||||
| High | 22 (11.3) | 37 (17.6) | 59 (14.6) | 57 (20.1) | 64 (17.9) | 121 (18.9) | |
| Normal | 173 (88.7) | 173 (82.4) | 346 (85.4) | 226 (79.9) | 294 (82.1) | 520 (81.1) | |
| HDL cholesterol | .001 | ||||||
| Low | 42 (21.5) | 41 (19.5) | 83 (20.5) | 100 (35.3) | 90 (25.1)* | 190 (29.6) | |
| Normal | 153 (78.5) | 169 (80.5) | 322 (79.5) | 183 (64.7) | 268 (74.9) | 451 (70.4) | |
| Blood pressure | .002 | ||||||
| High | 5 (2.6) | 8 (3.8) | 13 (3.2) | 34 (12.1) | 17 (4.8)* | 51 (8.0) | |
| Normal | 188 (97.4) | 202 (96.2) | 390 (96.8) | 248 (87.9) | 337 (95.2) | 585 (92.0) | |
| Fasting blood glucose | .462 | ||||||
| Elevated | 10 (8.1) | 6 (4.5) | 16 (6.2) | 14 (8.0) | 18 (7.5) | 32 (7.7) | |
| Normal | 113 (91.9) | 128 (95.5) | 241 (93.8) | 161 (92.0) | 221 (92.5) | 382 (92.3) | |
| Number of metabolic abnormalities |
|||||||
| 0 | 73 (59.3) | 89 (67.4) | 162 (63.5) | 79 (45.1) | 129 (54.9) | 208 (50.7) | .001 |
| ≥1 | 50 (40.7) | 43 (32.6) | 93 (36.5) | 96 (54.9) | 106 (45.1) | 202 (49.3) | .001 |
| ≥2 | 12 (9.8) | 22 (16.7) | 34 (13.3) | 38 (21.7) | 35 (14.9) | 73 (17.8) | .127 |
| ≥3 | 0 (0) | 2 (1.5) | 2 (0.8) | 17 (9.7) | 9 (3.8)* | 26 (6.3) | .001 |
*Indicates a statistically significant difference between boys and girls within age group.
3.4. Factors Associated with Clustering of MeS Components
MeS was significantly associated with BMI only. None of other variables were significantly associated with MeS. Children with overweight were 12.2 times more likely to have MeS (P-value =.042), and those with obesity were 69.5 times more likely to have MeS (P-value <.005).
Table 3 shows the factors that are associated with the three outcome variables: having at least one metabolic abnormality, having at least two metabolic abnormalities, and having at least three metabolic abnormalities. Compared to children aged ≤12 year, those who were older than 12 years had higher odds of having at least one metabolic abnormality (OR = 1.6) and having at least two metabolic abnormalities (OR = 17.2). Children living in north (OR = 2.3) and middle (OR = 2.6) were more likely to have at least one metabolic abnormality. Compared to those with normal BMI, children with obesity had higher odds of having at least one, two, or three metabolic abnormalities. Children with overweight were more likely to have at least one metabolic abnormality only. Exercise for more than 7 hours per week was associated with decreased odds of having at least three metabolic abnormalities.
Table 3.
Multivariate analysis of factors associated with having at least one metabolic abnormality, having at least two metabolic abnormalities, and having at least three metabolic abnormalities.
| ≥1 component | ≥2 components | ≥3 components | ||||
|---|---|---|---|---|---|---|
| Age (year) | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| ≤12 | 1 | 1 | ||||
| >12 | 1.6 (1.1, 2.4) | .010 | 17.2 (2.2, 30.7) | .006 | ||
| Body mass index | ||||||
| Normal | 1 | |||||
| Overweight | 2.2 (1.3, 3.7) | .005 | 1.8 (0.9, 3.5) | .083 | 1.3 (0.3, 4.7) | .727 |
| Obesity | 7.4 (3.6, 15.4) | <.005 | 6.2 (3.4, 11.4) | <.005 | 6.7 (2.6, 17.5) | <.005 |
| Region | ||||||
| North | 2.3 (1.4, 3.7) | <.005 | ||||
| Middle | 2.6 (1.4, 4.6) | .002 | ||||
| South | 1 | |||||
| Regular activities | ||||||
| ≤7 hours/week | 1 | |||||
| >7 hours/week | 0.3 (0.1, 0.8) | .013 | ||||
4. Discussion
Only few studies have assessed the prevalence of MeS in a population-based samples of young people [9, 22–24]. Although direct comparison across studies is difficult because of the differences in the definition of the syndrome, the overall prevalence of MeS in Jordanian children and adolescents (2.1%) was similar to that reported in subjects aged from 10 to 17 years in Turkey (2.2%) [25]. Using a definition similar to that proposed in ATP III criteria, a prevalence of 4.2% was reported among US adolescents aged 12 to 19 years who participated in the NHANES III [9]. Using the 2007 pediatric IDF definition among US adolescents aged 12–17 years, Ford et al. reported that the prevalence of the MeS was approximately 4.5% [24]. In northern Mexico children and adolescents aged 10–18 years, the prevalence varied between 3.8% to 7.8% according to the definition used [10]. Much higher prevalence rates were reported in Kuwaiti female adolescents aged 10–19 years, where the prevalence was 14.8% using IDF and 9.1% using the ATP III modified for age diagnostic criteria [26]. Furthermore, a prevalence of 9.2% was reported in subjects aged 12–19 years in Korea [27].
Although some of the previous studies suggest that the overall prevalence rates of the MeS in childhood are low, the perspective is very different in overweight and obese adolescents. In this study, the prevalence of the MeS was 0.3% in subjects with normal BMI, 3.0% in overweight subjects, and 15.1% in obese subjects. This finding is similar to that reported by others who reported that the prevalence is much higher among obese children and adolescents. In some studies, the prevalence approximated 30%–50% among obese children and adolescents [9, 13, 25, 27–29].
In the whole sample, the most commonly found abnormality was low HDL (26.1%) followed by increased TG (17.2%). In contrast, the prevalence rates were 7.2% for high fasting glucose, 6.2% for high blood pressure, and 5.8% for increased waist circumference. In US adolescents, the most common abnormality was high triglycerides and low HDL cholesterol [9]. In the same study, the prevalence rate of high fasting glucose was very low (1.5%).
In our study, BMI defined obesity was significantly associated with increased odds of clustering of metabolic abnormalities in the multivariate analysis. The role of obesity and insulin resistance in the etiology of the metabolic syndrome has been explored in children in other studies [14, 30]. Our finding is in agreement with that reported by others [31, 32]. Chu et al. [31] reported that the prevalence of two or more cardiovascular risk factors was four to five times greater in obese than in nonobese children.
Having regular physical activity of more than 7 hours per week was associated with lower odds of MeS. Low levels of physical activity were identified in other studies as a potential modifiable factor associated with metabolic risk in children, and habitual physical activity and aerobic fitness were shown to be inversely associated with the MeS score [33–35]. This association was shown in some studies to be independent of adiposity [36, 37].
This study showed that subjects living in the north or middle regions were more likely to have MeS compared to those living in the south. This finding might be explained by the differences in the socioeconomic and nutrition status between the three regions of Jordan. Previous reports supported the association between socioeconomic status and the MeS among children, and the potential mechanisms suggested were low birthweight, poor nutrition, and inadequate physical activity [38, 39].
The associations between studied variables and MeS should be interpreted with cautious since we cannot infer causality from our findings because this study is cross-sectional and some studied variables such as physical activity were self-reported.
In conclusion, our results indicate that although the prevalence of MeS is low in Jordanian children and adolescents, a large proportion of them had one or two metabolic abnormalities.
References
- 1.McGill HC, McMahan CA, Zieske AW, Malcom GT, Tracy RE, Strong JP. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation. 2001;103(11):1546–1550. doi: 10.1161/01.cir.103.11.1546. [DOI] [PubMed] [Google Scholar]
- 2.Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107(11):1562–1566. doi: 10.1161/01.cir.0000061521.15730.6e. [DOI] [PubMed] [Google Scholar]
- 3.Virkola K, Pesonen E, Åkerblom HK, Siimes MA. Cholesterol and carotid artery wall in children and adolescents with familial hypercholesterolaemia: a controlled study by ultrasound. Acta Paediatrica, International Journal of Paediatrics. 1997;86(11):1203–1207. doi: 10.1111/j.1651-2227.1997.tb14847.x. [DOI] [PubMed] [Google Scholar]
- 4.Cuomo S, Guarini P, Gaeta G, et al. Increased carotid intima-media thickness in children-adolescents, and young adults with a parental history of premature myocardial infarction. European Heart Journal. 2002;23(17):1345–1350. doi: 10.1053/euhj.2001.3111. [DOI] [PubMed] [Google Scholar]
- 5.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Journal of the American Medical Association. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. Journal of Pediatrics. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Srinivasan SR, Elkasabany A, Berenson GS. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. American Journal of Epidemiology. 1999;150(7):667–674. doi: 10.1093/oxfordjournals.aje.a010069. [DOI] [PubMed] [Google Scholar]
- 8.Raitakari OT, Porkka KVK, Ronnemaa T, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The cardiovascular risk in young Finns study. Diabetologia. 1995;38(9):1042–1050. doi: 10.1007/BF00402173. [DOI] [PubMed] [Google Scholar]
- 9.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Morán M, Salazar-Vázquez B, Violante R, Guerrero-Romero F. Metabolic syndrome among children and adolescents aged 10–18 years. Diabetes Care. 2004;27(10):2516–2517. doi: 10.2337/diacare.27.10.2516. [DOI] [PubMed] [Google Scholar]
- 11.Lambert M, Paradis G, O'Loughlin J, Delvin EE, Hanley JA, Levy E. Insulin resistance syndrome in a representative sample of children and adolescents from Quebec, Canada. International Journal of Obesity. 2004;28(7):833–841. doi: 10.1038/sj.ijo.0802694. [DOI] [PubMed] [Google Scholar]
- 12.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabetic Medicine. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. New England Journal of Medicine. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 14.Cruz ML, Weigensberg MJ, Huang TTK, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight hispanic youth and the role of insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2004;89(1):108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 15.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. 2005.
- 16.Zimmet P, Alberti GKMM, Kaufman F, et al. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatric Diabetes. 2007;8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51(1):204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 19.Alberti KGMM, Zimmet PZ, Shaw JE. The metabolic syndrome—a new worldwide definition from the international diabetes federation consensus. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. 2000 CDC growth charts: United States.
- 21.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 22.De Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N. Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999-2000 National Health and Nutrition Examination Surveys. Clinical Chemistry. 2006;52(7):1325–1330. doi: 10.1373/clinchem.2006.067181. [DOI] [PubMed] [Google Scholar]
- 23.Jolliffe CJ, Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the adult treatment panel III and International Diabetes Federation criteria. Journal of the American College of Cardiology. 2007;49(8):891–898. doi: 10.1016/j.jacc.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the international diabetes federation. Diabetes Care. 2008;31(3):587–589. doi: 10.2337/dc07-1030. [DOI] [PubMed] [Google Scholar]
- 25.Agirbasli M, Cakir S, Ozme S, Ciliv G. Metabolic syndrome in Turkish children and adolescents. Metabolism. 2006;55(8):1002–1006. doi: 10.1016/j.metabol.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Al-Isa A, Akanji AO, Thalib L. Prevalence of the metabolic syndrome among female Kuwaiti adolescents using two different criteria. British Journal of Nutrition. 2010;103(1):77–81. doi: 10.1017/S0007114509991425. [DOI] [PubMed] [Google Scholar]
- 27.Kim HM, Park J, Kim HOS, Kim DH. Prevalence of the metabolic syndrome in Korean adolescents aged 12–19 years from the Korean National Health and Nutrition Examination Survey 1998 and 2001. Diabetes Research and Clinical Practice. 2007;75(1):111–114. doi: 10.1016/j.diabres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Dhuper S, Cohen HW, Daniel J, et al. Utility of the modified ATP III defined metabolic syndrome and severe obesity as predictors of insulin resistance in overweight children and adolescents: a cross-sectional study. Cardiovascular Diabetology. 2007;6, article 4 doi: 10.1186/1475-2840-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen Y, Kandemir N, Alikasifoglu A, Gonc N, Ozon A. Prevalence and risk factors of metabolic syndrome in obese children and adolescents: the role of the severity of obesity. European Journal of Pediatrics. 2008;167(10):1183–1189. doi: 10.1007/s00431-007-0658-x. [DOI] [PubMed] [Google Scholar]
- 30.Sinaiko AR, Jacobs DR, Jr., Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. Journal of Pediatrics. 2001;139(5):700–707. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 31.Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Clustering of cardiovascular disease risk factors among obese schoolchildren: the Taipei children heart study. American Journal of Clinical Nutrition. 1998;67(6):1141–1146. doi: 10.1093/ajcn/67.6.1141. [DOI] [PubMed] [Google Scholar]
- 32.Csábi G, Török K, Molnár D, Jeges S. Presence of metabolic cardiovascular syndrome in obese children. European Journal of Pediatrics. 2000;159(1-2):91–94. doi: 10.1007/pl00013812. [DOI] [PubMed] [Google Scholar]
- 33.Brage S, Wedderkopp N, Ekelund U, et al. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS) Diabetes Care. 2004;27(9):2141–2148. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 34.Ekelund U, Anderssen SA, Froberg K, Sardinha LB, Andersen LB, Brage S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: the European youth heart study. Diabetologia. 2007;50(9):1832–1840. doi: 10.1007/s00125-007-0762-5. [DOI] [PubMed] [Google Scholar]
- 35.Ekelund U, Brage S, Froberg K, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European youth heart study. PLoS Medicine. 2006;3(12):2449–2457. doi: 10.1371/journal.pmed.0030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen LB, Harro M, Sardinha LB, et al. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368(9532):299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 37.Eisenmann JC, Katzmarzyk PT, Perusse L, Tremblay A, Després JP, Bouchard C. Aerobic fitness, body mass index, and CVD risk factors among adolescents: the Québec family study. International Journal of Obesity. 2005;29(9):1077–1083. doi: 10.1038/sj.ijo.0802995. [DOI] [PubMed] [Google Scholar]
- 38.Batty GD, Leon DA. Socio-economic position and coronary heart disease risk factors in children and young people: evidence from UK epidemiological studies. European Journal of Public Health. 2002;12(4):263–272. doi: 10.1093/eurpub/12.4.263. [DOI] [PubMed] [Google Scholar]
- 39.Lawlor DA, Harro M, Wedderkopp N, et al. Association of socioeconomic position with insulin resistance among children from Denmark, Estonia, and Portugal: cross sectional study. British Medical Journal. 2005;331(7510):183–186. doi: 10.1136/bmj.331.7510.183. [DOI] [PMC free article] [PubMed] [Google Scholar]