Abstract
Alzheimer's disease (AD) is an age-associated neurodegenerative disease characterized by the progressive loss of cognitive function, loss of memory and insomnia, and abnormal behavioral signs and symptoms. Among the various theories that have been put forth to explain the pathophysiology of AD, the oxidative stress induced by amyloid β-protein (Aβ) deposition has received great attention. Studies undertaken on postmortem brain samples of AD patients have consistently shown extensive lipid, protein, and DNA oxidation. Presence of abnormal tau protein, mitochondrial dysfunction, and protein hyperphosphorylation all have been demonstrated in neural tissues of AD patients. Moreover, AD patients exhibit severe sleep/wake disturbances and insomnia and these are associated with more rapid cognitive decline and memory impairment. On this basis, the successful management of AD patients requires an ideal drug that besides antagonizing Aβ-induced neurotoxicity could also correct the disturbed sleep-wake rhythm and improve sleep quality. Melatonin is an effective chronobiotic agent and has significant neuroprotective properties preventing Aβ-induced neurotoxic effects in a number of animal experimental models. Since melatonin levels in AD patients are greatly reduced, melatonin replacement has the potential value to be used as a therapeutic agent for treating AD, particularly at the early phases of the disease and especially in those in whom the relevant melatonin receptors are intact. As sleep deprivation has been shown to produce oxidative damage, impaired mitochondrial function, neurodegenerative inflammation, and altered proteosomal processing with abnormal activation of enzymes, treatment of sleep disturbances may be a priority for arresting the progression of AD. In this context the newly introduced melatonin agonist ramelteon can be of much therapeutic value because of its highly selective action on melatonin MT1/MT2 receptors in promoting sleep.
1. Introduction
Alzheimer's disease (AD), a major age-associated neurodegenerative disease, is characterized by progressive loss of cognitive function, loss of memory, impaired synaptic function, and a massive brain cell loss that ultimately results in premature death. Although the exact cause of the disease is under intense investigation, the prevailing hypothesis proposes that the deposition of amyloid β-protein- (Aβ-)containing senile plaques and of intracellular neurofibrillary tangles major etiological factors in AD [1]. Deposition of amyloid plaques causes cell death by inducing mitochondrial dysfunction and oxidative stress [2]. Aβ deposition initiates the flavo-enzyme-dependent increase of hydrogen peroxide (H2O2) and lipid peroxides that increase free radical generation [3, 4]. Neural tissues of AD patients exhibit increased levels of end products of peroxidation such as malondialdehyde, 4-hydroxynonenal, or carbonyls. Though Aβ contributes directly or indirectly to neuronal degeneration, its potential to cause AD depends on individual's susceptibility to Aβ-mediated toxicity [5].
Mitochondrial dysfunction plays an important role in AD and the link among impaired mitochondrial function, tau phosphorylation, and Aβ amyloidosis is increasingly recognized as a major phenomenon in AD physiopathology [2, 6, 7]. Aβ accumulation and neurofibrillary tangles composed of tau protein induce functional deficits of the respiratory chain complexes thereby resulting in mitochondrial dysfunction and oxidative stress (the “Aβ cascade hypothesis of AD”). It is interesting to note that women are more vulnerable to AD than men, presumably because the mitochondria are protected by estrogens against Aβ toxicity [8].
Indeed, aging and neurodegenerative diseases are accompanied by abnormal levels of oxidation of proteins, lipids, and nucleic acids [9–11]. Mechanisms such as chronic inflammation associated with the release of cytokines and trace element neurotoxicity have also been suggested as possible contributory factors underlying the physiopathologic events of AD [12–14]. Membrane disruption and induction of apoptosis by caspase enzymes have also been implicated [15].
In addition to cognitive and memory dysfunction, sleep-wake and other circadian rhythm dysregulation, are commonly seen in AD [16–19]. These circadian rhythm disturbances are associated with disturbed melatonin rhythmicity and decreased circulating and brain melatonin levels [20–22]. It is hypothesized that the decreased levels of melatonin, in fact, could contribute to the pathophysiology of AD in view than melatonin combines chronobiotic with effective antioxidant, anti-inflammatory, and antifibrillogenic properties [23].
Among the factors known to suppress the production of melatonin by the pineal gland, hypoxia deserves to be considered [24]. Reduced production of melatonin has been reported to occur in other ischemic conditions such as coronary artery disease or severe congestive heart failure [25–27]. Hypoxia may play a role in the pathogenesis of AD as it can induce formation of Aβ [28–30]. The role of hypoxia in potentiating AD is supported by the observation that patients suffering from cardiorespiratory disorders, cerebral ischemia or stroke are much more susceptible to development of dementias including AD [31]. It is remarkable that the daily administration of melatonin reduces the hypoxia induced Aβ generation in the rat hippocampus [32].
With this background, the replacement of brain melatonin levels has been suggested as a way arresting the progress of AD and for correcting the circadian and sleep-wake disturbances associated with the disease. As melatonin is a short-lived molecule having a limited duration of action (half life = 0.54–0.67 h [33]), analogs with a high affinity for melatonin receptors and a longer duration of action have been synthesized with a potential therapeutic efficacy to treat insomnia and psychiatric disorders like depression and bipolar affective disorder [34]. Ramelteon was the first of these molecules approved by the U.S. Food and Drug Administration to be used in the treatment of insomnia [35] and its potential use in AD together with that of melatonin is discussed in this review article.
2. Melatonin in AD
Melatonin is synthesized both in the pineal gland and in a number of peripheral organs and tissues by a process starting with tryptophan conversion to serotonin (reviewed in [36]). Serotonin is then acetylated to form N-acetylserotonin by the enzyme arylakylamine N-acetyltransferase while N-acetylserotonin is converted into melatonin by the enzyme hydroxyindole-O-methyl transferase [37, 38]. Once formed melatonin is not stored within the pineal gland it diffuses into the capillary blood and the cerebrospinal fluid (CSF) [39, 40]. CSF melatonin values are nearly 30 times higher than those in the blood; thus, the brain tissue has a higher melatonin concentration than any other tissue in the body [41].
Regional distribution of melatonin in different areas of the brain varies and early studies have shown that hypothalamic melatonin concentrations are nearly fifty times higher than in plasma [42–44]. While tissue melatonin only exhibits a moderate circadian variation, circulating melatonin exhibits most pronounced circadian rhythm with highest levels occurring at night and very low levels during daytime [36].
Circulating melatonin is metabolized mainly in the liver via hydroxylation in the C6 position by cytochrome P450 monooxygenases (CYP1A2;CYP1A1) [45]. It is thereafter conjugated with sulphate to form 6-sulfatoxymelatonin (aMT6S), the main metabolite of melatonin in urine. In the brain, melatonin is metabolized to kynuramine derivatives like N 1-acetyl-N 2-formyl-5-methoxykynuramine (AFMK) [46, 47]. In several tissues melatonin is also nonenzymatically metabolized to cyclic 3-hydroxy melatonin [48].
Melatonin is involved in the control of various physiological functions such as circadian rhythmicity [49, 50], sleep regulation [51, 52], immune function [53, 54], antioxidant defense [55, 56], control of reproduction [57–59], inhibition of tumor growth [60, 61], and control of human mood [62, 63]. Melatonin participates in many of these functions by acting through G-protein membrane receptors, the MT1 and MT2 melatonin receptors [64–66]. Nuclear melatonin receptors belonging to the RZR/ROR α receptor class have also been described [56, 67, 68]. Melatonin also acts directly on the cells without the intervention of any of these receptors by binding to intracellular proteins like calmodulin [69] or tubulin [70]. In general, the free radical scavenging action of melatonin does not involve receptors except for the induction of synthesis of some antioxidant enzymes like γ-glutamylcysteine synthase that involves RZR/ROR α receptors [71].
In view of the involvement of oxidative stress in AD, melatonin represents an interesting neuroprotective agent as it antagonizes oxidative stress both in a direct and in an indirect way [55, 56, 72, 73]. In the N2a murine neuroblastoma cell model Pappolla et al. [74] first demonstrated that coincubation of Aβ with melatonin significantly reduced several features of apoptosis like cellular shrinkage or formation of membrane blubs. In a number of studies melatonin prevented the death of neuroblastoma cells exposed to Aβ [5, 75, 76].
Several animal models of AD have been used to study the possible antoxidative and antiapoptotic actions of melatonin in arresting neuronal lesions. Okadaic acid induces physiological and biochemical changes similar to those seen in AD. Increased levels of 4-hydroxynonenal in cultured neuronal cells have been found following administration of okadaic acid [77]. After the administration of antioxidants like melatonin or vitamin C, the effects of okadaic acid on NIE 115 neuronal cells were prevented effectively [78]. Melatonin was more effective than vitamin C, since it not only prevented the free radical-induced damage with greater efficiency but also increased the activity of the antioxidant enzymes glutathione-S transferase and glutathione reductase [78].
Several studies indicate that the apoptosis of astrocytes contribute to the pathogenesis of AD (sees [79]). Astrocytes exhibit tau phosphorylation and activation of stress kinases as seen in AD pathology. They also produce apolipoprotein E4 (apoE4) that aggravates Aβ neurodegenerative effects [80, 81]. During interaction with Aβ, astrocytes lose control over NO production leading to the neurotoxic peroxynitrate formation. By treating the C6 astroglioma cells with melatonin, the increase in NO production induced by Aβ was effectively prevented [82].
3. Molecular Mechanisms of Melatonin's Anti-Amyloid Actions
Melatonin not only reduced apoptosis but also exerts its antiamyloid actions through additional mechanisms. One of them is by preventing Aβ-induced mitochondrial damage and disruption of respiration. Melatonin administration prevented Aβ action on mitochondrial DNA proteins and level of lipid peroxidation [75]. In this aspect it is interesting to note that melatonin's metabolite AFMK also offered protection from Aβ-induced mitochondrial oxidative stress [83] although a higher concentration was needed.
Melatonin inhibits the formation of amyloid fibrils as demonstrated by different techniques [84, 85]. The structural analog of melatonin indole-3-propionic acid not only shares the radical scavenging activity of melatonin [86] but also exhibits similar or even higher antifibrillogenic activity [87].
Several lipoproteins can modulate fibrillogenesis [88]. Melatonin was shown to reverse the profibrillogenic activity of apoE4 and to antagonize the neurotoxic combinations of Aβ and apoE4 or apoE3 [83]. ApoE4 is also produced by astrocytes and aggravates Aβ effects showing thereby the mutual interaction of Aβ protein and apo-E4 in the astrocyte-neuron interactions [81]. The antifibrillogenic effects of melatonin and its metabolites were observed not only in vitro but also in vivo in transgenic mouse models [84, 89, 90]. Protection from Aβ toxicity was observed, especially at the mitochondrial level.
As mentioned above, chronic intermittent hypoxia has been shown to induce Aβ protein generation by upregulating the APP processing enzymes BACE and PSEN-1 [28–30]. The daily administration of melatonin (10 mg/kg) prior to a short-term hypoxia prevented the generation of Aβ protein but it did not reduce the increase of HIF-1 transcription factor induced by hypoxia [32]. Hence it was suggested that melatonin's neuroprotective effect against amyloid-β-peptide was due to its direct free radical scavenging properties actions [32].
Another manifestation of AD studied in experimental models is the expression of protein hyperphosphorylation and cytoskeletal disorganization. Calyculin A, an inhibitor of protein phosphatases (PP), was used in neuroblastoma N2 cells to examine this point. Calyculin A resulted in activation of glycogen synthase kinase 3 (GSK-3), a redox-controlled enzyme involved in various regulatory mechanisms of the cell, and the consequent hyperphosphorylation of tau [91]. Melatonin administration decreased oxidative stress and tau hyperphosphorylation and reversed GSK-3 activation showing thereby that it not only acts as an antioxidant but also interferes with the phosphorylation system, particularly stress kinases [91].
The inhibition of PP-2A and PP-1 brought about by calyculin A caused hyperphosphorylation of tau and of neurofilaments, synaptophysin loss, and spatial memory retention impairment, an effect counteracted by the administration of melatonin i.p. for 9 days before calyculin injection [92]. Melatonin also partially reversed the phosphorylation of the catalytic subunit of PP-2A at tyrosine 307 (Y307) crucial site regulating the activity of PP-2A, and reduced malondialdehyde levels induced by calyculin A [92]. Melatonin also attenuated tau hyperphosphorylation induced by wortmannin [93, 94] and isoproterenol [95].
Tyrosine kinase (trk) receptors, important elements of the phosphorylation system, as well as neurotrophins, are affected by Aβ and other oxidotoxins and melatonin normalized in neuroblastoma cells trk and neurotrophin expression [96]. Recent studies using organotypic hippocampal studies confirmed that the presence of melatonin (25–100 μM) prevented the cell damage induced by exposure to Aβ reducing the activation of GSK-3β, the phosphorylation of tau protein, and the Aβ-induced increases of TNF-α and IL-6 levels [97]. The chronobiological aspects of melatonin- Aβ interaction are underlined by a study describing the protective effect of melatonin against the circadian changes produced by Aβ25–35 microinjection into the suprachiasmatic nuclei (SCN) of golden hamsters [98].
4. Potential Therapeutic Value of Melatonin in AD
A number of studies in AD patients have indicated that there is a profound disturbance in sleep/wake cycle associated with the progression of the disease. Cross-sectional studies reveal that sleep disturbances are associated with memory and cognitive impairment. [16–19]. A severe disruption of the circadian timing system occurs in AD as indicated by alterations in numerous overt rhythms like body temperature, glucocorticoids, and/or plasma melatonin [22, 99, 100]. The internal desynchronization of rhythms is significant in AD patients [101, 102].
“Sundowning” is a chronobiological phenomenon observed in AD patients in conjunction with sleep-wake disturbances, including symptoms like disorganized thinking, reduced ability to maintain attention to external stimuli, agitation, wandering, and perceptual and emotional disturbances, all appearing in late afternoon or early evening [99, 103, 104]. Chronotherapeutic interventions such as exposure to bright light and/or timed administration of melatonin in selected circadian phases alleviated sundowning symptoms like wandering, agitation and delirium and also improved sleep-wake patterns of AD patients [105].
A number of studies have revealed that melatonin levels are lower in AD patients as compared to age-matched control subjects [20–22, 106]. The decreased CSF melatonin levels of AD patients were attributed to decreased melatonin production. CSF melatonin levels decreased even in preclinical stages (Braak stages-1) when patients did not manifest cognitive impairment [107] suggesting thereby that reduction in CSF melatonin may be an early marker (and cause) for incoming AD. The decrease of melatonin levels in AD was attributed to a defective retinohypothalamic tract or SCN-pineal connections [108]. The impaired melatonin production at night correlates significantly with the severity of mental impairment of demented patients [109]. As AD patients have profound deficiency of endogenous melatonin, replacement of levels of melatonin in the brain could be a therapeutic strategy for arresting the progress of the disease. Melatonin's neuroprotective and vasoprotective properties would help in enhancing cerebral blood flow and would help to improve the clinical condition of AD patients [23].
Sleep disturbances exacerbate memory and cognitive impairment [110]. Therefore, optimization in management of sleep disturbances is of paramount importance in treating AD patients. In an initial study on 14 AD patients with 6–9 mg of melatonin given for 2-3 year period it was noted that melatonin improved sleep quality [111]. Sundowning, diagnosed clinically, was no longer detectable in 12 out of 14 patients. Reduction in cognitive impairment and amnesia was also noted. This should be contrasted with the significant deterioration of the clinical conditions expected from patients after 1–3 year of evolution of AD [111, 112].
Several studies support the efficacy of melatonin in treating sleep and chronobiologic disorders in AD patients (Table 1). The administration of melatonin (6 mg/day) for 4 weeks to AD patients reduced nighttime activity as compared to placebo [113]. An improvement of sleep and alleviation of sundowning were reported in 11 AD patients treated with melatonin (3 mg/day at bedtime) and evaluated by using actigraphy [114]. Improvement in behavioral signs was reported with use of 6–9 mg/day of melatonin for 4 months in AD patients with sleep disturbances [115].
Table 1.
Clinical studies on melatonin efficacy in AD.
| Design | Subjects (M, F) | Treatment | Study's duration | Measured | Results | Reference |
|---|---|---|---|---|---|---|
| Open-label study | 10 (6, 4) demented patients | 3 mg melatonin p.o./daily at bed time | 3 weeks | Daily logs of sleep and wake quality completed by caretakers | Seven out of ten dementia patients having sleep disorders treated with melatonin showed a significant decrease in sundowning and reduced variability of sleep onset time | [116] |
| Open-label study | 14 (8, 14) AD patients | 9 mg melatonin p.o./daily at bed time | 22 to 35 months | Daily logs of sleep and wake quality completed by caretakers. Neuropsychological assessment. | At the time of assessment, a significant improvement of sleep quality was found. Sundowning was not longer detectable in 12 patients and persisted, although attenuated in 2 patients. Clinically, the patients exhibited lack of progression of the cognitive and behavioral signs of the disease during the time they received melatonin. | [111] |
| Case report | Monozygotic twins with AD of 8 years duration | One of the patients was treated with melatonin 9 mg p.o./daily at bed time. | 36 months | Neuropsychological assessment. Neuroimaging. |
Sleep and cognitive function severely impaired in the twin not receiving melatonin as compared to the melatonin-treated twin. | [112] |
| Open-label, placebo-controlled trial | 14 AD patients | 6 mg melatonin p.o./daily at bed time or placebo | 4 weeks | Daily logs of sleep and wake quality completed by caretakers. Actigraphy | The 7 AD patients receiving melatonin showed a significantly reduced percentage of nighttime activity compared to a placebo group | [113] |
| Open-label study | 11 (3, 8) AD patients | 3 mg melatonin p.o./daily at bed time | 3 weeks | Daily logs of sleep and wake quality completed by the nurses. | Analysis revealed a significant decrease in agitated behaviors in all three shifts and a significant decrease in daytime sleepiness. | [117] |
| Open-label study | 45 (19, 26) AD patients | 6–9 mg melatonin p.o./daily at bed time | 4 months | Daily logs of sleep and wake quality completed by caretakers. Neuropsychological assessment. | Melatonin improved sleep and suppressed sundowning, an effect seen regardless of the concomitant medication employed to treat cognitive or behavioral signs of AD. | [115] |
| Randomized double blind placebo controlled cross over study | 25 AD patients | 6 mg of slow release melatonin p.o. or placebo at bed time | 7 weeks | Actigraphy | Melatonin had no effect on median total time asleep, number of awakenings, or sleep efficiency. | [118] |
| Double-blind, placebo-controlled study | 20 (3, 17) AD patients | Placebo or 3 mg melatonin p.o./daily at bed time | 4 weeks | Actigraphy. Neuropsychological assessment. | Melatonin significantly prolonged the sleep time and decreased activity in the night. Cognitive function was improved by melatonin. | [119] |
| Randomized, placebo-controlled clinical trial | 157 (70, 87) AD patients | 2.5 mg slow-release melatonin, or 10 mg melatonin or placebo at bed time | 2 months | Actigraphy. Caregiver ratings of sleep quality | Nonsignificant trends for increased nocturnal total sleep time and decreased wake after sleep onset were observed in the melatonin groups relative to placebo. On subjective measures, caregiver ratings of sleep quality showed improvement in the 2.5 mg sustained-release melatonin group relative to placebo. | [120] |
| Open-label study | 7 (4, 3) AD patients | 3 mg melatonin p.o./daily at bed time | 3 weeks | Actigraphy. Neuropsychological assessment. | Complete remission of daynight rhythm disturbances or sundowning was seen in 4 patients, with partial remission in other 2. | [114] |
| Randomized, placebo-controlled study | 17 AD patients | 3 mg melatonin p.o./daily at bed time (7 patients). Placebo (10 patients) | 2 weeks | Actigraphy. Neuropsychological assessment. | In melatonin-treated group, actigraphic nocturnal activity and agitation showed significant reductions compared to baseline. | [121] |
| Randomized, placebo-controlled study | 50 AD patients | Morning light exposure (2,500 lux, 1 h) and 5 mg melatonin (n = 16) or placebo (n = 17) in the evening. Control subjects (n = 17) received usual indoor light (150–200 lux). | 10 weeks | Night time sleep variables, day sleep time, day activity, day : night sleep ratio, and rest-activity parameters were determined using actigraphy. | Light treatment alone did not improve night time sleep, daytime wake, or rest-activity rhythm. Light treatment plus melatonin increased daytime wake time and activity levels and strengthened the rest-activity rhythm. | [122] |
| Case report | 68-year-old man with AD who developed rapid eye movement (REM) sleep behavior disorder | 5–10 mg melatonin p.o./daily at bed time. | 20 months | Polysomnography | Melatonin was effective to suppress REM sleep behavior disorder | [123] |
| Randomized, placebo-controlled study | 41 (13, 28) AD patients | Melatonin (8.5 mg immediate release and 1.5 mg sustained release) (N = 24) or placebo (N = 17) administered at 10 : 00 P.M. | 10 days | Actigraphy. | There were no significant effects of melatonin, compared with placebo, on sleep, circadian rhythms, or agitation. | [124] |
In a double blind study conducted on AD patients it was noted that 3 mg/day of melatonin significantly prolonged actigraphically evaluated sleep time, decreased activity in night, and improved cognitive functions [119]. In a multicenter, randomized, placebo-controlled clinical trial of a sample of 157 AD patients with sleep disturbances, melatonin or placebo was administered for a period of 2 months [120]. In actigraphic studies a trend to increased nocturnal total sleep time and decreased wake after sleep onset was noted in the melatonin-treated group. On subjective measures by caregiver ratings significant improvement in sleep quality was noted with 2.5 mg sustained release melatonin relative to placebo [120].
Negative results with the use of melatonin in fully developed AD were also published. For example, in a study in which melatonin (8.5 mg fast release and 1.5 mg sustained release) was administered at 10.00 PM for 10 consecutive nights to patients with AD, no significant difference was noticed with placebo on sleep, circadian rhythms and, agitation [124]. Although the lack of beneficial effect of melatonin in this study on sleep could be attributed to the short period of time examined, it must be noted that large interindividual differences between patients suffering from a neurodegenerative disease are not uncommon. It should be also taken into account that melatonin, though having some sedating and sleep latency-reducing properties, does not primarily act as a sleeping pill, but mainly as a chronobiotic.
Since the circadian oscillator system is obviously affected in AD patients showing severe sleep disturbances, the efficacy of melatonin should be expected to depend on disease progression. In a recent paper one of us summarized the published data concerning melatonin treatment of AD patients [125] (Table 1). Eight reports (5 open-label studies, 2 case reports) (N = 89 patients) supported a possible efficacy of melatonin: sleep quality improved and in patients with AD sundowning was reduced and cognitive decay showed less progression. In 6 double blind, randomized placebo-controlled trials (N = 210) sleep was objectively measured by wrist actigraphy and additionally neuropsychological assessment and sleep quality were subjectively evaluated. Sleep quality increased and sundowning decreased significantly and cognitive performance improved in 4 studies (N = 143) whereas there was absence of effects in 2 studies (N = 67) [125]. Therefore, the question whether melatonin has a causal value in preventing or treating AD, affecting disease progression of the neuropathology and the driving mechanisms, remains unanswered. Double-blind multicenter studies are needed to further explore and investigate the potential and usefulness of melatonin as an antidementia drug. Its apparent usefulness in symptomatic treatment, concerning sleep, sundowning, and so forth, even in a progressed state, further underlines the need for such decisive studies.
It has been shown that with degeneration of the SCN, the master body clock, there is a decrease in the expression of MT1 receptors so that strength of melatonin as a synchronizing agent is reduced [126]. Moreover the input of neural pathways involved in entrainment (synchronization) of the central clock may become dysfunctional or less sensitive during aging and even more so in AD [127]. In a large multicentre trial only a nonsignificant trend to improvement in the circadian rhythm disturbance of AD is when treatment was done using melatonin [120]. Because MT1 receptor expression in the SCN is decreased it is certainly possible that melatonin will be ineffective as a synchronizing agent although it is possible that a higher dose of melatonin or a more potent melatonin agonist such as ramelteon may be useful. Another strategy could be exposure to bright light [128] (see below).
5. Melatonin as a Therapeutic Agent for Mild Cognitive Impairment
As outlined, melatonin acts at different levels relevant to the development and manifestation of AD. The antioxidant, mitochondrial, and antiamyloidogenic effects may be seen as a possibility of interfering with the onset of the disease. Therefore, early beginning of treatment may be decisive [129].
Mild cognitive impairment (MCI) is an etiologically heterogeneous syndrome characterized by cognitive impairment shown by objective measures adjusted for age and education in advance of dementia [130]. Approximately 12% of MCI converts to AD or other dementia disorders every year. Since MCI may represent prodromal A,D it should be adequately diagnosed and treated. Indeed, the degenerative process in AD brain starts 20–30 years before the clinical onset of the disease [130]. During this phase, plaques and tangles loads increase and at a certain threshold the first symptom appears. As already mentioned, CSF melatonin levels decrease even in preclinical stages when the patients do not manifest any cognitive impairment (at Braak stages I-II), suggesting that the reduction in CSF melatonin may be an early trigger and marker for AD. Therefore, MCI could be an appropriate moment for initiating any melatonin treatment aiming to affect progression of the disease. Studies on melatonin effect on MCI are summarized in Table 2.
Table 2.
Clinical studies on melatonin efficacy in MCI.
| Design | Subjects (M, F) | Treatment | Study's duration | Measured | Results | Reference(s) |
|---|---|---|---|---|---|---|
| Double-blind, placebo-controlled, crossover study | 10 (4, 6) patients with mild cognitive impairment (MCI) | 6 mg melatonin p.o./daily at bed time | 10 days | Actigraphy. Neuropsychological assessment. | Enhanced the rest-activity rhythm and improved sleep quality (reduced sleep onset latency and in the number of transitions from sleep to wakefulness Total sleep time unaffected. The ability to remember previously learned items improved along with a significant reduction in depressed mood. | [131] |
| Double-blind, placebo-controlled pilot study | 26 individuals with age-related MCI | 1 mg melatonin p.o. or placebo at bed time | 4 weeks | Sleep questionnaire and a battery of cognitive tests at baseline and at 4 weeks | Melatonin administration improved reported morning “restedness” and sleep latency after nocturnal awakening and also improved scores on the California Verbal Learning Test-interference subtest. | [132] |
| Open-label, retrospective study | 50 (13, 37) MCI outpatients | 25 had received daily 3–9 mg of a fast-release melatonin preparation p.o. at bedtime. Melatonin was given in addition to the standard medication | 9–18 months | Daily logs of sleep and wake quality. Initial and final neuropsychological assessment. | Patients treated with melatonin showed significantly better performance in neuropsychological assessment. Abnormally high. Beck Depression Inventory scores decreased in melatonin-treated patients, concomitantly with an improvement in wakefulness and sleep quality. | [133] |
| Randomized, double blind, placebo-controlled study | 354 individuals with age-related cognitive decay | prolonged release melatonin (Circadin, 2 mg) or placebo, 2 h before bedtime | 3 weeks | Leeds Sleep Evaluation and Pittsburgh Sleep Questionnaires, Clinical Global Improvement scale score and quality of life. | PR-melatonin resulted in significant and clinically meaningful improvements in sleep quality, morning alertness, sleep onset latency, and quality of life | [134] |
| Long-term, double-blind, placebo-controlled, 2 × 2 factorial randomized study | 189 (19, 170) individuals with age-related cognitive decay | Long-term daily treatment with whole-day bright (1000 lux) or dim (300 lux) light. Evening melatonin (2.5 mg) or placebo administration | 1 to 3.5 years | Standardized scales for cognitive and noncognitive symptoms, limitations of activities of daily living, and adverse effects assessed every 6 months. | Light attenuated cognitive deterioration and also ameliorated depressive symptoms. Melatonin shortened sleep onset latency and increased sleep duration but adversely affected scores for depression. The combined treatment of bright light plus melatonin showed the best effects. | [105] |
| Prospective, randomized, double-blind, placebo-controlled, study | 22 (15, 7) individuals with age-related cognitive decay | Participants received 2 months of melatonin (5 mg o.o./day) and 2 months of placebo | 2 months | Sleep disorders were evaluated with the Northside Hospital Sleep Medicine Institute (NHSMI) test. Behavioral disorders were evaluated with the Yesavage Geriatric Depression Scale and Goldberg Anxiety Scale. | Melatonin treatment significantly improved sleep quality scores. Depression also improved significantly after melatonin administration. | [135] |
The first report on melatonin treatment of 10 MCI patients (6 mg/day for 10 days) indicated that besides enhancing the rest-activity rhythm and improved sleep quality the ability to remember previously learned items improved along with a significant reduction in depressed mood [131]. In another double-blind, placebo-controlled pilot study performed in 26 individuals with age-related MCI, the administration of 1 mg melatonin or placebo at bed time for 4 weeks resulted in improvement of sleep and of scores on the California Verbal Learning Test-interference subtest [132].
In a retrospective study of a group of 25 MCI patients who received melatonin (3–9 mg per day) for 9 to 18 months in comparison to a similar group of 25 MCI patients who did not receive it [133], patients treated with melatonin showed significantly better performance in a number of neuropsychological tests. Abnormally high Beck Depression Inventory scores decreased in melatonin treated patients, concomitantly with an improvement in wakefulness and sleep quality. The results suggested that melatonin could be a useful add-on drug for treating MCI in a clinic environment [133]. A follow up of that study has now been completed on a group of 35 MCI patients receiving melatonin for 9 to 24 months with essentially similar results [125].
A randomized controlled trial on the effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities was published [105]. The authors concluded that light has a benefit in improving some cognitive and noncognitive symptoms of MCI which was amplified by the conjoint administration of melatonin. In other two similar studies, one of them using the prolonged release preparation of melatonin (Circadin) recently approved by the European Medicines Agency, melatonin resulted in significant and clinically meaningful improvements of sleep quality, morning alertness, sleep onset latency and quality of life in old patients with mild cognitive impairment [134, 135]. In these studies melatonin treatment also improved mood. The evaluation of the published data concerning melatonin treatment of MCI that include 5 double blind, randomized placebo-controlled trials, and 1 open-label retrospective study (N = 651) all agreess in indicating that treatment with daily evening melatonin improves sleep quality and cognitive performance in MCI [125] (Table 2).
6. Use of Melatonin Agonist, Ramelteon in AD
As AD is associated with disturbed sleep/wake rhythms and circadian rhythm disturbances, a melatonin agonist with higher affinity to melatonin MT1 and MT2 receptors with a longer duration would theoretically be beneficial in tackling sleep-wake and circadian rhythm disturbances. In this aspect, ramelteon, which is the first melatonin receptor agonist approved by FDA with activity on MT1 and MT2 receptors, should be considered [136, 137].
The chemical structure of ramelteon is: (S)-N-[2-(1,6,7,8-tetrahydro-2Hindeno[5,4-b]furan-8-yl)ethyl] propionamide. This melatonin receptor agonist has a chemical formula C16 H21 NO2 with a molecular weight 259.34. Receptor binding studies indicated that ramelteon has high selectivity for MT1 and MT2 receptors, with little affinity for quinone reductase 2 binding [138]. The selectivity of ramelteon for MT1 has been found >1000-fold over that of MT2 receptors. It is well known that melatonin exerts its hypnotic effects through the activation of the MT1 and MT2 melatonin receptors [139]. Although both MT1 and MT2 receptors are involved in the regulation of sleep, the selectivity of MT1 receptors by ramelteon suggests that it targets sleep onset more specifically than melatonin [140]. Ramelteon has been found to have no affinity for benzodiazepine (BZP), dopamine, opiate, or serotonin receptor binding sites [138]. Hence ramelteon has advantages over other hypnotic drugs in not causing rebound insomnia, withdrawal symptoms, or dependence which is common with the activation of BZP, opiate, or dopamine receptors.
On oral administration, ramelteon is rapidly absorbed with a Tmax of less than 1 hour [141]. The absolute bioavailability of the oral formulation of ramelteon is less than 2% (range 0.5% to 12%) [141]. It is metabolized mainly in the liver via oxidation to hydroxyl and carbonyl groups and then conjugated with glucuronide. CYP1A2 is the major hepatic enzyme involved in ramelteon metabolism. Four principal metabolites ramelteon, that is, M-I, M-II, M-III, and M-IV, have been identified [141]. Among these, M-II has been found to occur in much higher concentration with systemic concentration being 20- to 100- fold greater than ramelteon.
Ramelteon is rapidly excreted and its elimination is significantly higher in elderly than in younger adults [142]. The influence of age and gender on the pharmacokinetics and pharmacodynamics of ramelteon has been evaluated in healthy volunteers following the administration of a single dose of 16 mg of ramelteon. When compared to young volunteers, ramelteon clearance was significantly reduced in elderly volunteers and its half life significantly increased. No significant effect of gender was observed [142]. The contribution of ramelteon's metabolites on the net pharmacologic activity was also evaluated. Among the four metabolites produced, the activity of M-II was to be about 30-fold lower than that of ramelteon, but its exposure exceeds exposure to ramelteon by a factor 30. It was thus suggested that M-II may contribute to net clinical activity of ramelteon [142].
The subjective efficacy of ramelteon was evaluated in clinical trials consisting of 829 elderly outpatients with chronic insomnia; 701 patients (128 patients discontinued) were treated for a period of 5-weeks with 4 mg and 8 mg ramelteon [143]. Patients in both ramelteon groups reported significant reductions in sleep onset latency (SOL) and increases in total sleep time (TST). Continuation of this study on 100 elderly patients established the efficacy of ramelteon in improving TST and decreasing SOL [144]. A number of studies have now established the efficacy of ramelteon in treating patients with chronic insomnia [145–147].
Concerning the safety and adverse effects with ramelteon, in a double blind placebo controlled study of rebound insomnia (sleep latency after treatment discontinuation) Roth and co-workers [143] evaluated each of the 7 nights of placebo run-out period. It was noted that during each of the 7 nights, patients in both ramelteon treatment groups (4 mg/day and 8 mg/day) maintained a similar or greater reduction in sleep latency from baseline as compared to those receiving placebo [143]. Withdrawal effects, as assessed by a BZP withdrawal symptom questionnaire, did not differ from the placebo group [143]. In another recent study it was noted that ramelteon did not affect alertness or the ability to concentrate, indicating no next-morning residual effects [148]. The incidence of adverse effects in ramelteon-treated patients in a 5 week study was found to be similar to that of placebo-treated patients. The adverse effects included mild gastrointestinal disturbances and nervous system effects such as dizziness, headache, somnolence, depression, fatigue, myalgia, and exacerbated eye pain [143].
Ramelteon not only has the potential in improving the sleep quality of AD and other neurodegenerative patients but can also offer neuroprotection as well in AD [149]. As ramelteon is a melatonin agonist with more potency and longer duration of action, it could act more efficiently than melatonin in its actions against neurotoxic effects involved in the pathogenesis of AD.
To what extent ramelteon reproduces the nonreceptor mediated effects of melatonin is not known. Ramelteon displays no relevant antioxidant capacity in the ABTS radical cation assay, as compared to luzindole or melatonin [150]. However, MT1/MT2 receptor-mediated effects on the upregulation of several antioxidant enzymes by physiological concentration of melatonin [151] such as glutathione peroxidase, glutathione reductase, γ-glutamylcysteine synthase, glucose-6-phosphate dehydrogenase, hemoperoxidase/catalase, Cu,Zn- and Mn-superoxide dismutases (reviewed in [152–155] can well give the basis for the use of ramelteon in AD. Since there are extensive data indicating a loss of melatonin receptors in AD patients, including the cerebral cortex and pineal gland (MT1 and MT2 receptors) [156], the hippocampus [157] and retina [158] (MT2 receptors) and the cerebrovascular system [159], and SCN [126, 128] (MT1 receptors), the chances of alleviating symptoms such as sundowning and disturbed sleep by giving the MT1/MT2 receptor agonist may vanish in late AD patients.
In addition, it has been suggested that melatonin and its receptors participate in neurodevelopment and regulation of neurotrophic factors [160]. In vitro studies have shown that melatonin promotes the viability and neuronal differentiation of neural stem cells and increases the production brain-derived neurotrophic factor (BDNF) by acting through MT1 receptors [161]. In mouse cerebellar granule cells in culture ramelteon increased the neural content of BDNF [162]. Therefore, if ramelteon treatment is capable of regulating brain BDNF levels, it could be used as a possible therapeutic agent in neurodegenerative diseases like AD for treating symptoms other than sleep disturbances.
7. Concluding Remarks
As AD disease involves a complex physiopathology, it has been suggested that monotherapy targeting early single steps in this complex cascade process may not be of much help [149]. Pleiotrophic drugs that can act independently by different routes including antioxidant, antiinflammatory, and antiamyloid effects would be much beneficial in the treatment of AD and other neurodegenerative disorders. Available evidence indicates suppression of GSK-3β overactivity; neuroinflammation and mitochondrial impairment are some of the combined strategies required in AD.
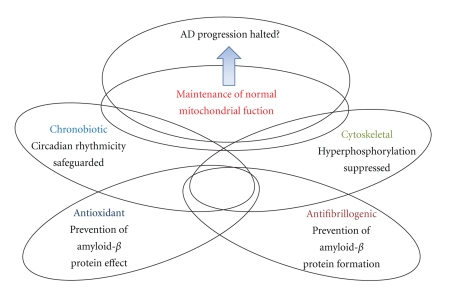
Melatonin is a pleiotropic molecule with antioxidant, antiinflammatory and antinitridergic properties [56, 154, 163]. It has also a role in sleep induction, and this is important in view that sleep deprivation is one of the cardinal features seen in AD and other neurodegenerative diseases. Sleep deprivation is associated with GSK-3β activation [164], altered proteosomal processing [165], oxidative damage [166], impaired mitochondrial integrity and function [167], and neurodegenerative inflammation [168]. Therefore, improvement of insomnia in neurodegenerative conditions and particularly in AD is a good practical approach for arresting the progression of the disease (Figure 1).
Figure 1.
Melatonergic agonists in AD. The multiple effects of melatonin discussed in the text and the different degree of overlap (interrelations and mutual influences) are indicated by the respective intersections in the scheme.
Melatonin and particularly ramelteon can be greatly beneficial in preventing the insomnia-induced damage of neuronal cells and can be of therapeutic value in treating AD. Owing to its potent effect on MT1 and MT2 receptors, ramelteon activates sleep onset by influencing the hypothalamic “sleep switch” downstream from the SCN more efficiently than melatonin itself [35]. Multicenter, placebo-controlled clinical trials using ramelteon are needed to prove the efficacy of this drug in arresting the progression or prevention of AD or remission in the early stages of AD such as MCI.
Acknowledgment
D. P. Cadinale is a Research Career Awardee from the Argentine National Research Council (CONICET), Argentina.
Conflict of Interest Statement and Disclosure Statement
S. R. Pandi-Perumal is a stockholder and the President and Chief Executive Office of Somnogen Inc., a New York Corporation. He declared no competing interests that might be perceived to influence the content of this article. All remaining authors declare that they have no proprietary, financial, professional, nor any other personal interest of any kind in any product or services and/or company that could be construed or considered to be a potential conflict of interest that might have influenced the views expressed in this manuscript.
References
- 1.Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature Neuroscience. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancuso M, Orsucci D, LoGerfo A, Calsolaro V, Siciliano G. Clinical features and pathogenesis of Alzheimer's disease: involvement of mitochondria and mitochondrial DNA. Advances in Experimental Medicine and Biology. 2010;685:34–44. doi: 10.1007/978-1-4419-6448-9_4. [DOI] [PubMed] [Google Scholar]
- 3.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 4.Hensley K, Carney JM, Mattson MP, et al. A model for β-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(8):3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappolla MA, Chyan YJ, Poeggeler B, et al. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer’s disease. Journal of Neural Transmission. 2000;107(2):203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. Journal of Alzheimer's Disease. 2010;20(supplement 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller WE, Eckert A, Kurz C, Eckert GP, Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease-therapeutic aspects. Molecular Neurobiology. 2010;41(2-3):159–171. doi: 10.1007/s12035-010-8141-5. [DOI] [PubMed] [Google Scholar]
- 8.Viña J, Lloret A. Why women have more Alzheimer's disease than men: gender and mitochondrial toxicity of amyloid-β peptide. Journal of Alzheimer's Disease. 2010;20(supplement 2):S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira BF, Nogueira-Machado JA, Chaves MM. The role of oxidative stress in the aging process. Scientific World Journal. 2010;10:1121–1128. doi: 10.1100/tsw.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hipkiss AR. Mitochondrial dysfunction, proteotoxicity, and aging: causes or effects, and the possible impact of NAD+-controlled protein glycation. Advances in Clinical Chemistry. 2010;50:123–150. [PubMed] [Google Scholar]
- 11.Calabrese V, Cornelius C, Mancuso C, Lentile R, Stella AM, Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods in Molecular Biology. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- 12.Candore G, Bulati M, Caruso C, et al. Inflammation, cytokines, immune response, apolipoprotein E, cholesterol, and oxidative stress in alzheimer disease: therapeutic implications. Rejuvenation Research. 2010;13(2-3):301–313. doi: 10.1089/rej.2009.0993. [DOI] [PubMed] [Google Scholar]
- 13.Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer’s disease. CNS and Neurological Disorders—Drug Targets. 2010;9(2):156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiala M. Re-balancing of inflammation and Aβ immunity as a therapeutic for Alzheimer’s disease-view from the bedside. CNS and Neurological Disorders—Drug Targets. 2010;9(2):192–196. doi: 10.2174/187152710791012044. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 16.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nature Reviews Neurology. 2009;5(12):649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. International Psychogeriatrics. 2009;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 18.Cochen V, Arbus C, Soto ME, et al. Sleep disorders and their impacts on healthy, dependent, and frail older adults. Journal of Nutrition, Health and Aging. 2009;13(4):322–329. doi: 10.1007/s12603-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 19.Vecchierini MF. Sleep disturbances in Alzheimer’s disease and other dementias. Psychologie et NeuroPsychiatrie du Vieillissement. 2010;8(1):15–23. doi: 10.1684/pnv.2010.0203. [DOI] [PubMed] [Google Scholar]
- 20.Skene DJ, Vivient-Roels B, Sparks DL, et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Research. 1990;528(1):170–174. doi: 10.1016/0006-8993(90)90214-v. [DOI] [PubMed] [Google Scholar]
- 21.Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-ε4/4 genotype. Journal of Clinical Endocrinology and Metabolism. 1999;84(1):323–327. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- 22.Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biological Psychiatry. 1999;45(4):417–421. doi: 10.1016/s0006-3223(97)00510-6. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan V, Pandi-Perumal SR, Cardinali DP, Poeggeler B, Hardeland R. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behavioral and Brain Functions. 2006;2, article 15 doi: 10.1186/1744-9081-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur C, Sivakumar V, Lu J, Ling EA. Increased vascular permeability and nitric oxide production in response to hypoxia in the pineal gland. Journal of Pineal Research. 2007;42(4):338–349. doi: 10.1111/j.1600-079X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 25.Sakotnik A, Liebmann PM, Stoschitzky K, et al. Decreased melatonin synthesis in patients with coronary artery disease. European Heart Journal. 1999;20(18):1314–1317. doi: 10.1053/euhj.1999.1527. [DOI] [PubMed] [Google Scholar]
- 26.Girotti L, Lago M, Ianovsky O, et al. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. Journal of Pineal Research. 2000;29(3):138–142. doi: 10.1034/j.1600-079x.2000.290302.x. [DOI] [PubMed] [Google Scholar]
- 27.Girotti L, Lago M, Ianovsky O, et al. Low urinary 6-sulfatoxymelatonin levels in patients with severe congestive heart failure. Endocrine. 2003;22(3):245–248. doi: 10.1385/ENDO:22:3:245. [DOI] [PubMed] [Google Scholar]
- 28.Peers C, Pearson HA, Boyle JP. Hypoxia and Alzheimer’s disease. Essays in Biochemistry. 2007;43:153–164. doi: 10.1042/BSE0430153. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmotto M, Aragno M, Autelli R, et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1α. Journal of Neurochemistry. 2009;108(4):1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Zhou K, Wang R, et al. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. Journal of Biological Chemistry. 2007;282(15):10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Le W. Pathological role of hypoxia in Alzheimer’s disease. Experimental Neurology. 2010;223(2):299–303. doi: 10.1016/j.expneurol.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Ng K-M, Lau C-F, Fung M-L. Melatonin reduces hippocampal β-amyloid generation in rats exposed to chronic intermittent hypoxia. Brain Research. 2010;1354(C):163–171. doi: 10.1016/j.brainres.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 33.Aldhous M, Franey C, Wright J, Arendt J. Plasma concentrations of melatonin in man following oral absorption of different preparations. British Journal of Clinical Pharmacology. 1985;19(4):517–521. doi: 10.1111/j.1365-2125.1985.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson SA, Rajaratnam SMW, Dawson D. Melatonin agonists and insomnia. Expert Review of Neurotherapeutics. 2010;10(2):305–318. doi: 10.1586/ern.10.1. [DOI] [PubMed] [Google Scholar]
- 35.Pandi-Perumal SR, Srinivasan V, Spence DW, et al. Ramelteon: a review of its therapeutic potential in sleep disorders. Advances in Therapy. 2009;26(6):613–626. doi: 10.1007/s12325-009-0041-6. [DOI] [PubMed] [Google Scholar]
- 36.Brown GM, Cardinali DP, Pandi-Perumal SR. Melatonin and mental illness. In: Pandi-Perumal SR, Kramer M, editors. Sleep and Mental Illness. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 37.Klein DC. Arylalkylamine N-acetyltransferase: "the timezyme". Journal of Biological Chemistry. 2007;282(7):4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 38.Klein DC, Bailey MJ, Carter DA, et al. Pineal function: impact of microarray analysis. Molecular and Cellular Endocrinology. 2010;314(2):170–183. doi: 10.1016/j.mce.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tricoire H, Locatelli A, Chemineau P, Malpaux B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology. 2002;143(1):84–90. doi: 10.1210/endo.143.1.8585. [DOI] [PubMed] [Google Scholar]
- 40.Leston J, Harthé C, Brun J, et al. Melatonin is released in the third ventricle in humans. A study in movement disorders. Neuroscience Letters. 2010;469(3):294–297. doi: 10.1016/j.neulet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Reiter RJ, Tan DX. Role of CSF in the transport of melatonin. Journal of Pineal Research. 2002;33(1, article 61) doi: 10.1034/j.1600-079x.2002.2e001.x. [DOI] [PubMed] [Google Scholar]
- 42.Pang SF, Brown GM. Regional concentrations of melatonin in the rat brain in the light and dark period. Life Sciences. 1983;33(12):1199–1204. doi: 10.1016/0024-3205(83)90025-5. [DOI] [PubMed] [Google Scholar]
- 43.Catalá MD, Quay WB, Timiras PS. Lower tryptophan:phenylalanine ratios in culture media increase medium: pineal melatonin ratios in early dark but not late light phase. Journal of Pineal Research. 1987;4(3):267–275. doi: 10.1111/j.1600-079x.1987.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 44.Cardinali DP, Rosenstein RE, Golombek DA, et al. Melatonin binding sites in brain: single or multiple? Advances in Pineal Research. 1991;5:159–165. [Google Scholar]
- 45.Facciolá G, Hidestrand M, von Bahr C, Tybring G. Cytochrome P isoforms involved in melatonin metabolism in human liver microsomes. European Journal of Clinical Pharmacology. 2001;56(12):881–888. doi: 10.1007/s002280000245. [DOI] [PubMed] [Google Scholar]
- 46.Hirata F, Hayaishi O, Tokuyama T, Senoh S. In vitro and in vitro formation of two new metabolites of melatonin. Journal of Biological Chemistry. 1974;249(4):1311–1313. [PubMed] [Google Scholar]
- 47.Hardeland R, Tan DX, Reiter RJ. Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. Journal of Pineal Research. 2009;47(2):109–126. doi: 10.1111/j.1600-079X.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 48.Tan DX, Manchester LC, Reiter RJ, et al. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochemical and Biophysical Research Communications. 1998;253(3):614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- 49.Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49(8):654–664. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- 50.Dawson D, Armstrong SM. Chronobiotics—drugs that shift rhythms. Pharmacology and Therapeutics. 1996;69(1):15–36. doi: 10.1016/0163-7258(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 51.Wurtman RJ, Zhdanova I. Improvement of sleep quality by melatonin. Lancet. 1995;346(8988):p. 1491. doi: 10.1016/s0140-6736(95)92509-0. [DOI] [PubMed] [Google Scholar]
- 52.Monti JM, Alvariño F, Cardinali D, Savio I, Pintos A. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Archives of Gerontology and Geriatrics. 1999;28(2):85–98. doi: 10.1016/s0167-4943(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 53.Carrillo-Vico A, Reiter RJ, Lardone PJ, et al. The modulatory role of melatonin on immune responsiveness. Current Opinion in Investigational Drugs. 2006;7(5):423–431. [PubMed] [Google Scholar]
- 54.Srinivasan V, Maestroni GJM, Cardinali DP, Esquifino AI, Pandi Perumal SR, Miller SC. Melatonin, immune function and aging. Immunity and Ageing. 2005;2, article 17 doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reiter RJ, Garcia JJ, Pie J. Oxidative toxicity in models of neurodegeneration: responses to melatonin. Restorative Neurology and Neuroscience. 1998;12(2-3):135–142. [PubMed] [Google Scholar]
- 56.Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Progress in Brain Research. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- 57.Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocrine Reviews. 1980;1(2):109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- 58.Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biology of Reproduction. 2009;81(3):445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, Brzezinski A. Melatonin and human reproduction: shedding light on the darkness hormone. Gynecological Endocrinology. 2009;25(12):779–785. doi: 10.3109/09513590903159649. [DOI] [PubMed] [Google Scholar]
- 60.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Current Topics in Medicinal Chemistry. 2002;2(2):113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 61.Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. Journal of Pineal Research. 2010;48(1):9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewy AJ. Circadian misalignment in mood disturbances. Current Psychiatry Reports. 2009;11(6):459–465. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 63.Pandi-Perumal SR, Trakht I, Brown GM, Cardinali DP. Melatonin, circadian dysregulation, and sleep in mental disorders. Primary Psychiatry. 2008;15(5):77–82. [Google Scholar]
- 64.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13(5):1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 65.Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel(1b) melatonin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacological Reviews. 2010;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carlberg C, Wiesenberg I. The orphan receptor family RZR/ROR, melatonin and 5-lipoxygenase: an unexpected relationship. Journal of Pineal Research. 1995;18(4):171–178. doi: 10.1111/j.1600-079x.1995.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 68.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Progress in Nucleic Acid Research and Molecular Biology. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 69.Benítez-King G. Melatonin as a cytoskeletal modulator: implications for cell physiology and disease. Journal of Pineal Research. 2006;40(1):1–9. doi: 10.1111/j.1600-079X.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 70.Cardinali DP, Freire F. Melatonin effects on brain. Interaction with microtubule protein, inhibition of fast axoplasmic flow and induction of crystaloid and tubular formations in the hypothalamus. Molecular and Cellular Endocrinology. 1975;2(5):317–330. doi: 10.1016/0303-7207(75)90019-2. [DOI] [PubMed] [Google Scholar]
- 71.Urata Y, Honma S, Goto S, et al. Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radical Biology and Medicine. 1999;27(7-8):838–847. doi: 10.1016/s0891-5849(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 72.Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, Cardinali DP. Role of melatonin in neurodegenerative diseases. Neurotoxicity Research. 2005;7(4):293–318. doi: 10.1007/BF03033887. [DOI] [PubMed] [Google Scholar]
- 73.Kaur C, Ling EA. Antioxidants and neuroprotection in the adult and developing central nervous system. Current Medicinal Chemistry. 2008;15(29):3068–3080. doi: 10.2174/092986708786848640. [DOI] [PubMed] [Google Scholar]
- 74.Pappolla MA, Sos M, Omar RA, et al. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. Journal of Neuroscience. 1997;17(5):1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pappolla MA, Chyan YJ, Poeggeler B, et al. Alzheimer β protein mediated oxidative damage of mitochondrial DNA: prevention by melatonin. Journal of Pineal Research. 1999;27(4):226–229. doi: 10.1111/j.1600-079x.1999.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 76.Pappolla MA, Simovich MJ, Bryant-Thomas T, et al. The neuroprotective activities of melatonin against the Alzheimer β-protein are not mediated by melatonin membrane receptors. Journal of Pineal Research. 2002;32(3):135–142. doi: 10.1034/j.1600-079x.2002.1o838.x. [DOI] [PubMed] [Google Scholar]
- 77.Pérez M, Hernández F, Gómez-Ramos A, Smith M, Perry G, Avila J. Formation of aberrant phosphotau fibrillar polymers in neural cultured cells. European Journal of Biochemistry. 2002;269(5):1484–1489. doi: 10.1046/j.1432-1033.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- 78.Benitez-King G, Túnez I, Bellon A, Ortíz GG, Antón-Tay F. Melatonin prevents cytoskeletal alterations and oxidative stress induced by okadaic acid in N1E-115 cells. Experimental Neurology. 2003;182(1):151–159. doi: 10.1016/s0014-4886(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 79.Ting KK, Brew B, Guillemin G. The involvement of astrocytes and kynurenine pathway in Alzheimer’s disease. Neurotoxicity Research. 2007;12(4):247–262. doi: 10.1007/BF03033908. [DOI] [PubMed] [Google Scholar]
- 80.Harris FM, Tesseur I, Brecht WJ, et al. Astroglial regulation of apolipoprotein E expression in neuronal cells: implications for Alzheimer’s disease. Journal of Biological Chemistry. 2004;279(5):3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- 81.Malchiodi-Albedi F, Domenici MR, Paradisi S, Bernardo A, Ajmone-Cat MA, Minghetti L. Astrocytes contribute to neuronal impairment in βA toxicity increasing apoptosis in rat hippocampal neurons. GLIA. 2001;34(1):68–72. doi: 10.1002/glia.1041. [DOI] [PubMed] [Google Scholar]
- 82.Feng Z, Zhang JT. Protective effect of melatonin on β-amyloid-induced apoptosis in rat astroglioma c6 cells and its mechanism. Free Radical Biology and Medicine. 2004;37(11):1790–1801. doi: 10.1016/j.freeradbiomed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 83.Poeggeler B, Miravalle L, Zagorski MG, et al. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the alzheimer amyloid Aβ peptidet. Biochemistry. 2001;40(49):14995–15001. doi: 10.1021/bi0114269. [DOI] [PubMed] [Google Scholar]
- 84.Matsubara E, Bryant-Thomas T, Quinto JP, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. Journal of Neurochemistry. 2003;85(5):1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 85.Cheng X, van Breemen RB. Mass spectrometry-based screening for inhibitors of β-amyloid protein aggregation. Analytical Chemistry. 2005;77(21):7012–7015. doi: 10.1021/ac050556a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poeggeler B, Pappolla MA, Hardeland R, et al. Indole-3-propionate: a potent hydroxyl radical scavenger in rat brain. Brain Research. 1999;815(2):382–388. doi: 10.1016/s0006-8993(98)01027-0. [DOI] [PubMed] [Google Scholar]
- 87.Chyan YJ, Poeggeler B, Omar RA, et al. Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. Journal of Biological Chemistry. 1999;274(31):21937–21942. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
- 88.Harris JR, Milton NG. Cholesterol in Alzheimer’s disease and other amyloidogenic disorders. Sub-Cellular Biochemistry. 2010;51:47–75. doi: 10.1007/978-90-481-8622-8_2. [DOI] [PubMed] [Google Scholar]
- 89.Feng Z, Chang Y, Cheng Y, et al. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. Journal of Pineal Research. 2004;37(2):129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 90.Olcese JM, Cao C, Mori T, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. Journal of Pineal Research. 2009;47(1):82–96. doi: 10.1111/j.1600-079X.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 91.Li XC, Wang ZEF, Zhang JX, Wang Q, Wang JZ. Effect of melatonin on calyculin A-induced tau hyperphosphorylation. European Journal of Pharmacology. 2005;510(1-2):25–30. doi: 10.1016/j.ejphar.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 92.Yang X, Yang Y, Fu Z, Jr, et al. Melatonin ameliorates alzheimer-like pathological changes and spatial memory retention impairment induced by calyculin A. doi: 10.1177/0269881110367723. Journal of Psychopharmacology. In press. [DOI] [PubMed] [Google Scholar]
- 93.Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacologica Sinica. 2005;26(5):519–526. doi: 10.1111/j.1745-7254.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 94.Liu SJ, Wang JZ. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacologica Sinica. 2002;23(2):183–187. [PubMed] [Google Scholar]
- 95.Wang XC, Zhang J, Yu X, et al. Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Acta Physiologica Sinica. 2005;57(1):7–12. [PubMed] [Google Scholar]
- 96.Olivieri G, Otten U, Meier F, et al. β-amyloid modulates tyrosine kinase B receptor expression in SHSY5Y neuroblastoma cells: influence of the antioxidant melatonin. Neuroscience. 2003;120(3):659–665. doi: 10.1016/s0306-4522(03)00342-7. [DOI] [PubMed] [Google Scholar]
- 97.Hoppe JB, Frozza RL, Horn AP, et al. Amyloid-β neurotoxicity in organotypic culture is attenuated by melatonin: involvement of GSK-3β, tau and neuroinflammation. Journal of Pineal Research. 2010;48(3):230–238. doi: 10.1111/j.1600-079X.2010.00747.x. [DOI] [PubMed] [Google Scholar]
- 98.Furio AM, Cutrera RA, Thea VC, et al. Effect of melatonin on changes in locomotor activity rhythm of Syrian hamsters injected with beta amyloid peptide 25-35 in the suprachiasmatic nuclei. Cellular and Molecular Neurobiology. 2002;22(5-6):699–709. doi: 10.1023/A:1021805023906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer's disease: a review. International Journal of Alzheimer's Disease. 2010;2010 doi: 10.4061/2010/716453. Article ID 716453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harper DG, Stopa EG, McKee AC, et al. Differential circadian rhythm disturbances in men with Alzheimer disease and frontotemporal degeneration. Archives of General Psychiatry. 2001;58(4):353–360. doi: 10.1001/archpsyc.58.4.353. [DOI] [PubMed] [Google Scholar]
- 101.van Someren EJW. Circadian and sleep disturbances in the elderly. Experimental Gerontology. 2000;35(9-10):1229–1237. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 102.van Someren EJW. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiology International. 2000;17(3):313–354. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 103.Klaffke S, Staedt J. Sundowing and circadian rhythm disorders in dementia. Acta Neurologica Belgica. 2006;106(4):168–175. [PubMed] [Google Scholar]
- 104.Pandi-Perumal SR, Seils LK, Kayumov L, et al. Senescence, sleep, and circadian rhythms. Ageing Research Reviews. 2002;1(3):559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 105.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, van Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. Journal of the American Medical Association. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 106.Ohashi Y, Okamoto N, Uchida K, Iyo M, Mori N, Morita Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biological Psychiatry. 1999;45(12):1646–1652. doi: 10.1016/s0006-3223(98)00255-8. [DOI] [PubMed] [Google Scholar]
- 107.Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. Journal of Pineal Research. 2003;35(2):125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 108.Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Experimental Gerontology. 2003;38(1-2):199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 109.Magri F, Locatelli M, Balza G, et al. Changes in endocrine circadian rhythms as markers of physiological and pathological brain aging. Chronobiology International. 1997;14(4):385–396. doi: 10.3109/07420529709001459. [DOI] [PubMed] [Google Scholar]
- 110.McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Medicine Reviews. 2000;4(6):603–628. doi: 10.1053/smrv.2000.0127. [DOI] [PubMed] [Google Scholar]
- 111.Brusco LI, Marquez M, Cardinali DP. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuroendocrinology Letters. 1998;19(3):111–115. [PubMed] [Google Scholar]
- 112.Brusco LI, Márquez M, Cardinali DP. Monozygotic twins with alzheimer’s disease treated with melatonin: case report. Journal of Pineal Research. 1998;25(4):260–263. doi: 10.1111/j.1600-079x.1998.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 113.Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiology International. 2000;17(3):419–432. doi: 10.1081/cbi-100101055. [DOI] [PubMed] [Google Scholar]
- 114.Mahlberg R, Kunz D, Sutej I, Kühl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. Journal of Clinical Psychopharmacology. 2004;24(4):456–459. doi: 10.1097/01.jcp.0000132443.12607.fd. [DOI] [PubMed] [Google Scholar]
- 115.Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer’s disease. Neuroendocrinology Letters. 2002;23(1):20–23. [PubMed] [Google Scholar]
- 116.Fainstein I, Bonetto AJ, Brusco LI, Cardinali DP. Effects of melatonin in elderly patients with sleep disturbance: a pilot study. Current Therapeutic Research—Clinical and Experimental. 1997;58(12):990–1000. [Google Scholar]
- 117.Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia—a preliminary study. Archives of Gerontology and Geriatrics. 2000;31(1):65–76. doi: 10.1016/s0167-4943(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 118.Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randodmised placebo controlled trial of low dose melatonin for sleep disorders in dementia. International Journal of Geriatric Psychiatry. 2002;17(12):1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 119.Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. Journal of Nippon Medical School. 2003;70(4):334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- 120.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26(7):893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mahlberg R, Walther S. Actigraphy in agitated patients with dementia: monitoring treatment outcomes. Zeitschrift fur Gerontologie und Geriatrie. 2007;40(3):178–184. doi: 10.1007/s00391-007-0420-z. [DOI] [PubMed] [Google Scholar]
- 122.Dowling GA, Burr RL, van Someren EJW, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. Journal of the American Geriatrics Society. 2008;56(2):239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anderson KN, Jamieson S, Graham AJ, Shneerson JM. REM sleep behaviour disorder treated with melatonin in a patient with Alzheimer’s disease. Clinical Neurology and Neurosurgery. 2008;110(5):492–495. doi: 10.1016/j.clineuro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with alzheimer disease. American Journal of Geriatric Psychiatry. 2009;17(2):166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Current Neuropharmacology. 2010;8(3):218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu YH, Zhou JN, van Heerikhuize J, Jockers R, Swaab DF. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiology of Aging. 2007;28(8):1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 127.Wu YH, Fischer DF, Kalsbeek A, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the "master clock". The FASEB Journal. 2006;20(11):1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 128.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Medicine. 2007;8(6):623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 129.Quinn J, Kulhanek D, Nowlin J, et al. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Research. 2005;1037(1-2):209–213. doi: 10.1016/j.brainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 130.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 131.Jean-Louis G, von Gizycki H, Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. Journal of Pineal Research. 1998;25(3):177–183. doi: 10.1111/j.1600-079x.1998.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 132.Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. American Journal of Geriatric Psychiatry. 2004;12(4):432–436. doi: 10.1176/appi.ajgp.12.4.432. [DOI] [PubMed] [Google Scholar]
- 133.Furio AM, Brusco LI, Cardinali DP. Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. Journal of Pineal Research. 2007;43(4):404–409. doi: 10.1111/j.1600-079X.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 134.Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Current Medical Research and Opinion. 2007;23(10):2597–2605. doi: 10.1185/030079907X233098. [DOI] [PubMed] [Google Scholar]
- 135.Garzón C, Guerrero JM, Aramburu O, Guzmán T. Effecf of melatonin administration on sleep, behavioral disorders and hypnotic drug discontinuation in the elderly: a randomized, double-blind, placebo-controlled study. Aging—Clinical and Experimental Research. 2009;21(1):38–42. doi: 10.1007/BF03324897. [DOI] [PubMed] [Google Scholar]
- 136.Miyamoto M. Pharmacology of ramelteon, a selective MT/MT receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neuroscience and Therapeutics. 2009;15(1):32–51. doi: 10.1111/j.1755-5949.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanchez-Barcelo EJ, Martinez-Campa CM, Mediavilla MD, et al. Melatonin and melatoninergic drugs as therapeutic agents: ramelteon and agomelatine, the two most promising melatonin receptor agonists. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery. 2007;1:142–151. [Google Scholar]
- 138.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48(2):301–310. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cellular and Molecular Life Sciences. 2007;64(10):1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cajochen C. TAK-375 Takeda. Current Opinion in Investigational Drugs. 2005;6(1):114–121. [PubMed] [Google Scholar]
- 141.Stevenson S, Bryson S, Amayke D, et al. Study to investigate the absolute bioavailability of a single oral dose of ramelteon (TAK-375) in healthy male subjects. Clinical Pharmacology and Therapeutics. 2004;75:p. P22. [Google Scholar]
- 142.Greenblatt DJ, Harmatz JS, Karim A. Age and gender effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. Journal of Clinical Pharmacology. 2007;47(4):485–496. doi: 10.1177/0091270006298602. [DOI] [PubMed] [Google Scholar]
- 143.Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Medicine. 2006;7(4):312–318. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 144.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon’s efficacy and safety in older adults with chronic insomnia. Current Medical Research and Opinion. 2007;23(5):1005–1014. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 145.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. Journal of Clinical Sleep Medicine. 2007;3(5):495–504. [PMC free article] [PubMed] [Google Scholar]
- 146.Zammit G, Schwartz H, Roth T, Wang-Weigand S, Sainati S, Zhang J. The effects of ramelteon in a first-night model of transient insomnia. Sleep Medicine. 2009;10(1):55–59. doi: 10.1016/j.sleep.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 147.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32(3):351–360. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kryger M, Wang-Weigand S, Roth T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep and Breathing. 2007;11(3):159–164. doi: 10.1007/s11325-006-0096-4. [DOI] [PubMed] [Google Scholar]
- 149.Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(1):8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- 150.Mathes AM, Kubulus D, Waibel L, et al. Selective activation of melatonin receptors with ramelteon improves liver function and hepatic perfusion after hemorrhagic shock in rat. Critical Care Medicine. 2008;36(10):2863–2870. doi: 10.1097/CCM.0b013e318187b863. [DOI] [PubMed] [Google Scholar]
- 151.Tan D, Reiter RJ, Chen L, Poeggeler B, Manchester LC, Barlow-Walden LR. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis. 1994;15(2):215–218. doi: 10.1093/carcin/15.2.215. [DOI] [PubMed] [Google Scholar]
- 152.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochimica Polonica. 2003;50(4):1129–1146. [PubMed] [Google Scholar]
- 153.Hardeland R, Pandi-Perumal SR. Melatonin, a potent agent in antioxidative defense: actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutrition and Metabolism. 2005;2, article 22 doi: 10.1186/1743-7075-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS Journal. 2006;273(13):2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 155.Hardeland R, Poeggeler B. Melatonin beyond its classical functions. Open Physiology Jounal. 2008;1:1–23. [Google Scholar]
- 156.Brunner P, Sözer-Topcular N, Jockers R, et al. Pineal and cortical melatonin receptors MT1 and MT1 are decreased in Alzheimer’s disease. European Journal of Histochemistry. 2006;50(4):311–316. [PubMed] [Google Scholar]
- 157.Savaskan E, Ayoub MA, Ravid R, et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. Journal of Pineal Research. 2005;38(1):10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 158.Savaskan E, Jockers R, Ayoub M, et al. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Current Alzheimer Research. 2007;4(1):47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- 159.Savaskan E, Olivieri G, Brydon L, et al. Cerebrovascular melatonin MT1-receptor alterations in patients with Alzheimer’s disease. Neuroscience Letters. 2001;308(1):9–12. doi: 10.1016/s0304-3940(01)01967-x. [DOI] [PubMed] [Google Scholar]
- 160.Niles LP, Armstrong KJ, Rincón Castro LM, et al. Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neuroscience. 2004;5, article 41 doi: 10.1186/1471-2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kong X, Li X, Cai Z, et al. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cellular and Molecular Neurobiology. 2008;28(4):569–579. doi: 10.1007/s10571-007-9212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Imbesi M, Uz T, Dzitoyeva S, Manev H. Stimulatory effects of a melatonin receptor agonist, ramelteon, on BDNF in mouse cerebellar granule cells. Neuroscience Letters. 2008;439(1):34–36. doi: 10.1016/j.neulet.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 163.Rosenstein RE, Pandi-Perumal SR, Srinivasan V, Spence DW, Brown GM, Cardinali DP. Melatonin as a therapeutic tool in ophthalmology: implications for glaucoma and uveitis. Journal of Pineal Research. 2010;49(1):1–13. doi: 10.1111/j.1600-079X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 164.Benedetti F, Serretti A, Colombo C, Lorenzi C, Tubazio V, Smeraldi E. A glycogen synthase kinase 3-β promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neuroscience Letters. 2004;368(2):123–126. doi: 10.1016/j.neulet.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 165.Sehgal A, Joiner W, Crocker A, et al. Molecular analysis of sleep: wake cycles in Drosophila. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72:557–564. doi: 10.1101/sqb.2007.72.018. [DOI] [PubMed] [Google Scholar]
- 166.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. European Respiratory Journal. 2009;33(6):1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 167.Cirelli C, Tononi G. Uncoupling proteins and sleep deprivation. Archives Italiennes de Biologie. 2004;142(4):541–549. [PubMed] [Google Scholar]
- 168.Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. Journal of Neurochemistry. 2006;98(5):1632–1645. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]