Abstract
Commencement of antiretroviral treatment (ART) in severely immunosuppressed HIV-infected persons is associated with unmasking of subclinical disease. The subset of patients that are diagnosed with tuberculosis (TB) disease while on ART have been classified as ART-associated TB. Few studies have reported the incidence of ART-associated TB and unmasking TB-IRIS according to the International Network for the Study of HIV-Associated IRIS (INSHI) consensus definition. To determine the incidence and predictors of ART-associated TB, we screened 219 patients commencing ART at the Infectious Diseases Clinic in Kampala, Uganda for TB by symptoms, sputum microscopy, and chest X-rays and followed them for one year. Fourteen (6.4%) patients were diagnosed with TB during followup. Eight (3.8%) patients had ART-associated TB (incidence rate of 4.3 per 100 person years); of these, three patients fulfilled INSHI criteria for unmasking TB-associated IRIS (incidence rate of 1.6 per 100 person years). A body mass index of less than 18.5 kg/m2 BMI (HR 5.85 95% CI 1.24–27.46, P = .025) and a C-reactive protein greater than 5 mg/L (HR 8.23 95% CI 1.36–38.33, P = .020) were risk factors for ART-associated TB at multivariate analysis. In conclusion, with systematic TB screening (including culture and chest X-ray), the incidence of ART-associated TB is relatively low in settings with high HIV and TB prevalence.
1. Introduction
Tuberculosis (TB) remains a leading cause of morbidity and mortality in sub-Saharan Africa with the HIV pandemic accounting for 31% of all new TB cases in adults [1, 2]. This is partly because available diagnostic tests are not sensitive enough for early detection of all TB cases [3–5]. Additionally, patients often seek medical care late when they already have advanced HIV disease or are very sick [6–8]. Sputum smear microscopy, the tool for TB screening in most resource-limited settings, has low sensitivity to diagnose TB disease, especially in HIV-infected patients with advanced immunodeficiency [9–11].
The World Health Organization (WHO) currently recommends routine screening for TB prior to antiretroviral treatment (ART) initiation [12]. Such screening generally targets symptomatic patients. Therefore, TB patients without symptoms or with atypical symptoms and subclinical disease are often not diagnosed. Intensive screening of HIV-infected patients for TB prior to ART regardless of symptoms has been demonstrated to increase the number of TB cases identified [13, 14]. In a recent study from Durban, South Africa, TB was detected by intensive screening in 158 (19%) of 825 patients undergoing ART preparation [13]. Only 52% of these patients reported cough. It has been demonstrated that patients with subclinical disease started on ART may rapidly progress to symptomatic TB disease as a result of immune reconstitution, and this risk is highest during the first three months of ART [15, 16].
Immune reconstitution inflammatory syndrome (IRIS) is a disorder commonly observed upon ART initiation in severely immune-compromised patients who have concomitant opportunistic infections. HIV/TB coinfection is a leading cause of IRIS. Two clinical types of TB-IRIS have been described: unmasking IRIS (an existing occult/subclinical infection becomes clinically evident after the start of ART) and paradoxical IRIS (worsening of a successfully treated infection following the introduction of ART) [17]. Whereas unmasking IRIS is postulated to result from an imbalanced and exuberant inflammatory response against a viable pathogenic organism in face of a rapidly reconstituting immunity, in paradoxical TB-IRIS, this dysregulated immune response is generally targeted against residual pathogenic antigens. Exact mechanisms for the dysfunctional restoration of pathogen-specific immune responses are not well understood, but defects in antigen-specific activated regulatory and effector CD4 T lymphocytes have been suggested by a number of studies [18–21]. Several studies have reported the incidence of paradoxical TB-IRIS, but relatively few studies have reported the incidence of unmasking and ART-associated TB-IRIS.
According to the International Network for the Study of HIV-Associated IRIS (INSHI), ART-associated TB refers to all TB diagnosed during ART, while the subset of patients who develop rapidly progressive signs and symptoms of TB, with exuberant inflammatory features, after initiation of ART are called “unmasking TB-associated IRIS” [22]. In programmes “rolling out” ART, ART-associated TB may be difficult to differentiate from multiple other opportunistic pathologies, thus further delaying diagnosis and appropriate treatment.
The purpose of this study was to determine the incidence and clinical manifestations of both ART-associated TB and unmasking TB-associated IRIS in an ambulatory HIV care setting in Uganda, where HIV prevalence is high [23]. A secondary objective was to determine the predictive baseline demographic, clinical, and laboratory parameters in patients who develop ART-associated TB.
2. Study Methodology
In a prospective cohort of HIV patients eligible for ART at the Infectious Disease Institute (IDI), Kampala, Uganda, we screened 247 patients for study entry. Inclusion criteria were (1) age >18 years, (2) documented HIV infection, (3) ART eligibility according to Uganda Ministry of Health guidelines [24] (CD4 < 250 cells/μL), (4) ART naïve, (5) no evidence of active TB disease by acid fast bacilli (AFB) smear microscopy and chest radiograph (CXR) and (6) willingness to participate in all followup visits and clinical examinations and to have blood drawn for clinical and immunological studies.
At study enrolment, the HIV serostatus was confirmed using a standard HIV testing algorithm [25], complete blood count with differential CD4 count (FACSCalibur, Becton Dickinson), and C-reactive protein (CRP) (COBAS C-Reactive Protein (Latex), Roche Diagnostics; normal range <5 mg/L) were also measured. Patients were initiated on ART according to the Uganda National ART guidelines [24]. TB screening was based on a standardized symptom questionnaire (presence and duration of cough, chest pain, fever, poor appetite, and weight loss; specifying the duration of each symptom) and physical examination. Patients who had a productive cough of greater than two weeks had sputum collected for TB investigations. Two expectorated sputum samples (early morning) were obtained and sent for microscopy by Ziehl Neelsen (ZN) stain and Fluorescence microscopy (FM); and mycobacterial cultures were performed at the national TB reference laboratory on Lowenstein-Jensen (LJ) culture media. A sputum smear was considered AFB positive if there were at least 10 bacilli per 100 fields. A positive mycobacterial culture was defined as any presence of Mycobacterium tuberculosis (M. tb) colonies within a period of 6 to 8 weeks of incubation on LJ media. A tuberculin skin test (TST) was administered by intradermal injection of 2TU of RT23 in the volar aspect of the left forearm, and all participants were instructed to come back for its reading 48–72 hours later. A positive TST was defined as a skin induration of at least 5 mm diameter. All patients had CXRs done, and these were examined by a radiologist and reported as normal (unlikely pulmonary tuberculosis (PTB)), possible PTB (infiltrates, nodules, or other abnormality), or probable PTB (with cavitation, adenopathy, pleural effusion, or miliary pattern). Patients judged to have active TB (by clinical assessment, sputum microscopy, or CXR) at ART commencement were started on TB treatment and excluded from the study. Asymptomatic participants with a positive TST and judged to be free of TB based on CXR and sputum culture results were offered isoniazid prophylaxis.
Study participants were followed up and regularly assessed for signs and symptoms suggestive of TB at 2, 4, 8, and 12 weeks after ART initiation, and quarterly thereafter up to one year. Further TB investigations were performed during followup if the patient developed new or worsening symptoms suggestive of TB. This included two sputum microscopy examinations for AFB and culture on LJ media, a CXR (which was compared to one at ART treatment start for evidence of worsening), and an abdominal ultrasound. Patients diagnosed with TB were started on TB treatment and censored from the study. Further routine followup for TB treatment was conducted at the national TB Leprosy programme clinic. The laboratory examinations (haematology, immunology) were performed at the Joint Clinical Research Centre (JCRC) and Makerere University-John Hopkins University (MU-JHU) Core laboratories in Kampala; microscopy examination for AFB and mycobacterial cultures was performed at the National TB Reference Laboratory in Kampala and JCRC TB laboratory.
2.1. Study Definitions
TB was diagnosed by a study medical officer according to WHO recommendations [26]. PTB diagnosis was based on at least one sputum AFB-positive result; a positive culture result or an abnormal CXR with a clinical improvement after initiation of TB treatment. For diagnosis of extrapulmonary TB, we considered one sample from an extrapulmonary site which was AFB positive or M.tb culture positive, or histological or strong clinical evidence of TB and decision to treat with a full course of TB treatment. Patients who developed signs and symptoms suggestive of TB during followup were evaluated by the study medical officer as TB-IRIS suspects using a standard questionnaire including the INSHI TB-IRIS criteria. Two of the study investigators (W. Worodria and R. Colebunders) later classified these patients as ART-associated TB with or without unmasking TB-IRIS according to the INSHI case definitions (see Table 1) [22]. TB was defined as prevalent if the participant had significant symptoms before the start of ART (a cough of at least two weeks with or without chest pain, fever, or poor appetite) with a positive AFB smear or M.tb culture, or radiological abnormality with response to TB, treatment. Patients who had none of the above features at ART initiation but developed them later during the course of treatment were classified as incident TB. As ART-associated TB, we included all patients diagnosed with TB after the start of ART regardless of the fact that after the start of ART a sputum culture obtained before the start of ART was found to be positive. We did not include ART-associated TB patients diagnosed after the start of ART if retrospective review of clinical files indicated that the diagnosis of TB was missed according to WHO guidelines.
Table 1.
The International Network for the Study of HIV-Associated IRIS (INSHI) case definition for ART-associated tuberculosis [22].
| ART-associated tuberculosis | |
| (i) Patient is not receiving treatment for tuberculosis when ART is initiated. | |
| (ii) Active tuberculosis is diagnosed after initiation of ART. | |
| (iii) The diagnosis of tuberculosis should fulfil WHO criteria for smear-positive pulmonary tuberculosis, smear-negative pulmonary tuberculosis, or extrapulmonary tuberculosis. | |
| Unmasking tuberculosis-associated IRIS* | |
| (i) Patient is not receiving treatment for tuberculosis when ART is initiated and then presents with tuberculosis within 3 months of starting ART. | |
| And one of the following criteria must be met: | |
| (ii) heightened intensity of clinical manifestations, particularly if there is evidence of a marked inflammatory component to the presentation. Examples include tuberculosis lymphadenitis or tuberculosis abscesses with prominent acute inflammatory features, presentation with tuberculosis that is complicated by respiratory failure due to adult respiratory distress syndrome, and those who present with a marked systemic inflammatory syndrome related to tuberculosis, | |
| (iii) once established on tuberculosis treatment, a clinical course that is complicated by a paradoxical reaction. | |
| ART: antiretroviral therapy; IRIS: immune reconstitution inflammatory syndrome |
*unmasking tuberculosis-associated IRIS is a subset of ART-associated tuberculosis.
2.2. Data Management and Statistical Analysis
Patient data was collected on case report forms, monitored by clinical research associates, double entered, validated and stored in an SQL database, and exported to STATA version 10.1 (STATA, College Station, TX, USA) for analysis. Baseline characteristics for patients with prevalent TB, incident TB, and without TB were compared. The predictive value of clinical and laboratory parameters for any diagnosis of TB and specifically for ART-associated TB was examined by Cox proportional hazards and reported as hazard ratios. The time from start of ART to all diagnosis of TB and ART-associated TB was represented as events on a Kaplan Meier curve. BMI was categorized according to WHO recommendations with underweight defined as BMI < 18.5 kg/m2 [27].
2.3. Ethics
Ethical approval for the study was obtained from the Infectious Disease Scientific Review Committee, Makerere Faculty of Medicine Ethics Committee, the Uganda National Council on Science and Technology, and the University of Antwerp Ethics Committee.
3. Results
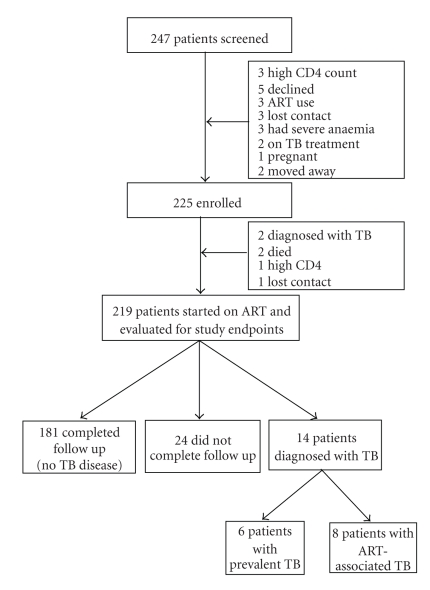
Of 247 patients evaluated for ART eligibility, 225 were enrolled, 17 were not eligible, and five were lost to followup (Figure 1). The median age of study participants was 35.7 years (interquartile range (IQR) 30.5–41.3 years) and 157 (70%) were females. The median CD4 count of the study participants was 130 cells/μL (IQR 62–170), and 99 (44%) of the patients were in WHO clinical stage 3 or 4 at enrolment. Sixty eight (30%) of 220 patients had a positive tuberculin skin test at enrolment. The median time from enrolment to ART initiation was 10 days (IQR 7–17). Nine (4.1%) patients who initiated ART died, two patients withdrew consent, and 7 (3.2%) moved out of the study area. Six (2.7%) patients were lost to followup and three were empirically started on TB treatment. Five of the 9 patients who died had CD4 counts less than 50 cells/μL, and these 5 died with clinical signs and symptoms suggestive of sepsis. One patient died of gastroenteritis with severe dehydration; in one patient, the cause of death was not established.
Figure 1.
Study enrollment and retention.
Of all patients screened for TB, 28 (13%) had cough of greater than two weeks' duration. Fifty (23%) patients had at least one symptom (cough, chest pain, fever, poor appetite, or weight loss) for greater than two weeks. Baseline characteristics of patients diagnosed with prevalent TB, incident TB, and without TB are presented in Table 2. Over one year of followup, 14 (6%) patients were diagnosed with TB after starting ART (incidence rate of 6.9 per 100 person years). Of these, 6 (43%) had prevalent TB (2 had positive TB cultures and 4 were sputum AFB smear negative but had abnormalities on CXR) (Table 3). TB in these patients was unrecognized and therefore untreated prior to ART start. The remaining 8 (57%) were considered to be ART-associated TB (incidence rate of 4.3 per 100 person years). Three of these patients (patients 2, 6, and 7 in Table 4) had symptoms consistent with unmasking TB-associated IRIS (incidence rate of 1.6 per 100 person years). In 5 of 8 patients with ART-associated TB and in 2 out of 3 patients with unmasking TB-associated IRIS, TB was diagnosed before an increment in absolute CD4 counts (Table 4). Isoniazid prophylaxis was provided to 23 (34%) of 68 eligible patients with normal CXR and no evidence of TB. There were no complications reported among patients on prophylaxis.
Table 2.
Baseline characteristics of 219 HIV-infected patients with prevalent TB, incident TB, and no TB.
| Characteristics | Prevalent TB (unrecognized) (n = 6) | Incident TB (n = 8) | No TB (n = 205) |
|---|---|---|---|
| Age (yrs), mean (SD) | 38.5 (5.3) | 38.3 (9.2) | 36.4 (8.2) |
| Gender (Female) (%) | 4 (67) | 6 (75) | 143 (70) |
| BMI (kg/m2), median (IQR) | 18.8 (16.6–19.7)* | 19.1 (17.9–21.2)** | 22.8 (20.4–25.7) |
| CD4 counts (cell/μL), median (IQR) | 131 (60–168) | 103 (23–140) | 131 (67–172) |
| Haemoglobin (g/dL), mean (SD) | 11.9 (1.9) | 10.5 (1.4)** | 12.4 (1.9) |
| C-reactive protein (mg/dL), median (IQR) | 24.9 (11.6–31.5)** | 19.4 (3.9–51.3)* | 2.2 (1.0–6.0) |
| TST positive at ART start (%) | 4 (67) | 1 (25) | 59 (30) |
ART: antiretroviral therapy; BMI, body mass index; IQR: interquartile range; SD: standard deviation; TB: tuberculosis; TST: tuberculin skin test.
P-values: *P < .05, **P < .001 compared with the no TB group.
Table 3.
Characteristics of patients with prevalent (undiagnosed) TB.
| Patient | Age (years) | Sex | Baseline CD4 counts (cells/μL) | Baseline clinical symptoms* | Baseline CXR findings | Time to TB diagnosis (days) | TB category | Basis of TB diagnosis | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 32 | F | 6 | cough, chest pain, weight loss, poor appetite | middle and lower lung zone infiltrates | 24 | PTB | culture+, worsening CXR | improved |
| Patient 2 | 43 | M | 168 | no symptoms | upper and middle lung zone infiltrates with cavitation | 35 | PTB | worsening CXR | improved |
| Patient 3 | 35 | F | 121 | cough, fever, weight loss, poor appetite | normal | 65 | PTB | culture+ | died |
| Patient 4 | 46 | F | 60 | cough, chest pain, poor appetite | upper lung zone infiltrates and left side pleural effusion | 0 | EPTB | abnormal CXR | improved |
| Patient 5 | 35 | F | 140 | cough | middle and lower lung zone infiltrates and pleural thickening | 43 | PTB | abnormal CXR | lost contact |
| Patient 6 | 41 | M | 180 | no symptoms | middle and lower lung zone infiltrates and bilateral cavities | 10 | PTB | abnormal CXR | improved |
Abbreviations: M: Male; F: Female; TB: tuberculosis; CXR: chest X-ray; PTB, pulmonary tuberculosis, AFB+: acid fast bacilli positive; AFB−: acid fast bacilli negative; IRIS: immune reconstitution inflammatory syndrome.
*included only clinical symptoms of greater than two weeks.
Table 4.
Characteristics of patients with ART-associated TB.
| Patient | Age (years) | Sex | CD4 counts | Baseline clinical symptoms* | Baseline CXR findings | Time to TB (days) | TB category | Basis of TB diagnosis | Basis of TB- IRIS diagnosis† | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (cell/μL) | TB (cell/μL) | ||||||||||
| Patient 1 | 53 | F | 156 | 148 | weight loss | Possible TB | 345 | TB pleura | cytology | NA | Improved |
| Patient 2 | 27 | F | 15 | 5 | weight loss, anorexia | Normal | 79 | Disseminated TB | abnormal CXR culture +ve | 2,4,5,6 | Improved |
| Patient 3 | 31 | F | 90 | 68 | cough, fever, weight loss, anorexia | Normal | 14 | PTB | culture +ve | NA | Improved |
| Patient 4 | 37 | M | 123 | — | no symptoms | Normal | 24 | PTB | culture +ve | NA | Lost contact |
| Patient 5 | 42 | M | 116 | 95 | cough, weight loss, anorexia | Possible TB | 54 | PTB | culture +ve | NA | Died |
| Patient 6 | 45 | F | 21 | 28 | weight loss | Normal | 17 | PTB | AFB+ | 2,5,6,7 | Improved |
| Patient 7 | 28 | F | 24 | 18 | no symptoms | Normal | 20 | TB adenitis | FNA AFB+ | 1,5,6 | Lost contact |
| Patient 8 | 43 | F | 206 | 313 | no symptoms | Normal | 100 | PTB | abnormal CXR | NA | Improved |
Abbreviations: M: Male; F: Female; TB: tuberculosis; CXR: chest X-ray; PTB: pulmonary tuberculosis; AFB+: acid fast bacilli positive; AFB−: acid fast bacilli negative; IRIS: immune reconstitution inflammatory syndrome.
*included clinical symptoms of greater than two weeks.
†criteria for TB-IRIS (new or worsening): 1: adenopathy; 2: CXR abnormalities; 3: central nervous system features of TB; 4: serositis; 5: constitutional symptoms (fever, night sweats, weight loss); 6: respiratory symptoms (cough, dyspnea, stridor); 7: abdominal pain with peritonitis, hepatomegaly, splenomegaly, or adenopathy; NA: not applicable.
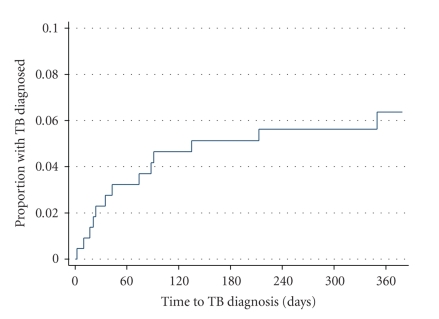
Participants diagnosed with TB had a lower BMI and higher levels of CRP protein compared to patients without TB. CRP greater than 5 mg/L and BMI < 18.5 kg/m2 were predictive of ART-associated TB in uni- and multivariate analysis (Table 5). When we included patients with unrecognized TB as cases of ART-associated TB, the same factors were also predictive (data not shown). The cumulative cases of all patients diagnosed with TB are shown in Figure 2.
Table 5.
Cox proportional hazards for baseline predictors of ART-associated TB in HIV-infected patients commencing ART.
| Baseline characteristics | Unadjusted HR (95% CI) | P-value | Adjusted† HR (95% CI) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| >40 | 1.42 (0.26–7.77) | .687 | 1.27 (0.21–7.80) | .798 |
| 30–39 | 0.43 (0.06–3.05) | .397 | 0.35 (0.05–2.64) | .308 |
| <30 | 1 | 1 | ||
| Sex | ||||
| Females | 1.19 (0.24–5.88) | .835 | 1.87 (0.34–10.21) | .468 |
| Males | 1 | 1 | ||
| Tuberculin skin test (TST) | ||||
| positive | 0.78 (0.16–3.88) | .764 | — | |
| negative | 1 | — | ||
| C-reactive protein (mg/L) | ||||
| ≥5 | 8.23 (1.66–40.82) | .010 | 7.23 (1.36–38.33) | .020 |
| <5 | 1 | 1 | ||
| Haemoglobin (g/dL) | ||||
| <12.5 | 6.66 (0.82–54.16) | .076 | 2.31 (0.23–23.43) | .477 |
| ≥12.5 | 1 | 1 | ||
| CD4 cell counts (cells/μL) | ||||
| <50 | 3.04 (0.72–12.74) | .129 | 2.22 (0.49–10.16) | .304 |
| ≥50 | 1 | 1 | ||
| Body mass index (kg/m2) | ||||
| <18.5 | 7.71 (1.92–30.89) | .004 | 5.85 (1.24–27.46) | .025 |
| ≥18.5 | 1 | 1 | ||
| WHO clinical stage | ||||
| 3 and 4 | 4.68 (0.94–23.23) | .065 | 1.82 (0.33–10.18) | .495 |
| 1 and 2 | 1 | 1 |
†adjusted for age, sex, baseline C-reactive protein result, baseline haemoglobin, baseline CD4 counts, body mass index, and WHO clinical stage.
Abbreviations: ART: antiretroviral therapy; TB: tuberculosis; HR: hazard ratio.
Figure 2.
Kaplan Meier curve of cumulative cases of tuberculosis diagnosed among ART naïve patients with HIV infection starting antiretroviral therapy.
3.1. Description of a Patient to Illustrate Unmasking TB-Associated IRIS
A 25-year-old HIV seropositive female (patient 2, Table 1) started ART (zidovudine/lamivudine/efavirenz) with a CD4 count of 15 cells/μL and viral load 128,056 copies/μL. At ART initiation, she complained of weight loss, reduced appetite, and a skin rash since 2 weeks but had no cough. Physical examination was normal; her BMI was 21.2 kg/m2. CXR was normal (Figure 3(a)), but CRP was elevated to 92.21 mg/L. Eleven weeks after starting ART, she developed a productive cough with fever, drenching night sweats, weight loss, and shortness of breath on exertion. On examination, there was moderate abdominal distension due to ascites. Sputum microscopy was AFB + on fluorescence microscopy. A repeat CXR demonstrated bilateral infiltrates in all lung zones (Figure 3(b)). An abdominal ultrasound confirmed the ascites, but there were no abdominal lymphadenopathies. Sputum and ascitic fluid cultures for M.tb were positive. At TB diagnosis, the CD4 count was 5 cells/μL and her viral load <400 copies/μL. She fully recovered on completion of TB treatment.
Figure 3.
4. Discussion
Of 213 patients commencing ART, only 8 (3.8%) developed ART-associated TB. This represents a lower incidence of TB than reported in previous studies and may be due to the systematic strategy used for TB screening: standardized questionnaire for TB-related symptoms, sputum microscopy, and culture, and CXR before commencement of ART. It may also be caused by referral bias as patients with obvious symptoms and signs of TB were not referred. Most previous studies on ART-associated TB and unmasking TB-IRIS have been retrospective [28–30], and few have been able to systematically screen for TB using CXR prior to the start of ART. This may have led to misclassification of prevalent TB disease and over diagnosis of ART-associated TB. Even in our research cohort, there were 6 additional TB cases that were not recognized at study enrolment. An earlier retrospective study performed at the IDI clinic (prior to the INSHI consensus definition) found that 26 (9.6%) of 271 ART naïve patients without a TB diagnosis at onset developed active TB within one year of ART start [31]. Thirty-one percent of these TB patients were diagnosed within 3 months of ART. A more recent study at the IDI clinic found that central nervous system infections and mycobacterial disease were the main causes of HIV-related mortality, especially in the first three months of ART [32]. Our data support systematic intensive screening for TB that includes clinical evaluation, CXRs, sputum microscopy, and culture [13, 14, 33]. Wide-scale implementation of this strategy, however, requires evaluation of cost effectiveness of the methods used and sufficient resources at a programmatic level.
Only 3 patients in our study developed a clinical picture that could be considered as unmasking TB-associated IRIS according to the INSHI definition (see Table 1). A study performed in South Africa did not find any case of TB-associated IRIS with an intensive pre-ART screening strategy for TB [33]. It is, however, difficult to define TB-associated IRIS in the absence of a biological marker and because of the subjectivity of quantifying the level of inflammatory response.
ART-associated TB represents a heterogeneous group of diseases that develop in the context of starting ART. One form is unmasking TB-associated IRIS which refers to patients without clinical, microbiological, and radiological evidence of TB at onset of ART who develop rapid and exuberant features of inflammation within three months of ART commencement. Another group are patients who were symptomatic before the start of ART but in whom a diagnosis of TB was missed due to lack of sensitive diagnostic tests. Careful followup of such patients with regular evaluation of their symptoms in addition to microbiological and radiological tests may lead to early detection of TB. The third category refers to patients with incident TB who were asymptomatic at the onset of ART but develop signs and symptoms of TB while on ART without the heightened signs and symptoms of inflammation. This may be due to a new infection or a reactivation of latent TB infection. Despite some immunological recovery on ART, patients with HIV infection still have an increased susceptibility to TB infection or reinfection albeit with reduced risk [34].
At multivariate analysis, a raised CRP (5 mg/L or greater) and low BMI (<18.5 kg/m2) were predictors of ART-associated TB (Table 3). CRP is a nonspecific direct quantitative measure of acute phase reaction [35, 36]. An increment in the level of CRP is independent of the stage of HIV infection and thus is useful for supporting a diagnosis of opportunistic infections in HIV-coinfected patients. This also makes it useful in monitoring TB treatment response [37]. Low BMI is a marker for poor prognosis in patients with HIV and has also been associated with increased risk of TB and death [38, 39]. Early initiation of TB treatment in HIV-infected patients with wasting and increased CRP levels prior to initiating ART may therefore alleviate the excess morbidity due to undiagnosed TB and ART-associated TB.
The mortality in this cohort of patients was minimal (9(4%) of 219 patients starting ART) compared to other reports. This could be due to the systematic screening and close followup of patients in a study setting with early and effective TB treatment and ART. In particular, TB-IRIS was not a significant cause of early mortality in agreement with retrospective cohort observations [32]. Several studies have demonstrated a survival benefit of early ART in HIV/TB-coinfected patients. In the South African SAPIT trial, a 56% reduction in mortality was observed in patients who started ART during TB treatment compared to those who started after completion of TB treatment [40]. More recently the CAMELIA trial in Cambodia showed that the initiation of ART 2 weeks after starting TB treatment significantly enhanced survival in TB/HIV-coinfected patients compared to starting ART at 8 weeks [41]. This further emphasizes the need to screen for TB prior to ART and to ensure early treatment for both TB and HIV.
Our study has several limitations. Firstly, the sample size was small. Secondly, 24 patients did not complete the one year followup and 9 of them died. Postmortem examinations were not performed, and therefore we possibly underestimated the burden of TB. Thirdly, in the absence of a biomarker for TB-IRIS, we relied only on clinical evaluation which may be subjective in defining patients with exuberant inflammation. Patients with mild-to-moderate degrees of inflammation were not considered as IRIS. In spite of our efforts to establish an accurate diagnosis of TB, definite diagnosis (sputum smear positivity or culture-positive results) could not be ascertained in 2 patients (patient 1 and patient 8 in Table 4) considered to have ART-associated TB. With sputum induction, bronchoscopy, liquid culture, and/or molecular techniques, more patients with subclinical TB may have been detected.
In conclusion, careful screening for TB before the start of ART and the continuous assessment of patients for signs and symptoms of TB after starting ART particularly among patients that are wasted and have a raised CRP will lead to an earlier TB diagnosis and ultimately to reduced morbidity and mortality.
Acknowledgments
This study received financial support of an EC FP6 Specific Targeted Research Project (STREP) no. LSHP-CT-2007-037659-TBIRIS. The authors thank the patients who participated in this study and the team which enrolled them, including Alfin Okullo, Harriet Nakuya, Teddy Nalwoga, Sarah Nansikombi, and Alfred Andama; Danstan Bagenda (School of Public Health-Makerere University) and Joris Menten (Institute of Tropical Medicine, Antwerp) for their expert statistical input. They also thank the IDI Administration for facilitating this research and INTERACT for the administrative and technical support and assistance with data management.
TB IRIS Study Group
Institute of Tropical Medicine, Antwerp, Belgium: Luc Kestens, Robert Colebunders, Pascale Ondoa, Marguerite Massinga Loembé; Infectious Disease Institute, Kampala, Uganda: Harriet Mayanja, William Worodria; Joint Clinical Research Centre: Harriet Mayanja; Universite Libre de Bruxelles, Belgium: Francoise Mascart; Birj Universiteit Brussels, Belgium: Rafael van den Bergh; Institut Pasteur de Lille, France: Camille Locht; Academic Medical Centre, Center for Poverty-related Communicable Disease and Amsterdam Institute for Global Health and Development, Amsterdam, The Netherlands: Peter Reiss, Frank Cobelens, Pascale Ondoa, Nadine Pakker; INTERACT, Kampala, Uganda: Roy Mugerwa, Harriet Mayanja, Nadine Pakker, William Worodria.
References
- 1.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Archives of Internal Medicine. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 2.Martinson NA, Karstaedt A, Venter WF, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21(15):2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369(9578):2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infectious Diseases. 2009;9(3):173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 5.Dorman SE. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clinical Infectious Diseases. 2010;50(supplement 3):S173–S177. doi: 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 6.Jacob ST, Moore CC, Banura P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS ONE. 2009;4(11, article e7782) doi: 10.1371/journal.pone.0007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kigozi IM, Dobkin LM, Martin JN, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. Journal of Acquired Immune Deficiency Syndromes. 2009;52(2):280–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerene D, Endale A, Hailu Y, Lindtjøorn B. Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infectious Diseases. 2006;6, article 136 doi: 10.1186/1471-2334-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease. 2000;4(2):97–107. [PubMed] [Google Scholar]
- 10.Siddiqi K, Lambert M-L, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infectious Diseases. 2003;3(5):288–296. doi: 10.1016/s1473-3099(03)00609-1. [DOI] [PubMed] [Google Scholar]
- 11.Kibiki GS, Mulder B, van der Ven AJAM, et al. Laboratory diagnosis of pulmonary tuberculosis in TB and HIV endemic settings and the contribution of real time PCR for M. tuberculosis in bronchoalveolar lavage fluid. Tropical Medicine and International Health. 2007;12(10):1210–1217. doi: 10.1111/j.1365-3156.2007.01907.x. [DOI] [PubMed] [Google Scholar]
- 12.WHO. WHO Three I's Meeting. Report of a Joint WHO HIV/AIDS and TB Department Meeting. Geneva, Switzerland: World Health Organization; 2008. http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. [Google Scholar]
- 13.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clinical Infectious Diseases. 2010;51(7):823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clinical Infectious Diseases. 2005;40(10):1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 15.Lawn SD, Myer L, Bekker L-G, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 16.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PloS One. 2010;5(5, article e10527) doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clinics in Chest Medicine. 2009;30(4):797–810. doi: 10.1016/j.ccm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Seddiki N, Sasson SC, Santner-Nanan B, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. European Journal of Immunology. 2009;39(2):391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 19.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20(2):F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 20.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis- associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. Journal of Infectious Diseases. 2009;200(11):1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli LR, Mahnke Y, Hodge JN, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. doi: 10.1182/blood-2010-05-285080. Blood. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infectious Diseases. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uganda Ministry of Health and ORC Macro. HIV/AIDS Sero-Behavioural Survey 2004-2005. Calverton, Md, USA: Ministry of Health (Uganda) and ORC Macro; 2006. [Google Scholar]
- 24.Uganda National Antiretroviral Treatment and Care Guidelines for Adults, Adolescents and Children. 2nd edition. Kampala, Uganda: Ministry of Health; 2008. http://www.who.int/hiv/amds/uganda_moh_treatment_guidelines.pdf. [Google Scholar]
- 25.Uganda Ministry of Health. Uganda National Policy Guidelines for HIV Counselling and Testing. Kampala, Uganda: Ministry of Health; 2003. [Google Scholar]
- 26.WHO. Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extrapulmonary Tuberculosis among Adults and Adolescents. Recommendations for HIV Prevalent and Resource-Constrained Settings. Geneva, Switzerland: World Health Organization; 2007. http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf. [Google Scholar]
- 27.WHO. WHO Technical Report Series. 894. Geneva, Switzerland: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf. [PubMed] [Google Scholar]
- 28.Breen RAM, Smith CJ, Cropley I, Johnson MA, Lipman MCI. Does immune reconstitution syndrome promote active tuberculosis in patients receiving highly active antiretroviral therapy? AIDS. 2005;19(11):1201–1206. doi: 10.1097/01.aids.0000176221.33237.67. [DOI] [PubMed] [Google Scholar]
- 29.Park WB, Choe PG, Jo JH, et al. Tuberculosis manifested by immune reconstitution inflammatory syndrome during HAART. AIDS. 2007;21(7):875–877. doi: 10.1097/QAD.0b013e3280f7751f. [DOI] [PubMed] [Google Scholar]
- 30.Valin N, Pacanowski J, Denoeud L, et al. Risk factors for 'unmasking immune reconstitution inflammatory syndrome' presentation of tuberculosis following combination antiretroviral therapy initiation in HIV-infected patients. AIDS. 2010;24(10):1519–1525. doi: 10.1097/qad.0b013e3283396007. [DOI] [PubMed] [Google Scholar]
- 31.Baalwa J, Mayanja-Kizza H, Kamya MR, John L, Kambugu A, Colebunders R. Worsening and unmasking of tuberculosis in HIV-1 infected patients after initiating highly active anti-retroviral therapy in Uganda. African Health Sciences. 2008;8(3):190–195. [PMC free article] [PubMed] [Google Scholar]
- 32.Castelnuovo B, Manaba YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clinical Infectious Diseases. 2009;49(6):965–972. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 33.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker L-G, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24(9):1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawn SD, Myer L, Edwards D, Bekker L-G, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collazos J, Martinez MM, Izquierdo F. Evaluation of acute-phase reactants, immunologic markers and other clinical and laboratory parameters in patients with pneumonia and non-pneumonic lower respiratory tract infections. American Journal of Infectious Diseases. 2007;3(1):42–45. [Google Scholar]
- 36.Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in west african patients receiving treatment for pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease. 2000;4(4):340–344. [PubMed] [Google Scholar]
- 37.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. International Journal of Tuberculosis and Lung Disease. 2006;10(1):31–38. [PubMed] [Google Scholar]
- 38.Hanrahan CF, Golub JE, Mohapi L, et al. Body mass index and risk of tuberculosis and death. AIDS. 2010;24(10):1501–1508. doi: 10.1097/QAD.0b013e32833a2a4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Sande MAB, Shim van der Loeff MF, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. Journal of Acquired Immune Deficiency Syndromes. 2004;37(2):1288–1294. doi: 10.1097/01.qai.0000122708.59121.03. [DOI] [PubMed] [Google Scholar]
- 40.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. New England Journal of Medicine. 2010;362(8):697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanc FX, Sok T, Laureillard D, et al. Significant enhancement in survival with early (2 weeks) vs. late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis. In: Proceedings of the 18th International AIDS Conference (IAC ’10); July 2010; Vienna, Austria. [Google Scholar]