Abstract
In experiment 1, six cochlear-implant (CI) listeners discriminated a stimulation pattern eliciting equal loudness for each electrode from a stimulation pattern in which the stimulation at one or more electrodes was increased (peak) or decreased (notch). Three cochlear locations and three bandwidths were tested, without and with level roving. Listeners could always detect peaks but not always notches. Increasing the bandwidth beyond two electrodes produced no improvement in just-noticeable differences (JNDs). JNDs for the basal location were higher than for the apical and middle locations, although listeners had highly individual tendencies. In experiment 2, listeners discriminated changes in the peak heights and notch depths. JNDs for higher peaks were better while JNDs for deeper notches were worse than for experiment 1. In experiment 3, listeners discriminated the electrode position of peaks or notches. JNDs were approximately one electrode. In experiment 4, the first three experiments were repeated with large amounts of level roving. There was no evidence that CI listeners performed an across-channel comparison in these tasks.
I. INTRODUCTION
The perception of complex spectral patterns is vitally important to basic auditory tasks. Speech recognition, in particular vowel identification, depends partially on the ability to identify spectral peak frequencies of speech sounds. The perception of the timbre of different musical instruments depends on which harmonics are emphasized in the spectrum. The localization of sound sources in a vertical plane depends on spectral peaks and notches introduced by direction-dependent filtering imposed by the head, pinnae, and torso of the listener. In this study, we investigated the ability of cochlear-implant (CI) users to discriminate different spectral patterns.
The discrimination of a flat-spectrum stimulus from a stimulus with a spectral peak or notch is considered a profile-analysis experiment. Profile analysis has been extensively studied for normal-hearing (NH) listeners (see Green, 1988 for an introduction). Profile-analysis experiments sometimes use wideband noise stimuli, but more often use complex tones of various spectral densities. It is generally accepted that profile analysis of broadband stimuli involves some form of spectral-shape discrimination process or across-channel comparison. Other processes that could be used for peak and notch detection are discrimination of overall intensity or discrimination of intensity within a single auditory channel. The most direct method of showing that a listener is performing a spectral profile analysis is to include overall level roving in the experiment. The first profile-analysis experiments were performed by Spiegel et al. (1981) and Spiegel and Green (1982). They measured the discrimination of a flat-spectrum stimulus from a stimulus with a spectral peak with large amounts of overall level roving. They found that just-noticeable differences (JNDs) increased by only 6 dB when the overall level was varied over a 60-dB range, thus supporting the idea that listeners were using an across-channel comparison to perform the task. For a two-up, one-down adaptive procedure, which tracks a detection probability level of 70.7%, it has been theoretically shown that JNDs that are less than 23.5% of the roving range cannot be achieved by intensity discrimination within a single channel alone (Green, 1988, Appendix A). This limit has sometimes been called the “level detection limit.”
Another method of showing that a listener is performing profile analysis is to compare JNDs for a target band without background, which is intensity discrimination, with JNDs with a background. If the JNDs are better for the stimuli with a background, the listener must have gained an advantage from an across-channel comparison. Studies by Green et al. (1984), Green and Mason (1985), and Versfeld and Houtsma (1991) have produced examples of JNDs becoming better when there is a stimulus background. We will call the improvement in discriminating the intensity of a target when a background is added the “background advantage.”
Yet another method of showing that a listener is performing profile analysis is to vary the bandwidth of the spectral peak or notch to include very large bandwidths. Heinz and Formby (1999) reported the performance of listeners in a profile-analysis experiment that used a variety of bandwidths for peak and notch detection. Their results showed that as the bandwidth of the peak or notch increased, performance improved until the target bandwidth reached a certain percentage of the full bandwidth of the stimulus with background. Thus a transition occurs where no across-channel comparison can be made and only overall intensity can be used as a cue. In this study, we focus on using the level detection limit and the background advantage methods to assess that profile analysis has been performed.
Only a few studies related to profile analysis have been done with CI users. Drennan and Pfingst (2006) tested the ability of CI listeners to detect a peak on a single electrode fixed in the center of the electrode array while varying the number of electrodes in the background in terms of the background spacing. They found that performance decreased as the number of electrodes increased, contrary to the effects of acoustic spectral range found in some studies with NH listeners. Therefore, there was no background advantage. However, this study was possibly confounded by presenting stimuli with a different number of components at different current levels to maintain a constant overall loudness. This is because single-electrode current-level sensitivity decreases with decreasing current level (Nelson et al., 1996; Drennan and Pfingst, 2005). No overall level roving was used for these experiments.
Henry and Turner (2003) and Henry et al. (2005) tested the spectral-resolution capabilities of CI listeners, which may be related to spectral profile analysis. They measured the just-resolvable spectral peak spacing in broadband noises with spectral ripples, which were either linearly or logarithmically spaced and had a 30-dB peak-to-valley ratio. The listeners' task was to discriminate between two spectra where the peak and valley positions were reversed. The stimuli were presented through the speech processor of the CI. Henry et al. (2005) showed that, on average, NH listeners' spectral peak resolution was 4.84 ripples/octave and eight times better than CI listeners' spectral peak resolution of 0.62 ripples/octave. In this experiment, the overall level was roved over a range of 8 dB, which was not sufficient to eliminate within-channel intensity cues.
Even though CI listeners seem to be much worse at spectral profile analysis than NH listeners, they have better intensity discrimination thresholds for stimuli delivered directly to the electrodes (Pfingst et al., 1983; Nelson et al., 1996). However, CI listeners often have smaller dynamic ranges (DRs) than NH listeners, and the better intensity discrimination only partially compensates for the smaller DR. Roughly speaking, CI listeners can discriminate intensity about as well as NH listeners. If the limiting factor in a profile-analysis task were intensity discrimination, one would expect CI listeners to perform approximately as well as NH listeners. However, if the limiting factor is the across-channel comparison, one would expect larger JNDs for CI listeners than for NH listeners. This is because the large spread of electrical excitation from a given electrode would effectively smear the spectral information, reducing the spectral contrast between channels, which would make the profile cue less salient. If sensitivity to spectral peaks and notches in the sound spectrum is particularly poor, this may be the reason why speech understanding is limited in CI listeners, which was postulated by Henry et al. (2005), and it would make vertical-plane sound localization difficult for CI listeners.
Our experiment follows the basic outline of a study by Moore et al. (1989), which we will refer to as simply Moore et al. They measured the sensitivity of NH listeners to spectral peaks and notches in noise stimuli. They tested at two center frequencies (1 and 8 kHz), with three different bandwidths, and performed several types of experiments, three of which we replicated with CI listeners. The first experiment was peak or notch detection, which is the discrimination of a stimulus with a spectral peak or notch from a stimulus with a flat background. The second experiment was peak height or notch depth discrimination, which is the detection of a change in height or depth of an existing peak or notch, respectively. The third experiment was frequency discrimination, which is the detection of the change of the center frequency (fc) of a peak or notch. A short summary of the findings of Moore et al. is that JNDs were higher for notches than peaks. The bandwidth of the peak or notch had a small effect on JNDs for peak or notch detection and peak height or notch depth discrimination, but there was no bandwidth effect for frequency discrimination. The JNDs were generally higher at higher fc. Overall level roving had relatively little effect on the JNDs.
The analogous experiments that we performed were peak or notch detection, peak height or notch depth discrimination, and electrode discrimination with a background where current levels were adjusted to elicit an equal loudness for each individual electrode. The goal of our experiments was to extend our knowledge of spectral-shape discrimination in electrical hearing and to determine to what extent CI listeners were able to perform across-channel comparisons, especially when level roving was included.
II. EXPERIMENT 1: PEAK AND NOTCH DETECTION
A. Listeners and equipment
There were six listeners in this experiment, each implanted with a MED-EL 12-electrode implant. Table I shows the etiology for each listener. The spacing of the electrodes for this type of implant is 2.4 mm. The numbering of electrodes starts at 1 for the apical-most, low-frequency electrode and ends at 12 for the basal-most, high-frequency electrode. All 12 electrodes were used for all six listeners.
TABLE I.
Information about the listeners.
| Subject | Etiology | Age (yr) |
Duration of deafness (yr) |
Implant use (yr) |
Hearing aid use (yr) |
Implant type |
|---|---|---|---|---|---|---|
| CI14 | Progressive | 67 | 11 | 6 | 15 | C40+ |
| CI16 | Progressive | 54 | 8 | 1 | 30 | Pulsar |
| CI17 | Morbus Meniere | 59 | 1 | 1.5 | 14 | Pulsar |
| CI18 | Progressive | 49 | 11 | 2 | 33 | Pulsar |
| CI23 | Progressive | 75 | 8 | 3 | 14 | C40+ |
| CI24 | Progressive | 45 | 5 | 3 | 7 | C40+ |
The current scaling is linear for this type of implant. For three listeners, one current unit (cu) corresponded to 7.71 μA for all 12 electrodes. For the other three listeners, some electrodes had different scalings; one cu corresponded to either 4.42 or 13.57 μA.
The stimuli were presented directly to the CI via a research interface box, developed at the University of Innsbruck, Austria. Responses were made via a gamepad attached to a personal computer.
B. Stimuli
The peak or notch detection task required the discrimination of a stimulation pattern eliciting equal loudness for each electrode from a stimulation pattern in which the stimulation at one or more electrodes led to a higher (peak) or lower (notch) loudness than for adjacent electrodes. Figure 1(a) shows a schematic of the stimuli for the peak or notch detection task. The background was presented at a comfortable overall level chosen by the listener. Each electrode was stimulated with a constant pulse rate of 1515 pulses per second (pps). The order of electrode activation corresponded to the clinically used order (1, 7, 2, 8,…, 6, 12), which minimizes channel interactions. The duration of the stimuli was 300 ms and the interleaved stimulation of all 12 electrodes occurred for the entire stimulus duration.
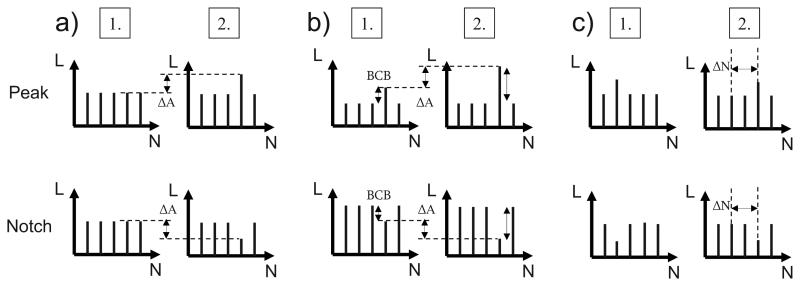
FIG. 1.
Schematic of stimuli for the three experiment types: (a) peak and notch detection (experiment 1), (b) peak height and notch depth discrimination (experiment 2), and (c) electrode discrimination of a peak or notch (experiment 3). The plots show the loudness (L) for each electrode (N). An equal-loudness background was used. All three panels show the reference condition for peaks or notches in the first interval. The second interval is the adapted target interval. In experiment 1, illustrated in (a), the height of the peak or depth of the notch, ΔA in cu, was varied. In experiment 2, illustrated in (b), the height of the peak or depth of the notch, ΔA, was varied while keeping the BCB fixed. In both of these experiments, the JND corresponds to average ΔA over several staircases. In experiment 3, illustrated in (c), the distance of the peak or notch between reference and target intervals in electrodes, ΔN, was varied for a fixed peak height or notch depth. The JND corresponds to the average ΔN over several staircases.
Three bandwidths (BW=1, 2, and 3 electrodes) and three cochlear locations of spectral peaks and notches were used. For BW=3, the apical location used electrodes 4–6, the middle location used electrodes 7–9, and the basal location used electrodes 10–12. For BW=2, the highest electrode in the band of three electrodes was omitted at each location (4–5, 7–8, and 10–11). For BW=1, only the center electrode was used (5, 8, and 11). Conditions were tested with overall level roving absent and present. For the conditions with level roving, the current level for each observation interval was varied over a range of 10 cu (±5 cu) according to a rectangular distribution with an integer step size, the change in cu being constant for all electrodes. In total, there were 36 different conditions [3 BW×3 locations×2 band types (peaks and notches)×2 roving types (absent and present)].
An additional type of stimulus was used after the 36 conditions were run, which is called the “band-only” stimulus. This stimulus had no background; the current levels at the electrodes not in the tested band were set to zero. Hence, the band-only stimuli provided intensity discrimination thresholds. Only level increments (peaks) were used and level roving was absent.
C. Procedure
1. Equal-loudness balancing
The DR was calculated in linear cu and was found for each electrode by manually determining the threshold level and maximum comfortable level. Afterwards, an iterative loudness-balancing experiment was performed so that stimuli were presented at a comfortable level and all 12 electrodes elicited the same loudness.
The loudness balancing consisted of two stages: (1) single-electrode balancing (SEB) and (2) overall-comfortable-level adjustment (OCL). Before the first SEB, a single electrode was adjusted to a comfortable level. This electrode was randomly chosen from electrodes 3 to 10 to avoid the apical- and basal-most electrodes, which are sometimes problematic for CI listeners (e.g., they often have the smallest DRs and are more likely to be deactivated). The current level applied to the electrode began at a random level which was 50%DR±5 cu. The listener then adjusted the single-interval presentation to a comfortable level. The adaptation step size started at 4 cu, and was reduced to 2 cu after at least one increase and one decrease in level were made. The listener ended the adjustment after at least two increases and two decreases in level by pressing a button. The final value of the level was recorded. After four repetitions of this single-electrode adjustment, the average comfortable level was calculated. The average level from the single-interval presentation was used as a reference for the pairwise level balancings performed in the first SEB.
In the first SEB, 24 balancings were performed (two repetitions for 12 electrodes, presented in random order). The interstimulus interval was 400 ms. In the first interval, the reference electrode was stimulated at the previously found comfortable level. In the second interval, one of the 12 electrodes was stimulated. The level of the second interval started at a random current level that was 50%DR±5 cu. The loudness of the second interval was adjusted in 4-cu steps, and this was reduced to 2-cu steps after at least one increase and one decrease in level were made. When the loudness of the second interval matched that of the first interval, after at least two increases and two decreases in level, the listener ended the task by pressing a button. The final value of the level was recorded. The average level of two balancings was calculated for each electrode.
After the first SEB, the first OCL used stimuli that activated all 12 electrodes with interleaved multi-channel stimulation. The current level for the electrodes was the average level found in the first SEB with a change of ±5 cu, constant across the electrodes. This stimulus was too loud for the listener to be comfortable (by 16 cu per electrode on average). The listener then adjusted the overall level of this stimulus to a comfortable loudness; the step size was 2 cu for all electrodes. The listener ended the adjustment after at least two increases and two decreases in level by pressing a button. Four repetitions of the adjustment were performed and an average level offset was calculated.
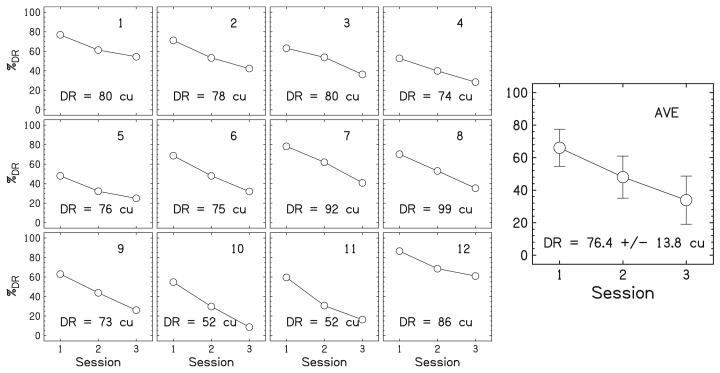
Since all the single electrodes were not necessarily at an equal loudness after the first OCL, a second SEB was performed, which was the same as before with one exception: the electrodes (including the reference electrode) were presented at random levels over a 10-cu range centered around the levels determined from the first OCL, not centered at 50%DR. The reference electrode was again randomly chosen from electrodes 3 to 10. After the second SEB, a second OCL was performed, which was similar to the first OCL except that it used the levels found in the second SEB. This iterative process continued until the listeners did not change the level of the 12-electrode stimulus in an OCL over the four repetitions (within ±3 cu on average). Listeners finished the level balancing in two to three blocks of SEB and OCL. The electrode levels determined in the last OCL were used for the main experiment. Figure 2 shows the results of the loudness balancing procedure for an example listener, CI14. From the figure, it can be seen that the curves have different slopes and shapes implying that the loudness growth curves are different for each electrode.
FIG. 2.
The results of the loudness-balancing procedure for a typical listener, CI14. The small panels on the left show the results for individual electrodes. Dynamic ranges (DRs) are reported in cu and correspond to 100%DR. Data points represent levels that are equally loud between electrodes for a given session. Session 3 shows the data for the final current level used for the equal-loudness stimulus in the main experiment. The large panel on the right shows the averaged %DR levels over all 12 electrodes. The error bars show ±1 standard deviation.
2. Main experiment
A three-interval, two-alternative forced-choice task was used to find the JND for discriminating an equal-loudness background from a background with a peak or notch. The first interval always contained the equal-loudness reference stimulus. The target stimulus with the peak or notch was randomly presented in the second or third interval. The remaining stimulus was the same as the reference. Listeners chose the interval that was different from the other two. The interstimulus interval was 400 ms. Listeners were provided with feedback after each trial.
A two-down, one-up staircase procedure was used to target the 70.7% correct point on the psychometric function. In the staircases, the current level of a band was varied. The level of the band was initially 20 cu different from the reference level for each electrode in the band. Before the first turnaround, the step size was 4 cu, to quickly converge to the approximate JND. After the first turnaround, the step size was reduced to 2 cu. The current level for notch staircases was allowed to go below the single-electrode threshold level, down to the lowest allowable current level (0 cu). Therefore, turnarounds and notch JNDs below the single-electrode threshold level could occur. If the staircase required a current level below 0 cu, the staircase was terminated with no measurement recorded. Staircases were not allowed to go above the single-electrode maximum comfortable level. The staircase had 16 turnarounds and the average current level in cu of the peak or notch with respect to the background was calculated over the last 12 turnarounds.
For each condition tested, there were at least three staircases. Staircases were monitored for converging characteristics, quantitatively measured by having a within-staircase standard deviation of less than six cu. Learning was also monitored, defined as having average levels monotonically decrease over the three staircases or having the average level of the last staircase be noticeably lower than the others. Additional staircases were performed if there was evidence of nonconvergence within a staircase or suspicion of learning over the staircases. The JND was calculated from the average current level of the last three converging staircases. If the standard deviation of the JND was greater than six cu, more staircases were performed, and the three staircases with the lowest within-staircase standard deviation were chosen for the calculation of the JND.
Before taking data, listeners performed several staircases to learn the task. They were trained until stable performance was obtained. For the actual data collection, the 36 conditions for experiment 1 were presented in random order within a single block. Since there were at least three repetitions for each condition, three full blocks were performed. In cases of nonconvergence, learning, or too large a standard deviation over staircases, additional repetitions were added after the end of the third block. After this, the band-only data were obtained similarly.
D. Results
The JNDs were converted from cu to %DR. For multiple-electrode bandwidths, the DR for the bandwidth was calculated as the average over the single electrodes.1 The appendix provides JNDs expressed as ten times the logarithm of the Weber fraction and as microamperes.
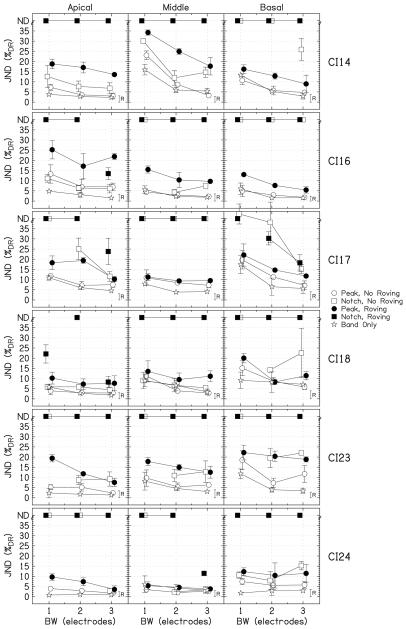
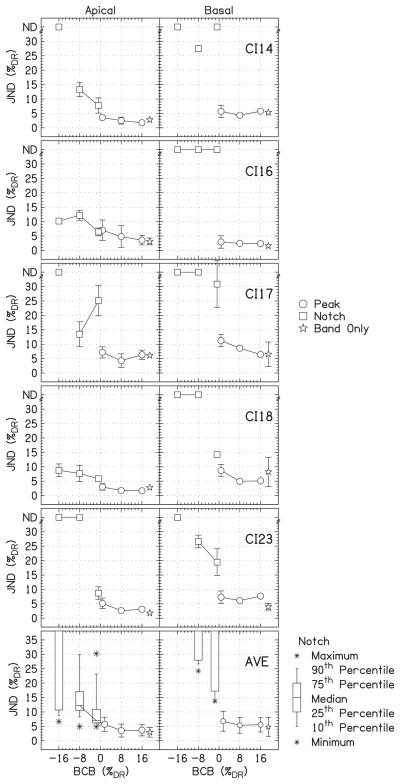
Figure 3 shows the JNDs for the individual listeners as a function of bandwidth. The height of the small bar, labeled “R,” represents the level detection limit for BW=3. Any JND for peaks or notches with roving above this bar may have been achieved by within-channel intensity cues, not by profile analysis. Notice that the listeners have highly individual tendencies, especially with respect to cochlear location. For example, CI14 has relatively small JNDs for the apical and basal locations, but relatively large JNDs for the middle location. CI17 shows the opposite pattern, with the lowest JNDs for the middle location. Hence it is important to plot the individual data.
FIG. 3.
The results of experiment 1, which show individual JNDs expressed as %DR for the individual listeners as a function of bandwidth. The circles show the data for the peaks, the squares show the data for the notches, and the stars show the band-only data. The filled symbols indicate the conditions with roving. For notches for which the threshold could not be measured, JNDs are placed at the top of the panels at “ND.” The error bars show ±1 standard deviation. The heights of the roving bars, labeled “R,” on right side of each panel show the level detection limit for BW=3.
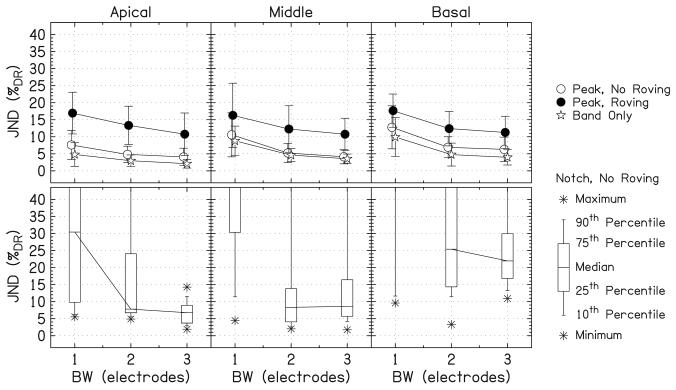
Figure 4 summarizes the data for all six listeners. The first row of panels shows the JNDs for the peaks averaged over listeners. The second row shows box-and-whisker plots for the notches without roving because JNDs could not be measured for all of the notches and the distribution of measurements was non-Gaussian. Data for notches with roving were omitted because only a few measurements could be made.
FIG. 4.
The average of the JNDs over listeners. The first row of panels shows the average JND for the peaks. The error bars show ±1 standard deviation. The second row shows the box-and-whisker plots for the notches without roving. The percentiles above the top of the plot represent unmeasurable JNDs. The lines connect the medians across bandwidth.
It was possible to measure all of the JNDs for the peaks, without and with roving. Averaged over location and bandwidth, the JND for peaks was 7.0±4.9%DR without roving and 13.5±6.5%DR with roving. The notches were more difficult to detect than the peaks. Without roving, JNDs could be measured for 69% of all notch conditions (78% apical, 67% middle, and 61% basal; 89% BW=3, 78% BW=2, and 39% BW=1). Averaged over location and bandwidth (for the points that could be measured), the JND for notches without roving was 13.1±10.1%DR. With roving, JNDs could be measured for only 13% of all notch conditions.
Separate three-way repeated-measures analyses of variance (RM ANOVA) for each feature type were conducted (factors: location, bandwidth, and roving). For the data for the peaks, there was a significant effect of location (p=0.021). In a post hoc test (all post hoc tests were Tukey's HSD), there was no difference between the apical and middle locations (p=0.87) and between the middle and basal locations (p=0.090), but there was a significant difference between the apical and basal locations (p=0.027). There was a significant effect of bandwidth (p<0.0001). There was a significant difference between BW=1 and the larger bandwidths (p<0.0001 for both), but not between BW=2 and 3 (p=0.096). There was a significant effect of roving (p<0.0001). There were no significant two-way interactions.
A second three-way RM ANOVA was performed on the data for the notches. Missing data for notches were omitted, so the conditions matrix was not full rank. The effect of location was significant (p=0.001). JNDs were significantly higher for the basal location than for the apical and middle locations (p<0.0001 for both) but there was no difference between the apical and middle locations (p=0.85). There was a significant effect of bandwidth (p<0.0001), which is easily seen from the box-and-whisker plots in Fig. 4. There was a significant difference between BW=1 and BW=2 (p=0.001) or BW=3 (p=0.006), but not between BW=2 and 3 (p=0.84), as was found for the data for the peaks. The effect of roving was significant (p=0.002). The significant two-way interactions were location×roving (p<0.0001) and bandwidth×roving (p=0.015). Because so few of the JNDs could be measured for the notches with roving, these interactions should be treated cautiously.
The data for the peaks without roving (open circles) can be compared to the band-only data (stars). Qualitatively, the band-only JNDs are smaller than or equal to the peak JNDs. In a three-way RM ANOVA [factors: location, bandwidth, and type (peak or band only)], all three main effects were significant (p<0.0001). Thus, there was a significant difference between the peak and band-only data. None of the interactions were significant. A correlation analysis between the peak and band-only data showed a highly significant correlation (r=0.81; p<0.0001; n=54). Focusing on combinations of individual listeners and individual locations (6 listeners×3 locations=18 conditions), there was a significant difference between the peaks and the band-only data for nine conditions at the 0.05 level, while there was no difference for the other nine.
A separate two-way RM ANOVA was performed on the band-only data. The results were very similar to those for peaks without roving. The effects of location and bandwidth were significant (p<0.0001 for both) and the interaction was significant (p=0.044). The JNDs for the apical location were lower than for the middle and basal locations (p<0.0001 for both), but there was no difference between the JNDs for the middle and basal locations (p=0.51). JNDs for BW=1 were higher than for the larger bandwidths (p<0.0001 for both), but there was no difference between the JNDs for BW=2 and BW=3 (p=0.71).
E. Discussion
Listeners could always detect sufficiently large spectral peaks, whether or not the level was roved. Notches were more difficult to detect and were sometimes not detectable. A possible reason for the poorer performance in the detection of notches compared to peaks for NH listeners was offered by Moore et al. They attributed the poorer notch detection to the spread of excitation from adjacent frequency components: “…a narrow notch in the spectrum of a noise will be represented by only a small dip in the excitation pattern evoked by that noise, even when the notch is infinitely deep.” Studies by Ellermeier (1996) and Heinz and Formby (1999) agree with Moore et al. The analogous process in CI listeners is the spread of current to the region of the electrode notch from adjacent electrodes. Current from these nearby electrodes effectively fills in the notch. This process would affect peak detection much less.
There was a large amount of interlistener variability in the JNDs for different cochlear locations. This is expected because CI listeners often show highly individualized data in psychophysical tasks, especially with respect to cochlear location. The reason for this is that a number of uncontrollable factors, such as surviving neural populations and placement of the electrode array in the cochlea, can affect the outcome of psychophysical tasks. However, Green and Mason (1985) and Versfeld and Houtsma (1991) also showed individual differences with respect to center frequency in profile-analysis tasks for NH listeners using complex-tone stimuli. Other studies on the effect of center frequency in profile analysis, for example Bernstein and Green (1987), show more uniform results with more extensively trained listeners. Thus, a potential explanation for the individual differences in our data was that the training provided did not give the listeners time to learn the optimal decision strategy for the task. Since we provided training before the experiment, feedback during the experiment (consisting of at least 108 staircases), and we monitored improvements during the experiment, we believe that the individual variability with respect to location of excitation is mostly due to physiological factors and less to procedural factors. This idea is further supported by the intensity discrimination JNDs for the band-only stimuli, which probably required much less training and were highly correlated with the spectral peak JNDs for individual listeners.
There was a significant effect of cochlear location of the peaks and notches, as indicated both by the values of the JNDs and by the number of notch JNDs that could be measured. The largest number of notch JNDs could be measured at the apical location, followed by the middle location, and then the basal location. The NH results of Moore et al. showed a corresponding deterioration of JNDs with increasing fc. One reason for the cochlear location effect for spectral notches in CI listeners could be the equal-loudness stimulus, which tended to have reference levels near threshold level at the basal location. As seen in the example in Fig. 2, electrodes 10 and 11 have equal-loudness currents that are very near threshold. This was typical for four of the six listeners. Thus it appears that our CI listeners tended to have steep loudness growth functions near the threshold level at basal locations.
There was a significant effect of bandwidth, which can be easily seen in Fig. 4. JNDs were significantly higher for BW=1 than for BW=2, but JNDs did not differ for BW=2 and 3. Thus two-electrode peaks and notches are sufficiently large for the detection of peaks and notches by CI users. A similar trend, especially for notches, was shown for NH listeners by Moore et al.
Drennan and Pfingst (2006) performed a peak detection task with Nucleus 24-electrode implant listeners. For the 11-electrode configuration, the peak placed at a single center electrode, and 250 pps per channel (compared to our 1515 pps per channel), the JND, expressed as ten times the logarithm of the Weber fraction, was −7±5 dB. In another experiment using a seven-electrode configuration, the authors found an increase of the JND to −4.9 dB when switching from the 250 pps condition to the conditions most close to our rate (1200–1800 pps). It should be noted that the effect of pulse rate was highly variable across listeners and this approximation represents the average trend. Converting our JNDs for peak detection to the same measure yields −7.9±1.9 dB for BW=3, −7.1±2.3 dB for BW=2, and −4.6±2.6 dB for BW=1. Thus, the JND for the one-electrode condition is similar to that found by Drennan and Pfingst (2006), despite the several differences between the two studies. The most important differences include the stimulation mode (our monopolar versus their bipolar), the number of electrodes in the background, and the level profile (our equal-loudness background versus their background fixed at 70%DR).
Drennan and Pfingst (2005) showed no effect of cochlear location on intensity discrimination using electrodes 16 and 8 of the Nucleus24 implant, which correspond to our apical and middle locations, respectively, assuming standard insertion depths. They found an average JND of 4.1%DR for a reference current at 50%DR (monopolar condition). In contrast, for our single-electrode band-only condition, we found significantly higher sensitivity at the apical location (4.8%DR) than at the middle (8.8%DR) and basal (9.9%DR) locations. The worse performance of our subjects at the middle location, which is at odds with the results of Drennan and Pfingst (2005), may be due to a violation of our assumption of standard insertion depth. Our middle location electrodes might be placed more basally than electrode 8 in Drennan and Pfingst's (2005) study.
Our finding that, for half of the conditions tested, JNDs were significantly larger with a background (data for the peaks) than without (band-only data) indicates the relevance of channel interactions, which is consistent with the findings of Drennan and Pfingst (2006). Channel interactions can be viewed as masking from the background electrode currents on the target electrode currents. Moore et al. showed for NH listeners that intensity discrimination for a noise band alone worsens when a noise background is added. Hence, both studies showed no background advantage. In contrast, Green and Mason (1985) and Versfeld and Houtsma (1991) showed that profile-analysis JNDs for NH listeners were often better than intensity-discrimination JNDs. The different outcomes may be due to the fact that these studies used complex-tone stimuli whose components fell essentially in separate auditory filters, whereas Moore et al. used noise bands with continuous spectra. Therefore, it seems appropriate to consider the stimuli used in this experiment as more comparable to noise bands than to complex tones.
There was a significant effect of level roving, with an increase in the JNDs of approximately half of the roving range on average. This increase is much greater than that shown for NH listeners by Moore et al. Also in contrast to our results, many other profile-analysis experiments with NH listeners showed small effects of level roving (Spiegel et al., 1981; Kidd et al., 1989; Drennan and Watson, 2001; Lentz, 2005). The data with level roving can be compared to the bars in Fig. 3, which show the level detection limit for the 10-cu roving range converted to %DR. For most listeners, the JNDs are considerably higher than the bars. Listener CI24 shows JNDs for peaks with roving at the apical and middle locations for the larger bandwidths that are only marginally higher than the level detection limit. Interestingly, many of the JNDs presented in Moore et al. are also above the level detection limit. For example, approximately one-third of the peaks for peak detection could have been achieved by using intensity cues within a channel. Therefore, although level roving is extremely detrimental to profile analysis in CI listeners, finding no JNDs below the level detection limit is not necessarily inconsistent with the NH listener results in Moore et al.
Because the listeners showed neither a background advantage nor JNDs below the level detection limit, the results raise the concern that the CI listeners did not perform profile analysis, but instead used only intensity cues within a channel. There are several possible explanations for these results, which will be presented in the general discussion. Nevertheless, there remains the possibility that CI listeners can perform profile analysis for stimuli that are not roved in level. When roving is used, it may distract listeners or introduce undesired perceptual changes that cause listeners to perform nonoptimally. NH listeners seem to make nonoptimal use of absolute level when the level is randomized (Kidd et al., 1986, Appendix). The fact that level roving leads to impaired performance does not prove that profile analysis is not performed. Therefore, in the next two experiments, it was useful to assess what performance can be achieved when level is not roved. In experiment 4, all three experiments were rerun with roving ranges of 20 and 30 cu.
III. EXPERIMENT 2: PEAK HEIGHT AND NOTCH DEPTH DISCRIMINATION
A. Methods
In experiment 2, the task of the listener was to identify the interval with the higher peak or deeper notch. The reference intervals had an equal-loudness background with a peak or notch. The height of the peak was either 8 or 16%DR. These values were chosen because 8%DR is larger than the average JND for the peak conditions without level roving in experiment 1, thus resulting in a detectable peak for most listeners. The depth of the notches equaled the size of the peaks. These reference peak heights and notch depths will be called the band current with respect to the background (BCB). No level roving was applied.
In the target intervals, the height of the peaks or depth of the notches was varied with respect to the BCB. The peaks and notches had a two-electrode bandwidth and two cochlear locations were tested, apical (4–5) and basal (10–11). The two-electrode bandwidth was chosen because beyond that bandwidth no significant improvement in the JND occurred in experiment 1. Figure 1(b) shows an example of a reference stimulus and a target stimulus. The procedure was the same as for experiment 1. The listeners were the same except that listener CI24 did not participate.
B. Results
The results are shown in Fig. 5. Data from experiment 1 were replotted at BCB=0%DR and were included in the analyses. As for experiment 1, all of the peak JNDs could be measured. The JNDs at the apical location (3.9±2.4%DR) were significantly lower than those at the basal location (6.0±2.8%DR), as shown by a two-way RM ANOVA (factors: location and BCB; p<0.0001 for both). There was no interaction between the factors (p=0.99). Post hoc tests showed that JNDs for BCB=0%DR were significantly higher than those for BCB= +8 and +16%DR (p=0.002 and 0.004, respectively). The JNDs for BCB= +8%DR were not significantly different from those for BCB= +16%DR (p=0.97).
FIG. 5.
Individual and average listener data from the peak height or notch depth discrimination task, experiment 2. The JNDs, expressed as %DR, are plotted for different band currents with respect to the background (BCB). The error bars show ±1 standard deviation. Data for BCB=0%DR and band-only data are replotted from experiment 1. JNDs for notches that were unmeasurable are placed at the top of the panels at “ND.” The notch JNDs are summarized with a box-and-whisker plot.
An additional two-way RM ANOVA combined the data for the peaks with the band-only data from experiment 1 (factors: location and BCB), where the band-only data represent an additional level of factor BCB. The main effects and interactions were similar to those for the previous RM ANOVA. A post hoc test showed no difference between results for the band-only condition and the BCB= +8 and +16%DR conditions (p=0.89 and p=0.78, respectively). Looking at individual pairs of data points, there are three JNDs that are significantly lower than the band-only JNDs as measured by a two-sample t-test: the +16%DR apical location for CI14 (p=0.025), the +8%DR apical location for CI18 (p=0.009), and the +16%DR apical location for CI18 (p=0.004).
As for experiment 1, only a portion of the JNDs for the notches could be measured, 40% of the total. Of these, six could be measured at the apical location and two could be measured at the basal location. In a two-way RM ANOVA for the notches (factors: location and BCB) the effects of location and BCB were significant (p<0.0001 and p=0.020, respectively), and the interaction was significant (p=0.025). Increasing the depth of the notch made the task more difficult, especially at the basal location.
C. Discussion
The data from experiment 2 are similar in pattern to those from experiment 1 with notches being more difficult to discriminate than peaks. All the JNDs for the peaks were measurable while only 40% of the JNDs for the notches were. As in experiment 1, the basal notches were more difficult to discriminate than the apical notches.
Discrimination of peaks became better with increasing reference peak height and JNDs with BCB greater than 0%DR were not different from those for band-only conditions. This indicates that the detrimental effect of having a background can be compensated by increasing the reference peak height.
Discrimination of notches became worse with increasing notch depth. Reasons for this could be that across-channel interactions effectively reduce the difference in excitation pattern between reference and target stimuli and/or the deeper notches are closer to the electrode threshold level (where no perception is elicited) and 0 cu (where the adaptive procedure terminates). Moore et al. also showed that the discrimination of peaks becomes better with increasing peak height and discrimination of notches becomes worse with increasing notch depth for NH listeners.
There is an alternative explanation for the patterns observed in experiment 2. Assuming listeners were not performing profile analysis, but rather using only intensity cues, the task was effectively to detect a change in loudness in which only 2 of 12 electrodes were altered. The overall loudness change therefore depends on the relative loudness of the varied electrodes compared to the remainder of the stimulus. Loudness summation for multiple electrodes has been studied by McKay et al. (2001). They found that a louder component (i.e., a higher peak) will dominate the overall loudness and changes in it produce salient overall loudness changes, whereas a softer component (i.e., a deeper notch) contributes negligibly to the overall loudness and changes in it produce nonsalient overall loudness changes. This could explain the better JNDs for higher peaks and worse or unmeasurable JNDs for notches.
IV. EXPERIMENT 3: PEAK AND NOTCH ELECTRODE DISCRIMINATION
A. Methods
In experiment 3, the task was to discriminate the cochlear location of the peak or notch for a fixed peak height or notch depth. An equal-loudness background was present. All three cochlear locations were used as the reference location and the bandwidth was two electrodes. For the apical reference location (4–5), target peaks and notches basal to the reference electrodes were used. For the middle reference location (7–8), target peaks and notches basal and apical to the reference electrodes were used. For the basal reference location (10–11), target peaks and notches apical to the reference electrodes were used. Peak heights and notch depths were always 12%DR averaged over all the electrodes more basal or more apical in location, depending on the target peak or notch location. No level roving was applied.
A schematic of the stimuli is shown in Fig. 1(c). The separation between the electrodes with the peak or notch in the reference and target intervals started at three electrodes. The two-down, one-up staircase procedure decreased the separation by one electrode after two correct answers, and increased the separation by one electrode after an incorrect answer. The location of the peak or notch was bounded by the very edge of the electrode array. If more than four incorrect answers were obtained at the very edge of the electrode array, the staircase was terminated with no measurement recorded. All other procedural aspects were similar to those for experiments 1 and 2. All six listeners participated in this experiment.
B. Results
The results are shown in Fig. 6. The JNDs were measured as electrode separation. For the staircase procedure, the smallest achievable JND is 0.5 electrodes. There were 14 of a total of 48 JNDs that had values very close to 0.5 electrodes. All 24 JNDs for peaks could be measured. However, there were 4 of a total of 24 JNDs for notches that could not be measured, for different listeners, locations, and directions. The average data are arithmetic means calculated over the listeners (the data at “ND” were ignored). The mean JNDs for peaks and notches were 0.9±0.4 and 1.3±0.8 electrodes, respectively. In terms of tonotopic distance, this translates to 2.2 and 3.1 mm, respectively.
FIG. 6.
Individual and average listener data from the electrode discrimination task, experiment 3. Peaks and notches tested at locations basal to the reference location are plotted as positive JNDs and have open symbols. Peaks and notches tested at locations apical to the reference electrodes are plotted as negative JNDs and have filled symbols. The error bars show ±1 standard deviation. JNDs for notches that were unmeasurable are placed at the top and bottom of the panels at “ND.”
A three-way RM ANOVA [factors: location, direction (from apical and basal), and type (peak and notch)] was performed. There was no effect of direction (p=0.63). JNDs for the notches were significantly larger than JNDs for the peaks (p<0.0001). There was a significant effect of location (p<0.0001). A post hoc test showed no difference between the JNDs for the apical and middle locations (p=0.44), whereas the JNDs for the basal location were significantly worse than the JNDs for the apical (p=0.006) and the middle (p<0.0001) locations. The only significant interaction was location×type (p=0.001). The peaks and notches led to similar JNDs for the middle location, but notches led to higher JNDs than peaks for the apical and basal locations.
C. Discussion
Listeners could perform the electrode discrimination task for both peaks and notches. Several JNDs were near 0.5 electrodes, the minimum JND measurable in this experiment. It has been shown that pitch percepts intermediate to those elicited by the individual electrodes are possible by varying the level ratio of two neighboring electrodes stimulated simultaneously (Donaldson et al., 2005; Koch et al., 2007; Firszt et al., 2007) or sequentially (McDermott and McKay, 1994; Kwon and van den Honert, 2006). Thus, future studies could measure electrode discrimination for peaks and notches using dual-electrode level adjustment to measure JNDs below the limit of 0.5 electrodes imposed in the present study.
Our data on electrode discrimination for peaks can be compared to the data from electrode discrimination studies without a background. A study by Laneau and Wouters (2004) using Nucleus24 implants in monopolar mode found JNDs for electrode discrimination ranging from 0.34 to 0.61 electrodes for four individual subjects,2 which translates to 0.26 and 0.46 mm, respectively. They observed no effect of bandwidth when changing the bandwidth from one to eight electrodes. Our mean JND for peaks was 2 mm, which is four to six times larger. However, several JND measurements were at the lower limit of measurement as given by the electrode spacings in the two studies, which confounds the comparison because the physical spacings of electrodes of the two implants are quite different. Nevertheless, the comparison for the majority of measurements not affected by floor effects indicates that adding a background makes electrode discrimination more difficult. This is in agreement with the results for NH listeners in Moore et al. where frequency discrimination of peaks and notches was found to be considerably worse than the discrimination of bands of noise or pure tones.
As for the previous experiments, notches were more difficult to discriminate than peaks and JNDs at the basal location were higher than JNDs at the apical and middle locations. Other studies have shown poorer electrode discrimination performance at basal locations without a background (e.g., Zwolan et al., 1997; Pfingst et al., 1999; Henry et al., 2000).
V. EXPERIMENT 4: LARGE LEVEL ROVING
Once the parameter spaces for the three types of experiments were explored, selected conditions from the previous experiments were rerun with large amounts of overall level roving. The purpose of including large roving ranges was to clarify if CI listeners can perform profile analysis.
A. Methods
Experiments 1, 2, and 3 were performed again with overall level roving ranges of 0, 20, and 30 cu. Three of the listeners from the previous experiments participated: CI16, CI17, and CI23.
All three cochlear locations were used. Only peaks were used. For the repetition of experiment 1, peak detection, BW=2 and 3 were used. For the repetition of experiment 2, peak height discrimination, BW=2 and BCB=8 and 16%DR were used. For the repetition of experiment 3, electrode discrimination, BW=2 and 3 and BCB=12%DR were used. For the basal reference location, peaks were tested at apical target locations. For the middle and apical reference locations, peaks were tested at basal target locations.
B. Results
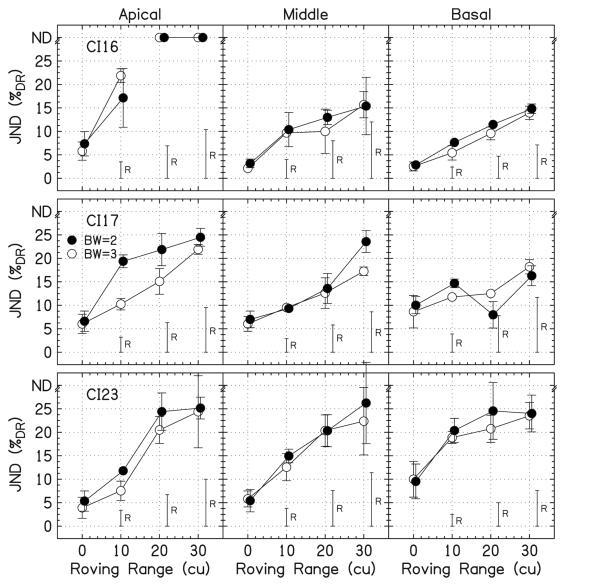
Figure 7 shows the data for peak detection from this experiment and experiment 1. Because there was no significant difference in the data between the experiments for the 0-cu roving range (p=0.48), they were combined. All of the data points are well above the level detection limit except one point, the BW=2, basal, 20-cu roving range for CI17, which was only slightly above the level detection limit.
FIG. 7.
Individual JNDs for peak detection. Data from experiments 1 (roving range=0 and 10 cu) and 4 (roving range=0, 20, and 30 cu) were combined. The open symbols are for BW=3 and the filled symbols are for BW=2. The height of the roving bars, labeled “R,” shows the level detection limit. JNDs that were unmeasurable are placed at the top of the panels at “ND.” The error bars show ±1 standard deviation.
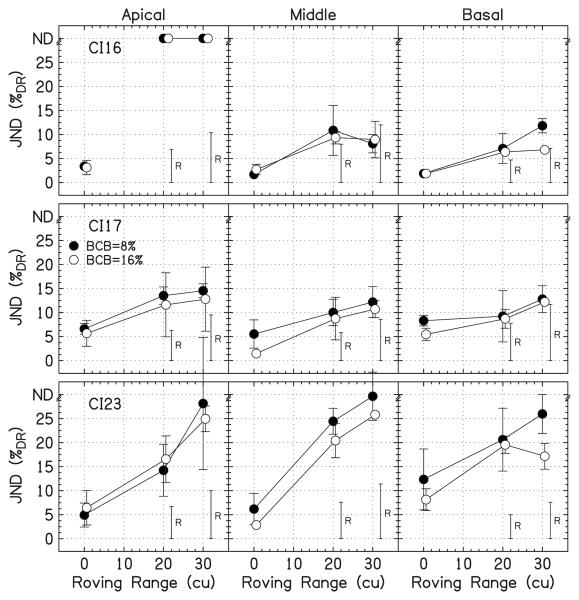
Figure 8 shows the data for peak height discrimination with overall level roving. The JNDs for CI16 and CI17 are close to the level detection limit. For CI16, there are three data points below the level detection limit for the middle and basal locations for the 30-cu roving range, but none statistically lower than the level detection limit. The JNDs for CI23 are well above the level detection limit.
FIG. 8.
Individual JNDs for peak height discrimination with overall level roving. The open symbols are for BCB=16%DR and the filled symbols are for BCB=8%DR. The height of the roving bars, labeled “R,” shows the level detection limit. JNDs that were unmeasurable are placed at the top of the panels at “ND.” The error bars show ±1 standard deviation.
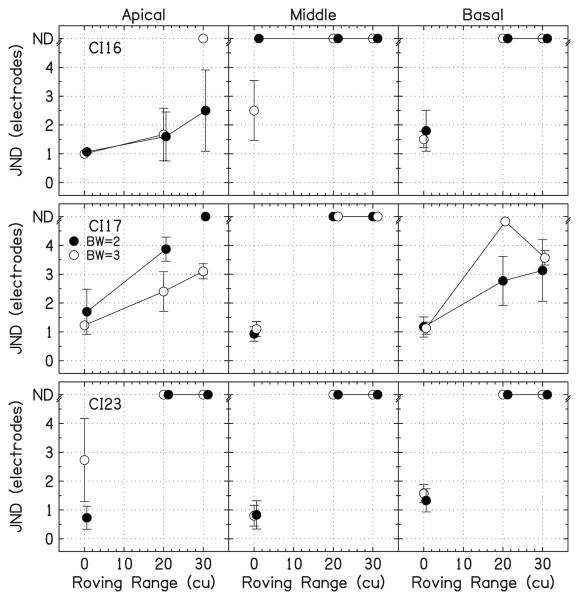
Figure 9 shows the data for electrode discrimination with overall level roving. Most JNDs were unmeasurable. Only 28% of the JNDs could be measured with a nonzero roving range. The JNDs with roving are larger than those without roving. Note that, for these conditions, the use of within-channel cues cannot be excluded because the reference peak height of 12%DR was always larger than the level detection limit.
FIG. 9.
Individual JNDs for electrode discrimination with overall level roving. The open symbols are for BW=3 and the filled symbols are for BW=2. JNDs that were unmeasurable are placed at the top of the panels at “ND.” The error bars show ±1 standard deviation.
C. Discussion
Experiments 1, 2, and 3 were repeated with large amounts of level roving to check if listeners were performing intensity discrimination or profile analysis in these tasks. It appears that listeners were using only intensity cues because the JNDs were so large or because the JNDs could not be determined when roving was used. However, there are still some indications that profile analysis may be possible. In particular, the JNDs for peak height discrimination approached a level where they could not be achieved solely by monitoring the intensity within a channel. For NH listeners, Green and Kidd (1983) found that a “pedestal” minimizes masking effects while still allowing for profile analysis. In this task, when the BCB is larger, the across-channel interactions cause less masking of the target. Thus, these relatively small JNDs for peak discrimination compared to the JNDs for peak detection may be an indication of how severely the across-channel current spread affects the listeners' performance. Therefore, future profile-analysis experiments in CI listeners may want to focus on peak discrimination.
VI. GENERAL DISCUSSION
An important cue for NH listeners to detect changes in spectral profiles is the comparison of levels between bands (e.g., Green, 1988). One aim of this study was to determine whether CI listeners can do the same. CI listeners' sensitivity was measured to current increments and decrements on three or less electrodes presented with an equal-loudness background. The results showed many similarities in effects of cochlear location, bandwidth, and band current (level) with respect to the background between CI listeners and NH listeners; the analogous experiments with NH listeners were performed by Moore et al. However, in general, the data did not show that CI listeners could perform a profile analysis. The data showed a substantial increase in JNDs when roving was added, which is much different from most data for NH listeners, who show little effect of overall level roving. JNDs higher than 23.5% of the roving range can be achieved by using within-channel intensity cues only (Green, 1988, Appendix A), which is called the level detection limit. Most of our JNDs for conditions with roving were much higher than this limit. Also, intensity-discrimination JNDs for conditions without a background (band-only) were compared to JNDs for conditions with a background to see if there was an advantage due to the background, which has been shown for NH listeners (Green et al., 1984; Green and Mason, 1985; Versfeld and Houtsma, 1991). In general, our data did not show a background advantage.
One question is why CI listeners cannot utilize across-channel comparisons to limit the detrimental effect of overall level roving while NH listeners can. It may be that CI listeners are inherently limited in some way. It is possible that channel interactions cause excessive blurring of the spectral shape, making it difficult to compare levels across different electrodes, which would explain many facets of our data. Drennan and Pfingst (2006) also performed an experiment using CI listeners, where the peak height on the center electrode was incremented while the number of electrodes and electrode density were varied. It was found that each electrode added to the background worsened the performance, even with large spacings between activated electrodes. (Note that this result may be confounded by the level being decreased to obtain a comfortable loudness each time more electrodes were added.) This is an interesting result, as NH listeners can show decreasing JNDs when energy outside the critical band is added for complex-tone stimuli, which indicates a background advantage. For NH listeners, the optimal log-spaced ratio of frequencies of the adjacent components is between 1.1 and 1.3 (Green, 1988). Denser spacings cause components to mask the target component and increase JNDs.
Kidd and Mason (1992) postulated that hearing-impaired (HI) listeners would be especially susceptible to level roving because of poorer frequency selectivity than NH listeners and loudness recruitment; these problems, in a way, are similar to those in CI listeners. Surprisingly, Lentz and Leek (2002, 2003) and Lentz (2006) showed no difference in performance between HI and NH listeners in profile-analysis tasks. The reason for this, as suggested by Lentz and Leek (2003), may be that NH and HI listeners achieved similar performance by utilizing different parts of the spectrum to perform the task. The NH listeners may use frequencies near the spectral feature to be detected and the HI listeners may use frequencies remote from the spectral feature. It is possible that CI listeners, with even poorer frequency selectivity than HI listeners because of channel interactions, cannot benefit from using the edges of the spectrum and are thus hindered more than both NH and HI listeners in profile-analysis tasks.
Loudness is a potential cue in profile-analysis tasks and it is worth considering differences of loudness perception between acoustic and electric hearing. Loudness summation across electrodes in CI listeners is different from summation across frequencies for NH listeners. In models for acoustic hearing (Zwicker and Scharf, 1965; Moore and Glasberg, 1997), a signal is passed through a bank of overlapping bandpass filters to determine the cochlear excitation. This excitation is converted to specific loudness via a compressive power function. In a model for electrical hearing (McKay et al., 2001, 2003), the excitation at each location in the cochlea is converted to specific loudness via an expansive power function because basilar membrane mechanics are bypassed in electrical stimulation. The overall loudness is calculated from the integration of specific loudness across cochlear location. As mentioned in the discussion of experiment 2, the loudness of components higher in level than the background (peak) will dominate the loudness perception, while components lower in level than the background (notch) will contribute negligibly to the loudness perception. Therefore by bypassing the compressive cochlear mechanics and altering the process by which loudness sums, the loudness could become the dominant perceptual feature for these stimuli, not the spectral shape.
It is possible that our lack of evidence for profile analysis in CI listeners is due to the specific properties of the stimuli used. There is evidence from the literature that temporal cues can contribute to profile analysis. Particularly for relatively narrowband spectra, pitch differences between the target and comparison sounds resulting from the evaluation of the temporal waveforms are important cues (e.g., Feth and Stover, 1987; Berg et al., 1992). For very narrowband spectra, profile analysis may be mediated via detection of changes in the temporal envelope (Kidd and Mason, 1992; Green et al., 1992). Encoding of spectral shape based on phase locking to temporal signal aspects seems to occur over the entire dynamic range, as has been shown with vowel stimuli (Young and Sachs, 1979). Hence, temporal cues seem to be resistant to overall level roving of the stimulus. The stimuli presented to the CI listeners in the present study contained no useful temporal cues, since all electrodes were stimulated at the same pulse rate. This may be a reason for the lower sensitivity of CI listeners in our profile-analysis task compared to NH listeners, particularly with level roving. The results of Moore et al. using noise stimuli, which provide less regularity than stimuli with discrete sinusoidal components, support this idea. At 1 kHz, where noise stimuli provide some temporal cues, they found higher JNDs compared to studies using tonal stimuli. At 8 kHz, where possibly neither type of stimulus provides useful temporal cues, they found no difference in JNDs. Moore et al. conjectured that this may also explain why level roving had a larger effect at 8 kHz than at 1 kHz. Interestingly, our data with level roving do not show more degradation of performance by level roving at the most basal place, consistent with the idea that the lack of temporal cues is the reason for the generally large JNDs with roving in electric hearing. Future CI studies could test the potential of temporal stimulus aspects in profile analysis with CIs by using stimuli that have temporal cues.
Several researchers have conducted profile-analysis experiments where the entire spectrum was altered, not just small portions of the spectrum (Bernstein and Green, 1987; Kidd and Mason, 1992; Lentz and Richards, 1997; Lentz and Leek, 2003). The advantage of such a technique is that it produces smaller JNDs, which reduces the roving range necessary to yield JNDs below the level detection limit. Such stimuli have not been used yet with CI listeners.
Since CI users can discriminate loudness-equated steady-state vowels (Laback et al., 2004), this may indicate that they can perform some sort of profile analysis, ignoring possible within-channel cues. However, we did not test the electrodes that are probably most important for vowel discrimination. The tonotopic location of our lowest-frequency/most-apical target started at electrode 4, which normally receives information bandpass filtered between 700 and 900 Hz. It is possible that CI listeners can perform profile analysis when the peaks and notches are on the apical-most electrodes, electrodes 1–3. Continuing along this line of thought, there is no study known to the authors that tested spectral vowel discrimination with level roving. Based on the present study, it would not be surprising if vowel discrimination performance would decrease dramatically with roving.
Lastly and possibly the largest problem with our stimuli is that differences in loudness growth functions across electrodes could alter the background substantially from equal loudness when level roving was included. This is because we roved the overall level in cu instead of in loudness units. Kidd et al. (1986) showed that using stimuli that had a background with randomly varied amplitudes across frequency, frozen over 50 trials, increased JNDs by between 2.5 and 9.5 dB, depending on the range of the variation in the background. Larger increases in JNDs were seen if the background was varied from trial to trial. The variation of the background due to different growth of loudness curves at different electrodes may cause an effect similar to a random-amplitude background, which causes a large increase in JNDs. Future experiments could compensate for this limitation by using loudness growth curves for the level roving.
As mentioned before, Drennan and Pfingst (2006) performed a profile-analysis experiment with CI users, although they did not try to determine if the listeners were using an across-channel comparison as rigorously as we have. Their study did not show a background advantage. Henry and Turner (2003) and Henry et al. (2005) also tested CI listeners' sensitivity to spectral profiles with a spectral ripple reversal test. However, given that our results do not conclusively confirm that CI listeners are performing profile analysis, could the listeners in Henry et al. (2005) also have just monitored intensity within a single channel? The experiment used an 8-dB roving range, much smaller than the range necessary to eliminate the possibility of using intensity cues for a 30-dB peak-to-valley ratio. They admitted that the task could have been done without profile analysis. It seems that much more work is necessary to adequately address the question of profile analysis in electrical hearing.
VII. CONCLUSION
We assessed whether CI listeners can perform profile analysis. We know that CI listeners probably can perform some form of spectral-shape discrimination because they can discriminate vowels. However, our data showed no evidence of CI listeners performing across-channel comparisons in three basic types of profile-analysis experiments. We have suggested reasons for our results, both due to properties of CIs per se (spectral image blurring from channel interactions and different nature of loudness summation) and due to the specific stimuli used in this study (lack of temporal cues, stimuli where the entire spectrum is altered were not used, the lowest electrodes were not tested, and roving was not done in loudness units but in current units). Future experiments should consider these factors carefully.
ACKNOWLEDGMENTS
We would like to thank Mr. M. Mihocic for running experiments, our listeners, and the MED-EL Corp. for providing the equipment for direct electrical stimulation. We thank the associate editor, Brian Moore, and two anonymous reviewers for greatly improving this manuscript. This study was funded by the Austrian Science Fund (FWF Project No. P18401-B15).
APPENDIX
To increase the accessibility to the data, we provide all of the JNDs from experiments 1, 2, and 4 expressed as ten times the logarithm of the Weber fraction (WF) and as microamperes. The results of the experiments can be found in Tables II-IV. The WF was used by Pfingst et al. (1983) and Drennan and Pfingst (2006), and for CI listeners is
where I is the reference current and ΔI is the change in current. The WF has been shown to be an appropriate measure for electric stimulation since the variance across repeated measures at different base levels was shown to be constant (Nelson et al., 1996). A majority of the results and all of the interpretations did not change using this measure, so it was omitted from the discussion. The data in microamperes are provided because they are a direct physical measure.
TABLE II.
The average JNDs over listeners from experiment 1 expressed as %DR, WF, and μA. Conditions for which no JND was measured are labeled “…”.
| Cochlear location | Apical |
Middle |
Basal |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BW (electrodes) | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | |
| Peak | %DR | 4.07±2.56 | 4.80±2.44 | 7.58±4.26 | 4.12±2.08 | 5.23±2.82 | 10.56±6.47 | 6.28±3.58 | 6.91±3.09 | 12.76±6.32 |
| No roving | WF (dB) | −7.62±1.92 | −6.75±2.26 | −5.12±2.52 | −7.91±1.91 | −7.12±2.33 | −4.58±2.64 | −6.89±1.73 | −5.71±1.24 | −3.35±1.38 |
| μA | 38.58±22.85 | 42.17±22.22 | 65.23±43.93 | 34.55±9.44 | 42.78±18.93 | 78.65±48.90 | 32.53±14.71 | 37.10±18.32 | 62.29±32.53 | |
| Peak | %DR | 10.74±6.23 | 13.33±5.61 | 16.93±6.13 | 10.73±4.72 | 12.27±6.85 | 16.29±9.43 | 11.27±4.75 | 12.38±5.06 | 17.65±4.90 |
| 10-cu roving | WF (dB) | −4.03±2.71 | −2.59±2.58 | −1.48±2.62 | −4.33±2.31 | −3.83±2.84 | −2.59±3.05 | −4.36±1.56 | −3.27±1.31 | −1.51±1.28 |
| μA | 87.48±63.51 | 99.23±46.04 | 130.90±69.12 | 78.73±31.45 | 89.80±51.30 | 117.95±74.90 | 56.99±26.96 | 68.16±35.96 | 94.17±55.28 | |
| Notch | %DR | 6.83±3.64 | 10.77±7.89 | 9.88±4.21 | 8.74±5.06 | 7.22±4.42 | 19.55±11.81 | 20.99±7.07 | 20.06±12.69 | 26.44±17.64 |
| No roving | WF (dB) | −8.09±3.60 | −5.93±3.19 | −5.91±3.37 | −7.47±4.60 | −8.56±4.53 | −3.13±5.01 | −2.92±2.16 | −3.57±3.42 | −1.91±2.65 |
| μA | 35.80±20.01 | 56.18±43.92 | 62.36±37.67 | 47.03±29.53 | 37.54±29.21 | 136.98±94.52 | 62.56±32.69 | 73.31±65.79 | 112.57±69.65 | |
| Notch | %DR | 15.22±7.86 | … | 22.09±4.48 | 11.44±0.81 | … | … | 18.25±3.97 | 30.30±3.23 | … |
| 10-cu roving | WF (dB) | −4.58±3.10 | … | −2.93±1.06 | −4.92±0.38 | … | … | −3.60±1.10 | −0.86±0.55 | … |
| μA | 95.48±48.46 | … | 106.40±23.13 | 139.95±80.23 | … | … | 74.02±17.78 | 131.84±14.87 | … | |
| Band only | %DR | 2.08±1.27 | 2.95±1.69 | 4.77±3.47 | 3.52±1.38 | 4.60±1.93 | 8.79±4.34 | 3.95±2.24 | 4.76±3.36 | 9.90±5.71 |
| WF (dB) | −9.49±1.37 | −8.47±1.32 | −7.38±2.04 | −8.29±1.40 | −7.37±1.66 | −5.02±2.30 | −8.39±1.66 | −7.35±2.02 | −4.54±2.41 | |
| μA | 23.09±7.33 | 28.35±11.15 | 39.95±19.99 | 31.91±8.65 | 38.65±12.35 | 67.53±33.42 | 27.78±10.43 | 32.80±16.43 | 61.21±30.96 | |
TABLE III.
The average JNDs over listeners from experiment 2 expressed as %DR, WF, and μA. Conditions for which no JND was measured are labeled “…”.
| Cochlear location | Apical |
Basal |
|
|---|---|---|---|
| BW (electrodes) | 2 | 2 | |
| Peak | %DR | 3.19±2.13 | 5.29±2.46 |
| +8%DR-BCB | WF (dB) | −9.43±2.20 | −7.88±0.90 |
| μA | 30.15±20.74 | 36.54±10.92 | |
| Peak | %DR | 3.28±1.96 | 5.46±2.31 |
| +16%DR-BCB | WF (dB) | −10.07±1.91 | −8.60±1.05 |
| μA | 29.79±14.75 | 36.82±8.54 | |
| Notch | %DR | 11.64±3.53 | 27.03±1.61 |
| −8%DR-BCB | WF (dB) | −6.08±2.52 | −1.98±0.33 |
| μA | −67.35±28.12 | −103.59±6.47 | |
| Notch | %DR | 9.47±1.69 | … |
| −16%DR-BCB | WF (dB) | −7.83±1.84 | … |
| μA | −59.72±23.60 | … | |
TABLE IV.
The average JNDs over listeners from experiment 4 expressed as %DR, WF, and μA.
| Cochlear location | Apical |
Middle |
Basal |
||||
|---|---|---|---|---|---|---|---|
| BW (electrodes) | 3 | 2 | 3 | 2 | 3 | 2 | |
| Peak and Notch detection |
%DR | 4.87±1.56 | 6.43±2.39 | 4.03±1.97 | 4.82±1.95 | 7.22±4.10 | 7.77±4.49 |
| WF (dB) | −6.45±2.01 | −5.53±2.01 | −7.67±1.97 | −6.85±1.71 | −6.12±1.59 | −5.75±1.64 | |
| No roving | μA | 42.64±14.57 | 54.60±26.27 | 33.36±6.72 | 39.01±8.99 | 47.89±16.21 | 50.64±11.36 |
| %DR | 17.79±3.88 | 23.13±3.61 | 14.31±5.73 | 15.63±4.31 | 14.27±5.28 | 14.66±8.28 | |
| 20-cu roving | WF (dB) | −1.00±2.77 | 0.28±2.37 | −2.83±2.55 | −2.13±2.15 | −2.99±1.23 | −2.87±2.65 |
| μA | 106.01±17.85 | 135.82±18.83 | 95.69±25.84 | 104.41±17.24 | 93.97±36.78 | 105.98±55.72 | |
| %DR | 23.14±5.09 | 24.83±1.93 | 18.43±4.93 | 21.74±7.31 | 18.55±4.51 | 18.37±4.84 | |
| 30-cu roving | WF (dB) | 0.24±2.38 | 0.65±2.09 | −1.51±1.71 | −0.71±2.29 | −1.70±0.83 | −1.48±1.36 |
| μA | 136.21±26.03 | 145.33±10.59 | 124.93±29.07 | 141.07±34.36 | 125.04±55.95 | 133.08±62.04 | |
| Cochlear location | Apical |
Middle |
Basal |
||||
| BCB | +8%DR | +16%DR | +8%DR | +16%DR | +8%DR | +16%DR | |
|
| |||||||
| Peak and notch Discrimination |
%DR | 4.97±1.99 | 5.08±2.81 | 4.82±2.91 | 3.75±2.07 | 7.52±5.60 | 5.16±3.04 |
| WF (dB) | −7.78±1.42 | −8.82±2.27 | −8.45±2.34 | −9.92±1.61 | −7.27±2.45 | −8.73±1.70 | |
| No roving | μA | 40.52±10.84 | 39.99±13.66 | 37.43±11.79 | 33.20±13.59 | 43.87±12.89 | 36.55±8.04 |
| %DR | 13.88±3.63 | 14.09±5.87 | 16.28±6.94 | 13.80±6.36 | 12.31±7.74 | 11.54±6.24 | |
| 20-cu roving | WF (dB) | −3.64±1.49 | −4.65±2.66 | −3.54±2.28 | −5.26±2.60 | −4.98±2.69 | −5.44±2.09 |
| μA | 81.47±19.51 | 83.78±30.42 | 106.39±27.71 | 90.97±25.70 | 77.93±36.42 | 77.34±23.82 | |
| %DR | 21.34±11.48 | 18.88±8.06 | 17.43±9.98 | 15.85±8.57 | 16.87±7.31 | 12.06±4.69 | |
| 30-cu roving | WF (dB) | −1.98±2.77 | −3.37±2.77 | −3.46±2.85 | −4.79±2.95 | −3.23±1.86 | −5.10±1.25 |
| μA | 116.42±45.99 | 107.55±34.93 | 108.27±30.84 | 100.97±34.32 | 110.53±48.65 | 81.41±19.76 | |
Footnotes
The maximum DR from the group of two or three electrodes was also used to analyze the data, not just the average DR. It was found that the DRs were similar enough across different cochlear locations that a majority of the results and all of the interpretations did not change using this measure, so it was omitted.
The fitting procedure used by Laneau and Wouters (2004) could lead to electrode discrimination JNDs less than 0.5 electrodes. The value of 0.34 corresponds to perfect pitch ranking and electrode discrimination in their task.
References
- Berg BG, Nguyen QT, Green DM. Discrimination of narrow-band spectra. I: Spectral weights and pitch cues. J. Acoust. Soc. Am. 1992;92:1911–1918. doi: 10.1121/1.405238. [DOI] [PubMed] [Google Scholar]
- Berstein LR, Green DM. Detection of simple and complex changes of spectral shape. J. Acoust. Soc. Am. 1987;82:1587–1592. doi: 10.1121/1.395147. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Cochlear location-pitch discrimination of single- versus dual-electrode stimuli by cochlear implant users. J. Acoust. Soc. Am. 2005;118:623–626. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Drennan WR, Pfingst BE. Current-level discrimination using bipolar and monopolar electrode configurations in cochlear implants. Hear. Res. 2005;202:170–179. doi: 10.1016/j.heares.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Drennan WR, Pfingst BE. Current-level discrimination in the context of interleaved, multi-channel stimulation in cochlear implants: Effects of number of stimulated electrodes, pulse rate, and electrode separation. J. Assoc. Res. Otolaryngol. 2006;7:308–316. doi: 10.1007/s10162-006-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, Watson CS. Sources of variation in profile analysis. II. Component spacing, dynamic changes, and roving level. J. Acoust. Soc. Am. 2001;110:2498–2504. doi: 10.1121/1.1408311. [DOI] [PubMed] [Google Scholar]
- Ellermeier W. Detectability of increments and decrements in spectral profiles. J. Acoust. Soc. Am. 1996;99:3119–3125. [Google Scholar]
- Feth LL, Stover LJ. Demodulation processes in auditory perception. In: Yost WA, Watson CS, editors. Auditory Processing of Complex Sounds. Erlbaum; Hillsdale, NJ: 1987. pp. 76–86. [Google Scholar]
- Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol. Neurotol. 2007;28:629–636. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Green DM. Profile Analysis: Auditory Intensity Discrimination. University Press; New York: 1988. Oxford Psychology Series No. 13. [Google Scholar]
- Green DM, Kidd G. Further studies of auditory profile analysis. J. Acoust. Soc. Am. 1983;73:1260–1265. doi: 10.1121/1.389274. [DOI] [PubMed] [Google Scholar]
- Green DM, Mason CR. Auditory profile analysis: Frequency, phase, and Weber's law. J. Acoust. Soc. Am. 1985;77:1155–1161. doi: 10.1121/1.392179. [DOI] [PubMed] [Google Scholar]
- Green DM, Berg BG, Dai H, Eddins DA, Onsan Z, Nguyen Q. Spectral shape discrimination of narrow-band sounds. J. Acoust. Soc. Am. 1992;92:2586–2597. doi: 10.1121/1.404431. [DOI] [PubMed] [Google Scholar]
- Green DM, Mason CR, Kidd G. Profile analysis: Critical bands and duration. J. Acoust. Soc. Am. 1984;75:1163–1167. doi: 10.1121/1.390765. [DOI] [PubMed] [Google Scholar]
- Heinz MG, Formby C. Detection of time- and bandlimited increments and decrements in a random-level noise. J. Acoust. Soc. Am. 1999;106:313–326. doi: 10.1121/1.428039. [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, Clark GM. The relationship between speech perception and electrode discrimination in cochlear implants. J. Acoust. Soc. Am. 2000;108:1269–1280. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J. Acoust. Soc. Am. 2003;113:2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. J. Acoust. Soc. Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Kidd G, Mason CR. A new technique for measuring spectral shape discrimination. J. Acoust. Soc. Am. 1992;91:2855–2864. doi: 10.1121/1.402966. [DOI] [PubMed] [Google Scholar]
- Kidd G, Mason CR, Green DM. Auditory profile analysis of irregular sound spectra. J. Acoust. Soc. Am. 1986;79:1045–1053. doi: 10.1121/1.393376. [DOI] [PubMed] [Google Scholar]
- Kidd G, Mason CR, Brantley MA, Owen GA. Roving-level tone-in-noise detection. J. Acoust. Soc. Am. 1989;86:1310–1317. doi: 10.1121/1.398745. [DOI] [PubMed] [Google Scholar]
- Koch DB, Downing M, Osberger MJ, Litvak L. Using current steering to increase spectral resolution in CII and HiRes 90K users. Ear Hear. 2007;28:38S–41S. doi: 10.1097/AUD.0b013e31803150de. [DOI] [PubMed] [Google Scholar]
- Kwon BJ, van den Honert C. Dual-electrode pitch discrimination with sequential interleaved stimulation by cochlear implant users. J. Acoust. Soc. Am. 2006;20:EL1–6. doi: 10.1121/1.2208152. [DOI] [PubMed] [Google Scholar]
- Laback B, Deutsch WA, Baumgartner W-D. Coding of vowellike signals in cochlear implant listeners. J. Acoust. Soc. Am. 2004;116:1208–1223. doi: 10.1121/1.1772398. [DOI] [PubMed] [Google Scholar]
- Laneau J, Wouters JJ. Multi-channel cochlear location pitch sensitivity in cochlear implant recipients. J. Assoc. Res. Otolaryngol. 2004;5:285–294. doi: 10.1007/s10162-004-4049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz JJ. Profile analysis: the effects of rove on sparse spectra. J. Acoust. Soc. Am. 2005;118:2794–2797. doi: 10.1121/1.2062187. [DOI] [PubMed] [Google Scholar]
- Lentz JJ. Spectral-peak selection in spectral-shape discrimination by normal-hearing and hearing-impaired listeners. J. Acoust. Soc. Am. 2006;120:945–956. doi: 10.1121/1.2216564. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, Leek MR. Decision strategies of hearing-impaired listeners in spectral shape discrimination. J. Acoust. Soc. Am. 2002;111:1389–1398. doi: 10.1121/1.1451066. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, Leek MR. Spectral shape discrimination by hearing-impaired and normal-hearing listeners. J. Acoust. Soc. Am. 2003;113:1604–1616. doi: 10.1121/1.1553461. [DOI] [PubMed] [Google Scholar]
- Lentz JJ, Richards VM. Sensitivity to changes in overall level and spectral shape: An evaluation of a channel model. J. Acoust. Soc. Am. 1997;101:3625–3635. doi: 10.1121/1.418323. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM. Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea. J. Acoust. Soc. Am. 1994;96:155–162. doi: 10.1121/1.410475. [DOI] [PubMed] [Google Scholar]
- McKay CM, Henshall KR, Farrell RJ, McDermott HJ. A practical method of predicting the loudness of complex electrical stimuli. J. Acoust. Soc. Am. 2003;113:2054–2063. doi: 10.1121/1.1558378. [DOI] [PubMed] [Google Scholar]
- McKay CM, Remine MD, McDermott HJ. Loudness summation for pulsatile electrical stimulation of the cochlea: Effects of rate, electrode separation, level, and mode of stimulation. J. Acoust. Soc. Am. 2001;110:1514–1524. doi: 10.1121/1.1394222. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR. A model of loudness perception applied to cochlear hearing loss. Aud. Neurosci. 1997;3:289–311. [Google Scholar]
- Moore BCJ, Oldfield SR, Dooley GJ. Detection and discrimination of spectral peaks and notches at 1 and 8 kHz. J. Acoust. Soc. Am. 1989;85:820–836. doi: 10.1121/1.397554. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Schmitz JL, Donaldson GS, Viemeister NF, Javel E. Intensity discrimination as a function of stimulus level with electric stimulation. J. Acoust. Soc. Am. 1996;100:2393–2414. doi: 10.1121/1.417949. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Burnett PA, Sutton D. Intensity discrimination with cochlear implants. J. Acoust. Soc. Am. 1983;73:1283–1292. doi: 10.1121/1.389277. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Holloway LA, Zwolan TA, Collins LM. Effects of stimulus level on electrode-place discrimination in human subjects with cochlear implants. Hear. Res. 1999;134:105–115. doi: 10.1016/s0378-5955(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Spiegel MF, Green DM. Signal and masker uncertainty with noise maskers of varying duration, bandwidth, and center frequency. J. Acoust. Soc. Am. 1982;71:1204–1210. doi: 10.1121/1.387769. [DOI] [PubMed] [Google Scholar]
- Spiegel MF, Picardi MC, Green DM. Signal and masker uncertainty in intensity discrimination. J. Acoust. Soc. Am. 1981;70:1015–1019. doi: 10.1121/1.386951. [DOI] [PubMed] [Google Scholar]
- Versfeld NJ, Houtsma AJM. Perception of spectral changes in multi-tone complexes. Q. J. Exp. Psychol. 1991;43:459–479. doi: 10.1080/14640749108400982. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibres. J. Acoust. Soc. Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- Zwicker E, Scharf B. A model of loudness summation. Psychol. Rev. 1965;72:3–26. doi: 10.1037/h0021703. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J. Acoust. Soc. Am. 1997;102:3673–3685. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]