Abstract
BACKGROUND
Disease-specific estimates of medical costs are important for health policy decision making.
OBJECTIVE
To identify predictors of health care costs associated with hepatitis C virus (HCV) seropositivity across disease phases.
METHODS
HCV laboratory tests from the BC Centre for Disease Control were linked to administrative data pertaining to health services and drugs dispensed to estimate costs among case subjects and controls. The case group comprised HCV seropositive individuals (n=20,001), and the control group comprised single-tested, HCV seronegative persons (n=70,752) identified between January 1997 and December 2004. Subject observation time was assigned to the three following disease phases: initial phase (after diagnosis), late phase (late-stage liver disease) and predeath phase (12 months before death). Case subjects and controls were matched for age, sex and a propensity score within each phase to determine the net cost attributable to HCV seropositivity, and were adjusted for demographic and clinical factors.
RESULTS
Costs increased with disease progression, with hospitalization being the highest cost component in all phases. Initial and late phase net costs (2005 Canadian dollars) were $1,850 and $6,000 per patient per year, respectively. Costs among case subjects were driven by age, comorbidities, mental illness, illicit drug use and HIV coinfection. While predeath case subject and control costs were virtually the same, costs were high and case subjects died at a younger age.
CONCLUSION
HCV seropositivity is associated with increased medical costs driven by viral sequelae and medicosocial vulnerabilities (ie, mental illness, illicit drug use and HIV coinfection). Cost mitigation and health outcome improvements will require broad-based prevention programming to reduce vulnerabilities and HCV treatment to prevent disease progression, respectively.
Keywords: Cost of illness, Health economics, Hepatitis C, Liver disease, Net costs, Vulnerable populations
Abstract
HISTORIQUE
Il est important d’évaluer les coûts médicaux liés à une maladie donnée pour prendre des décisions en matière de politiques de santé.
OBJECTIF
Déterminer les prédicteurs des coûts de santé associés à la séropositivité au virus de l’hépatite C (VHC) lors des diverses phases de la maladie.
MÉTHODOLOGIE
Les tests de laboratoire du VHC du Centre for Disease Control de la Colombie-Britannique étaient liés à des données administratives relatives aux services de santé et aux médicaments dispensés pour évaluer les coûts chez les sujets atteints et les sujets témoins. Le groupe atteint se composait de personnes séropositive au VIH (n=20 001) et le groupe témoin, de personnes séronégatives au VHC ayant subi un seul test de dépistage (n=70 752) dépistées entre janvier 1997 et décembre 2004. La période d’observation des sujets était divisée en trois phases pathologiques : phase initiale (après le diagnostic), phase tardive (maladie hépatique de phase tardive) et phase terminale (12 mois avant le décès). Les sujets atteints et les sujets témoins étaient appariés selon l’âge, le sexe et un indice de propension dans chaque phase pour déterminer les coûts nets attribuables à la séropositivité au VHC, le tout rajusté selon des facteurs démographiques et cliniques.
RÉSULTATS
Les coûts augmentaient avec l’évolution de la maladie, l’hospitalisation constituant l’élément de coût le plus élevé à toutes les phases. Les coûts nets de la phase initiale et de la phase tardive (en dollars canadiens de 2005) s’élevaient à 1 850 $ et à 6 000 $ par patient par année, respectivement. Les coûts chez les sujets dépendaient de l’âge, des comorbidités, de la maladie mentale, de la consommation de drogues illicites et de la co-infection par le VIH. Les coûts liés aux sujets en phase terminale et aux sujets témoins étaient virtuellement les mêmes, mais les coûts étaient élevés et les sujets atteints mouraient plus jeunes.
CONCLUSION
La séropositivité au VHC s’associe à une augmentation des coûts médicaux attribuable aux séquelles virales et à des vulnérabilités médicosociales (c’est-à-dire, maladie mentale, consommation de drogues illégales et co-infection par le VIH). Pour atténuer les coûts et améliorer les issues de santé, il faudra respectivement instaurer des programmes de prévention généralisés afin de réduire les vulnérabilités et traiter le VHC de manière à éviter l’évolution de la maladie.
An estimated 243,000 Canadians are infected with hepatitis C virus (HCV), approximately 20% remain undiagnosed and approximately 7900 are newly infected each year mostly as a result of illicit drug use (1,2). Three-quarters of those who acquire HCV become chronically infected, and 14% to 19% will develop cirrhosis within 20 years, leading to liver failure, hepatocellular carcinoma and death (3,4). The burden of HCV is expected to increase because new infections and the progression of liver disease in those already infected outpace the rate of spontaneous and treatment-induced viral clearance (5).
The direct costs of HCV infection are associated with physician services, hospitalization, diagnostic testing, antiviral therapy and treatment of liver disease; these costs vary according to disease stage (5–7). Published studies (7–9) have reported high costs for HCV-related hospital-based services, particularly among patients with comorbid illnesses such as HIV infection. To date, cost estimates have been limited by the lack of HCV uninfected control groups required to determine the HCV-related or net costs of infection. In addition, HCV costing has not addressed cost differences according to disease stage. Net costing and phase of care approaches have been used extensively in measuring cancer costs, and comprehensive cost estimates are equally important for planning HCV prevention and care programs (10–13).
Using linked laboratory and administrative data, we estimated the net costs of HCV infection over three phases of illness: the initial phase (after diagnosis), late phase (late-stage liver disease) and predeath phase (12 months before death) among residents of British Columbia (BC) undergoing serological testing for HCV. The net costs were calculated as the mean costs for HCV antibody-positive cases minus those of matched anti-HCV-negative controls. We also determined predictors of costs among the case subjects to elucidate health care cost drivers.
METHODS
The costs of care among individuals undergoing serological testing for HCV were determined from the three following data sources: HCV laboratory testing data from the BC Centre for Disease Control (BCCDC, Vancouver, BC); the BC Linked Health Database, which stores information on publicly insured physician services, inpatient hospital services (including hospital discharge abstract data), outpatient diagnostic and laboratory services, outpatient clinics and same-day surgery; and data regarding prescription drug use from PharmaNet, which captures prescriptions dispensed from community and hospital outpatient pharmacies in BC for which at least a portion was publicly funded.
Data linkage followed a multistep, anonymized process as outlined by the BC Ministry of Health and College of Pharmacists of BC (14,15). The present study was approved by the University of BC (Vancouver) and the University of Toronto (Toronto, Ontario) ethics review boards.
Since April 1, 1992, 95% of all of BC’s HCV antibody tests (anti-HCV) have been performed at the BCCDC laboratory (16). Anti-HCV testers were eligible if they underwent at least one anti-HCV test during the study period (January 1, 1997 to December 31, 2004), had a valid personal health number, and provided their sex and date of birth. Case subjects were selected from seropositive individuals, while controls were selected from seronegative individuals who were tested only once within the study period. Observation time began at the time of the first positive anti-HCV test for case subjects, and the single negative anti-HCV test for controls, and ended either at death or the end of the study period.
Using the perspective of the BC Ministry of Health, the major components of publicly insured direct medical costs were used. These included physician services, inpatient and outpatient hospital services, outpatient diagnostic and laboratory testing, and outpatient prescription drugs. All costs were based on publicly paid service fees on the date of service delivery adjusted to 2005 Canadian dollars using the Statistics Canada consumer price index for BC.
The services of physicians practising in settings that do not submit encounter data, laboratory tests performed by the BCCDC laboratory, cancer treatments, costs related to continuing care (extended care and homecare) or emergency services were not available in the present linked dataset. Also unavailable were medications provided in other settings (eg, physician offices, clinics or emergency departments), those administered to hospitalized patients; those used to treat HIV/AIDS, cancer, transplant or renal disease; over-the-counter medications; and prescriptions for federally insured patients (eg, federal employees, persons in correctional institutions and Aboriginal peoples) (14).
Three phases of HCV infection were defined based on disease natural history: initial, late and predeath. Case subjects whose observation time was not associated with hospital procedures or diagnostic codes for late-stage liver disease or death were assigned to the initial phase; case subject observation time associated with a hospital diagnosis or procedure code (5) relating to late-stage liver disease (decompensated cirrhosis, liver cancer, variceal bleeding, encephalopathy, ascites or transplant [Appendix 1]) were assigned to the late phase; and the predeath phase was the 12 months preceding death from any cause. The phased approach considered costs and patterns of care at clinically meaningful points, and was appropriate because health care needs and services change with disease progression (13). However, these phases do not represent precise clinical disease stages.
Appendix 1.
Late-phase conditions
| Charlson comorbidity index | CCP code | ICD-10 code | ICD-9 code | Description |
|---|---|---|---|---|
| 1NA13BA | 1006 | B190 | 0706 | Unspecified viral hepatitis with hepatic coma |
| 1NA13BABD | 1006 | C220 | 1550 | Malignant neoplasm of liver, primary |
| 1NA13BAFA | 5421 | C229 | 1552 | Malignant neoplasm of liver, not specified as primary or secondary |
| 1NA13BAX7 | 5421 | D695 | 2874 | Secondary thrombocytopenia |
| 1OA59DAGX | 6219 | D696 | 2875 | Thrombocytopenia, unspecified |
| 1OA59DAX7 | 6219 | D731 | 2894 | Hypersplenism |
| 1OA59HAX7 | 6294 | G934 | 3483 | Encephalopathy, unspecified |
| 1OA59LAAD | 6219 | I81 | 452 | Portal vein thrombosis |
| 1OA59LAGX | 6219 | I850 | 4560 | Esophageal varices with bleeding |
| 1OA85LAXXK | 624 | I859 | 4561 | Esophageal varices without mention of bleeding |
| 1OA85WLXXJ | 6241 | I864 | 4568 | Varicose veins of other sites |
| 1OA85WLXXK | 6249 | K703 | 5712 | Alcoholic cirrhosis of liver |
| 1OA87LA | 6249 | K704 | 5728 | Other sequelae of chronic liver disease |
| 1OA87LAAZ | 6249 | K720 | 570 | Acute and subacute necrosis of liver |
| 1OT52HA | 6212 | K721 | 5728 | Other sequelae of chronic liver disease |
| 3OT20WE | 6212 | K729 | 5728 | Other sequelae of chronic liver disease |
| 3OT40WC | 6691 | K766 | 5723 | Portal hypertension |
| 251 | K767 | 5724 | Portal hypertension | |
| 276 | R161 | 7892 | Splenomegaly | |
| R162 | 7891 | Hepatomegaly | ||
| R17 | 7824 | Jaundice unspecified, not of newborn | ||
| R18 | 7895 | Ascites | ||
| T86400 | 9968 | Complications of transplanted organ | ||
| T86401 | 9968 | Complications of transplanted organ | ||
| T86402 | 9968 | Complications of transplanted organ | ||
| T869 | 9968 | Complications of transplanted organ | ||
| Z944 | V427 | Organ or tissue replaced by transplant – liver |
CCP Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures; ICD International Classification of Diseases (9th and 10th Revisions)
Because persons at risk for HCV infection are often concurrently at risk for other social, economic and health-related problems (ie, mental illness, substance-use disorders, poverty, and coinfection with other blood-borne and sexually transmitted infections), it is important to control for the effect of these factors on resource use (17–19). Case subjects were matched with up to four control subjects based on age, sex and propensity score (20).
Propensity scores for each subject were calculated based on a general comorbidity score (Deyo-Charlson comorbidity index), socioeconomic quintile, rural residence and disease-specific comorbidities (21,22). Disease-specific comorbidities were defined by the presence of medical services plan or hospital discharge diagnoses, or service/procedure codes associated with HIV, hemophilia, illicit drug use, alcohol use and mental illness in the year before cohort entry (Appendix 2). The propensity score was then used a priori to match cases and controls to reduce bias (23).
Appendix 2.
Disease-specific comorbidities
| ICD-10 code | Description |
|---|---|
| Alcohol abuse | |
| Z714 | Alcohol abuse counselling and surveillance |
| Y573 | Alcohol deterrents causing adverse effect in therapeutic use |
| Z502 | Alcohol rehabilitation |
| Y919 | Alcohol involvement, not otherwise specified |
| Z721 | Alcohol use |
| I426 | Alcoholic cardiomyopathy |
| K703 | Alcoholic cirrhosis of liver |
| K700 | Alcoholic fatty liver |
| K702 | Alcoholic fibrosis and sclerosis of liver |
| K292 | Alcoholic gastritis |
| K704 | Alcoholic hepatic failure |
| K701 | Alcoholic hepatitis |
| K709 | Alcoholic liver disease, unspecified |
| G721 | Alcoholic myopathy |
| G621 | Alcoholic polyneuropathy |
| K860 | Alcohol-induced chronic pancreatitis |
| X65 | Intentional self-poisoning by and exposure to alcohol |
| F100 | Mental and behavioural disorders due to use of alcohol, acute intoxication |
| F101 | Mental and behavioural disorders due to use of alcohol, harmful use |
| F102 | Mental and behavioural disorders due to use of alcohol, dependence syndrome |
| F103 | Mental and behavioural disorders due to use of alcohol, withdrawal state |
| F104 | Mental and behavioural disorders due to use of alcohol, withdrawal state with delirium |
| F105 | Mental and behavioural disorders due to use of alcohol, psychotic disorder |
| F106 | Mental and behavioural disorders due to use of alcohol, amnesic syndrome |
| F107 | Mental and behavioural disorders due to use of alcohol, residual and late-onset psychotic disorder |
| F108 | Mental and behavioural disorders due to use of alcohol, other mental and behavioural disorders |
| F109 | Mental and behavioural disorders due to use of alcohol, unspecified mental and behavioural disorder |
| Z8640 | Personal history of alcohol abuse |
| HIV | |
| Z717 | HIV counselling |
| B24 | HIV disease |
| R75 | Laboratory evidence of HIV |
| Z21 | Asymptomatic HIV infection status |
| F024 | Dementia in HIV disease |
| Hemophilia | |
| D66 | Hereditary factor VIII deficiency |
| D67 | Hereditary factor IX deficiency |
| Drug abuse | |
| Z715 | Drug abuse counselling and surveillance |
| Z722 | Drug use |
| Z8641 | Personal history of drug abuse |
| F110–149 | Mental and behavioural disorders due to opioids, cannabinoids, sedatives, hypnotics, cocaine, various presentations |
| F160–169 | Mental and behavioural disorders due to stimulants, hallucinogens, various presentations |
| F190–199 | Mental and behavioural disorders due to multiple drug use and use of psychoactive substances, various presentations |
| R782 | Finding of cocaine in blood |
| R783 | Finding of hallucinogen in blood |
| R781 | Finding of opiate drug in blood |
| R784 | Finding of other drugs of addictive potential in blood |
| R788 | Finding of other specified substances, not normally found in blood |
| R785 | Finding of psychotropic drug in blood |
| R789 | Finding of unspecified substance, not normally found in blood |
| T407 | Poisoning by cannabis (derivatives) |
| T405 | Poisoning by cocaine |
| T401 | Poisoning by heroin |
| T408 | Poisoning by lysergide (LSD) |
| T403 | Poisoning by methadone |
| T400 | Poisoning by opium |
| T406 | Poisoning by other and unspecified narcotics |
| T409 | Poisoning by other and unspecified psychodysleptics (hallucinogens) |
| T402 | Poisoning by other opioids |
| T404 | Poisoning by other synthetic narcotics |
| T436 | Poisoning by psychostimulants with abuse potential |
| Mental illness | |
| F04 | Organic amnesic syndrome, not induced by alcohol and other psychoactive substances |
| F050 | Delirium not superimposed on dementia, so described |
| F051 | Delirium superimposed on dementia |
| F058 | Other delirium |
| F059 | Delirium, unspecified |
| F060–F066 | Organic mental disorders, various (not drug induced) |
| F070 | Organic personality disorder |
| F071 | Postencephalitic syndrome |
| F072 | Postconcussional syndrome |
| F078 | Other organic personality and behavioural disorders due to brain disease, damage and dysfunction |
| F079 | Unspecified organic personality and behavioural disorder due to brain disease, damage and dysfunction |
| F09 | Unspecified organic or symptomatic mental disorders |
| F21 | Schizotypal disorder |
| F24 | Induced delusional disorder |
| F28 | Other nonorganic psychotic disorders |
| F29 | Unspecified nonorganic psychosis |
| F39 | Unspecified mood (affective) disorder |
| F54 | Psychological and behavioural factors associated with disorders or diseases classified elsewhere |
| F55 | Abuse of nondependence-producing substances |
| F59 | Unspecified behavioural syndromes associated with physiological disturbances and physical factors |
| F61 | Mixed and other personality disorders |
| F69 | Unspecified disorder of adult personality and behaviour |
| F99 | Mental disorder, not otherwise specified |
| F200–F209 | Schizophrenia, various presentations |
| F220 | Delusional disorder |
| F228 | Other persistent delusional disorders |
| F229 | Persistent delusional disorder, unspecified |
| F230 | Acute polymorphic psychotic disorder without symptoms of schizophrenia |
| F231 | Acute polymorphic psychotic disorder with symptoms of schizophrenia |
| F232 | Acute schizophrenia-like psychotic disorder |
| F233 | Other acute predominantly delusional psychotic disorders |
| F238 | Other acute and transient psychotic disorders |
| F239 | Acute and transient psychotic disorder, unspecified |
| F300 | Hypomania |
| F301 | Mania without psychotic symptoms |
| F302 | Mania with psychotic symptoms |
| F308 | Other manic episodes |
| F309 | Manic episode, unspecified |
| F310–F319 | Bipolar affective disorders, various presentations |
| F319–F323 | Depressive disorders, by severity, various presentations |
| F328–F334 | Other depressive episodes, by frequency, various presentations |
| F338–F339 | Other recurrent depressive disorders, various presentations |
| F340 | Cyclothymia |
| F341 | Dysthymia |
| F348 | Other persistent mood (affective) disorders |
| F349 | Persistent mood (affective) disorder, unspecified |
| F380 | Other single mood (affective) disorders |
| F381 | Other recurrent mood (affective) disorders |
| F388 | Other specified mood (affective) disorders |
| F400–F402, F408–F409 | Phobias, various |
| F410 | Panic disorder (episodic paroxysmal anxiety) |
| F411–F413, F418–F419 | Anxiety disorders, various |
| F420–F422, F428–F429 | Obsessive compulsive disease, various |
| F430–F432, F438–F439 | Acute stress disorders, various |
| F440–F449 | Dissociative disorders, various |
| F450–F454, F458–F459 | Somatoform disorders, various |
| F480–F481, F488–F489 | Neurotic disorders, various |
| F500–F509 | Eating disorders, various |
| F515–F529 | Sleeping and sexual disorders, various |
| F530–F531, F538–F539 | Puerperal mental disorders, various |
| F600–F609 | Personality disorders, various presentations |
| F620–F621, F628–F629 | Enduring personality change, various |
| F630–F633, F638–F639 | Habit and impulse disorder, various |
| F640–F642, F648–F649 | Gender identity disorders, various |
| F650–F659 | Multiple disorders of sexual preference |
| F660–F662, F668–F669 | Psychosexual relational disorders, various |
| F680–F681, F688 | Other specified disorders of adult personality and behaviour |
| F900–F901, F908–F909 | Hyperkinetic disorders, various |
| F910–F913, F918–F919 | Conduct disorders, various |
| F920 | Depressive conduct disorder |
| F928 | Other mixed disorders of conduct and emotions |
| F929 | Mixed disorder of conduct and emotions, unspecified |
| X60–X84 | Intentional self-harm, various methods |
| X7400–X7401, X7408–X7409 | Intentional self-harm, various methods |
ICD International Classification of Diseases, 10th Revision
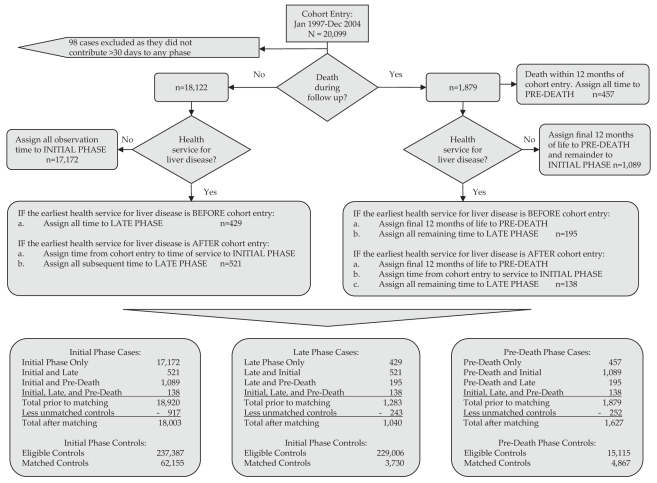
Case subjects in each phase were ‘greedy matched’ with up to four controls based on the propensity score, sex and age (±5 years) (24). Each phase consisted of a unique cohort and was analyzed separately. Case subjects who contributed observation time to multiple phases were rematched to controls at entry into each phase (Figure 1). While some subjects may have contributed observation time to multiple phases, there was no overlap or duplication of case or control observation time and costs across phases. Controls were not matched to more than one case subject within a given phase. The quality of the match between case subjects and controls was evaluated using descriptive statistics on all variables for each phase and compared using standardized differences (25).
Figure 1.
Outcome of phase allocation and case-control matching. Dec December; Jan January
Because case subjects and controls were matched, cost differences represent the net cost or cost attributable to HCV seropositivity adjusted for demographic and clinical factors. Generalized estimating equation models, in which case-control pairs were treated as clusters, were used to generate mean and net case and control costs per 100 days for each phase and cost category. Service component and total costs for each subject in each phase were divided by the subject’s observation time in the disease phase, standardized to a cost per 100 patient days and converted to annual costs. Finally, for HCV cases, predictors of total cost were identified using multiple linear regression with logarithmically transformed cost data to correct for skewness (26).
RESULTS
Phase allocation and case-control matching resulted in 20,001 unique HCV cases (Figure 1). Of the case subjects, 18,058 (90%) contributed time to only one disease phase, 1805 (9%) to two phases and 138 (1%) to all three phases. The final numbers of matched cases were 18,003, 1040 and 1627 for the initial, late and predeath phases, respectively. Thus, the vast majority of observation time was related to the initial phase. Many case subjects who contributed person-time to the initial, late and predeath phases (917, 243 and 252, respectively) could not be matched with suitable controls. Unmatched cases were younger and had very high propensity scores related to multiple markers of vulnerability (ie, HIV, poverty, flags for addictions, mental illness and high Deyo-Charlson comorbidity index).
Table 1 summarizes sociodemographic and clinical characteristics, and overall matching between case subjects and controls across the disease phases. Despite intensive efforts to evenly match cases and controls, several attribute variables could not be evenly distributed and demonstrated standardized differences (sd) of greater than 0.10. For example, codes for mental health were higher among cases than controls across all phases (initial phase sd=0.20; late phase sd=0.19; and predeath phase sd=0.17). Comorbidities were higher in late phase cases than in controls (sd=0.58). Flags for illicit drug use were higher among cases than controls in the initial phase and predeath phase (initial phase sd=0.31; predeath phase sd=0.30). Finally, the mean ages of the initial, late and predeath phase cases were 43, 55 and 56 years, respectively. Predeath phase cases were younger than their matched controls (56.1 years versus 60.7 years, sd=0.26).
TABLE 1.
Baseline characteristics of matched cases and controls, and unmatched cases
| Disease phase |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial |
Late |
Predeath |
|||||||
| Characteristic | Cases | Controls | Nonmatched | Cases | Controls | Nonmatched | Cases | Controls | Nonmatched |
| Subjects | 18,003 (100) | 62,155 (100) | 917 (100) | 1040 (100) | 3730 (100) | 242 (100) | 1627 (100) | 4867 (100) | 252 (100) |
| Follow-up, days (mean ± SD) | 1735±776 | 1714±780 | 2146±736 | 1478±808 | 1314±805 | 966±834 | 361±26 | 361±25 | 356±43 |
| Age, years | |||||||||
| Mean ± SD | 42.9±11.7 | 43.1±11.9 | 41.3±7.0 | 55.1±15.0 | 55.6±15.3 | 48.4±11.7 | 56.1±16.1 | 60.7±16.3 | 44.0±7.6 |
| Median | 43 | 43 | 42 | 52 | 53 | 48 | 52 | 60 | 45 |
| 0–10 | 106 (0.6) | 417 (0.7) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 2 (0.1) | 1 (0.0) | 0 (0.0) |
| 11–20 | 233 (1.3) | 947 (1.5) | 1 (0.1) | 3 (0.3) | 12 (0.3) | 1 (0.4) | 0 (0.0) | 7 (0.1) | 0 (0.0) |
| 21–30 | 1868 (10.4) | 6244 (10.0) | 63 (6.9) | 33 (3.2) | 126 (3.4) | 10 (4.1) | 41 (2.5) | 131 (2.7) | 12 (4.8) |
| 31–40 | 5097 (28.3) | 17,406 (28.0) | 304 (33.0) | 94 (9.0) | 360 (9.7) | 39 (16.1) | 202 (12.4) | 413 (8.5) | 61 (24.2) |
| 41–50 | 7214 (40.1) | 23,999 (38.6) | 485 (53.0) | 350 (33.7) | 1219 (32.7) | 99 (40.9) | 462 (28.4) | 827 (17) | 139 (55.2) |
| 51–60 | 2264 (12.6) | 8699 (14.0) | 58 (6.3) | 214 (20.6) | 709 (19.0) | 62 (25.6) | 359 (22.1) | 1160 (23.8) | 36 (14.3) |
| 61–70 | 727 (4.0) | 2705 (4.4) | 4 (0.4) | 136 (13.1) | 502 (13.5) | 16 (6.6) | 183 (11.3) | 820 (16.8) | 3 (1.2) |
| ≥71 | 494 (2.7) | 1738 (2.8) | 0 (0.0) | 210 (20.2) | 802 (21.5) | 14 (5.8) | 378 (23.2) | 1508 (31) | 1 (0.4) |
| Sex | |||||||||
| Female | 6467 (35.9) | 22,929 (36.9) | 254 (28.0) | 386 (37.1) | 1456 (39.0) | 77 (31.8) | 464 (28.5) | 1634 (33.6) | 78 (31.0) |
| Male | 11,536 (64.1) | 39,226 (63.1) | 663 (72.0) | 654 (62.9) | 2274 (61.0) | 165 (68.2) | 1163 (71.5) | 3233 (66.4) | 174 (69.0) |
| Income quintile | |||||||||
| 1 (low) | 5907 (32.8) | 19,016 (30.6) | 454 (50.0) | 318 (30.6) | 1274 (34.2) | 111 (45.9) | 578 (35.5) | 1349 (27.7) | 133 (52.8) |
| 2 | 3704 (20.6) | 12,406 (20.0) | 200 (21.3) | 221 (22.0) | 768 (20.6) | 48 (19.8) | 333 (20.5) | 1023 (21.0) | 42 (16.7) |
| 3 | 2786 (15.5) | 10,360 (16.7) | 77 (13.8) | 143 (8.4) | 556 (14.9) | 28 (11.6) | 212 (13.0) | 810 (16.6) | 22 (8.7) |
| 4 | 2523 (14.0) | 9619 (15.5) | 78 (15.2) | 158 (8.5) | 516 (13.8) | 20 (8.3) | 224 (13.8) | 779 (16.0) | 22 (8.7) |
| 5 (high) | 1856 (10.3) | 7313 (11.8) | 42 (13.1) | 136 (4.6) | 433 (11.6) | 21 (8.7) | 163 (10.0) | 637 (13.1) | 16 (6.3) |
| Missing | 1227 (6.8) | 3441 (5.5) | 66 (6.2) | 64 (7.2) | 183 (4.9) | 14 (5.8) | 117 (7.2) | 269 (5.5) | 17 (6.7) |
| Rural flag | |||||||||
| No | 15,646 (86.9) | 54,453 (87.6) | 847 (92.4) | 892 (85.8) | 3,143 (84.3) | 207 (85.5) | 1434 (88.1) | 4241 (87.1) | 242 (96.0) |
| Yes | 2357 (13.1) | 7702 (12.4) | 70 (7.6) | 148 (14.2) | 587 (15.7) | 35 (14.5) | 193 (11.9) | 626 (12.9) | 10 (4.0) |
| Index year | |||||||||
| 1996 | – | – | – | – | – | – | 51 (3.1) | 145 (3.0) | 7 (2.8) |
| 1997 | 4071 (22.6) | 13,827 (22.2) | 427 (47.0) | 169 (16.3) | 641 (17.2) | 20 (8.3) | 147 (9.0) | 410 (8.4) | 21 (8.3) |
| 1998 | 3184 (17.7) | 10,520 (16.9) | 262 (29.0) | 135 (13.0) | 510 (13.7) | 25 (10.3) | 174 (10.7) | 542 (11.1) | 22 (8.7) |
| 1999 | 2640 (14.7) | 8831 (14.2) | 91 (9.9) | 151 (14.5) | 561 (15.0) | 24 (9.9) | 188 (11.6) | 607 (12.5) | 24 (9.5) |
| 2000 | 2243 (12.5) | 7778 (12.5) | 47 (5.1) | 153 (14.7) | 570 (15.3) | 29 (12.0) | 222 (13.6) | 628 (12.9) | 43 (17.1) |
| 2001 | 2190 (12.2) | 7811 (12.6) | 39 (4.3) | 102 (9.8) | 380 (10.2) | 32 (13.2) | 290 (17.8) | 896 (18.4) | 31 (12.3) |
| 2002 | 1992 (11.1) | 7177 (11.5) | 40 (4.4) | 136 (13.1) | 480 (12.9) | 32 (13.2) | 272 (16.7) | 846 (17.4) | 39 (15.5) |
| 2003 | 1683 (9.3) | 6211 (10.0) | 11 (1.2) | 131 (12.6) | 401 (10.8) | 45 (18.6) | 283 (17.4) | 793 (16.3) | 65 (25.8) |
| 2004 | – | – | – | 63 (6.1) | 187 (5.0) | 35 (14.5) | – | – | – |
| Measures of comorbidity | |||||||||
| Deyo-Charlson comorbidity index | |||||||||
| 0 | 17,498 (97.2) | 61,150 (98.4) | 864 (94.0) | 564 (54.2) | 3001 (80.5) | 27 (11.2) | 1270 (78.1) | 3648 (75.0) | 163 (64.7) |
| 1 | 272 (1.5) | 556 (0.9) | 16 (1.7) | 139 (13.4) | 233 (6.2) | 24 (9.9) | 99 (6.1) | 357 (7.3) | 14 (5.6) |
| 2 | 129 (0.7) | 303 (0.5) | 3 (0.3) | 92 (8.8) | 293 (7.9) | 20 (8.3) | 107 (6.6) | 427 (8.8) | 5 (2.0) |
| ≥3 | 104 (0.6) | 146 (0.2) | 34 (3.7) | 245 (23.6) | 203 (12.4) | 171 (70.7) | 151 (9.3) | 435 (8.9) | 70 (27.8) |
| Disease-specific services flags (hepatitis C-related comorbidities) | |||||||||
| HIV | 137 (0.8) | 250 (0.4) | 86 (9.4) | 12 (1.2) | 55 (1.5) | 52 (21.5) | 21 (1.3) | 42 (0.9) | 96 (38.1) |
| Mental health | 6340 (35.2) | 16,467 (26.6) | 894 (97.5) | 366 (35.2) | 1670 (44.8) | 206 (85.1) | 545 (33.5) | 1252 (30.1) | 234 (92.9) |
| Illicit drug use | 2930 (16.3) | 4513 (7.3) | 901 (98.3) | 127 (12.2) | 539 (14.5) | 141 (58.3) | 183 (11.2) | 206 (4.2) | 223 (88.5) |
| Alcohol use | 1140 (6.3) | 2179 (3.5) | 185 (20.2) | 129 (12.4) | 541 (14.5) | 152 (62.8) | 154 (9.5) | 303 (6.2) | 66 (26.2) |
| Hemophilia | 49 (0.3) | 101 (0.2) | 2 (0.2) | 24 (2.3) | 41 (1.1) | 6 (2.5) | 30 (1.8) | 112 (2.3) | 4 (1.6) |
Data presented as n (%) unless indicated otherwise
Table 2 summarizes health resource use. Total costs increased across disease phases, largely due to hospitalization. Increases in hospitalization correlated with reduced prescription drug costs from 26% of total costs in the initial phase to 4% in the predeath phase, in which drug costs while in hospital were included in overall hospitalization costs. Therefore, BC spent approximately $1,068/100 patient-days or $3,900/person/year and $3,013/100 patient-days or $11,000/person/year for initial and late phase patient care, respectively. Approximately one-half of these costs relate to HCV infection or related risks. For cases in the predeath phase, BC spent $10,281/100 patient-days or $37,530/person/year, which was virtually identical to the health-related costs of the controls during their final year of life.
TABLE 2.
Mean health care costs* among cases and controls according to cost category and disease phase
| Disease phase |
||||||
|---|---|---|---|---|---|---|
| Initial |
Late |
Predeath |
||||
| Cost category | Cases | Controls | Cases | Controls | Cases | Controls |
| n | 18,003 | 62,155 | 1040 | 3730 | 1627 | 4867 |
| Total drug cost†, $ | 377 | 165 | 616 | 355 | 561 | 570 |
| Nonpublicly paid portion, $ | 104 | 83 | 219 | 124 | 130 | 197 |
| Publicly paid portion, $ (%) | 273 (25.6) | 82 (14.7) | 397 (13.2) | 231 (17.0) | 431 (4.2) | 373 (3.6) |
| MSP cost (physician and outpatient clinic services; outpatient diagnostic and laboratory services), $ (%) | 307 (28.7) | 203 (36.5) | 687 (22.8) | 338 (24.8) | 1,073 (10.4) | 1,124 (10.9) |
| Hospital cost (acute inpatient), $ (%) | 446 (41.8) | 232 (41.7) | 1,712 (56.8) | 721 (53.0) | 8,667 (84.3) | 8,707 (84.0) |
| Same-day surgery cost, $ (%) | 42 (4.0) | 39 (7.0) | 216 (7.2) | 72 (5.3) | 110 (1.1) | 157 (1.5) |
| Total cost‡, $ (%) | 1,068 (100) | 556 (100) | 3,013 (100) | 1,361 (100) | 10,281 (100) | 10,361 (100) |
Mean health care costs are expressed in 2005 $CAD per 100 patient days; 2005 $1 CAD = $0.83 USD;
PharmaNet files report two cost components: total drug cost (the full drug and dispensing fee) and the publicly paid portion (the portion of total drug cost that is paid by the provincial PharmaCare program). The remaining cost (nonpublicly paid portion) is generally paid by the patient at the time of receipt of the drug. It may also be paid at either the point of purchase or later reimbursed to the patient by a third-party payer. Both components are displayed to provide a more complete description of drug costs. However, only the publicly paid portion is included in these costing estimates;
Total cost includes only bolded categories, excluding nonpublicly paid portion of drug costs. MSP Medical services plan
Table 3 summarizes the net costs and CIs according to disease phase and service category. Net costs increased from $507/100 patient-days (95% CI $473 to $540) or $1,850/year in the initial phase, to $1,642/100 patient-days (95% CI $1,302 to $1,983) or $6,000/year in the late phase. Predeath costs were $22/100 patient-days or $80/year lower in case subjects than controls (95% CI –$972 to $929); however, given the CIs, no cost differences were observed for this disease phase.
TABLE 3.
Health care costs* attributable to hepatitis C according to cost category and disease phase
| Disease phase |
|||
|---|---|---|---|
| Cost category | Initial | Late | Predeath |
| Total drug cost | 210 (200 to 219) | 259 (199 to 319) | −9 (−55 to 38) |
| Publicly funded drug cost | 190 (182 to 198) | 165 (121 to 209) | 58 (17 to 99) |
| MSP cost (physician and outpatient clinic services; outpatient diagnostic and laboratory services) | 101 (96 to 107) | 348 (288 to 408) | −50 (−126 to 26) |
| Hospital cost (acute inpatient) | 213 (185 to 241) | 987 (703 to 1,270) | 14 (−885 to 912) |
| Same-day surgery cost | 3 (1 to 5) | 145 (113 to 176) | −47 (−69 to −24) |
| Net cost (hepatitis C-related cost)† | 507 (473 to 540) | 1,642 (1,302 to 1,983) | −22 (−972 to 929) |
| Net cost as a percentage of the mean total cost in cases, % | 47.5 | 54.5 | −0.2 |
Health care costs expressed in 2005 Canadian dollars ($1 CAD = $0.83 USD) per 100 days (95% CI) unless indicated otherwise;
Table 4 reports independent predictors of total costs among cases. Age and the Deyo-Charlson comorbidity index were significant cost predictors in all phases, although the pattern varied. Illicit drug use had an effect on initial and late phase costs, but not on predeath costs; mental illness was a significant predictor of costs only for the initial phase; and HIV infection was associated with increased costs in all three disease phases. There were no significant cost differences for unmatched cases in the adjusted model of costs.
TABLE 4.
Predictors of total health cars costs in persons with hepatitis C (HCV)
| Disease phase |
||||||
|---|---|---|---|---|---|---|
| Initial |
Late |
Predeath |
||||
| Characteristic | eβ* | 95% CI | eβ | 95% CI | eβ | 95% CI |
| Age, years | ||||||
| ≤30 | 0.954 | 0.925–0.983 | 0.973 | 0.819–1.157 | 0.79 | 0.644–0.968 |
| 31–40 (referent) | 1.00 | – | 1.00 | – | 1.00 | – |
| 41–50 | 1.057 | 1.034–1.081 | 1.099 | 0.99–1.22 | 1.169 | 1.054–1.297 |
| 51–60 | 1.205 | 1.169–1.243 | 1.148 | 1.023–1.288 | 1.362 | 1.214–1.529 |
| 61–70 | 1.428 | 1.36–1.50 | 1.244 | 1.095–1.413 | 1.437 | 1.25–1.652 |
| ≥71 | 1.537 | 1.45–1.63 | 1.23 | 1.09–1.389 | 1.291 | 1.143–1.457 |
| Sex | ||||||
| Male (referent) | 1.00 | 1.00 | 1.00 | |||
| Female | 1.115 | 1.094–1.136 | 1.061 | 0.997–1.128 | 1.199 | 1.115–1.289 |
| Deyo-Charlson comorbidity index | ||||||
| 0 (referent) | 1.00 | – | 1.00 | – | 1.00 | – |
| 1 | 1.464 | 1.361–1.575 | 1.245 | 1.134–1.366 | 1.355 | 1.177–1.559 |
| 2 | 1.603 | 1.44–1.785 | 1.359 | 1.22–1.515 | 1.363 | 1.185–1.569 |
| 3+ | 1.407 | 1.249–1.584 | 1.352 | 1.252–1.459 | 1.375 | 1.226–1.542 |
| Income quintile | ||||||
| 1–low (referent) | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.984 | 0.96–1.009 | 0.943 | 0.871–1.022 | 1.07 | 0.978–1.171 |
| 3 | 0.973 | 0.946–1 | 0.999 | 0.91–1.096 | 0.941 | 0.846–1.047 |
| 4 | 0.956 | 0.929–0.984 | 0.969 | 0.884–1.062 | 0.948 | 0.854–1.053 |
| 5–high | 0.944 | 0.914–0.975 | 0.961 | 0.873–1.059 | 0.929 | 0.825–1.046 |
| Missing | 0.95 | 0.915–0.986 | 1.058 | 0.93–1.203 | 0.906 | 0.794–1.033 |
| Index year | ||||||
| 1996 | – | – | – | 1.00 | – | |
| 1997 | 1.00 | – | 1.00 | – | 0.914 | 0.75–1.114 |
| 1998 | 0.984 | 0.957–1.012 | 1.089 | 0.975–1.217 | 0.974 | 0.801–1.184 |
| 1999 | 0.966 | 0.938–0.995 | 1.037 | 0.931–1.156 | 0.945 | 0.778–1.148 |
| 2000 | 0.98 | 0.95–1.012 | 1.026 | 0.921–1.144 | 0.981 | 0.811–1.187 |
| 2001 | 0.966 | 0.936–0.998 | 1.032 | 0.916–1.161 | 0.906 | 0.75–1.093 |
| 2002 | 0.931 | 0.9–0.962 | 0.915 | 0.815–1.026 | 0.909 | 0.75–1.1 |
| 2003 | 0.97 | 0.936–1.005 | 0.967 | 0.862–1.085 | 0.811 | 0.67–0.981 |
| 2004 | – | – | 1.293 | 1.127–1.484 | – | – |
| Rural flag | 0.968 | 0.942–0.994 | 0.955 | 0.878–1.038 | 0.978 | 0.879–1.088 |
| Disease-specific use of health services (HCV-related comorbidities) | ||||||
| Alcohol related | 1.034 | 0.996–1.073 | 1.031 | 0.943–1.128 | 1.01 | 0.902–1.131 |
| Hemophilia related | 1.353 | 1.14–1.607 | 1.08 | 0.894–1.305 | 1.381 | 1.083–1.76 |
| HIV related | 1.38 | 1.255–1.518 | 1.175 | 1.01–1.367 | 1.288 | 1.088–1.524 |
| Illicit drug related | 1.23 | 1.193–1.268 | 1.219 | 1.109–1.339 | 1.084 | 0.96–1.224 |
| Mental health related | 1.224 | 1.195–1.255 | 1.075 | 0.996–1.161 | 1.046 | 0.958–1.141 |
| Nonmatched cases | 0.993 | 0.947–1.041 | 0.925 | 0.823–1.04 | 1.006 | 0.874–1.159 |
| HCV RNA testing (polymerase chain reaction testing) | ||||||
| HCV RNA negative (referent) | 1.00 | – | 1.00 | – | 1.00 | – |
| HCV RNA positive | 1.08 | 1.044–1.116 | 0.985 | 0.882–1.1 | 1.048 | 0.881–1.247 |
| No HCV RNA test | 0.812 | 0.787–0.839 | 0.866 | 0.775–0.967 | 0.848 | 0.721–0.998 |
eβ refers to the exponential of the regression coefficient interpreted as the relative change in median cost with a one-unit increase in predictor variable
A subset of case subjects had undergone HCV-RNA testing to determine whether their HCV infection was active (Table 4). In the natural history of HCV, approximately 25% of HCV infected individuals spontaneously clear HCV RNA but remain anti-HCV positive, indicating resolved infection. Among the cases, there were 8892, 627 and 450 subjects with HCV-RNA testing in initial, late and predeath phases, respectively. Of these, 81%, 84% and 81% were RNA positive, across the respective disease phases. While these individuals were classified as case subjects based on their positive anti-HCV status, those who were HCV RNA negative are known to be at very low risk of viral-related sequelae (27,28). Case subjects who did not undergo HCV RNA testing had lower costs (19% less in the initial, 13% less in the late and 15% less in the pre-death disease phase).
DISCUSSION
During the initial and late disease phases, BC spent an estimated $1,850/person/year and $6,000/person/year, respectively, on direct HCV-related health care. Costs increase with disease progression and hospitalization was the largest cost component across all disease phases, followed by medical services and publicly funded drugs. While no increase in the net cost was observed for the predeath phase, two limitations need to be considered. First, PharmaNet does not capture medications used in HIV/AIDS, cancer, transplant or renal disease, and the BC Linked Health Database files do not capture cancer care costs. Thus, capture of costs relating to known causes of death in individuals infected with HCV is incomplete (29). Second, while predeath costs for cases and controls were similar, case subjects died at a significantly younger age, suggesting potential years of life lost due to HCV-related illness not accounted for by direct costing (4,30). Our findings align with previous work (8,29,31) showing higher costs and earlier mortality among HCV monoinfected and HCV-HIV coinfected individuals.
Approximately 14% to 19% of individuals chronically infected with HCV develop cirrhosis within 20 years, leading to liver failure, hepatocellular carcinoma and death (3,4). Thus, late and predeath disease phase case subjects reflect the relatively small proportion of HCV patients requiring medically cost-intensive services. These cases represent a missed opportunity to prevent chronic HCV sequelae by using potentially curative treatment (32).
In contrast, initial phase case subjects have a special significance when one considers that the majority of prevalent cases will spend decades in this phase. Initial phase case costs increased with age, comorbid conditions, HIV infection, illicit drug use and mental illness. Nguyen et al (31) reported that physician and hospital service costs among HCV patients tended to be highest in the year following diagnosis, largely related to mental health services. Using methods similar to the present study, a Canadian research group found mental health and drug-related services to be important predictors of initial phase HCV costs for the province of Ontario (M Patterson and M Krahn, unpublished data, 2009).
In the initial phase, mental illness and illicit drug use are both risk factors for HCV acquisition and contribute to health care costs. It remains challenging to separate costs of the medical sequelae of HCV infection from acquisition-related risks and costs. Sulkowski and Thomas (19) reviewed the complex inter-relationship of HIV/HCV coinfection, illicit drug use, and mental illness and its impact on the delivery of medical care for both infections. They determined that the higher rates of illicit drug use, mental illness and poverty confounded the assessment of the relative impact of mono- or coinfection and this was likely the case in our study. Future studies based on RNA status can further differentiate these costs. For example, the lower costs in subjects who did not undergo HCV RNA testing suggest that this test may also be a marker for access to care and treatment. In addition, we found only minor differences in costs between HCV RNA-positive or -negative cases suggesting that a substantial proportion of costs reflects the impact of mental health and addictions rather than viral sequelae. While intriguing, it is important not to over interpret these findings because the present study was not designed to assess the impact of HCV RNA status on costs.
In BC, the cost of treating HCV infection with antivirals is estimated to range from $11,000 to $20,000 per completed patient course of treatment, depending on the genotype and number of weeks of treatment (33). During the study period, a very limited number of cases underwent antiviral treatment; approximately 1% of initial and late-phase case subjects and 0.2% of predeath phase case subjects received treatment during the costing period. Overall, antiviral treatments represented 0.7% of the reported case costs; however, this is an underestimate because PharmaNet data does not provide information about treatment starts in clinical trials, prison, or via federal or private payers. It is also important to note that pegylated interferon and ribavirin only became publicly funded in BC in May 2003. While treatment-related drug costs were a relatively trivial proportion of the HCV-attributable costs in our study, these costs would be expected to rise substantially with widespread treatment.
The two main limitations of the current study are that the predeath cost estimates did not capture all of the costs that are related to the recognized causes of HCV mortality, and that mental illness, addictions and behaviours known to correlate with the risk of HCV acquisition confound our ability to tease apart the HCV-attributable costs that relate to the risk of acquiring infection versus the consequences of the infection itself. The limitation in our ability to accurately quantify the impact of social vulnerability on costs occurs at two levels. First, valid personal health number identifiers are required for data linkage, and 14% of testers in the present study could not be linked to their administrative data; thus, a proportion of those most vulnerable were excluded from data linkage. In addition, certain case subjects could not be matched to controls because of their profound vulnerabilities (ie, HIV, poverty, flags for addictions, mental illness and high Deyo-Charlson comorbidity index). These unmatched case subjects had multiple markers of vulnerability suggesting that generalizability of the net costs to those most vulnerable is limited. The challenges in matching cases and controls speak to the nature of HCV positive testers as individuals with multiple comorbidities with a high level of health and social vulnerability. Limited capture of the costs of those most vulnerable combined with the use of seronegative single testers as controls, who might have some risk of HCV infection to justify serological testing, tends to make our cost estimates conservative.
The present study also has important strengths. The cohort was drawn from a large, comprehensive sample of anti-HCV testers in the province of BC. Detailed matching of cases and controls for such a large number of subjects would not be possible using traditional case-by-case follow-up. Both the serological data and the administrative health data were longitudinal, which enabled assessment of health resource use across time and the disease phases. Finally, we were able to base cost estimates on several sectors – not just hospitalization – and the use of control group matching provided a first estimate of HCV net costs.
HCV-related health care costs in BC are considerable and likely on par with annual provincial spending on HIV-related direct medical costs. While there are few studies with estimates of direct costs of HIV/AIDS, in 2006, Levy et al (34) and, in 2003, Krentz et al (35) reported that the total direct costs for treating HIV/AIDS in Canada was $11,196/person/year (2001 US dollars), not stratified according to disease phase. BC has reported 12,966 HIV-positive cases since 1989 (36). Not accounting for mortality or migration, this would suggest BC spends approximately $145 million/year on HIV/AIDS care, with about two-thirds of costs related to treatment.
A similar gross estimate can be made for HCV. Remis (1) estimated 9% of those Canadians living with HCV in 2007 had cirrhosis or liver failure. In BC, there were 62,214 HCV antibody positive cases reported in the Integrated Public Health Information System as of December 31, 2008 (BCCDC, unpublished data). If we apply this to the estimated $1,850/person/year and $6,000/person/year for initial and late-phase net costs, respectively, provincial spending on HCV-related health care approaches $136 million/year (assuming 89% are in initial phase [55,371×$1,850 = $102 million] and 9% are late phase [5,600×$6,000=$34 million] and 2% are in predeath phase [with no identified net costs]). Compared with HIV direct costs, a much lower proportion of costs are drug related. Future research on lifetime cost estimates of HCV is required to accurately gauge provincial and national spending on HCV.
HCV seropositivity is correlated with substantial increases in direct health care costs. Accurate costing of HCV infection will require refinements in assessing costs related to viral sequelae, and adjusting for underlying risk factors and related comorbidities. It is clear that prevention aimed at mental health and addictions, as well as HCV treatment are required to mitigate the costs and health outcomes in this population.
ACKNOWLEDGEMENTS
The authors acknowledge Darrel Cook and Dr Gail Butt for their advice on the manuscript.
REFERENCES
- 1.Remis RS. Modelling the incidence and prevalence of hepatitis C infection and its sequelae in Canada, 2007: Final Report. Ottawa: Public Health Agency of Canada; 2009. [Google Scholar]
- 2.Zou S, Tepper M, El Saadany S. Prediction of hepatitis C burden in Canada. Can J Gastroenterol. 2000;14:575–80. doi: 10.1155/2000/642707. [DOI] [PubMed] [Google Scholar]
- 3.Thein H-H, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology. 2008;48:418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 4.Wise M, Bialek S, Finelli L, Bell B, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology. 2008;47:1128–35. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C-virus infected persons in the United States: A multiple cohort model of HCV prevalence and disease. Gastroenterology. 2010;138:513–21. e6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Leigh JP, Bowlus CL, Leistikow BN, Schenker M. Costs of hepatitis C. Arch Intern Med. 2001;161:2231–7. doi: 10.1001/archinte.161.18.2231. [DOI] [PubMed] [Google Scholar]
- 7.Myers RP, Liu M, Shaheen AAM. The burden of hepatitis C virus infection is growing: A Canadian population-based study of hospitalizations from 1994 to 2004. Can J Gastroenterol. 2008;22:381–7. doi: 10.1155/2008/173153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong EP, Charland SL. Burden of illness of hepatitis C from a managed care organization perspective. Curr Med Res Opin. 2004;20:671–9. doi: 10.1185/030079904125003485. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg CL. Health care, treatment patterns and cost of services for patients infected with chronic hepatitis C virus in a large insured New England population. J Vir Hepatit. 2000;7:361–7. doi: 10.1046/j.1365-2893.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni RP, Riley GFM, Ramsey SDMDP, Brown MP. Measuring Costs: Administrative claims data, clinical trials, and beyond. Med Care. 2002;40:III-63–72. [PubMed] [Google Scholar]
- 11.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Nat Canc Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 12.Brown MLP, Riley GFM, Potosky ALP, Etzioni RDP. Obtaining long-term disease specific costs of care: Application to Medicare enrollees diagnosed with colorectal cancer. Med Care. 1999;37:1249–59. doi: 10.1097/00005650-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Baker MSP, Kessler LGS, Urban NS, Smucker RCBA. Estimating the treatment costs of breast and lung cancer. Med Care. 1991;29:40–9. doi: 10.1097/00005650-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 14.College of Pharmacists of British Columbia. PharmaNet Stewardship Committee: PharmaNet Data Disclosure. [Accessed April 2009]. < http://www.bcpharmacists.org/pharmanet/pharmanet_data_disclosure.php>.
- 15.Population Data BC. Health Services Data Holdings. [Accessed on November 8, 2010]. < http://www.popdata.bc.ca/dataaccess/process>.
- 16.Krajden M, Shivi R, Gunadasa K, et al. Evaluation of the core antigen assay as a second-line supplemental test for diagnosis of active hepatitis C virus infection. 2004;42:4054–9. doi: 10.1128/JCM.42.9.4054-4059.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg S, Goodman L, Osher F, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Pub Health. 2001;91:31–7. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg SD, Drake RE, Brunette MF, Wolford GL, Marsh BJ. Hepatitis C virus and HIV co-infection in people with severe mental illness and substance use disorders. AIDS. 2005;19(Suppl):S26–S33. doi: 10.1097/01.aids.0000192067.94033.aa. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS, Thomas DL. Perspectives on HIV/hepatitis C virus co-infection, illicit drug use and mental illness. AIDS. 2005;19(Suppl):S8–12. doi: 10.1097/01.aids.0000192064.09281.48. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, Mamdani MM. A comparison of propensity score methods: A case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical co-morbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson co-morbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Parsons LS. Reducing bias in a propensity score matched pair sample using greedy matching techniques. Cary: SAS Institute; 2005. [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioural sciences. Hillsdale: Academic Press; 1988. [Google Scholar]
- 26.Austin PC, Ghali WA, Tu JV. A comparison of several regression models for analysing cost of CABG surgery. Stat Med. 2003;22:2799–815. doi: 10.1002/sim.1442. [DOI] [PubMed] [Google Scholar]
- 27.Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502. doi: 10.1053/jhep.2003.50329. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyasu Y, Yuki N, Nagaoka T, et al. Histological improvement of chronic liver disease after spontaneous serum hepatitis C virus clearance. J Med Virol. 2003;69:41–9. doi: 10.1002/jmv.10250. [DOI] [PubMed] [Google Scholar]
- 29.Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: A large community-based linkage study. Lancet. 2006;368:938–45. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 30.Buti M, San Miguel R, Brosa M, et al. Estimating the impact of hepatitis C virus therapy on future liver-related morbidity, mortality and costs related to chronic hepatitis C. J Hepatol. 2005;42:639–45. doi: 10.1016/j.jhep.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen T-H, Jacobs P, Hanrahan A, et al. Health care costs of persons with newly diagnosed hepatitis C virus: A population-based, observational study. J Viral Hepat. 2008;15:634–40. doi: 10.1111/j.1365-2893.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- 32.Ghany MG, Strader DB, Thomas DL, Seeff LB. AASLD Practice Guidelines. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Services. Clinical Practice Guidelines & Protocols: Clinical Management of Chronic Hepatitis C. [Accessed on October 1, 2004]. < http://www.bcguidelines.ca/gpac/>.
- 34.Levy AR, James D, Johnston KM, et al. The direct costs of HIV/AIDS care. Lancet Infect Dis. 2006;6:171–7. doi: 10.1016/S1473-3099(06)70413-3. [DOI] [PubMed] [Google Scholar]
- 35.Krentz HB, Gill MJ, Auld MC. The changing direct costs of medical care for patients with HIV/AIDS, 1995–2001. CMAJ. 2003;169:106. [PMC free article] [PubMed] [Google Scholar]
- 36.Public Health Agency of Canada. Year-End Report 2007. Ottawa: Public Health Agency of Canada, Surveillance and Risk Assessment Division; 2008. HIV and AIDS in Canada. Surveillance Report to December 31, 2007. [Google Scholar]