Abstract
Patients with diabetes mellitus (DM) are at high risk for fractures. However, the relationship between diabetes and osteoporosis is not yet completely understood. Many factors such as type of diabetes, type of population and co-morbidities may influence the type and severity of bone abnormalities in these patients.
The aim of this study was to evaluate which factors may explain the risk of fractures in a homogeneous population of postmenopausal women with type 2 DM.
Twenty-one consecutive postmenopausal women with type 2 DM were enrolled. Serum and urinary markers of bone metabolism as well as the biochemical markers of glucose homeostasis and diabetes severity were evaluated. Bone mineral density and prevalence of vertebral fractures were evaluated by using MOC DXA and spine radiography, respectively.
The measurement of 25-hydroxyvitamin D serum levels revealed a condition of deficiency in 67% and insufficiency in 28% of patients. Vertebral and femoral neck T-scores were -1.1±1.1 and -0.8±1.0, respectively, while Z-scores were 0.1±1.1 and 0.1±0.9, respectively. Twenty-four % of patients showed ≥1 vertebral fractures. There was a direct correlation between occurrence of fractures and PTH levels (p<0.05), and an inverse correlation between fractures and deficiency of 25-hydroxyvitamin D (p<0.05).
In conclusion, although bone mineral density is comparable with that of age-matched normal subjects, patients with type 2 DM have an increased risk of fracture which appears to be associated with vitamin D deficiency and secondary increase of PTH.
Keywords: Bone Mineral Density; 25-hydroxyvitamin D; Type 2 Diabetes Mellitus; Hyperglycemia, Insulin Resistance.
Introduction
Type 2 diabetes mellitus (DM) is a pandemic metabolic disease with elevated morbidity and mortality. It is characterized by hyperglycemia secondary to peripheral insulin resistance with a variable degree of hyperinsulinemia and insulin secretion impairment. Hyperglycemia may have several adverse effects on bone metabolism especially in patients with poorly controlled diabetes. Glucose is the main energy source for osteoclasts and is able to dose-dependently enhance avian osteoclast activity in vitro (1). In addition, hyperglycemia leads to nonenzymatic glycosylation of various bone proteins, including type 1 collagen, which may impair bone quality (2).
The currently available data on bone metabolism and fracture risk in patients with DM are partly conflicting and inconclusive due to inhomogeneous study population and design. Patients with DM have various skeletal disorders, including osteopenia or osteoporosis, Charcot’s arthropathy and the diabetic foot syndrome (3). An increased prevalence of type 2 DM has been described in vitamin D-deficient individuals, and insulin synthesis and secretion have been shown to be impaired in beta cells from vitamin D-deficient animals (4-6). Glucose tolerance is restored when vitamin D levels return to normal (7). In type 1 DM an increased fracture risk has been shown and is associated to a decreased bone mineral density (8-10). The demineralization process involves especially the trabecular bone, and the decrease in bone mass is more significant in the first five years after the onset of the disease (11). On the contrary, in type 2 DM, the most of studies highlight normal or elevated (11-16) bone mineral density, and these results are surprising in consideration of the increased fracture risk which occurs also in type 2 DM patients (17-19). The reasons for this discrepancy are not fully understood.
In the general population, it has been demonstrated that bone mineral density and risk of fractures is inversely correlated to the body mass index (BMI) (20). Since the BMI is higher in type 2 than in type 1 DM, this may represent a possible explanation for the normal bone mineral density seen in type 2 DM patients. As a consequence, it is likely that type 2 DM subjects are characterized by an altered bone quality regardless from the bone mineral density.
The aim of this study was to evaluate which factors may explain the risk of fractures in a homogeneous population of postmenopausal women with type 2 DM.
Patients & Methods
Patients
A total of 27 consecutive women with type 2 DM referred to the Unity of Diabetology and Endocrinology, Hospital of Aosta, between March and October 2009, were enrolled in this study. The study protocol was approved by the local ethics committee institutional board and was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki as revised in 2000. This study included women who were diagnosed with type 2 DM according to American Diabetes Association (ADA) criteria (21). The exclusion criteria were patients affected with diseases associated with bone impairment such as Cushing’s syndrome, hypothyroidism, hyperthyroidism, prolactinomas, hyperparathyroidism, renal failure, malabsorption, chronic alcohol intake or heavy smoking, and medications that might affect bone and mineral metabolism. After the exclusion of three patients with hyperthyroidism and three patients with primary hyperparathyroidism, 21 patients, aged 43-70 years (mean 63 ± 10 SD years) were studied. The duration of diabetes varied 1-30 years. The anti-DM treatment was diet in 1 patient, hypoglycemic drugs in 15 patients, insulin in 4 patients, hypoglycemic drugs plus insulin in 1 patient. All patients were postmenopausal subjects and were not taking any hormone replacement therapy. All patients underwent a clinical, biochemical and bone mineral density examination.
The clinical, biochemical and bone mineral density characteristics of the 21 patients are shown in Table 1.
Table I.
- Clinical, biochemical and instrumental parameters.
| Age (yrs) | 63.4±13.8 | 43 – 70 |
| Systolic blood pressure (mmHg) | 132.1±13.8 | 100 – 160 |
| Diastolic blood pressure (mmHg) | 78±8.8 | 70 – 100 |
| Body mass index (kg/m2) | 29.3±4.7 | 21 - 36.7 |
| Disease duration (yrs) | 8±6.5 | 1 – 30 |
| Serum fasting glucose (mg/dl) | 162±28.8 | 122 – 227 |
| Glycated hemoglobin (%) | 7.7±1 | 6 - 10.9 |
| Total cholesterol (mg/dl) | 214±53.2 | 127 – 346 |
| HDL-cholesterol (mg/dl) | 57±17.7 | 31 – 87 |
| Triglycerides (mg/dl) | 171±137.9 | 71 – 600 |
| Uremia (mg/dl) | 4.9±1.1 | 2.9 - 6.8 |
| Serum albumin (g/L) | 42.7±3.7 | 38 - 48.7 |
| Serum creatinine (mg/dl) | 0.79±0.1 | 0.5 - 0.93 |
| Creatinine clearance (ml/min) | 97.2±20.9 | 68 – 142 |
| Microalbuminuria (µg/min) | 20.8±26 | 2.3 – 100 |
| 25-hydroxyvitamin D (ng/ml) | 18±6.4 | 7.8 - 32.4 |
| Total alkaline phosphatase (U/L) | 184±45.8 | 124 – 252 |
| Parathyroid hormone (pg/ml) | 61.6±29.6 | 18 – 154 |
| Serum calcium (mg/dl) | 9.6±0.4 | 8.7 - 10.2 |
| Serum phosphorus (mg/dl) | 3.4±0.5 | 2.3 - 4.1 |
| Urinary calcium (mg/24h) | 136.9±95.2 | 54 – 420 |
| Urinary phosphorus (mg/24h) | 635.4±315.1 | 320 - 1120 |
| (Lumbar spine) T-score | -1.1±1.1 | 0.6 - -2.8 |
| (Lumbar spine) Z-score | 0.1±1.1 | 2.5 - -2.9 |
| (Femoral neck)T-score | -0.8±1.0 | 0.8 - -2.6 |
| (Femoral neck) Z-score | 0.1±0.9 | 1.5 - -1.8 |
Methods
Clinical assessment
Height, weight, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP) were evaluated by standard methods. BMI was measured as the ratio between the weight and the square of the height. A BMI between 25 and 30 Kg/m2 was considered the index of over-weight, whereas BMI greater than 30 Kg/m2 was considered the index of obesity (22). Blood pressure was measured in the right arm, with the subjects in a relaxed sitting position. The average of 6 measurements (3 taken by each of 2 examiners) with a mercury sphygmomanometer was used. Hypertension was diagnosed when DBP values were ≥ 85 mmHg and SBP values were ≥ 130 mmHg in line with Adult Treatment Panel III (23).
Biochemical assessment
Glycemia, glycated hemoglobin (HbA1c), total cholesterol, HDL-cholesterol, triglycerides, uremia, creatinine, calcium, phosphorus, albumin, total alkaline phosphatase (ALP) were determined on serum samples at fasting by automated techniques (Roche Modular System). Intact parathyroid hormone (PTH) and fibrinogen were measured by electrochemiluminescence immunoassay concentraction and with the Clauss method, respectively. Serum levels of 25-hydroxyvitamin D (25-OHD) were measured with direct radioimmunoassy. Hypovitaminosis D insufficiency was defined as a serum concentration of 25-OHD between 20-30 ng/ml and hypovitaminosis D deficiency was defined as a serum concentration of 25-OHD below 20 ng/ml (24,25). The urinary albumin excretion rate was measured from a single 24-h urine collection. A urinary albumin excretion rate of 30 to 300 µg per min was defined as microalbuminuria; a rate grater than 300 µg per min was defined as macroalbuminuria. Urinary calcium, phosphorus and creatinine were also measured on 24-h urine samples.
Instrumental investigation
Bone mineral density was measured by dual-energy x-ray absorptiometry technique (DXA) at the spine and femoral neck. DXA measures areal BMD in g/cm2 by using ionizing radiation with photon beams of two different energy levels, T-score, the SD from the mean value obtained in 30 year old normal subjects, and Z-score, the SD from the mean value obtained in subjects of the same age and sex. A T-score -1 SD or grater was considered normal, between -1 and -2.5 SD was consistent with osteopenia, lower than -2.5 SD was consistent with osteoporosis and lower than -2.5 SD or less with a fragility fracture was consistent with severe osteoporosis (26, 27).
A lumbar spine radiography was performed in antero-posterior and latero-lateral in all patients to assess the existence of vertebral fractures.
Statistical Analysis
The statistical analysis was performed by SPSS for Windows version 10 (SPSS, Inc., Chicago, IL). Data were expressed as mean±SD. The linear regression analysis by calculating the Pearson’s coefficient was used to study the correlation between numerical data and the logistic regression analysis was used to study the correlation between numerical data and risk of fractures. The p values were given for these analysis. The significance was set at 5%.
Results
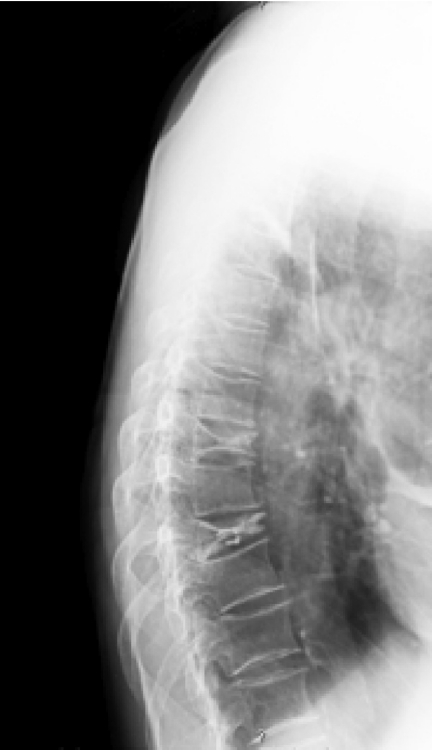
Twenty of 21 subjects showed low levels of 25-OHD (mean 18±6.4 ng/ml). In particular 14 subjects (67%) showed a deficit of 25-OHD, and 6 subjects (28%) showed insufficient 25-OHD levels, only 1 patient (5%) had 25-OHD in the normal range. PTH levels were at the higher limits of the normal range and were not associated with serum calcium levels increased (Table 1). The percentage of patients with normocalcaemic hyperparathyroidism was 28%. At the MOC DXA examination, T-score revealed a condition of osteopenia at the lumbar spine while the Z-score was in accordance with age of patients both at lumbar and femoral neck level (Table 1). At the radiological assessment, 24% of patients showed one o more vertebral fractures (1 fracture was found in 15% and 2 or more fractures in 9%), (Figure 1).
Figure 1.
- Vertebral radiography in a patient with type 2 diabetes mellitus: vertebral fractures are visible in D7 e D9.
There was a direct correlation between the presence of fractures and the PTH serum levels (p<0.05), while an inverse correlation was observed between the presence of fractures and vitamin D levels (p<0.05). A mild but not significant direct correlation there was between fractures and both serum glucose levels (p=0.06) and blood pressure values (p=0.08). At the linear correlation analysis, 25-OHD levels were not correlated neither with blood glucose levels nor with disease duration.
Discussion
Metabolic disorders including DM are now well known to affect the bone metabolism and may result in osteoporosis. However, the effects of DM on bone mineral density is not yet clear. Both the increase in bone turnover markers and the decrease in bone formation have been reported in diabetic populations (11). Hyperinsulinaemia is suggested to be associated with increased bone mineral density in subjects with diabetes (28) and without diabetes (29). Data from several studies have shown that hypovitaminosis D might play an important role in pathogenesis of type 2 DM. An increased prevalence of type 2 DM has been described in vitamin D-deficient individuals, and insulin synthesis and secretion is impaired in beta cells from vitamin D-deficient animals (4-6). Glucose tolerance is restored when vitamin D levels return to normal (7). For instance, prolonged treatment of osteomalacia with vitamin D increases insulin secretion and improves glucose tolerance (30,31).
The prevalence of hypovitaminosis D in diabetic population is still controversial (5,32) and recent findings suggest that the lower limit of normal value of serum 25-OHD concentrations should be revaluated (33-36). For these reason, in this study we defined 25-OHD insufficiency as values between 20-30 ng/ml and 25-OHD deficiency as values below 20 ng/ml (24,25). In this study, 95% of patients showed low 25-OHD levels; in particular 67% of patients had a 25-OHD deficiency and 28% of them had a 25-OHD insufficiency. This condition of hypovitaminosis D was likely responsible for a mild normocalcaemic hyperparathyroidism found in the DM patients under evaluation in this study. Vitamin D stimulates insulin secretion by pancreatic beta cells but inhibits PTH synthesis (37).
Type 2 DM has been also associated with an increased risk of fractures at any skeletal site (9,10,17,19,38). Nicodemus et al. reported a higher risk of hip fractures in postmenopausal women with type 2 DM than in women without diabetes. In addition, a longer duration of diabetes and the use of insulin or oral diabetes medications in women with type 2 DM were associated with a higher rate of hip fractures (9). The same results were observed by Schwartz et al. (17), Ottenbacher et al. (19), Vestergaard et al. (38) and Bonds et al. (39). These authors have found vertebral and non vertebral fractures in patients with type 2 DM. The Rotterdam study, conducted on 792 elderly patients with type 2 DM (483 women and 309 men, mean age 74 years) confirmed an increased fracture risk despite a higher bone mineral density at femur neck and lumbar spine (40). In our study the prevalence of fractures, assessed at the lumbar spine, was 24% of patients and was associated with hyperparathyroidism.
In type 1 DM an increased fracture risk have been shown to occur in parallel with a reduced bone mass (9,10). On the contrary, in type 2 DM, several but not all cross-sectional studies highlighted normal (11,12) or elevated (13-16) bone mass, and these results are surprising given the increased fracture risk associated with type 2 DM (17-19). The reasons for this discrepancy are not fully understood. A possible explanation is that type 2 diabetic patients have alterations in bone quality regardless from the bone mineral density.
In line with these data, in this study the bone mineral density was comparable between type 2 diabetic patients and normal subjects of comparable age as assessed by evaluating the Z-score. This finding was still more surprising if we consider that the study population included postmenopausal females. Therefore, the high rate of vertebral fractures (24%) we found could be attributed to a reduced bone quality. The mechanism resulting in bone alteration and high fracture risk in these patients may involve a deregulation in PTH secretion in a context of generalized vitamin D deficiency. Hypovitaminosis D could be a condition predisposing to fractures in all patients affected with type 2 DM, without correlation with glycemic control and duration of diabetes. The secondary impairment in PTH secretion could be the triggering factor resulting in a fracture event.
In women with DM, the risk of fractures has been reported to increase with the fasting blood glucose levels (41). In line with this observation, we found a tendency toward a correlation between hyperglycemia as well as hypertension and occurrence of fractures, which needs to be confirmed in a larger series of patients.
In conclusion, the risk of vertebral fractures is increased in postmenopausal females with type 2 DM in spite of normal values of bone mineral density. Hypovitaminosis D and consequent hyperparathyroidism are clear metabolic alterations which correlate with the high risk of fractures in these patients.
References
- 1.Williams JP, Blair HC, Mc Donald JM, et al. Regulation of osteoclstic bone resorption by glucose. Biochem Biophys Res Commun. 1997;235:646–651. doi: 10.1006/bbrc.1997.6795. [DOI] [PubMed] [Google Scholar]
- 2.Vashishth D, Gibson GJ, Khoury JI, et al. Influence of nonenzymatic glycation on bio-mechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AV. Diabetes mellitus: Does it affect bone? Calcif Tissue Int. 2003;73:515–519. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 4.Boucher BJ, Mannan N, Noonan K, et al. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38:1239–1245. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 5.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 6.Chiu KC, Chu A, Go VL, et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Davies M, Zakaria Y, et al. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Prostgrad Med J. 1994;70:440–443. doi: 10.1136/pgmj.70.824.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbloom AL, Lezotte DC, Weber FT. Skeletal and joint manifestations of childhood diabetes. Pediatr Clin North Am. 1984;31:569–589. doi: 10.1016/s0031-3955(16)34607-7. [DOI] [PubMed] [Google Scholar]
- 9.Nicodemus KK, Folsom AR. Type 1 and 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24:1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 10.Forsen L, Meyer HE, Midthjell K, et al. Diabetes mellitus and incidence of hip fracture: Results from the Nord-Trondelag Health Survey. Diabetologia. 1999;42:920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 11.Isaia GC, Ardissone P, Di Stefano M, et al. Bone metabolism in type 2 diabetes mellitus. Acta Diabetol. 1999;36:35–38. doi: 10.1007/s005920050142. [DOI] [PubMed] [Google Scholar]
- 12.Tuominen JT, Impivaara O, Puukka P, et al. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 13.Van Daele PL, Stolk RP, Burger H, et al. Bone density in non insulin-dependent diabetes mellitus. The Rotterdam Study. Ann Intern Med. 1995;122:409–414. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Hanley DA, Brown JP, Tenenhouse A, et al. Canadian Multicentre Osteoporosis Study Research Group. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-selectional results from the Canadian Multicentre Osteoporosis Study. J Bone Min Res. 2003;18:784–790. doi: 10.1359/jbmr.2003.18.4.784. [DOI] [PubMed] [Google Scholar]
- 15.Lunt M, Masaryk P, Scheidt-Nave C, et al. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: the EVOS Study. Osteoporosis Int. 2001;12:688–698. doi: 10.1007/s001980170069. [DOI] [PubMed] [Google Scholar]
- 16.Barret-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non insulin-dependent diabetes mellitus. JAMA. 1992;268:3333–3337. [PubMed] [Google Scholar]
- 17.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Study of Osteoporotic Features Research Group. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 18.Meyer HE, Tverdal A, Falch JA. Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol. 1993;137:1203–1211. doi: 10.1093/oxfordjournals.aje.a116622. [DOI] [PubMed] [Google Scholar]
- 19.Ottenbacher KJ, Ostir GV, Peek MK, et al. Diabetes mellitus as a risk factor for hip fracture in Mexican-American older adults. J Gerontol A Biol Sci Med Sci. 2002;57:M648–653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- 20.De Laet C, Kanis JA, Odèn A, et al. Body mass index as a predictor of fractures risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 21.Expert Committee on the Diagnosis and Classification of diabetes mellitus. Report of the expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 22.Obesity: A report of the Royal College of Physicians. J R Coll Physicians Lond. 1983;17:5–65. [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Food and Nutrition Board (Standing Committee on Scientific Evaluation of Dietary Reference Intake) Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, DC. 1999 [Google Scholar]
- 25.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva, Switzerland. WHO. 1994 [PubMed] [Google Scholar]
- 27.Khan AA, Brown JP, Kendler DL, et al. The 2002 Canadian bone densitometry recommendations: take-home messages. CMAJ. 2003;21(168 (2)):149. [PMC free article] [PubMed] [Google Scholar]
- 28.Fukunaga Y, Minamikawa J, Inoue D, et al. Does insulin use increase bone mineral density in patients with non-insulin-dependent diabetes mellitus? Arch Intern Med. 1997;157:2668–2669. doi: 10.1001/archinte.157.22.2668a. [DOI] [PubMed] [Google Scholar]
- 29.Barret-Connor E, Kritz-Silverstein D. Does Hyperinsulinemia preserve bone? Diabetes Care. 1996;19:1388–1392. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- 30.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 31.Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome “X”. Br J Nutr. 1998;79:315–327. doi: 10.1079/bjn19980055. [DOI] [PubMed] [Google Scholar]
- 32.Ishida H, Senio Y, Matsukura S, et al. Diabetic osteopenia and circulating levels of vitamin D metabolism in type 2 (noninsulin-dependent) diabetes. Metabolism. 1985;30:797–801. doi: 10.1016/0026-0495(85)90101-5. [DOI] [PubMed] [Google Scholar]
- 33.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 34.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 35.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 36.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin. D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 37.Bikle DD. Clinical counterpoint: vitamin D: new actions, new analogs, new therapeutic potential. Endocr Rev. 1992;13:765–784. doi: 10.1210/edrv-13-4-765. [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–9. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 39.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J clin Endocrinol Metab. 2006;91:3404–10. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 40.de Liefde II, van der Klift M, de Laet CE, et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 41.Ivers RQ, Cumming RG, Mitchell P, et al. Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care. 2001;24:1198–203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]