Abstract
In the cocaine self-administering rat, individual nucleus accumbens (NAcc) neurons exhibit phasic changes in firing rate within minutes and/or seconds of lever presses (i.e. slow phasic and rapid phasic changes, respectively). To determine whether neurons that demonstrate these changes during self-administration sessions are differentially distributed in the NAcc, rats were implanted with jugular catheters and microwire arrays in different NAcc subregions (Core, Dorsal Shell, Ventromedial Shell, Ventrolateral Shell or Rostral Pole). Neural recording sessions were typically conducted on Day 13 - 17 of cocaine self-administration (0.77 mg/kg/0.2mL infusion; fixed-ratio 1 schedule of reinforcement; 6 hr daily sessions). Pre-press rapid phasic firing rate changes were greater in lateral accumbal (core, ventrolateral shell) relative to medial accumbal (dorsal shell, rostral pole shell) subregions. Slow phasic pattern analysis revealed that reversal latencies of neurons that exhibited change + reversal patterns differed mediolaterally: medial NAcc neurons exhibited more early reversals and fewer progressive/late reversals than lateral NAcc neurons. Comparisons of firing patterns within individual neurons across time bases indicated that lateral NAcc pre-press rapid phasic increases were correlated with tonic increases. Tonic decreases were correlated with slow phasic patterns in individual medial NAcc neurons, indicative of greater pharmacological sensitivity of neurons in this region. On the other hand, the bias of the lateral NAcc towards increased pre-press rapid phasic activity coupled with a greater prevalence of tonic increase firing may reflect particular sensitivity of these neurons to excitatory afferent signaling and perhaps differential pharmacological influences on firing rates between regions.
Keywords: addiction, drug abuse, neurophysiology, reward, ventral striatum
The nucleus accumbens' (NAcc) putative role in reward and drug-taking behavior has prompted numerous studies in recent years that measured various physiological changes in the NAcc during drug self-administration. Several laboratories have investigated whether NAcc neural firing patterns during cocaine self-administration are temporally linked to events such as drug infusion or appetitive behavior (Carelli et al, 1993; Chang et al, 1994; Peoples & West, 1996, Woodward et al, 1999; Carelli, 2002). Two main categories of lever press-related firing patterns have been identified: slow phasic and rapid phasic.
Slow phasic patterns reflect changes in firing rate over minutes, and their time course approximates the inter-infusion interval. The pattern of locomotor behavior cannot account for slow phasic firing patterns, but the temporal pattern of approach to the lever per se nonetheless correlates with reversing slow phasic firing rate changes: approaches to the lever decrease after the infusion and then increase in likelihood as the inter-infusion interval proceeds (Peoples et al, 1998). This is consistent with the idea that conditioned incentive signals (via limbic afferents) likely decrease immediately after an infusion, then gradually increase as the time for the next infusion approaches (Wise, 1995). The reversing slow phasic firing pattern may alternatively or additionally reflect the influence of pharmacological factors on firing rate (Nicola & Deadwyler, 2000). Specifically, the post-infusion pharmacokinetic decline in synaptic drug levels could result in gating of afferent signals such that their ability to influence accumbens firing immediately after an infusion is minimal, but increases as the interval proceeds.
Rapid phasic patterns (Carelli & Deadwyler, 1996; Peoples et al, 1997) occur within seconds of the cocaine-reinforced instrumental response. On the basis of timing alone, a relation to pharmacological factors is unlikely and we have demonstrated that firing can be dissociated from any aspect of the cocaine infusion per se, including viscerosensory feedback (Peoples et al, 1997). Instead, rapid phasic patterns appear to be correlated with the animal's instrumental response (i.e. drug seeking) and/or the tone and light cues synchronized with cocaine infusion (Carelli & Deadwyler, 1996; Peoples et al, 1997; Carelli, 2000).
Evidence for a functional dichotomy between medial and lateral accumbal subregions continues to mount (Corbit et al., 2001; Rodd-Hendricks et al. 2002; Ghitza et al., 2003, 2004; Ikemoto et al., 2005). Aside from earlier reports, with regional assessments that were by design not intended to be comprehensive (Uzwiak et al, 1997; Carelli & Wondolowski, 2006; Ghitza et al., 2006), no prior investigations which employed single-unit recording have systematically tested whether phasic firing patterns are differentially distributed among accumbens subregions. In the present study, microwires were implanted throughout the NAcc to survey whether electrophysiological differences exist across subregions that may reflect functional differences with respect to the accumbens' role in drug-taking behavior. In addition, an analysis of the distribution of phasic firing patterns among neurons exhibiting tonic (see Fabbricatore et al., 2009) changes in firing was conducted in order to help parse the relative influences of pharmacologic factors versus cortical-limbic signaling on firing rate changes across time bases.
Materials and Methods
Subjects
Male Long-Evans rats (n = 32; Charles River Laboratories, USA) were individually housed with a 12:12 hr light/dark cycle (lights on at 10:00 A.M.), handled daily and food restricted to maintain target body weights between 330 - 350 g (≥90% adult body weight). Rats were approximately 120 -150 days old over the course of training and testing. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication) and approved by the Rutgers University Animal Care & Facilities Committee.
Surgical procedures and drug self-administration training were described in detail previously (Fabbricatore et al., 2009). Briefly, animals were outfitted with a jugular catheter which exited a scalp incision and was affixed to a chronically implanted microwire array headstage anchored to the skull with acrylic cement and stainless steel screws. Catheter patency was maintained during recovery and between training and testing sessions by quarter hourly infusions (0.2mL) of dilute heparinized saline solution delivered by a timer-equipped, motor driven syringe pump (Razel Scientific Instruments, Stamford, CT).
During training, each reinforced lever press (RLP) resulted in a 0.2 mL intravenous infusion of cocaine hydrochloride (National Institutes on Drug Abuse, Research Triangle Park, NC) solution, a 7.5 second tone which corresponded with the duration of syringe pump operation and a 20 second time-out period during which the cue light was off and lever presses had no programmed consequence. Training sessions (FR1 schedule) were conducted seven days per week, each limited to 80 infusions or 6 hours, whichever was first attained. The average cocaine dose administered, given differences in subjects' body weights, ranged between 0.70 - 0.91 mg/kg/infusion with a mean drug dose of 0.77 ± 0.01(S.E.M.) mg/kg/infusion. This resulted in an inter-infusion interval with a median of 7.36 ± 0.01(S.E.M.) minutes. After acquisition, subjects were trained for 12 - 18 self-administration sessions before neural recordings commenced.
Electrophysiological Recording Sessions
Neural recordings began 30 minutes before the start of the self-administration session and continued for 1 hour after the session. The neural signal from individual microwires was led through a field effect transistor in the headset of an electronic harness (NB Labs, Denison, TX; Dr. Volodymyr Prokopenko), then through the Airflyte electronic swivel. From the swivel the signal continued to a preamplifier (Riverpoint Electronics, Goldsboro, NC) where it was differentially amplified against another microwire that exhibited no neural waveforms. The signal was then conducted through a bandpass (roll-off below 1000 Hz = 1.5 dB/octave and above 11000 Hz = 6 dB/octave; gain = 700) filter/amplifier (Riverpoint Electronics). The amplified signal was then sent to a remote computer where, using Datawave Technologies (Longmont, CO) software and hardware, each waveform larger than threshold value (approximately 120% of the 25 μV noiseband) was digitized (50 kHz sampling frequency per recorded wire), time stamped (0.1 msec resolution) and stored for off line analysis.
Data Analysis
Post-hoc analyses of the neural data were conducted using cluster analysis software (Datawave Technologies) to isolate neural waveforms as described previously (Peoples & West, 1996). Perievent time histograms (PETHs) were generated on two time bases: one which displayed the approximate inter-infusion interval (slow phasic time frame, in minutes) and another which displayed the approximate time frame of the instrumental response and drug infusion (rapid phasic time frame, in seconds). Thus, in the case of phasic histograms, periods of minutes (±6) or seconds (±12) were considered when evaluating increases or decreases in firing rate in relation to the lever press. For both slow phasic and rapid phasic PETHs, the node was the electronic offset of the reinforced lever press. For each experiment, the first 10 lever presses (load-up) were excluded before generating PETHs to assure stable behavior and drug levels and ensure consecutive lever presses did not overlap in the histogram.
Histological procedures were described in detail previously (Fabbricatore et al., 2009). Briefly, after fixing, slicing, mounting and Nissl staining, the precise location of each wire tip was estimated to be at the center of individual lesion marks in the brain. An independent observer, who was blind to the data recorded from each wire, evaluated the histological placement of microwires. A stringent criterion required that a lesion center within 150 μm of any border be characterized as a “border” neuron. Those that bordered extra-accumbal structures were eliminated from the study. Border neurons that lay between core and shell were treated as separate categories: those found at or ventral to -7.6 mm D-V were considered ventral border neurons while those dorsal to -7.6 mm D-V were considered dorsal border neurons.
The shell was subdivided into dorsal, ventromedial and ventrolateral regions. Wires placed in the ventromedial shell subregion were confined to the area: 1) at or posterior to 2.3 mm and anterior to 0.7 mm A-P, 2) ventral to -7.3 mm D-V, and 3) medial to 1.4 mm M-L. Wire tips within shell regions dorsal to this area were categorized as dorsal shell wires, while those located lateral to this region were categorized as ventrolateral shell wires. Rostral Pole neurons were defined by tip locations anterior to 2.3 mm A-P.
Statistical Analysis of Firing Patterns
Slow Phasic Patterns
Certain neurons exhibited changes in firing rate over minutes relative to the lever press i.e. slow phasic neurons. Analytical procedures which evaluated shifts in firing rate that occurred over the course of the inter-infusion interval were described previously (Peoples et al., 1998). Slow phasic analyses were sensitive to firing rate change latencies relative to lever pressing and were categorized accordingly (see Results for category definitions).
Rapid Phasic Patterns
Neurons whose firing rates changed within seconds of lever pressing i.e. rapid phasic neurons, were assessed on the basis of the magnitude of perievent firing rate change as compared to the baseline period which preceded it. During the 3 sec pre-press period, the first of 3 consecutive 200 msec bins that exhibited firing rates which exceeded +/ - 20% baseline firing defined the onset of the pre-press firing window for a given neuron. Offset was defined by the first of 3 consecutive bins in which firing returned to rates observed during the 9 second baseline period. If no firing rate offset occurred by the time of the lever press, the end of the firing window was defined as the time of the lever press. Using the same 9 second baseline period, a similar analysis was conducted to determine the post-press firing window. During the initial 3 sec post-press period, the onset of the post-press firing window was defined by the first of 3 consecutive 200 msec bins that exhibited firing rates which exceeded +/ - 20% of firing during the baseline period. Offset times were defined as the first of 3 consecutive bins in which a return to baseline firing occurred, up to the limit of the post-press period (12 sec). The requirement for 3 consecutive bins exhibiting 20% change provided a reliable and sensitive assessment of perievent firing rate changes while excluding sporadic fluctuations in activity. The raster of every neuron was visually inspected to ensure that the pattern of discharges which comprised each neuron's perievent time histogram was representative of the discharge pattern of individual trials depicted in the raster. These firing rates were then used to calculate a ratio B/(A+B), where A is equal to the mean baseline firing rate, while B is equal to the mean firing rate during the perievent (pre- or post-) firing window.
Average onset times and average offset times of pre- and post-press firing windows were calculated for neurons that met the >20% change rule. These averages were then applied to any neuron for which a pre-press and/or post-press window was not defined using the above criteria (i.e. did not exhibit an identifiable change). This allowed all neurons in the study to undergo pre- and post-press firing rate analyses.
After assignment of firing windows, all neurons were subjected to firing rate analyses (B/A+B). In a few cases (see Results) neurons exhibited particularly low firing rates during baseline and within a firing window. These neurons were assigned a B/A+B value of 0.5 to more accurately reflect the lack of a difference in leverpress firing relative to baseline firing.
The evaluation of firing rate changes with the B/A+B ratio employed in the present study has several advantages. First, it normalizes firing rate changes across neurons with different firing rates. Second, it provides a scale which equally weighs decreases and increases in firing. Third, the B/A+B ratio provides a scale on which the magnitude of firing rate change can be compared for every neuron in the sample. Fourth, this approach provides for a more comprehensive assessment of the various magnitudes of firing rate change of the entire sample of neurons in a particular region or category than an approach which selects only robust responses for analysis.
Rapid Phasic Duration Analysis
Analyses of response onset and offset times were not possible for neurons that exhibited little or no firing rate changes proximal to the lever press. Accordingly, durations of firing rate changes were calculated for neurons which exhibited substantial (i.e. 2-fold) increases or decreases in firing within seconds of the lever press. Pre-press and post-press firing durations were defined as the difference between offset and onset times.
Subregional Comparisons of Firing Patterns
Slow Phasic Firing Patterns
Chi-square tests (2×2; α = 0.05; Runyon et al., 1996) were employed to determine whether differences exist in the prevalence of responsive (as described in Peoples & West, 1996) neurons between subterritories. The proportion of responsive neurons from each subregion of the shell (i.e. dorsal, ventromedial and ventrolateral) was compared to the proportion of responsive neurons from the core. The prevalence of responsive neurons in the core was also compared to the proportion of responsive neurons in the dorsal and ventral border regions within the accumbens. For the purpose of this analysis, differences (e.g. reversal latency, post-press directionality) in the firing topographies of phasic patterns were collapsed, allowing for a two category analysis: responsive (any phasic pattern) vs. non-responsive.
A second, more qualitative, analysis evaluated the prevalence of slow phasic categories among subregions. As reported earlier, the majority of slow phasic neurons exhibit reversal patterns (Peoples et al, 1998), comprising either increases or decreases post-press that show reversals at various latencies as the inter-infusion interval proceeds. The possibility that these different categories of slow phasic patterns reflect distinct functional correlates warranted an analysis of their subregional distribution. Specifically, the major slow phasic categories, i.e. change plus reversal neurons, were compared between medial and lateral accumbens subregions.
Rapid Phasic Firing Patterns
Planned subregional comparisons of rapid phasic patterns were undertaken using two-tailed Mann-Whitney U tests (alpha 0.05; Castellan & Siegel, 1988), which assessed the magnitude of firing rate changes between core and each of the other subregions. To distinguish potentially behaviorally relevant neural activity (e.g. approach behavior versus processing the completion of the lever press), separate comparisons were made between subregions using pre- and postpress periods, as defined above.
Correlations of Firing Patterns between Time Bases
Differences among subregions in the prevalence of tonic firing patterns (i.e. average firing rate throughout the whole experiment) among accumbal subregions have been reported (Fabbricatore et al., 2009). In the present investigation, a third analysis utilized Spearman's Rho correlations (α = 0.05) of B/(A+B) values to compare relative magnitudes of individual neurons' firing rate changes between time bases within each subregion. This non-parametric test was used since the data did not exhibit a normal distribution. Analyses included separate calculations for each neuron's: 1) rapid phasic versus tonic firing rates, 2) slow phasic versus tonic firing rates and 3) rapid phasic versus slow phasic firing rates.
Results
Following acquisition of the task, behavior during experiments was typically characterized by an initial, brief period of rapid lever pressing, followed for the remainder of the self-administration phase by self-infusions at regular intervals, which ranged from 6 - 8 min. Between cocaine-reinforced lever presses animals typically engaged in focused stereotypy, consisting of nose poking, forelimb treading and repetitive manual-orofacial behaviors (Pickens & Thompson, 1968). Focused stereotypy typically spanned the majority of the inter-infusion interval with, in some cases, circling behavior oriented towards the lever during the last half minute before the subsequent lever press. Drug accumulation curves constructed according to the method of Yokel & Pickens (1974) showed stable drug levels during self-administration (Fabbricatore et al., 2009).
The recorded NAcc neural waveforms exhibited amplitudes which ranged between 100 - 300 μV. The average signal-to-noise ratio was 3.08 ± 0.08 (S.E.M.) with the majority (130/137; 95%) of waveform amplitudes greater than 200% of the respective noiseband (range: 1.82 - 6.90). The mean accumbal firing rate during the pre-drug phase (i.e. baseline recording) was 0.53 ± 0.12 (S.E.M.) Hz. Mean baseline firing rates did not differ among subregions [F(6,135) = 0.236, p = 0.96].
Anatomical Distribution of Accumbal Neurons
From a total of 297 basal forebrain neurons recorded in 32 subjects, 137 were from wires (n = 124) histologically confirmed to be located in the nucleus accumbens. Tonic firing patterns recorded from these same 124 wires were recently reported (Fabbricatore et al., 2009).
Accumbal Subterritorial Distribution
Seventy six percent (104/137) of the accumbal neurons were, according to 150 μm border criterion analysis (described in Methods), unequivocally placed in one of the three subterritories of the NAcc. Among these, the majority were placed in the shell (n = 74), while the remainder were distributed between core (n = 25) and rostral pole (n = 5). All rostral pole neurons proved to be in the shell subregion (Jongen-Relo et al., 1994; Paxinos & Watson, 1997).
Accumbal Shell Subregional Distribution
The majority of neurons recorded from the shell were localized to the ventrolateral shell (n = 49). The dorsal and ventromedial shell subregions yielded 12 and 13 of the recorded accumbal neurons, respectively.
Accumbal Border Neurons
Thirty three accumbal neurons could not be assigned a subterritorial designation due to their proximity to the boundary between shell and core. They were instead considered as a dorsal border group (n = 19) and a ventral border group (n = 14). As such, a total of 7 NAcc compartments were evaluated in the present study: core, dorsal shell, ventromedial shell, ventrolateral shell, rostral pole shell, dorsal border and ventral border.
Slow Phasic Firing Patterns
Neurons that exhibited changes in the minutes before and after lever presses, i.e. slow phasic changes, represented 55% (75/137) of the total recorded from the NAcc. Patterns which showed firing rate changes that approached pre-press levels within the first four minutes post-press were considered early reversal patterns, while those that reversed after four minutes post-press were considered progressive reversal neurons (Peoples & West, 1996). In rare cases (n = 3), neurons exhibited increases or decreases in firing rate that were more temporally proximal to the lever press, often beginning within the minute before the press and ending soon after it. Analyses related to phasic firing rate change categorization were conducted prior to and independently from the above histological procedures. All categories of slow phasic firing patterns had been observed in a separate data pool (Peoples et al., 1998).
Slow Phasic Category Analysis
The decrease + progressive reversal pattern was exhibited by a majority of slow phasic neurons (60%; 45/75) (Fig. 2a). Of the remaining slow phasic categories (Fig. 2b), none represented more than 15% (11/75) of the total (Table 1). Therefore, in subregional comparison analyses, slow phasic categories were collapsed into a single group and evaluated with respect to neurons within that same region that did not exhibit slow phasic firing rate changes.
Figure 2.
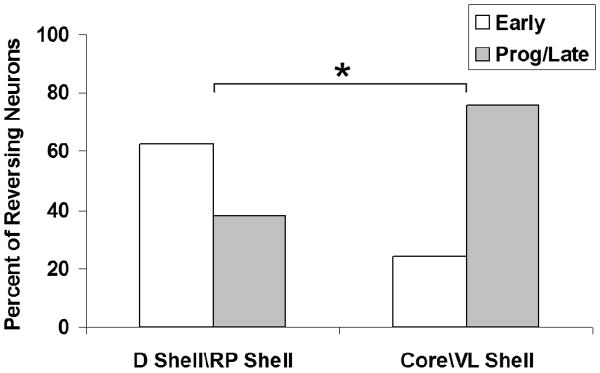
Mediolateral comparison of slow phasic reversal categories. Neurons that were histologically confirmed to be in either the lateral nucleus accumbens (NAcc) (i.e. core\ventrolateral shell; n = 74) or the medial NAcc (i.e. dorsal shell\rostral pole shell; n = 17) were evaluated in terms of whether they exhibited: (i) progressive or late reversal patterns (n = 32); or (ii) early reversal patterns (n = 14). To determine whether reversal categories are differentially expressed in the NAcc, a chi-square analysis was conducted. The percentage of early-reversing neurons was greater in the medial NAcc, whereas progressive/late-reversing neurons were more prevalent in the lateral NAcc [χ2(exact) = 4.70, degrees of freedom = 1, *P < 0.05). A post hoc odds ratio analysis confirmed that the differential probability of observing the two different reversal categories between regions was significant (oddsPLR/oddsER = 5.37, *P < 0.05). PLR, progressive/late-reversing; ER, early-reversing; D, dorsal; RP, rostral pole; VL, ventrolateral
Table 1. Slow phasic categories distribution.
| Categories of responsive neurons | No. (% neurons) | |
|---|---|---|
| Postpress Change | Reversal | |
| Decrease | Progressive | 45 (60) |
| Decrease | Early | 11 (15) |
| Decrease | Late | 1 (1) |
| Increase | Progressive | 8 (11) |
| Increase | Early | 6 (8) |
| Increase | Late | 1 (1) |
| No Change low pre- and postpress | - | 2 (3) |
| No Change high pre- and postpress | - | 1 (1) |
| 75 (100) | ||
Number of responsive neurons: 75 (of 137 total neurons)
In an analysis of the subregional prevalence of slow phasic patterns, the core was compared to shell subregions and none approached statistical significance (p > 0.05). The dorsal and ventral border regions were each compared to the core and no significant differences were shown between subregions (p > 0.05). Thus, the prevalence of slow phasic neurons did not differ among accumbal subregions.
Slow Phasic Reversals Analysis
Among slow phasic neurons, the majority (72/75; 96%) exhibited post-press change + reversal patterns. These included decrease + progressive reversal, decrease + early reversal, increase + progressive reversal, and increase + early reversal patterns (Table 1).
Given the recent evidence for accumbal mediolateral functional dichotomy (reported and reviewed in Fabbricatore et al., 2009), neurons that were histologically confirmed to be in either lateral NAcc (n = 74; core/ventrolateral shell) or medial NAcc (n = 17; dorsal shell/rostral pole shell) were evaluated in terms of whether they: 1) exhibited progressive/late reversal patterns, 2) exhibited early reversal patterns or 3) failed to exhibit reversal slow phasic patterns (Table 2). Lateral NAcc neurons exhibited more than twice the percentage of progressive or late reversal patterns compared to medial NAcc (Fig. 2). In contrast, the opposite trend was observed for early reversal patterns, with more than twice the percentage found in medial compared to lateral NAcc. An analysis of these reversal patterns confirmed that they are differentially expressed mediolaterally (χ2[exact] = 4.70, df=1, p < 0.05). Thus, over the inter-infusion interval, a longer latency in the reversal of post-press firing rates was observed in lateral accumbal as compared to medial accumbal neurons. Rare instances in which a subject yielded simultaneous medial and lateral recordings that exhibited early and late reversals, respectively, indicated that the different reversal latencies did not depend on differences in pharmacokinetic profiles between subjects. Of further note, all 7 ventral border neurons that exhibited slow phasic patterns were progressive reversals, consistent with the above-reported trend observed elsewhere (i.e. ventrolateral shell, core) in lateral accumbens. The dorsal border group, whose distinct anatomical and physiological characteristics render it difficult to assign in terms of mediolateral categorization (Fabbricatore et al., 2009), exhibited one early reversal firing pattern and 10 progressive reversal patterns among its 11 neurons with slow phasic reversal activity.
Table 2.
| D Shell\RP Shell | Core\VL Shell | |
|---|---|---|
| Early Reversals | 5 (29) | 9 (12) |
| Prog/Late Reversals | 3 (18) | 29 (39) |
| NON reversals | 9 (53) | 36 (49) |
| Total n: | 17 | 74 |
Rapid Phasic Firing Patterns
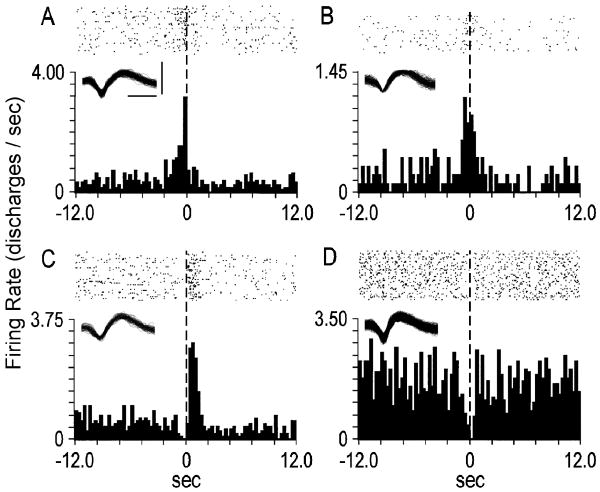
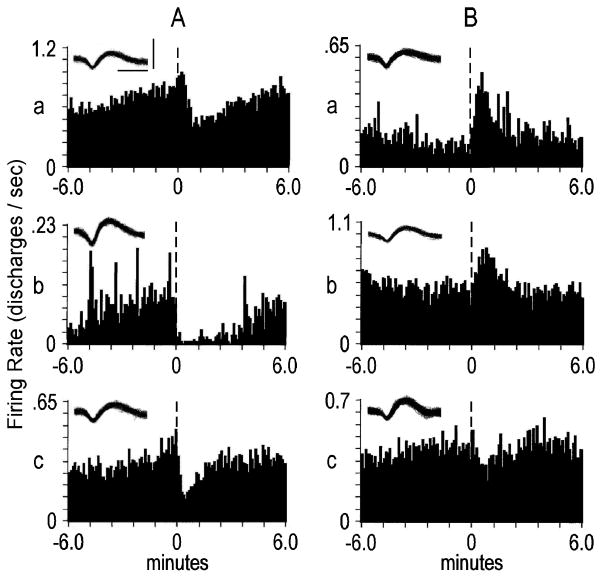
Neurons that exhibited firing rate changes in the seconds proximal to the lever press either: 1) spanned the lever press or 2) were exclusive pre- or post-press changes (Fig. 3). Mean durations of pre-press firing rate decreases (n=23) and increases (n=17) were 1.50 and 1.31 sec, respectively. Mean durations of post-press firing rate decreases (n=18) and increases (n=20) were 5.30 and 2.51 sec, respectively. Of the 19 neurons whose firing rate changes spanned the lever press, 8 were decreases, 7 were increases and 4 were mixed (i.e. exhibited post-press firing rate change opposite that of pre-press change).
Figure 3.
Examples of rapid phasic firing patterns. Each peri-event time histogram displays the firing pattern of a different neuron during the seconds before and after the lever press. The ordinate of each histogram displays average firing rate (i.e. average discharges/s calculated as a function of 0.2-s bins). Time 0 (vertical dashed line) on the abscissa marks the occurrence of the cocaine-reinforced lever press, and corresponds with the raster display above it. (A) A pre-press firing rate increase. (B) A lever press increase. (C) A post-press increase. (D) A lever press decrease. Insets depict corresponding neural waveforms. Calibrations (bars in A) of waveforms: insets A, B, and C, 0.25 ms, 0.20 mV; inset D, 0.25 ms, 0.15 mV
Rapid Phasic Category Analysis
Comparison of firing rate changes of core neurons to those of each other subregion revealed that the magnitude of change in pre-press firing was greater in core than both dorsal shell (p=0.007) and rostral pole shell (p=0.006). No other differences were observed (p>0.05) (Data not shown).
Since the magnitude of firing rate changes in the core did not differ from ventrolateral shell, while medial shell subregions exhibited marked differences from core, we replicated the subregional comparison employed in Fig. 2 to assess whether rapid phasic patterns show evidence of mediolateral compartmentalization, as well. Analysis of variance conducted for rapid phasic firing rate changes revealed that lateral NAcc (core/ventrolateral shell) neurons exhibited greater increases in pre-press firing rate than medial NAcc (dorsal shell/rostral pole shell) neurons (t[26]=1.71, p=0.023). (Fig. 4) Furthermore, this difference was maintained when ventral border neurons were included in the analyses (t[24]=1.71, p=0.018). No other differences were observed (p>0.05).
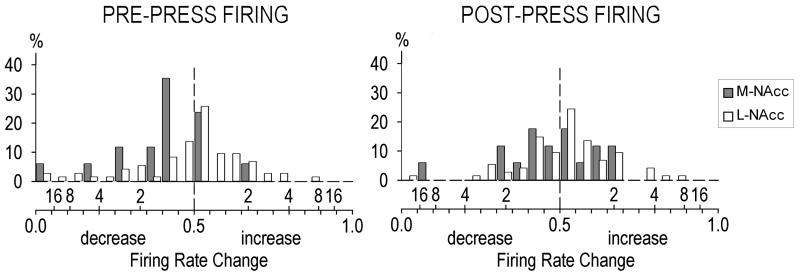
Figure 4.
Frequency distributions of pre-press (left panel) and post-press (right panel) rapid phasic firing rate change magnitudes in the medial nucleus accumbens (M-NAcc) vs. the lateral NAcc (L-NAcc). The magnitude of firing rate change is expressed as B/(A + B) (bottom side of x-axis). Values from 0.49 to 0 and from 0.51 to 1.00 reflect increasingly larger firing rate decreases and increases, respectively. Values of 0.50 reflect no change from baseline firing rate (vertical dashed line). The top side of the x-axis indicates twofold, fourfold, etc. increases above, or decreases below, baseline firing rate values. The ordinate scale indicates percentages of the total neurons in the medial (N = 17) and lateral (N = 74) NAcc. The distribution of pre-press B/A + B increases was greater in lateral NAcc (core/ventrolateral shell) neurons than in medial NAcc (dorsal shell/rostral pole shell) neurons [t26 = 1.71, P = 0.02).
Prevalence of Neurons Exhibiting Firing Rate Changes on More Than One Time Base
Phasic correlates within Tonic Groups
Slow phasic firing patterns were exhibited by 48% (30/63) of neurons exhibiting a tonic decrease and 81% (17/21) of neurons exhibiting a tonic increase (see Fabbricatore et al., 2009 for further details on tonic patterns). A more refined analysis involving particular categories of slow phasic patterns within tonic groups is described below. Consistent with our earlier report (Ghitza et al., 2006), rapid phasic patterns were not exclusive to any particular tonic category.
Tonic correlates within Phasic Groups
Neurons exhibiting a tonic decrease or a tonic increase represented 41% (30/74) and 23% (17/74) of neurons exhibiting slow phasic changes, respectively.
Comparisons of Firing Rate Changes between Time Bases by Subregion
Correlational analyses compared the relative magnitude of firing rate changes between time bases exhibited by individual neurons of each subregion. For each neuron, firing rate change values (B/A+B) for two time bases were entered into the analysis.
Rapid phasic versus tonic
Pre-press firing rate changes were positively correlated with tonic changes in the core. No other subregions exhibited correlations in firing rate between these two time bases (Table 3a).
Table 3. Individual neurons exhibiting firing rate changes on more than one time base.
| Regions | A RAPID PHASIC VS. TONIC |
B SLOW PHASIC VS.TONIC |
C RAPID PHASIC VS. SLOW PHASIC |
|||||
|---|---|---|---|---|---|---|---|---|
| Cell N | Corr. Coeffs: | Cell N | Corr. Coeffs: | Cell N | Corr. Coeffs: | |||
| Pre-press | Post-Press | Lever Press FR Change | Pre-press | Post-Press | ||||
| CORE | 24 | 0.48* | 0.31 | 17 | 0.56* | 17 | 0.20 | -0.21 |
| D SHELL | 12 | -0.05 | 0.09 | 6 | 0.94** | 6 | -0.09 | 0.20 |
| VM SHELL | 13 | 0.24 | 0.19 | 6 | -0.26 | 6 | -0.71 | -0.09 |
| VL SHELL | 48 | 0.01 | 0.10 | 21 | 0.43 | 21 | 0.23 | -0.04 |
| RP SHELL | 5 | 0.72 | 0.20 | 2 | - | 2 | - | - |
| D BORDER | 19 | 0.32 | 0.29 | 13 | 0.79** | 13 | 0.39 | 0.18 |
| V BORDER | 14 | -0.01 | -0.04 | 7 | 0.00 | 7 | -0.14 | -0.50 |
| N (total) | 135 | 72 | 72 | |||||
Significant at 0.05 level (2-tailed)
Significant at 0.01 level (2-tailed)
Slow phasic versus tonic
Firing rate change magnitudes were directly and significantly correlated between slow phasic and tonic time bases for dorsal shell and dorsal border neurons (Table 3b). Interestingly, of neurons exhibiting slow phasic decreases, tonic firing rate changes in 100% of the dorsal border neurons (13/13) and 83% of the dorsal shell neurons (5/6) were also decreases. Firing rate change magnitudes were positively correlated between slow phasic and tonic time bases in the core, as well, albeit to a lesser extent than dorsal shell and dorsal border. Unlike in the dorsomedial accumbens, the slow phasic/tonic relationship in core neurons was not unique to any particular directionality of firing rate change for either time base (i.e. both tonic decreases and increases exhibited slow phasic changes) (see Fig. 5).
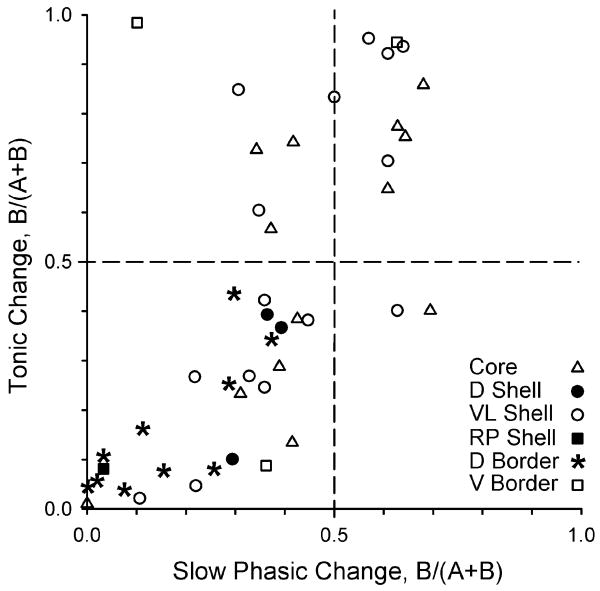
Figure 5.
Tonic firing changes in neurons that exhibit slow phasic firing patterns are differentially expressed among nucleus accumbens (NAcc) subregions. The graph depicts tonic firing data for neurons that exhibited slow phasic firing changes. Symbols indicate subregional placement of histologically confirmed NAcc microwires (see key). B/A + B values from 0.49 to 0 and from 0.51 to 1.00 reflect increasingly larger firing rate decreases and increases, respectively. A rather broad distribution of increases and decreases of slow phasic and tonic firing magnitudes is revealed for lateral NAcc neurons (e.g. core and ventrolateral shell), including opposite signs in firing rate change between time bases (i.e. symbols in upper left and lower right quadrants). For the medial NAcc (i.e. dorsal shell and rostral pole shell) and dorsal border regions, it is notable that slow phasic changes were exclusively decreases in neurons that also exhibited tonic decreases (lower left quadrant). D, dorsal; VL, ventrolateral; RP, rostral pole; V, ventral.
Rapid phasic versus slow phasic
No relationship existed in any subregion between the magnitude of firing rate changes on the order of seconds compared to minutes relative to lever presses (Table 3c).
Slow Phasic Categories Within Tonic Groups
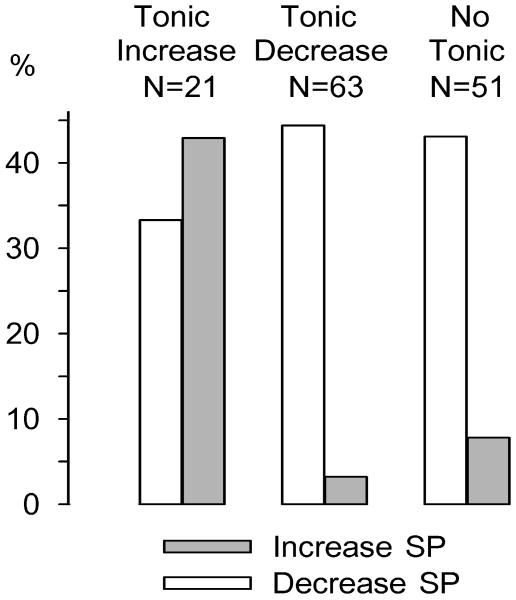
Specific categories of neurons exhibiting slow phasic changes were additionally analyzed with respect to their prevalence among tonic decrease and tonic increase groups. Interestingly, a similar distribution of decrease + progressive reversal patterns was observed within groups exhibiting tonic increases (29%, 6/21) or tonic decreases (37%, 23/63). What is more, the prevalence of decrease + early reversal patterns was approximately the same between tonic increase (5%, 1/21) and tonic decrease (6%, 4/63) groups. All slow phasic accumbens neurons were analyzed in terms of whether they demonstrated post-press firing rate increases (SP increase) or decreases (SP decrease) as a function of tonic category: increase, decrease or no tonic change. The analysis revealed that the prevalence of SP decreases was not markedly different among tonic increase, tonic decrease, and no tonic change groups (33%, 44%, and 43%, respectively). SP increases, on the other hand, exhibited striking differences in prevalence between tonic groups; forty-three percent (9/21) of neurons that showed tonic increases also demonstrated SP increases compared to only 2% (1/63) of tonic decrease neurons (Fig. 6). Like the tonic decrease group, SP increases comprised a relatively small percentage (8% [4/51]) of no tonic change neurons.
Figure 6.
Percentage of slow phasic (SP) increases and SP decreases among tonic categories. All tonic neurons (n = 135) were evaluated by category (increase, decrease, and non-responsive) for the prevalence of SP increases and decreases within them. SP decreases were observed in 33% of tonic increase neurons, 44% of tonic decrease neurons, and 43% of no tonic change neurons. SP increases were observed in 43% of tonic increase neurons, 2% of tonic decrease neurons, and 8% of no tonic change neurons. A 2 × 3 chi-square test revealed that SP increase and decrease reversal patterns were differentially expressed across tonic categories [χ2(2) = 17.3338, P < 0.001). An odds ratio analysis confirmed a differential prevalence of SP increase and decrease patterns between the tonic increase and decrease categories: odds ratio (95% confidence interval) = 0.0311 (0.0033–0.2892).
Discussion
Analyses of rapid and slow phasic firing patterns yielded complementary findings to tonic firing analyses, which together support functional heterogeneity in the accumbens. The more comprehensive subregional analyses in the present investigation, which expanded neural sampling into the intra-accumbal border regions and further refined subregional sampling of the shell, provided evidence of a more general mediolateral compartmentalization of accumbal firing patterns extending beyond our prior report (Ghitza et al., 2006). The 2006 report, in which neural recordings from rats tested under discriminative stimulus conditions were combined with part of the present FR1 data set, provided evidence for functional differences between medial shell and core that were similar between the two different reinforcement schedules and therefore potentially common to self-administered cocaine reinforcement. In addition to extending medial shell - core differences into broader mediolateral accumbens differences, the present updated observations show remarkable agreement with an emerging literature which describes the shell as a functionally heterogeneous structure (see below).
Slow Phasic Patterns
The cycle of post-press stereotypy followed, in the later minutes of the interinfusion interval, by lever-oriented approach behavior tempts speculation about the possible involvement of slow phasic patterns in the execution of the locomotor response. We have previously reported that slow phasic patterns are present in the accumbens even when all periods of locomotion are excluded from firing rate analyses (Peoples et al., 1998). Instead, slow phasic firing changes are likely associated with the timing of appetitive responding as cocaine, hence elevated dopamine, levels decline. Indeed, evidence has been reported that phasic activity over minutes is sensitive to the systemic administration of DA receptor antagonists eticlopride and SCH23390 (Nicola & Deadwyler (2000). The authors found that both receptor sub-types are implicated in the modulation of intertrial NAcc firing rates, but likely via distinct mechanisms. Subsequent reports have confirmed the synergistic relationship between D1 and D2 receptor activation in the processing of drug reinforced behavior (reviewed in Di Chiara et al., 2004). The present findings, in which different phasic reversal latencies were commonly found among simultaneous recordings within the same rat, are consistent with the above reports and focus attention on the processing of reward relevant firing changes associated with drug metabolism over the interinfusion interval.
Differences in the prevalence of early versus progressive/late reversal slow phasic patterns suggest that the firing rates of neurons in the medial (dorsal shell/rostral pole shell) versus lateral (core/ventrolateral shell) accumbens may be differentially modulated by dopamine/drug levels over the course of the inter-infusion interval. This is consistent with an abundant literature that provides evidence for subregional differences in several accumbens dopamine-related parameters: differential dopamine immunohistochemistry (Voorn et al., 1989), higher basal dopamine concentrations/dopaminergic innervation in the shell (Deutch & Cameron, 1992), and a greater medial shell dopamine response to cocaine using both chronoamperometry (David et al., 1998) and microdialysis (Pontieri et al., 1995; Barrot et al., 1999). This elevated shell dopamine response was recently reported to be associated with an increase in the number of phasic release events that was shown to be sensitive to inactivation of the dopaminergic ventral tegmental area projection to NAcc by GABA agonist administration (Aragona et al., 2008). In addition, the relative (co)expression of D1 and D2 dopamine receptor subtypes has been shown to differ between medial shell and core (Bertran-Gonzalez et al., 2008). Taken together with the report that dopamine receptor subtypes have been differentially implicated in phasic neural activity occurring over the interresponse interval during cocaine self-administration (Nicola & Deadwyler, 2000), differences in dopamine neuromodulation are likely between medial and lateral accumbens. This may account for differential drug level thresholds affecting slow phasic reversal latencies between medial and lateral accumbal subregions. Differences in reversal latencies may reflect the electrophysiological consequences of differential pharmacological sensitivity between neuronal populations; progressive reversal patterns may reflect neuronal processing in which a lower threshold must be attained by declining drug/dopamine levels before firing rates reverse over the inter-infusion interval, while early reversal patterns, sensitive to pharmacologic shifts at a higher threshold, reverse with a shorter latency.
Recent insights into the complex nature of striatal dopamine signaling may account for these observed differences in reversal latencies. Dopamine functions not only on the basis of the DA receptor sub-type to which it binds, but according to the activity of local glutamatergic synapses coupled with the timing of terminal DA release and diffusion. DA can act extrasynaptically as a volume transmitter and has been hypothesized to modulate excitatory input by regulating current flow through the heads of spines in MSNs (Arbuthnott & Wickens, 2007). Excitatory neurotransmission is a critical component in striatal synaptic plasticity, as it has been shown that the magnitude and the direction of time dependent shifts in excitatory post-synaptic potentials depend critically on glutamatergic interactions with DA signaling between DA receptor subtypes (Shen et al., 2008). While D1 and D2 receptor activation have commonly been implicated in opposite roles in electrochemical signaling (Uchimura et al., 1986, Pennartz et al., 1994), the consequence of dopamine binding a particular receptor subtype can be more complicated. For example, depending on the temporal sequence of binding, D1 receptor activation can either promote a continued hyperpolarized state or facilitate the maintenance of a depolarized state (Arbuthnott & Wickens, 2007). Furthermore, the extent to which D1 receptors remain activated differs relative to D2 receptors as a function of dopamine concentration, with D2 receptor signaling favored at lower concentrations (Richfield et al., 1989), which may occur later in the cocaine self-administration interinfusion interval as drug, hence dopamine, levels decline. The trend toward earlier reversal latencies in the medial relative to lateral accumbens is perhaps accounted for by subtle differences in the mechanism of dopamine-mediated neurotransmission, including differential activation of receptor subtypes (see above), or by differential mesencephalic glutamate co-transmission, for which evidence has recently been found medially, but not laterally, in the accumbens (Chuhma et al., 2009).
Rapid Phasic Patterns
The core subregion exhibited greater magnitude pre-press firing rate changes than the dorsal shell and rostral pole shell. These differences persisted when the core was expanded into the “lateral NAcc” by the inclusion of ventrolateral shell. We have previously reported medial shell - core differences in perievent firing using a separate set of subjects that self-administered cocaine in a discriminative stimulus paradigm (Ghitza et al., 2004) which these continuous reinforcement schedule data corroborate and expand upon by including ventrolateral shell.
Recently, Carelli & Wondolowski (2006) extended their investigations of rapid phasic firing patterns associated with natural versus cocaine reinforcement (Carelli et al, 2000; Carelli & Ijames, 2001; Carelli & Wondolowski, 2003) into a subterritorial analysis of these correlates between shell and core. At first glance, it appeared that our report of differential subregional prevalence of rapid phasic firing was contrary to their findings, which had suggested that patterned discharges of accumbens neurons associated with cocaine reinforcement are distributed evenly between shell and core. However, a closer inspection of their wire placements revealed that virtually all of their shell wires were lateral placements and consequently their subterritorial comparisons were between lateral NAcc regions. Therefore, their finding an even distribution is consistent with what would be predicted by the present results, which show core and ventrolateral shell exhibit similar firing pattern prevalences. Whether primary versus drug reinforcement neural correlates differ mediolaterally in the accumbens would be an intriguing follow-up study and would test the contribution of cocaine pharmacologic effects on observed mediolateral differences.
Correlations Between Time Bases
Many neurons exhibited significant changes in firing on more than one time base. Whether a neuron that exhibits a change in firing rate on the order of seconds also does so over hours or minutes might reveal clues as to the factors that influence its activity. Such comparisons might permit a further elaboration of the relative contributions of afferent signaling versus pharmacologic influences on the firing patterns commonly observed within the three time bases.
Slow Phasic versus Tonic Patterns
Neurons in both the dorsal shell and dorsal border regions exhibited highly significant correlations between the magnitudes of slow phasic and tonic firing rate changes. Tonic decreases predominate in the medial NAcc, particularly in the dorsal border region (Fabbricatore et al., 2009). Interestingly, most of the dorsal shell (83%) and all of the dorsal border (100%) neurons in this analysis also exhibited slow phasic decreases. Both tonic decreases and slow phasic post-press decreases are firing patterns that are consistent with classic primary dopaminergic effects on firing rate.
Core neurons also exhibited a correlation between the magnitudes of slow phasic and tonic firing rate changes, but this trend was not restricted to any particular tonic/phasic category. It is noteworthy that tonic increase neurons are virtually exclusive to lateral NAcc during cocaine self-administration (Fabbbricatore et al., 2009) and that, unlike for tonic decrease neurons, both slow phasic increases and decreases are prevalent among them.
As stated earlier, the decrease + progressive reversal firing pattern was the predominant slow phasic category among accumbal neurons. A closer inspection of its prevalence among tonic categories revealed that it was similarly proportioned between tonic increase and tonic decrease neurons (6/21 [29%] and 23/63 [37%], respectively). Moreover, decrease + early reversal patterns also exhibited similar prevalence between tonic categories: 1/21 (5%) for tonic increase and 4/63 (6%) for tonic decrease neurons. This observation prompted a similar comparison for post-press increase slow phasic neurons.
Among the 63 tonic decrease neurons, the slow phasic pattern of only one neuron (2%) was a post-press increase, compared to 43% (9/21) among tonic increase neurons. Tonic increase neurons shared with tonic decrease and no tonic change categories similar percentages of post-press decrease slow phasic patterns. Returning to the question of whether pharmacologic factors influence tonic firing rates, additional evidence is herein presented which suggests that drug effects alone cannot account for the firing pattern profiles observed over the course of the self-administration session. If the sole influence on firing rate were strictly pharmacologic, then tonic increase neurons would be expected to exhibit only post-press increase slow phasic patterns, consistent with the directionality of tonic firing rate change during the self-administration phase of the experiment. The fact that a substantial percentage of tonic increase neurons show post-press decrease slow phasic patterns suggests that some other factor, perhaps mediated by afferent signaling, is effecting this opposite firing rate directionality. As mentioned earlier, tonic increase neurons are restricted to lateral NAcc regions, therefore the influence of these “non-pharmacological” factors on firing rate are not homogeneously distributed in the NAcc, but are specific to lateral regions.
Rapid Phasic versus Slow Phasic Patterns
No accumbal subregion exhibited a significant correlation between the magnitudes of rapid and slow phasic firing rate changes of individual neurons. Rapid phasic patterns do not likely reflect pharmacological influences on firing rate (Carelli & Deadwyler, 1996; Peoples et al., 1997), whereas the time course of firing rate changes over the inter-infusion interval is not incompatible with pharmacologic contributions (Wise et al., 1995; Nicola & Deadwyler, 2000).
Rapid Phasic versus Tonic Patterns
Core neurons exhibited a positive correlation between the magnitudes of rapid phasic and tonic firing rate changes. Not surprisingly, tonic increase core neurons accounted for this correlation: among all NAcc subregions, tonic increases were most predominant in the lateral accumbens, the core in particular (see Fig.6 in Fabbricatore et al., 2009). The finding that pre-press firing increases and tonic increases co-exist preferentially in lateral accumbens, where pharmacologic firing rate suppression appears mitigated relative to medial accumbens (see above), tempts speculation that one or both firing patterns reflect afferent processing related to the instrumental response.
Floresco and colleagues (2003) reported that independent tonic and phasic modulation may occur at the level of the ventral tegmental area (VTA), wherein population firing and bursting firing (and hence tonic and phasic accumbal DA efflux, respectively) are regulated by distinct afferent pathways. This and more direct cortico-limbic signaling (Goto & Grace, 2005) are means by which the mesolimbic DA system and its target neurons can be influenced simultaneously by both pharmacological effects and signaling related to limbic-motor processing.
We have previously presented evidence of non-pharmacologic effects in both tonic increase and decrease categories (Fabbricatore et al., 1998) in experiments similar to those mentioned above, but with an extinction phase replacing the post-self-administration phase. Interestingly, evidence for non-pharmacologic effects on firing rate among neurons which exhibited tonic decreases appeared during late extinction, when calculated drug levels had waned. Late extinction reversals in firing rate which paralleled changes in response rate, but not declining drug levels, were present in more than one third of both tonic categories. Thus, in the case of neurons exhibiting reduced tonic firing, the throughput of behavioral signaling may be impeded by pharmacological factors during self-administration but become manifest in late extinction when drug levels are low.
A review by Di Chiara (2002) examined the impact of differential dopamine transmission between core and shell. The author described the differential responsivity to dopamine transmission in the shell and core (tolerance and sensitization, respectively) after chronic drug exposure, and concluded that each neuroadaptation is a likely mechanism in driving the persistent drug-seeking behavior which ultimately leads to addiction. The present findings, derived from animals with regular, 6 hr daily drug access, are consistent with this interpretation: the virtual absence in medial NAcc versus the relative abundance in lateral NAcc of firing rate increases across time bases reveals that suppression of medial accumbal output is a consequence of chronic, extended daily drug exposure. The resulting disinhibition of the primary output structure of the accumbens, the ventral pallidum, has been described as a common neurophysiological event in the administration of most, if not all drugs of abuse (Nestler, 2005; Wise, 2002). The acute effects of cocaine medially in the accumbens may critically disinhibit downstream structures which drive lateral accumbal output and consequent behavioral activation via dorsal striatum. Over time, chronic extended daily drug exposure may render medial NAcc tolerant to chronically high DA levels, while elevated lateral NAcc firing rates contribute to driving its target circuitry, which may be associated with enhanced behavioral sensitization (Di Chiara, 2002) and ultimately, compulsive drug-seeking (Porrino et al., 2007). Indeed, convergent evidence implicates dysregulated accumbal DA in the etiology of drug addiction (Everitt & Wolf, 2002; Di Chiara et al., 2004; Volkow et al., 2007).
The present findings show remarkable agreement with an emerging literature which 1) describes the shell compartmentally, in terms of its position and connectivity in basal forebrain circuitry (described in Fabbricatore et al., 2009), and 2) advances the notion that shell compartmentalization subserves functional heterogeneity. For example, Ikemoto and colleagues (2005, 2003) have demonstrated using intracranial self-administration that drug (cocaine, amphetamine) self-administration is maintained by medial, but not lateral, shell infusions, extending earlier reports (Carlezon et al., 1995; Rodd-Henricks et al., 2002; Sellings & Clarke, 2003) that implicated the medial shell in the primary reinforcing effects of psychomotor stimulant administration. Core was similar to lateral shell, in that self-infusions were not maintained in either region.
Conclusions
Mediolateral firing rate differences likely can be attributed to both 1) distinct subterritorial connectivities, in light of the finding that rapid phasic (hence, non-pharmacologic) pre-press increases are elevated in lateral NAcc and not elsewhere, and 2) pharmacological differences, since reversal latencies are shorter in medial NAcc, possibly the result of differential dopamine neurotransmission in this region. Lateral NAcc tends to exhibit more excitation, with greater prevalences of both tonic increases and rapid phasic pre-press increases, while the medial NAcc exhibits more inhibitory firing patterns across time bases, possibly reflecting higher neuronal drug sensitivity.
These data extend our previous observation (Fabbricatore et al., 1998) that direct pharmacological factors alone are insufficient to account for longer time frame firing patterns: opposing firing rate changes within individual neurons when tonic and slow phasic categories are evaluated together suggests that both pharmacologic and non-pharmacologic factors contribute to signaling in neurons critical to drug self-administration behavior. During drug self-administration, pharmacologic factors apparently predominate in medial NAcc, given the ubiquitous firing rate suppression observed here in both tonic and slow phasic firing patterns. The interaction of pharmacological effects and afferent signaling likely mediates lateral NAcc firing changes during drug self-administration, since opposite signs among tonic and slow phasic firing patterns are observed here. Future investigations into the capacity for chronic disinhibition of medial NAcc targets to drive lateral NAcc output and any resultant neuroplastic changes in basal forebrain circuitry which may occur over prolonged drug exposure may be crucial in understanding the shift from casual use to drug addiction.
Figure 1.
Examples of slow phasic reversal firing patterns. Each peri-event time histogram (PETH) displays the firing pattern of one neuron during the minutes before and after the lever press. The ordinate of each histogram displays the average firing rate (i.e. average discharges/s calculated as a function of 0.1-min bins). Time 0 (vertical dashed line) on the abscissa marks the occurrence of the cocaine-reinforced lever press. (A) Examples of decrease + progressive reversal firing patterns. (B) Examples of other categories of slow phasic patterns. Top, increase + progressive reversal; middle, increase + early reversal; bottom, decrease + early reversal. For each PETH, the inset depicts the corresponding neural waveform. Calibrations (bars in A, top) of waveforms: 0.25 ms, 0.10 mV (top left waveform); 0.25 ms, 0.20 mV (all other waveforms).
Acknowledgments
Research supported by NIDA grant DA 006886. Thanks to Jean Fugate, Don MacNeil, Linda King, Tony Pawlak and Sisi Ma for technical assistance. This article is dedicated to the memory of our friend and colleague, Volodymyr Fedorovitch Prokopenko (1947-2006). His scientific skills and insights, as well as his generous and good-natured disposition, are missed by any who had the pleasure to know him.
Supported by: NIDA DA006886
Literature Cited
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J, Neurosci. 2008;28(35):8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30(2):62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11:1155–1166. doi: 10.1046/j.1460-9568.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dual factors controlling activity of nucleus accumbens cell-firing during cocaine self-administration. Synapse. 1996;24:308–311. doi: 10.1002/(SICI)1098-2396(199611)24:3<308::AID-SYN14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci. 2000;20(11):4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907(1-2):156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76(3):379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23(35):11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse. 2006;59(2):69–73. doi: 10.1002/syn.20217. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Castellan NJ, Jr, Siegel S. Nonparametric statistics for the behavioral sciences. McGraw-Hill Inc.; Academic, New York: 1988. [Google Scholar]
- Chang J, Sawyer SF, Lee R, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14(3):1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Choi WY, Mingote S, Rayport S. Dopamine neuron glutamate cotransmission: frequency-dependent modulation in the mesoventromedial projection. Neuroscience. 2009;164(3):1068–1083. doi: 10.1016/j.neuroscience.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Zahniser NR, Hoffer BJ, Gerhardt GA. In vivo electrochemical studies of dopamine clearance in subregions of rat nucleus accumbens: differential properties of the core and shell. Exp Neurol. 1998;153:277–286. doi: 10.1006/exnr.1998.6898. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Beh Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbricatore AT, Uzwiak AJ, West MO, Peoples LL. Comparisons of firing rates of rat nucleus accumbens neurons during cocaine self-administration and extinction. Soc Neurosci Abstr. 1998;24:1736. [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. Eur J Neurosci. 2009;30:2387–2400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminitive-stimulus task. J Neurophysiol. 2004;92(3):1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience. 2006;137:1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature Neuroscience. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23(28):9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25(20):5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Relo AL, Docter GJ, Jonker AJ, Vreugdenhil E, Groenewegen HJ, Voorn P. Differential effects of dopamine depletion on the binding and mRNA levels of dopamine receptors in the shell and core of the rat nucleus accumbens. Mol Brain Res. 1994;25:333–343. doi: 10.1016/0169-328x(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd. Academy Press; New York: 1997. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42(6):719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci. 1996;16(10):3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. Operant behavior during sessions of intravenous cocaine infusion is necessary and sufficient for phasic firing of single nucleus accumbens neurons. Brain Res. 1997;757:280–284. doi: 10.1016/s0006-8993(97)00299-0. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998;18(18):7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161(1):122–129. [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared to the “core” of the rat nucleus accumbens. Proc Natl Acad Sci. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(8):1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30(3):767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of wistar rats. J Pharmacol Exp Ther. 2002;303(3):1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Runyon RP, Haber A, Pittenger DJ, Coleman KA. In: Fundamentals of Behavioral Statistics. McKean BL, Holton T, editors. McGraw Hill; New York: 1996. pp. 592–594. [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, Higashi H, Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986;375(2):368–72. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- Uzwiak AJ, Guyette FX, West MO, Peoples LL. Neurons in accumbens subterritories of the rat: Phasic firing time-locked within seconds of intravenous cocaine self-infusion. Brain Res. 1997;767:363–369. doi: 10.1016/s0006-8993(97)00752-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: Immunohistochemical distribution of enkephalin, substance P, dopamine and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Chang JY, Janak P, Azarov A, Anstrom K. Mesolimbic neuronal activity across behavioral states. Ann NY Acad Sci. 1999;877:91–112. doi: 10.1111/j.1749-6632.1999.tb09263.x. [DOI] [PubMed] [Google Scholar]
- Yokel AR, Pickens R. Drug level of D- and L-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]