Abstract
A literature review and new data are presented to evaluate the influence of intervertebral disc (IVD) injury on biomechanics, cellularity, inflammation, and biosynthesis. Literature and new experimental evidence support the hypothesis that localized injury in the disc can lead to immediate and long-term organ level changes in biomechanics and biology of the IVD. Biomechanical properties defining motion segment bending behaviors sensitive to injuries that affect anulus fibrosus (AF) integrity and nucleus pulposus (NP) pressurization. Axial mechanics and IVD height measurements show sensitivity to puncture and other injuries that reduce NP pressurization. Torsional biomechanics are strongly affected by the extent and location of AF lesions but are less sensitive to reduced NP pressurization. IVD injuries such as puncture and stab incisions may also lead to a cascade of biological changes consistent with degeneration, including loss of cellularity, altered biosynthesis and inflammation. New results on effects of 25G needle injection of saline into a bovine IVD organ culture model demonstrated a loss of cellularity and down-regulation of matrix gene expression, providing a specific example of how a minor injury affects the IVD organ response. We conclude that localized injuries in the IVD can induce an organ level degenerative cascade through biomechanical and biological mechanisms, and their interactions. Attempts at IVD repair should target the dual biomechanical roles of the anulus of maintaining nucleus pressurization and transmitting loads across the vertebrae. Biologically, it remains important to maintain IVD cellularity and biosynthesis rates following injury to prevent downstream degenerative changes.
Keywords: Disc degeneration, Needle puncture, Intervertebral disc biology, Spine biomechanics, Mechanobiology, Saline injection, Delamination, Degenerative cascade
INTRODUCTION
Intervertebral disc (IVD) degeneration is an aberrant, cell-mediated response to progressive structural failure.5 Early degeneration refers to accelerated age-related changes in a structurally intact disc, and degenerative disc disease refers to a degenerate IVD that is also painful.5 IVD degeneration strongly correlates with aging, yet rather than a necessary consequence of aging, it can be a distinct process characterized by matrix damage and loss of functionality. 9 While patient age correlates strongly with disc degeneration, there are many noteworthy exceptional cases of premature degeneration (or alternatively persons of advanced age not presenting degenerative changes) where degenerative grade does not correlate with age (Fig. 1). These IVDs are prone (or resistant) to degeneration, which may be accelerated (or protected) by genetic, biomechanical, and nutritional factors.12,44,61,65 Genetic factors are considered among the strongest determinants of degeneration, as heredity alone explains much of the variation in the reported prevalence of specific degenerative findings with aging.12 The genetic mechanisms underlying predisposition to disc degeneration are not fully known, however, some evidence suggests they may be associated with gene polymorphisms in the disc.19,35,53,60 For example, collagen polymorphisms are associated with distinct structural and biomechanical changes in IVDs.6,16 Mechanical and biological factors are also suspect in the context of genetic factors affecting IVD degeneration. For example, altered structural components may be caused by an underlying biological or genetic mechanism, but will contribute to weaker IVD tissue resisting daily loading activities, predisposing the IVD to injury.
FIGURE 1.
Diagram exhibiting a strong correlation between aging and degeneration (black squares) with representative images. A notable exception (pink triangle) exhibits degeneration substantially accelerated from what would be expected due to normal aging. Accelerated degeneration may be associated with genetic, biomechanical, or nutritional factors. The images and correlation were taken from specimens used in Costi et al.20 and the Thompson degeneration scale64 was modified for transverse sections as described1.
Despite the strong genetic contribution to disc degeneration, a substantial percentage of the reported variation in the prevalence of disc degeneration remain unexplained, which may be partly related to challenges quantifying risk factors with enough specificity. For example, occupational loading as quantified in epidemiology studies may not include biomechanical episodes such as participation in sports or intense activities that can lead to micro-injury, or acute trauma which may be important in the initiation and progression of degeneration. Mechanical forces on IVDs can accelerate degenerative changes with evidence pointing toward a ‘safe window’ for disc stresses, with immobilization reducing IVD cell metabolism and overloading and immobilization implicated in degenerative remodeling and damage.61 Structural damage and injury in the IVD are associated with inflammation, innervation, and hypomobility in animal models where current evidence suggests loss of NP pressure and volume is likely the root of degenerative changes in the disc, while pathology of the endplate and peripheral AF are more likely the source of pain.44
The IVD is the largest avascular structure in the body, and consequently may be particularly sensitive to trauma. Large diffusion distances decrease transport of nutrients and waste into and away from the IVD, which affect the ability of IVD cells to repair damaged tissue. IVD cells must endure, and adapt to, low levels of oxygen and glucose, along with high levels of metabolites such as lactic acid which are present especially in the center of the disc.65 IVD degeneration is associated with calcification of the endplates as well as loss of water content and increased fiber density in both the anulus fibrosus (AF) and nucleus pulposus (NP);13,51,52 both factors will further decrease diffusion rates.26 Consequently, IVD degeneration displays reduced cell viability and metabolic rates.30 Inflammation in the IVD may also contribute to the development of chronic and painful disc pathologies. IVD injury has been shown to induce an immediate inflammatory response, potentially through the immediate change in disc mechanics resulting from anular compromise.59
Genetic, biomechanical, and nutritional changes provide a reasonable context for understanding the pathogenesis of IVD degeneration, yet the complex relationships between these factors must be appreciated to understand how specific injuries may result in progression of a degenerative cascade which must be inhibited to prevent degeneration and to promote repair. The importance of nutritional factors in IVD degeneration highlights the challenges for tissue repair, but is intimately linked to IVD mechanics. For example, several important endplate changes such as Schmorl’s nodes are common in IVD degeneration,13 likely have mechanical and genetic origins, and strongly affect mechanical stress distributions and motion segment mechanics.4 Damage to the IVD may result in altered loading patterns, which could further contribute to cell death or decreases in cell biosynthesis rates as forces transmitted to the local cell microenvironment change.
The first aim of this study is to review the literature to describe the effects of acute mechanical injury on intervertebral disc biomechanics. The second aim is to evaluate the effects of these injuries on IVD cellularity, inflammation, and biosynthesis. New data on how needle injection of saline affects cellularity and matrix gene expression in a bovine IVD organ culture model is also presented to further describe how IVD injury affects its response. Saline injection is considered a minor injury of the IVD, as it is a similar procedure to that used for discography and often used as a control treatment in growth factor injection studies. However, little is known of whether saline injection affects the IVD cellular response. Potential mechanisms for organ level changes are also provided as we explore the hypothesis that localized and minor injuries in the IVD can result in organ-level changes in IVD structure and function. The IVD possesses a remarkably robust fiber reinforced composite structure, yet sufficient damage or removal of part of the structure will affect the behavior of the whole IVD. Therefore, the fundamental question is not whether the IVD’s mechanical behavior is altered by an injury, but we might ask by how much, under what circumstances, and what are the consequences?
STRUCTURAL AND COMPOSITIONAL CHANGES IN IVD DEGENERATION
An extensive study on IVD composition defined four stages of compositional changes: growth, maturation, aging, and degeneration.10 Research has largely been focused on degenerative changes in the IVD, as this is the time when pathology may be associated with painful conditions. IVD degeneration, distinct from age related remodeling, involves mechanical damage accumulation in the form of herniation and delamination, as well as enzymatic degradation of collagen and aggrecan.10,18,52,61 Extracellular matrix components are altered in degenerated IVDs, with studies noting changes in type and aggregating quality of proteoglycans, decreases in tissue water content, and a change in the proportion of denatured collagen type II to total collagen.52 Structural changes of the AF include increased layer thickness and number of fiber discontinuities, and alterations in fiber angle.18,61 IVD degeneration results in specific mechanical changes in IVD tissues that are associated with altered composition and structure.15,32,33,49 Biomechanical changes on the tissue level include increased compressive stiffness and reduced AF failure strengths.3,33,61 On the motion segment size scale, the functional impairment in flexibility parameters (range of motion, neutral zone, and neutral zone ratio, Fig. 2) correlated most strongly with degeneration grading systems based on microscopic findings. These specific relationships between functional biomechanics and microscopic degeneration provide the first evidence that localized injury leads to organ level changes and we investigate the mechanisms for these changes by clarifying how specific acute IVD disruptions can alter the motion segment biomechanics. Late stage degeneration is associated with more substantial disc fissures, calcification of the endplates and low transport rates,51 making it the time with largest need and lowest potential for repair.
FIGURE 2.
Schematic of motion segment with overview of loading modes and injury types.
CHARACTERIZING ACUTE MECHANICAL INJURIES ON INTERVERTEBRAL DISC BIOMECHANICS
Localized injury to the IVD has a significant impact on the biomechanical properties, compromising both IVD structure and function. An understanding of how injury might lead to mechanical instability of the vertebral motion segment by altering its load carriage properties is of particular relevance when considering how acute injury may lead to progressive degeneration. In the course of daily activities, the IVD undergoes combinations of several principal deformation modes (Fig. 2); axial tension/compression, anterior/posterior and lateral shear, flexion/extension and lateral bending, and torsion. These motions have previously been grouped together by dominance of fluid flow mechanisms (compression, bending, and flexion–extension) or intrinsic tissue solid mechanisms (anterior/posterior/lateral shear and torsion) in defining the IVD’s mechanical behaviors.20 To varying degrees, each of these motions makes use of the AF’s dual mechanical roles: containing NP fluid pressure and transmission of loads between vertebrae. It follows that the manner in which a particular type of injury diminishes the ability of the AF to fulfill one or both of these two roles will affect the whole IVD’s ability to function in the principal deformation modes.
In order to provide as near a comprehensive picture as possible of the impact of localized AF injury on IVD biomechanics, a literature review was performed focusing on experimental studies in which biomechanical measurements were made following acute injuries in the IVD in which tissue was not removed. Studies were classified by type of injury (Puncture, Concentric Tear, Radial Tear, Transverse Tear at Mid-Plane, and Transverse Tear at Rim, Fig. 2) and mode of biomechanical testing (Compression/Tension, Flexion/Extension/Bending, or Torsion) (Table 1). It is also likely that minimally invasive injection of a biologic agent at early time points may be more successful in preventing or altering the time course of degeneration, as has been demonstrated in animal models. 24,42,45 Since the pathway for many proposed repair strategies involves injection with small needles, puncture injury is included as an important IVD disruption deserving improved understanding.
TABLE 1.
Biomechanical effects of injury on disc height, stiffness (S), hysteresis (H), neutral zone length (N), neutral zone stiffness (SN), tensile stiffness (ST), and compressive stiffness (SC).
| Injury | Author | Species | Study type | Disc height |
Axial | Bending | Torsion |
|---|---|---|---|---|---|---|---|
| Rim lesion | de Visser22 | Sheep | C | S↓ | |||
| Thompson63 | Human | C | S↑ | S↓ | |||
| Thompson62 | Sheep | C | S↓ H↓ | S↓ H- | |||
| Mid-plane tear | Holm29 | Pig | L | P↓ | |||
| Kaigle34 | Pig | L | S- | ||||
| Keller36 | Pig | L | H↑* | ||||
| Radial tear | Thompson63 | Human | C | S↑ | S- | ||
| Schmidt55 | Human | C | S↓ | S↓ | |||
| Thompson62 | Sheep | C | S- H↓ | S- H- | |||
| Puncture | Korecki38 | Cow | C/L | ↓ | S↓ | ||
| Miyamoto47 | Rabbit | L | ↓ | ||||
| Aoki11 | Rabbit | L | ↓ | ||||
| Kim37 | Rabbit | L | ↓ | ||||
| Sobajima57 | Rabbit | L | ↓ | ||||
| Boxberger15 | Rat | C | SC- ST- N- | ||||
| Elliott23 | Rat | C | ST- SC- SN- N↑ | ||||
| Hsieh31 | Rat | C/L | S↓ H↓* | ||||
| Michalek46 | Rat | C | S↓ | ||||
| Fazzalari25 | Sheep | C/L | S- | S- | |||
| Elliott23 | Sheep | C | SC- ST- | ||||
| Concentric tear | Thompson63 | Human | C | S↑ | S↓ | ||
| Thompson62 | Sheep | C | S- H↓ | S- H- | |||
| Fazzalari25 | Sheep | C/L | S↓ | S- |
Study type is classified as live (L), cadaveric (C), or organ culture or live intervention and cadaveric mechanical testing (C/L)
(Inferred from model fit parameters).
Biomechanical outcomes were identified as stiffness (S), hysteresis (H), neutral zone stiffness (SN), and neutral zone length (N), and either increased (↑), decreased (↓), or remained unchanged (−) by anular injury. In addition to studies of the immediate impact of injury, the review included in vivo degenerative studies to help better understand how the biological response to acute injury leads to a new mechanical steady-state.
EFFECTS OF ACUTE MECHANICAL INJURY ON INTERVERTEBRAL DISC BIOMECHANICS
There is clear evidence that acute and localized injuries have immediate, measurable effects on localized IVD tissue but they also lead to biomechanical changes on the motion segment level in highly specific ways (Table 1). Torsional biomechanical properties are strongly affected by the extent and location of AF lesions but are generally less sensitive to reduced NP pressurization. Structural biomechanical properties defining bending (i.e., flexion, extension, and lateral bending) behaviors are perhaps the most sensitive as they are affected by alterations in AF integrity and NP pressurization. Axial mechanics and IVD height measurements are quite sensitive to IVD disruptions such as puncture injuries which have the capacity to reduce NP pressurization with relatively minor effects on vertebral connectivity.
The location of the AF disruption was more important than the relative amount of AF disruption in impacting IVD biomechanics under torsional loading, which is resisted almost exclusively by anular fibers40 with minimal nuclear pressurization.66 Radial tears did not always reduce torsional stiffness (Schmidt et al.55 but not Thompson et al.62,63), which is presumably due to the relative size of the tear. There is also evidence that sensitivity of torsional stiffness measurements depends on load magnitude.55 Concentric tears showed no change acutely,25 but correlated with decreased stiffness in cadaveric IVDs,63 suggesting a role in degenerative tissue changes. Rim lesions were universally found to decrease torsional stiffness. 62,63 These findings appear to be consistent with the hypothesis that torsion is resisted primarily by the helical arrangement of AF collagen fiber bundles. However, Thompson et al.62 found that torsional stiffness was reduced by rim lesion and not radial tears even though more than 90% uninterrupted AF fibers were retained in both injury types.62 This notable difference in torsional behaviors between rim lesions and radial tears stands in stark contrast with work done at the tissue scale suggesting a linear relationship between AF stiffness and mean fiber length.2 In torsion, therefore, it is likely that interlamellar connections play a strong role in minimizing the effects of injuries near the disc mid-plane, but that integrity of AF connections to the vertebrae (which are disrupted in rim lesions) are most important in determining AF torsional biomechanical behaviors.
An immediate decrease in bending hysteresis was observed following rim lesions, concentric tears, and radial tears with rim lesions always resulting in an additional loss of stiffness.62 Less severe injuries produced less consistent results, with stiffness decreasing following radial and concentric tears in some cases,25 but not in others.62 The rim lesion was shown to result in decreased stiffness regardless of whether the injured side of the disc was placed in tension or compression,22 suggesting a fundamental change in joint kinematics and stability, which may be associated more with loss of pressurization than with the disruption of load transmission between vertebrae.
Puncture injuries did not consistently result in changes in bending mechanics. In human cadaveric studies radial tears correlated with either an increase or decrease in bending stiffness depending on the study, while rim lesions and concentric tears were correlated with an increase in bending stiffness.55,63 These findings point strongly toward a biomechanical process by which the disc adapts and compensates for the initial change in mechanics following the acute injury. The discrepancy in findings regarding chronic response to radial tears may be associated with the extent of the radial tear, which may propagate with loading and can increase the risk of prolapse in IVDs with radial tears.17,48
Puncture injuries have the ability to depressurize the NP with relatively minor AF disruption (depending on needle gage size). Thus far, studies of needle puncture injuries have focused on changes in axial mechanics and disc height. Disc height was decreased by puncture in all cases both acutely15,23,38 and with time passing after injury.11,25,47,58 In cases where the puncture was relatively large (>40% of disc height), a decrease in disc axial stiffness was observed to be independent of needle size.38,46 Below this threshold size, changes in cyclic axial mechanics were either insignificant14 or evident only in neutral zone length.23 Under static loading, however, only a puncture injury over 90% of disc height showed an effect on creep time constant.31 Further supporting the hypothesis that injuries act predominantly to alter nuclear pressurization under axial load are the findings that needle puncture only results in disc height loss if it completely penetrates the AF,11 and that intradiscal pressure under axial compression decreases following a mid-plane tear which penetrates to the nucleus.29 Following a mid-plane tear injury, significant changes have been observed under static loading,36 but not dynamic loading,34 suggesting that the influence of altered NP pressurization increases with time scale of loading and interacts with the total amount of fluid entering or exiting the disc.
The effects of injury on axial mechanics of the disc generally support the hypothesis that this mode of deformation is heavily dependent on NP pressurization. Loss of disc height and stiffness are exhibited only when an injury fully penetrates the AF, and is large enough to not be “plugged” by NP material. The importance of pressurization to disc axial stiffness may be surmised from the lack of injury size dependence in some studies. This suggests a pressure vent phenomenon, by which an injury can provide an alternate pathway for fluid to enter and exit the NP, and at any given rate of deformation will cease to provide substantial resistance to fluid flow above a certain injury size. This pressure vent phenomenon may also have a profound impact on disc nutrition, by severely diminishing flow via the vertebral endplates.
EFFECTS OF INJURY ON CELL DEATH, BIOSYNTHESIS RATES, AND INFLAMMATION
Many studies have demonstrated that injury to the IVD influences biological homeostasis within the IVD in terms of cell viability, anabolic/catabolic mechanisms, and initiation of inflammatory processes.28,54,57,58 As a consequence, injury is often used as a mechanism to investigate the pathophysiology of IVD degeneration in animal models.7,8,44 The biological responses to injury are diverse, and dependent on the mechanism by which injury has been induced (puncture vs. anular tear) as well as the number of injuries (acute vs. chronic).
Cells within the IVD are sparse and exist in a harsh tissue environment even in the healthy condition with limited nutrition, and low pH and oxygen. Hence cell death induced by injury may have a large, long-term impact on the overall cellularity of the IVD. Court et al.21 used acute static bending at an angle of 42° to induce injury in the IVD of a mouse tail animal model. They observed decreases in anular cellularity on the compressed side of the IVD, which were irrecoverable after 3 months, with little change in the NP or AF on the uncompressed side, suggesting that cellular damage accumulation may be an important injury mechanism that is distinct from acute mechanical failure of the IVD. Decreases in cell viability were also observed by Haschtmann et al., in a rabbit model of vertebral endplate fracture, where up-regulation of lactate dehydrogenase activity (a method of detecting cell damage), coupled with an increase in the expression caspase 3 and FasL (pro-apoptotic factors) were noted in NP and AF regions. The authors concluded they could not discern whether the post-traumatic changes were consequences of physical damage to the IVD or associated only with NP depressurization.28 Studies investigating effects of needle puncture in a bovine organ culture model demonstrated little change in cell viability except for the needle insertion site where increased cell death was observed. Minimal changes in overall IVD cell viability suggested that overall cell viability was not immediately affected by the altered disc mechanics associated with the needle puncture injury.38 Thus, different methods of injury or insult can result in varying levels of IVD cell death.
Injury to the IVD can also alter cell metabolism and biochemical composition in favor of catabolic processes. Up-regulation of the gene expression of matrix enzymes such as matrix-metalloproteinase 1 (MMP-1), -3, and -13 combined with down-regulation of their inhibitors, TIMPs has been observed in the NP and AF of rabbit IVD models of injury.28,57 This correlated with a down-regulation of matrix proteins such as aggrecan and type II collagen and up-regulation of type I collagen in the NP. Changes have also been observed in the gene expression of anabolic factors such as BMPs and TGFβ suggestive of tissue remodeling effects.57 It is reasonable to assume that an imbalance between anabolic and catabolic processes within the injured IVD may perpetuate the immediate effects of injury observed on the biomechanical properties to further compromise IVD structure and function. Changes observed at the gene expression level support injury induced alterations in the biochemical composition of the IVD. Kim et al.37 created four rabbit IVD injury models using injection of an apoptotic agent (23G needle), nucleus aspiration (21G needle), 3 anular punctures (21G), and 1 anular puncture (18G). The most dramatic biochemical changes were observed with nucleus aspiration with decreases in both NP water content and sulfated glycosaminoglycan (GAG) content 12 weeks post injury. Anular puncture led to alterations in NP water content only, suggesting that physical injury to the NP has a greater impact on the overall biochemical composition of the IVD rather than anular puncture. Miyamoto et al.47 also induced injury in a rabbit IVD anular puncture model with an 18G needle, however, unlike the findings of Kim et al.,37 found decreases in GAG coupled with an increase in collagen content in the NP after 4 weeks. Little change was observed in the AF.47 Studies by Han et al.27 using a rat tail anular percutaneous puncture model (20G) demonstrated little change in total water or collagen content with both half and full penetration of the anulus yet significant decreases in GAG content 4 weeks post injury, which may represent early degenerative changes to injury. Korecki et al.38 did not find a change in water content or GAG in bovine caudal IVD explants 1 week after needle injury with either a 25G or 14G needle, but found alterations in biomechanical properties and suggested larger compositional changes may occur downstream of these organ level biomechanical changes.
A number of studies demonstrated that proinflammatory cytokines can influence cell viability, biochemical composition, cell metabolism, and the expression of pain-related factors in cells of the IVD.28,41,50,56 Injury can be used as a means of inducing degeneration in the IVD and the degenerate phenotype is often characterized by inflammation including an up-regulation of pro-inflammatory cytokines such as TNF-α and IL-1β.41 In spite of this, few studies have focused on the effect of injury of the IVD in terms of inflammation and pain. Haschtmann et al.28 demonstrated a correlation between increased cell death and the expression of TNF-α in the NP of a vertebral endplate injury model, suggesting that up-regulation of TNF-α may lead to continued apoptosis induced by Fas and TNF-α receptor bearing cells. A rabbit anular stab model observed changes in the levels of IL-1β expression with an initial increase at 3 weeks followed by another peak at 24 weeks.58 To examine the effects of injury on cytokine expression in an IVD rat tail model, Rousseau et al. stabbed the IVD with a number 11 blade54 and found an increase in IL-1β expression on Day 4 however little change in TNF-α or IL-6. However, in a triple stab IVD rat tail model, increases in IL-1β, TNF-α, and IL-8 gene expression were observed.54 It appears clear that inflammation induced by injury in the IVD is influenced by the method of injury and whether the injury is acute vs. chronic. However, the literature on the pathophysiology of localized injury on the IVD is limited and further work is necessary to gain a better insight into the mechanisms associated with injury induced inflammation and pain and also those impacts on the biological and biomechanical properties of whole IVD.
EFFECTS OF NEEDLE INJECTION ON GENE EXPRESSION AND VIABILITY IN BOVINE ORGAN CULTURE
At this point, it is clear that needle puncture and injection causes biomechanical and biological changes to the IVD. Needle injection also remains a promising pathway for delivery of biologic treatments into the IVD and saline injection into the IVD is often used as a control to normalize the effects of growth factor injection. However, the effects of needle puncture or needle injection on IVD cell viability and mRNA expression are not that clear. A study was performed to assess how saline injection in a bovine caudal IVD organ culture model influenced qRT-PCR measurements in the AF and NP regions, and influenced semi-quantitative measurements of cell viability in these regions. Needle puncture has been demonstrated to have significant effects on the gene expression of anabolic and catabolic matrix proteins within the IVD,54 therefore we examined a selection of genes representing important matrix proteins, a relevant matrix metalloproteinase, and an aggrecanase (aggrecan, collagen type II, MMP-1, and ADAMTS-4, respectively).
Bovine caudal IVDs were orientated using sterile forceps and the sterile 25G needle tip attached to a 1 mL syringe was inserted through the AF laterally into the NP of the IVDs (10–15 mm depth depending on IVD diameter). A volume of 100 µL of PBS/0.1%BSA was slowly injected into the NP of the IVD. The needle tip was removed and the injection site marked with India ink. The injected IVDs were set up in organ culture chambers as described previously with a static load of 0.2 MPa and cultured for 24 h with 5% CO2 at 37 °C (n = 7).39 Un-injected bovine caudal IVDs were also set up in culture chambers to serve as controls (n = 7). After 24 h in culture, IVDs were removed, RNA isolated from tissue, cDNA synthesized, and SYBR green QRT-PCR carried out using bovine specific primers for 18s, aggrecan, collagen type II, MMP-1 and ADAMTS-4, and the comparative Ct method normalizing to 18s and un-injected controls.43 Statistical analysis was performed using a Student’s t test of the ΔΔCt values with hypothesized mean = 0 (ΔΔCt for PBS injected and ΔΔCt = 0 for un-injected controls).
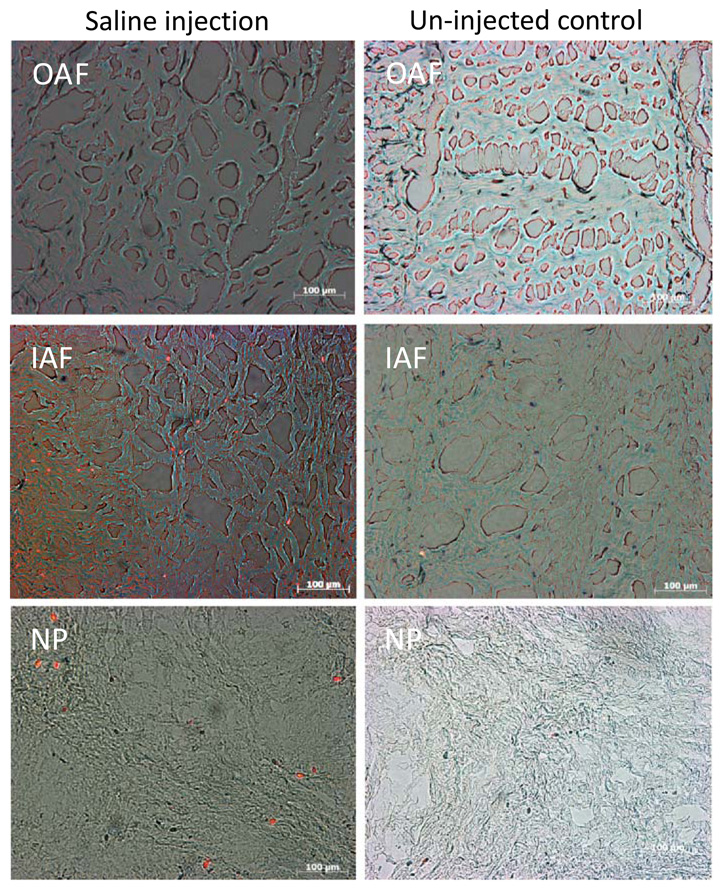
To assess the effects of saline injection on cell viability both saline injected (n = 5) and un-injected IVDs (n = 4), were incubated in 1 mg/mL of 3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT: Sigma) and Ethidium Homodimer-1 (ETH: Invitrogen) in PBS for 3 h.39 Following incubation, IVD tissue was washed and frozen at −20 °C, and three sections, and 3 × 10 µm thick sections of IVD tissue spaced 100 µm apart were cut to include AF, IAF, and NP regions using a cryotome. Bright field (MTT: detection of live cells) and fluorescent images (ETH: detection of dead cells) for each region of the IVD were captured at 20× magnification and merged. Cell viability was assessed using the scoring system (1 = all alive, 2 = mostly live, 3 = half alive, 4 = mostly dead, 5 = all dead) previously described.39
Saline injection resulted in a general down regulation of the matrix proteins including aggrecan and collagen type II, particularly in the NP (Fig. 3). Significant decreases of >10-fold (p < 0.05) were observed in the NP for both matrix proteins with changes of <4-fold (p > 0.05) in the AF. No significant changes were observed in MMP-1 or ADAMTS-4 expression for both the NP and AF. At the gene expression level, strong down-regulation of anabolic gene expression may favor a degenerative phenotype, particularly with respect to the NP. Two additional IVDs used Calcein-AM injection with 100 µL of fluid into the NP region of intact bovine caudal IVDs to verify that the injection diffused to both AF and NP regions and that the calcein was taken up by cells in both regions. Therefore, that greater changes were observed in the NP than the AF suggests that NP cells may be more sensitive to changes in solute concentration/fluid flow in combination with localized needle injury than the AF cells. Furthermore, there was some suggestion of greater loss of cell viability in the NP and IAF regions of IVDs with PBS injection compared to un-injected control IVDs (mean ± SEM scores of 3.4 ± 0.3 and 2.9 ± 0.3, respectively, for the NP region; and 2.8 ± 0.4 and 1.6 ± 0.2, respectively, for the IAF region) (Fig. 4). The OAF region showed no clear trends of viability with saline injection (mean ± SEM scores for saline injected and un-injected IVDs of 1.9 ± 0.3 and 2.3 ± 0.4, respectively, for OAF region). These results support the concept that saline injection may be injurious and should be used with caution.
FIGURE 3.
Fold changes in mRNA levels relative to 18s and un-injected controls (mean ± SEM) for anabolic and catabolic matrix genes in a bovine IVD organ culture model (n = 7). The effect of saline injection using a 25G needle resulted in a general down-regulation of mRNA expression for Aggrecan and Collagen type II. Little change was observed for MMP-1 and ADAMTS-4 (* p < 0.05).
FIGURE 4.
Viable and dead cells in the IAF and NP of IVD tissue injected with saline including un-injected control IVDs. MTT and ETH, which stain live cells blue and dead cells red/orange, respectively, were used to assess cell viability in healthy and saline injected bovine IVD caudal explants tissue. There were some non-significant trends of fewer viable cells in the IAF and NP of saline injected IVD tissue compared to un-injected controls.
Mechanisms for the reduction in matrix gene expression observed from saline injection may be associated with a number of factors including reduced NP pressure, transport, and loss of cell viability. We are not able to discern needle puncture effects from saline injection with the current study design, yet by comparing with the literature we may infer some distinct effects of needle puncture and saline injection. Needle puncture alone causes localized cell death in an organ culture model38 and reduces anabolic gene expression in the rabbit in vivo needle puncture model.59 Saline injection, as reported in this study, appears to causes a more generalized loss of cellularity with some similar reductions in anabolic gene expression as needle puncture alone. Taken together, we infer that needle puncture has a larger effect on gene expression while saline injection may have a larger effect on cell viability. Based on this review, it is reasonable to speculate that reduced pressurization due to needle puncture causes a profound loss of pressurization which may account for the reduced matrix gene expression observed particularly in the NP. These findings and review highlight that further work is necessary to more specifically identify mechanisms for biological and biomechanical alterations following minor injuries such as needle puncture and injection, and their regional effects. Such findings have important implications for growth factor/cell injection therapy.
CONCLUSIONS
IVD injuries, defined as rim lesions, mid-plane tears, circumferential tears, radial lesions, and puncture injuries all had specific and sensitive effects on IVD biomechanics based largely on how they influence NP pressurization and AF load transmission. IVD injuries also resulted in cell death, altered biosynthesis rates and inflammation. These biological effects lead to downstream changes on the protein and structural level, which can also lead to altered biomechanical behaviors. The new results on effects of needle injection on IVD gene expression and viability provide a specific example of how even minor injuries can influence IVD biology, mechanics, and their interactions.
In conclusion, biomechanical alterations were injury specific, with puncture and other tears that penetrate the AF leading to loss of NP pressurization and rim lesions most strongly implicated in transmitting loads between vertebrae. IVD injuries also reduce cellularity and biosynthesis rates while increasing inflammation. Interestingly, even a minor injury such as needle puncture or saline injection with a 25G needle can reduce matrix gene expression. Therefore, specific localized injuries in the IVD have the strong capacity to induce a degenerative cascade on the organ level through biomechanical and biological mechanisms. Biomechanically, attempts at IVD repair should target the dual AF biomechanical roles of maintaining NP pressurization and transmitting loads across the vertebrae. Biologically, it remains important to retain IVD cellularity and biosynthesis rates following injury to prevent downstream biochemical and structural changes. Most importantly, the interacting biological and biomechanical factors required for IVD maintenance and function must be addressed to repair degenerating IVDs.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institute of Health (R01 AR051146), the AO Foundation, and The NASA Vermont Space Grant Consortium (NNX07AK92A).
REFERENCES
- 1.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20(24):2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Adams M. Laboratory model of lumbar disc protrusion: fissure and fragment. Spine. 1994;19(17):2015–2017. [PubMed] [Google Scholar]
- 3.Adams MA, Dolan P. Spine biomechanics. J. Biomech. 2005;38(10):1972–1983. doi: 10.1016/j.jbiomech.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Adams MA, McNally DS, Dolan P. ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Joint Surg. Br. 1996;78(6):965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 5.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 6.Aladin DM, Cheung KM, Chan D, Yee AF, Jim JJ, Luk KD, Lu WW. Expression of the Trp2 allele of COL9A2 is associated with alterations in the mechanical properties of human intervertebral discs. Spine. 2007;32(25):2820–2826. doi: 10.1097/BRS.0b013e31815b75c5. [DOI] [PubMed] [Google Scholar]
- 7.Alini M, et al. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An HS, Masuda K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J. Bone Joint Surg. Am. 2006;88 Suppl 2:88–94. doi: 10.2106/JBJS.E.01272. [DOI] [PubMed] [Google Scholar]
- 9.An HS, et al. Introduction: disc degeneration: summary. Spine. 2004;29(23):2677–2678. doi: 10.1097/01.brs.0000147573.88916.c6. [DOI] [PubMed] [Google Scholar]
- 10.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki Y, Akeda K, An H, Muehleman C, Takahashi K, Moriya H, Masuda K. Nerve fiber ingrowth into scar tissue formed following nucleus pulposus extrusion in the rabbit anular-puncture disc degeneration model: effects of depth of puncture. Spine. 2006;31(21):E774–E780. doi: 10.1097/01.brs.0000238681.71537.41. [DOI] [PubMed] [Google Scholar]
- 12.Battie MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J. Bone Joint Surg. Am. 2006;88 Suppl 2:3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 13.Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur. Spine J. 2005;14(1):27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boxberger JI, Auerbach JD, Sen S, Elliott DM. An in vivo model of reduced nucleus pulposus glycosaminoglycan content in the rat lumbar intervertebral disc. Spine. 2008;33(2):146–154. doi: 10.1097/BRS.0b013e31816054f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boxberger JI, Sen S, Yerramalli CS, Elliott DM. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J. Orthop. Res. 2006;24(9):1906–1915. doi: 10.1002/jor.20221. [DOI] [PubMed] [Google Scholar]
- 16.Boyd LM, Richardson WJ, Allen KD, Flahiff C, Jing L, Li Y, Chen J, Setton LA. Early-onset degeneration of the intervertebral disc and vertebral end plate in mice deficient in type IX collagen. Arthritis Rheum. 2008;58(1):164–171. doi: 10.1002/art.23231. [DOI] [PubMed] [Google Scholar]
- 17.Brinckmann P, Porter RW. A laboratory model of lumbar disc protrusion. Fissure and fragment. Spine. 1994;19(2):228–235. doi: 10.1097/00007632-199401001-00019. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989;23(1):75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 19.Cheung KM, et al. Association of the Taq I allele in vitamin D receptor with degenerative disc disease and disc bulge in a Chinese population. Spine. 2006;31(10):1143–1148. doi: 10.1097/01.brs.0000216530.41838.d3. [DOI] [PubMed] [Google Scholar]
- 20.Costi JJ, Stokes IA, Gardner-Morse MG, Iatridis JC. Frequency-dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine. 2008;33(16):1731–1738. doi: 10.1097/BRS.0b013e31817bb116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Court C, Chin JR, Liebenberg E, Colliou OK, Lotz JC. Biological and mechanical consequences of transient intervertebral disc bending. Eur. Spine J. 2007;16(11):1899–1906. doi: 10.1007/s00586-007-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Visser H, Rowe C, Pearcy M. A robotic testing facility for the measurement of the mechanics of spinal joints. Proc. Inst. Mech. Eng. [H] 2007;221(3):221–227. doi: 10.1243/09544119JEIM175. [DOI] [PubMed] [Google Scholar]
- 23.Elliott DM, Yerramalli CS, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33(6):588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 24.Evans C. Potential biologic therapies for the intervertebral disc. J. Bone Joint Surg. Am. 2006;88 Suppl 2:95–98. doi: 10.2106/JBJS.E.01328. [DOI] [PubMed] [Google Scholar]
- 25.Fazzalari NL, Costi JJ, Hearn TC, Fraser RD, Vernon-Roberts B, Hutchinson J, Manthey BA, Parkinson IH, Sinclair C. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine. 2001;26(23):2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 26.Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: effect of porosity. Ann. Biomed. Eng. 2004;32(12):1710–1717. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 27.Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, Lin M, Wang J, Chen QX. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33(18):1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 28.Haschtmann D, Stoyanov JV, Gedet P, Ferguson SJ. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur. Spine J. 2008;17(2):289–299. doi: 10.1007/s00586-007-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm S, Ekstrom L, Kaigle Holm A, Hansson T. Intradiscal pressure in the degenerated porcine intervertebral disc. Vet. Comp. Orthop. Traumatol. 2007;20(1):29–33. [PubMed] [Google Scholar]
- 30.Horner HA, Urban JP. Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26(23):2543–2549. doi: 10.1097/00007632-200112010-00006. 2001. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine. 2009;34(10):998–1005. doi: 10.1097/BRS.0b013e31819c09c4. [DOI] [PubMed] [Google Scholar]
- 32.Iatridis JC, MacLean JJ, Ryan DA. Mechanical damage to the intervertebral disc annulus fibrosus subjected to tensile loading. J. Biomech. 2005;38(3):557–565. doi: 10.1016/j.jbiomech.2004.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johannessen W, Elliott DM. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine. 2005;30(24):E724–E729. doi: 10.1097/01.brs.0000192236.92867.15. [DOI] [PubMed] [Google Scholar]
- 34.Kaigle A, Holm S, Rostedt M, Hansson T. In vivo dynamic stiffness of the porcine lumbar spine exposed to cyclic loading: influence of load and degeneration. J. Spinal Disord. 1998;11(1):65–70. [PubMed] [Google Scholar]
- 35.Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24(23):2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Keller TS, Holm SH, Hansson TH, Spengler DM. Volvo Award in experimental studies. The dependence of intervertebral disc mechanical properties on physiologic conditions. Spine. 1990;15(8):751–761. doi: 10.1097/00007632-199008010-00004. 1990. [DOI] [PubMed] [Google Scholar]
- 37.Kim KS, Yoon ST, Li J, Park JS, Hutton WC. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine. 2005;30(1):33–37. doi: 10.1097/01.brs.0000149191.02304.9b. [DOI] [PubMed] [Google Scholar]
- 38.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine. 2008;33(3):235–241. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korecki CL, MacLean JJ, Iatridis JC. Characterization of an in vitro intervertebral disc organ culture system. Eur. Spine J. 2007;16(7):1029–1037. doi: 10.1007/s00586-007-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krismer M, Haid C, Rabl W. The contribution of anulus fibers to torque resistance. Spine. 1996;21(22):2551–2557. doi: 10.1097/00007632-199611150-00004. [DOI] [PubMed] [Google Scholar]
- 41.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 2005;7(4):R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levicoff EA, et al. Safety assessment of intradiscal gene therapy II: effect of dosing and vector choice. Spine. 2008;33(14):1509–1516. doi: 10.1097/BRS.0b013e318178866c. (discussion 1517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J. Bone Joint Surg. Am. 2006;88 Suppl 2:76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 45.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29(23):2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 46.Michalek AJ, Funabashi KL, Iatridis JC. The interactive effects of needle puncture injury and compressive overload on the biomechanics of rat intervertebral discs. 55th Annual Meeting of the Orthopaedic Research Society.2009. [Google Scholar]
- 47.Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GB, An HS. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6(6):692–703. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Natarajan RN, Andersson GB, Patwardhan AG, Verma S. Effect of annular incision type on the change in biomechanical properties in a herniated lumbar intervertebral disc. J. Biomech. Eng. 2002;124(2):229–236. doi: 10.1115/1.1449906. [DOI] [PubMed] [Google Scholar]
- 49.Perie DS, Maclean JJ, Owen JP, Iatridis JC. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Ann. Biomed. Eng. 2006;34(5):769–777. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res. Ther. 2008;10(4):R99. doi: 10.1186/ar2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21(4):415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 52.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 53.Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, Antoniou J. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur. Cell. Mater. 2006;11:1–7. (discussion 7) [PubMed] [Google Scholar]
- 54.Rousseau MA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. Stab incision for inducing intervertebral disc degeneration in the rat. Spine. 2007;32(1):17–24. doi: 10.1097/01.brs.0000251013.07656.45. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt TA, An HS, Lim TH, Nowicki BH, Haughton VM. The stiffness of lumbar spinal motion segments with a high-intensity zone in the anulus fibrosus. Spine. 1998;23(20):2167–2173. doi: 10.1097/00007632-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 56.Seguin CA, Bojarski M, Pilliar RM, Roughley PJ, Kandel RA. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25(7):409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Sobajima S, Kim JS, Gilbertson LG, Kang JD. Gene therapy for degenerative disc disease. Gene Ther. 2004;11(4):390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 58.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2005;30(1):15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 59.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 60.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, Ala-Kokko L, Riihimaki H. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur. Spine J. 2006;15(5):613–619. doi: 10.1007/s00586-005-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson RE, Pearcy MJ, Barker TM. The mechanical effects of intervertebral disc lesions. Clin Biomech (Bristol, Avon) 2004;19(5):448–455. doi: 10.1016/j.clinbiomech.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Thompson RE, Pearcy MJ, Downing KJ, Manthey BA, Parkinson IH, Fazzalari NL. Disc lesions and the mechanics of the intervertebral joint complex. Spine. 2000;25(23):3026–3035. doi: 10.1097/00007632-200012010-00010. [DOI] [PubMed] [Google Scholar]
- 64.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 65.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 66.van Deursen DL, Snijders CJ, Kingma I, van Dieen JH. In vitro torsion-induced stress distribution changes in porcine intervertebral discs. Spine. 2001;26(23):2582–2586. doi: 10.1097/00007632-200112010-00011. [DOI] [PubMed] [Google Scholar]