Abstract
Given the increasing research emphasis on putative accumbal functional compartmentation, we sought to determine whether neurons that demonstrate changes in tonic firing rate during cocaine self-administration are differentially distributed across subregions of the NAcc. Rats were implanted with jugular catheters and microwire arrays targeting NAcc subregions (Core, Dorsal Shell, Ventromedial Shell, Ventrolateral Shell, Rostral Pole Shell). Recordings were obtained after acquisition of stable cocaine self-administration (0.77 mg/kg/0.2mL infusion; fixed-ratio 1 schedule of reinforcement; 6 hour daily sessions). During the self-administration phase of the experiment, neurons demonstrated either: 1) tonic suppression (or decrease), 2) tonic activation (or increase) or 3) no tonic change in firing rate with respect to rates of firing during pre- and post-drug phases. Consistent with earlier observations, tonic decrease was the predominant firing pattern observed. Differences in the prevalence of tonic increase firing were observed between the core and the dorsal shell and dorsal shell-core border regions, with the latter two areas exhibiting a virtual absence of tonic increases. Tonic suppression was exhibited to a greater extent by the dorsal shell-core border region relative to the core. These differences could reflect distinct subregional afferent processing and/or differential sensitivity of subpopulations of NAcc neurons to cocaine. Ventrolateral Shell firing topographies resembled those of core neurons. Taken together, these observations are consistent with an emerging body of literature that differentiates the accumbens mediolaterally and further advances the likelihood that distinct functions are subserved by NAcc subregions in appetitive processing.
Keywords: addiction, drug abuse, neurophysiology, reward, ventral striatum
The nucleus accumbens (NAcc), commonly regarded as the brain’s limbic-extrapyramidal motor interface (Nauta & Domesick,1978; Mogenson et al., 1980), has been shown to be involved in the processing of natural rewards (Hernandez & Hoebel, 1988; Smith & Sneider, 1988; Robbins et al., 1989; Schultz et al., 1992; Damsma et al., 1992; Young et al., 1992; Salamone, 1992) and drug taking behaviors (Caine et al., 1995; Carlezon & Wise, 1996; Di Chiara, 1998; Cornish & Kalivas, 2000; Robinson & Berridge, 2000; Nicola et al., 2000). Substantial advances have begun to resolve physiological distinctions between the two principal compartments of the NAcc, the core and shell, and their differential involvement in drug reward (Deutsch & Cameron, 1992; Pennartz et al., 1992; Robledo & Koob, 1993; Jongen-Relo et al., 1994b; Pontieri et al., 1994; Voorn et al., 1994; O’Donnell & Grace, 1995; Ikemoto et al., 1995; Pontieri et al., 1995; Meredith, 1999; Rodd-Henricks et al., 2002). Measurements of the physiological changes that occur in the NAcc in acute preparations (DeFrance et al., 1985a,b; White & Wang, 1986; White, 1987, 1990; Boeijinga et al., 1990, 1993; White et al., 1993; O’Donnell & Grace, 1993; White et al., 1995; Nicola et al., 1996; Blaha et al., 1996: Nicola & Malenka, 1997; Floresco et al., 2001a) or during drug self-administration (Pettit & Justice, 1989, 1991; Pettit et al., 1990; Carelli et al., 1993, 2000; Chang et al., 1994, 2000; Gratton & Wise, 1994; Kiyatkin & Stein, 1995; Wise et al., 1995; Peoples et al., 1998; Peoples et al., 1999; Ghitza et al., 2003, 2004) have provided critical insight into the mechanisms by which its ensembles of neurons are implicated in drug-taking behavior.
The present study investigated accumbens output signals in the rat using the single-unit extracellular recording technique, wherein microwires were placed throughout the NAcc in order to determine whether electrophysiological differences exist across its subregions. These neural recordings yielded tonic firing patterns, i.e., firing rates over the duration of a cocaine self-administration experiment, which consisted of: 1) a 30 minute pre-drug baseline period, 2) a six hour self-administration session and 3) a 60 minute post-drug period. The tonic decrease pattern was defined by firing rates which decrease relative to pre-drug levels during the self-administration phase of the experiment and typically return to or exceed pre-drug levels in the post-drug phase. Conversely, neurons which demonstrated elevated firing rates during the self-administration phase relative to pre- and post-drug phases were described as exhibiting tonic increases.
Until now, few studies have addressed whether tonic decrease and tonic increase firing patterns are differentially expressed throughout accumbal subregions (Ghitza et al., 2006) and none has comprehensively evaluated the full extent of shell, which has recently been shown to exhibit differential sites in primary drug reinforcement (Ikemoto, 2005). Differences in the prevalence of tonic firing patterns may provide further evidence of functional differences between core and shell subterritories and hence indicate more precise targets for pharmaceutical therapies aimed at treating drug addiction and other neuropathologies involving corticostriatopallidothalamic circuits.
Materials and Methods
Surgery
Male Long-Evans rats (n=32; Charles River Laboratories, USA) were individually housed with a reversed 12 hr light/dark cycle (lights on at 7:00 A.M.), handled daily and food restricted to maintain target body weights between 330 - 350 g (≥90% adult body weight). Rats were anesthetized with an injection of sodium pentobarbital (50 mg/kg body weight, i.p.; Abbot Laboratories, North Chicago, IL, USA). Animals were also injected with atropine methyl nitrate (10 mg/kg i.p.; Sigma, St. Louis, MO, USA) and Penicillin G (75,000 i.u./0.25 mL i.m.; Wyeth Laboratories, Philadelphia, PA, USA). Rats were kept on a heating pad to maintain body temperature while anesthetized. Periodic injections of sodium pentobarbital (5 - 10 mg/kg, i.p.) or ketamine hydrochloride (60 mg/kg, i.p.; Fort Dodge Laboratories, Fort Dodge, IA, USA) were administered to maintain anesthesia over the course of surgery. A jugular catheter was fed through a spring leash and affixed to a skull-anchored headstage via a J-shaped cannula (Weeks, 1962). Microwire array headstages (Shaptech Services, Heightstown, NJ, USA) consisted of two attached rows of miniature connector strips soldered to two rows of eight quad-teflon-coated 50 μm stainless steel microwires (California Fine Wire, Grover City, CA, USA) separated by 0.3 – 0.5 mm. Microwire placements targeted the scope of accumbal subregions (targeted range [mm]: 0.7 to 3.0 A-P, 0.6 to 2.6 M-L, D-V: −6.0 to −8.5; Paxinos & Watson, 1997). Precautions were taken throughout catheterization, stereotaxic surgery and post-surgical housing of rats to maintain aseptic conditions. The treatment of animals was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication) and approved by the Rutgers University Animal Care & Facilities Committee.
Post-operative
Subjects were individually housed in a steel-grid chamber for a minimum of seven days. Thereafter, and at least three days prior to the start of self-administration training, animals were transferred into Plexiglas chambers (38 cm l × 20 cm w × 29 cm h) which henceforth served to accommodate housing, training and testing of subjects. Each Plexiglas chamber was enclosed within one of four ventilated, sound-attenuating isolation chambers (63.5 × 63.5 × 63.5 cm) in an experiment room to which white noise was provided continuously in order to minimize the likelihood of extraneous noises influencing the subjects’ behavior. In order to maintain catheter patency when not engaged in self-administration sessions, rats were infused every 15 min with heparinized bacteriostatic saline (0.2 mL/hour) by a timer-equipped, motor driven syringe pump (Razel Scientific Instruments, Stamford, CT, USA). Two types of swivels were used to maintain continuous intravenous access and animal mobility: an electronic swivel (Airflyte, Inc., Bayonne, NJ, USA), which was used during recording sessions, and a simple fluid swivel (Brown et al., 1976), used when experiments were not in progress. Occasionally, an ultra-short acting barbiturate (methohexital sodium, 10 mg/kg, i.v.; Eli Lilly, Indianapolis. IN, USA) was utilized to anesthetize subjects during the attachment of the electronic swivel or to confirm catheter patency. A tethering system, consisting of the J-shaped stainless steel cannula and metal spring leash, was used to protect the catheter, transfer rotational force to the swivel and provide strain relief for the electrical harness and catheter.
Training
Immediately before the start of a self-administration session, food and water were removed from the chamber and a non-retractable, black Plexiglas response lever was installed on the side wall via a set screw which fixed the lever’s stainless steel post to an aluminum mounting block, maintaining the lever approximately 5 cm above grid floor and 1 cm from the wall. The onset of the session was signaled by the illumination of a stimulus light mounted above the response lever. Each reinforced lever press resulted in a 0.2 mL intravenous infusion of cocaine hydrochloride (NIDA) solution, a 7.5 second tone which corresponded with the duration of syringe pump operation and a 40 second time-out period during which stimulus light was off and lever presses had no programmed consequence. Training sessions were conducted seven days per week, each limited to 80 infusions or 6 hours, whichever was first attained. The average cocaine dose administered, given differences in subjects’ body weights, ranged between 0.70 - 0.91 mg/kg/infusion with a mean drug dose of 0.77 ± 0.01(S.E.M.) mg/kg/infusion. This resulted in an inter-infusion interval with a median of 7.36 ± 0.01(S.E.M.) minutes, excluding the loading phase, which consisted of the first 8 - 10 presses, characteristically at short intervals (Pickens & Thompson, 1968). Each day, drug accumulation curves were evaluated to confirm that stable lever press behavior, and hence drug levels, were maintained throughout the self-administration session. Assuming first-order pharmacokinetics, calculated drug levels were determined over successive infusions by the equation Bn = (Bn-1 + D)e−KTn (Yokel & Pickens, 1974), in which Tn = the time since previous cocaine infusion (min), D = infusion dose (mg/kg), Bn-1 = cocaine level at time of last infusion (mg/kg) and K = rate constant (0.693/t1/2) reflecting the metabolic half-life for cocaine (Nayak et al., 1976). Thus, by plotting calculated drug levels at each cocaine-reinforced lever press, drug curves were generated yielding each subject’s within-session pharmacologic profile. Daily drug curves confirmed the acquisition and maintenance of consistent, uninterrupted lever press behavior. After acquisition, subjects were trained for 12 - 18 self-administration sessions before neural recordings commenced.
Electrophysiological Recording Sessions
Neural recordings began 30 minutes before (i.e. pre-drug phase) the start of the self-administration phase and continued, upon removal of the response lever, for 1 hour afterward (i.e. post-drug phase). The neural signal from individual microwires was led through a field effect transistor in the headset of an electronic harness (NB Labs, Denison, TX, USA), then through the Airflyte electronic swivel. From the swivel the signal continued to a preamplifier (Riverpoint Electronics, Goldsboro, NC, USA) where it was differentially amplified against another microwire that exhibited no neural waveforms. The signal was then conducted through a bandpass (roll-off below 1000 Hz = 1.5 dB/octave and above 11000 Hz = 6 dB/octave; gain = 700) filter/amplifier (Riverpoint Electronics). The amplified signal was then sent to a remote computer where the signal was digitized (Datawave Technologies [Longmont, CO, USA]; 50 kHz sampling frequency per recorded wire), time stamped (0.1 msec resolution) and stored for off line analysis.
Data Analysis
Post-hoc analyses of the neural data were conducted using cluster analysis software (Datawave Technologies) to isolate neural waveforms as described previously (Peoples & West, 1996). During the self-administration session animals exhibited a predictable operant behavioral pattern which resulted in elevated drug levels that remained stable until drug access was terminated. Mean firing rates during baseline and post-drug phases of the experiment were compared to those during the self administration phase.
Analysis of Firing Patterns
The Wilcoxon Matched pairs test (Siegel, 1956; Schultz et al., 1992) was used in the comparison of each neuron’s firing rates between any two blocks of time: baseline, drug phase, or post-drug phase. Baseline firing rate was taken from the last 20 min before the start of the self-administration phase, and post-drug firing rate was taken from the last 20 min of the post-drug phase. Firing rates during two twenty minute periods were evaluated during the self-administration phase, immediately after the first hour (i.e. after drug loading) and the final twenty minutes of the drug phase. These two periods were chosen to assure any within-phase differences in firing were not attributable to differences in drug level. The two 20 minute periods were used to compare firing rates during the drug phase with pre-drug and post-drug phases. Neurons that exhibited both a criterion decrease in firing rate (see below) during the two drug periods relative to baseline firing and a criterion increase in firing during the post-drug period (relative to the second drug period) were operationally defined as showing “tonic decrease” firing. Neurons whose firing rate increased during the two drug periods relative to baseline firing and subsequently decreased in firing during the post-drug period (relative to the second drug period) were categorized as showing “tonic increase” firing. Therefore, all tonic responsive neurons exhibited : 1) post-load firing rate changes that persisted into late drug phase and 2) a reversal of any self-administration phase firing change within an hour of withdrawal of drug availability. The above comparisons between experiment phases were made for every neuron by dividing each block into 40 consecutively numbered bins (0.5 minute bin width) and evaluating the number of discharges from comparably numbered bins which were entered into the test as matched pairs. The alpha level was set at 0.05 (unidirectional) as the criterion in order to be sensitive to changes that were consistent across bins. Thus, the test was used not for drawing inferential statistical conclusions, but rather because of its rigor in identifying consistency in individual neurons’ changes in firing rate. Analyses of test validity (not shown) revealed that 85% of neurons identified as demonstrating a tonic change exhibited the same direction of change on ≥ 80% of bins entered as matched pairs.
Histology
Subjects were injected with a lethal dose of sodium pentobarbital before anodal current (50 mA, 4 seconds) was passed through each of the microwires in the array. Rats were then transcardially perfused with a 10% formalin solution, decapitated and the brains removed and soaked in a formalin/sucrose solution. Fifty μm coronal sections of the brains were then mounted on slides and treated with a 5% solution of potassium ferrocyanide and 10% HCl to stain iron deposits left by the wire tips (Green, 1958). The sections were then counterstained with a 0.2% solution of Neutral Red and coverslipped. Tissue lesions were then analyzed using light microscopy to locate the precise anatomical location of each wire tip on its closest corresponding atlas plate (Paxinos & Watson, 1997). Lesion marks that could not be traced to the wire track identifying the exact wire number in the array were discarded.
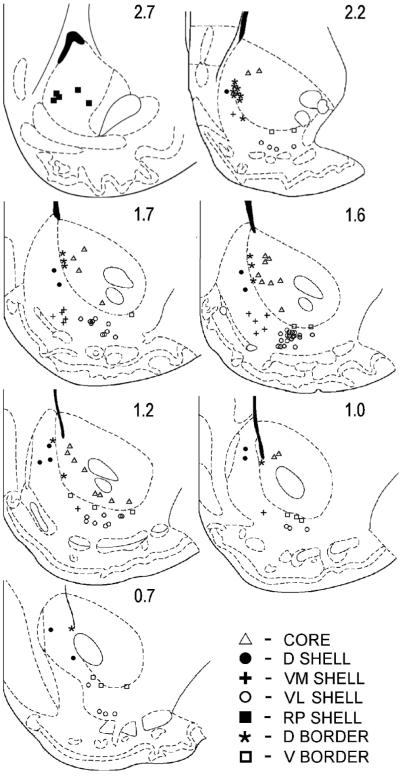
Lesions were localized to the subterritories of the nucleus accumbens as described by Zahm & Brog (1992). The precise location of the wire tip was estimated to be at the center of a lesion mark. An independent observer, blind to whether unitary data had been recorded from any particular wire, evaluated the histological placement of microwires. A stringent criterion required that any lesion center that was within 150 μm of any border be characterized as a “border” neuron. Those that bordered extra-accumbal structures were eliminated from the data pool. Border neurons that lay between core and shell were treated as separate categories: those found at or ventral to −7.6 mm D-V were considered ventral border neurons while those dorsal to −7.6 mm D-V were considered dorsal border neurons.
The shell was subdivided into dorsal, ventromedial and ventrolateral regions. Wires placed in the ventromedial shell subregion were confined to the area: 1) at or posterior to 2.3 mm and anterior to 0.7 mm A-P, 2) ventral to −7.3 mm D-V, and 3) medial to 1.4 mm M-L. Wire tips within shell regions dorsal to this area were categorized as dorsal shell wires, while those located lateral to this region were categorized as ventrolateral shell wires. Rostral Pole neurons were defined by tip locations anterior to 2.3 mm A-P. This additional compartment of the shell reflects the only difference in anatomical boundary designations from our earlier report (Ghitza et al, 2006), which incorporated portions of the present data with neural recordings from animals tested with a discriminative stimulus protocol to compare the firing rates of medial shell with core. Because ventral shell was not a target of the discriminative stimulus investigation, it was not the subject of regional comparisons in the 2006 report, nor were intra-accumbal border neurons. Thus, analyses of the seven subregions described above are herein reported for the first time.
While our goal was a complete assessment of all sectors of the accumbens, we were compelled to accept some limitations. Efforts were made to survey the extent of the core, with the exception of its ill-defined border with the ventral sector of the dorsal striatum (Heimer & Alheid, 1991; Zaborszky et al., 1985) and lateral regions in which placements into the anterior commissure are probable. Unlike the shell, which has received considerable attention in recent years related to its putative functional heterogeneity (Ikemoto, 2003, 2007; Ikemoto et al., 2005), the core survey in the present analyses was treated as a single region.
Statistical Analysis of Firing Pattern Prevalence
Chi-square tests (2×2; α = 0.05; Runyon et al., 1996 were employed to determine whether differences existed in the prevalence of responsive neurons between subterritories for both tonic increase and tonic decrease categories. The percentage of responsive neurons from each of the subregions of the shell (i.e. dorsal, ventromedial and ventrolateral) was compared to the percentage of responsive neurons from the core. The prevalence of responsive neurons in the core was also compared to the percentage of responsive neurons found in the rostral pole shell and dorsal and ventral border regions within the nucleus accumbens. Between-region analyses considered either tonic decrease (or not, including non-responsive and increased) or tonic increases (or not, including non-responsive and decreased). In addition, a histological record of all recorded microwires was maintained (regardless of whether neural data was recorded from a given wire) to determine whether differences exist in the likelihood of recording neural data across subregions.
Results
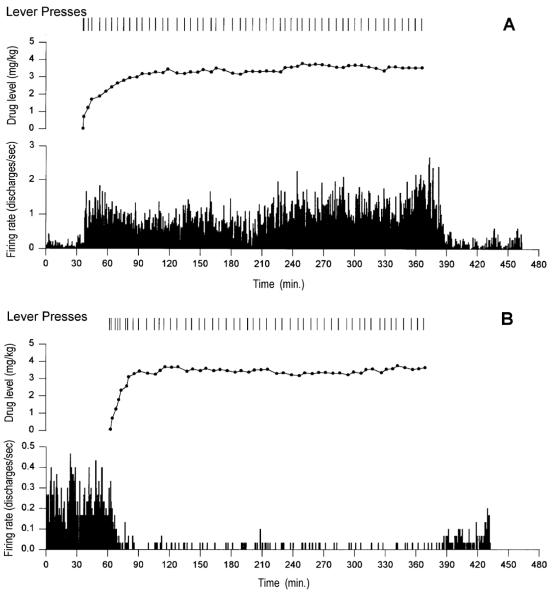
In trained animals, behavior prior to the start of the self-administration phase of experiments was typically characterized by quiescence with occasional grooming. Once the lever was installed and operant behavior commenced, an initial brief period of rapid lever pressing (i.e. load-up) was followed for the remainder of the self-administration phase by regularly spaced self-infusions, which ranged between 6 - 8 minutes (Fig. 1). Between cocaine-reinforced lever presses animals engaged in focused stereotypy, consisting of nose poking, forelimb treading and repetitive head movement. Focused stereotypy typically spanned most of the inter-infusion interval with, in some cases, circling behavior oriented towards the lever occurring in the last half minute before the subsequent lever press. Behavior during the early minutes of the post-drug phase was characterized by locomotion about the chamber, followed, as drug levels declined, by behavior similar to that observed during the pre-drug phase of the experiment.
Figure 1.
Examples of slow phasic reversal firing patterns. Each peri-event time histogram (PETH) displays the firing pattern of one neuron during the minutes before and after the lever press. The ordinate of each histogram displays the average firing rate (i.e. average discharges/s calculated as a function of 0.1-min bins). Time 0 (vertical dashed line) on the abscissa marks the occurrence of the cocaine-reinforced lever press. (A) Examples of decrease + progressive reversal firing patterns. (B) Examples of other categories of slow phasic patterns. Top, increase + progressive reversal; middle, increase + early reversal; bottom, decrease + early reversal. For each PETH, the inset depicts the corresponding neural waveform. Calibrations (bars in A, top) of waveforms: 0.25 ms, 0.10 mV (top left waveform); 0.25 ms, 0.20 mV (all other waveforms).
In order to record neural activity at approximately the same point in the animals’ drug training experience (i.e. after substantial self-administered drug exposure), rats were exposed to long daily access (6h) for a minimum of 2 weeks before neural recordings were conducted. The relatively high cumulative cocaine intake prior to recordings showed little variance across subjects (491.687 + 19.203[S.E.M.] mg/kg). Training experience also differed minimally (86.681 + 0.870[S.E.M.]hours) over two weeks leading up to recording days.
Accumbens neural waveforms exhibited amplitudes which ranged between 100 - 300 μV. The average signal-to-noise ratio was 3.08 ± 0.08 (S.E.M.) with the majority (130/137; 95%) of waveform amplitudes exceeding respective noisebands by 200% (S:N range = 1.82 - 6.90). Neither mean waveform amplitudes nor signal-to-noise ratios differed among accumbal subregions. Waveforms of all neurons met the minimal criteria for admission as single units into the present data pool, including an absence of discharges in the first 2 msec of the inter-spike interval histogram (Moore et al., 1966; Kosobud et al., 1994), consistent with the natural refractory period of a single neuron. The overall mean pre-drug (i.e. baseline) firing rate for accumbal neurons was 0.53 ± 0.12 (S.E.M.) Hz. Mean firing rates during the pre-drug phase did not differ among subregions [F(6,130) = 0.236, P = 0.96] (Table 1).
Table 1.
Electrophysiological Data by Subregion
| SUBREGION | N | MEAN AMPLITUDE (mV) | MEAN SIGNAL:NOISE | BASELINE FIRING RATE |
|---|---|---|---|---|
| Core | 24 | 92 ± 4.1 | 2.91 ± 0.12 | 0.42 ± 0.13 |
| Shell - Dorsal | 12 | 91 ± 4.6 | 2.64 ± 0.12 | 0.44 ± 0.16 |
| Shell - Ventromedial | 13 | 83 ± 4.6 | 2.85 ± 0.18 | 0.63 ± 0.29 |
| Shell - Ventrolateral | 48 | 103 ± 4.2 | 3.20 ± 0.15 | 0.59 ± 0.29 |
| Shell - Rostral Pole | 5 | 104 ± 9.3 | 3.31 ± 0.37 | 0.15 ± 0.05 |
| Dorsal Border | 19 | 109 ± 8.1 | 3.10 ± 0.20 | 0.40 ± 0.09 |
| Ventral Border | 14 | 102 ± 9.5 | 3.43 ± 0.34 | 0.82 ± 0.46 |
| 135 |
Anatomical Distribution of Accumbal Neurons
A total of 512 wires implanted in 32 subjects yielded recordings of 297 basal forebrain neurons, 137 of which were from wires (n = 124) histologically confirmed to be located in the nucleus accumbens. No wires were recorded more than once; above totals comprise autocorrelogram-confirmed single-unit data from solitary FR1 cocaine self-administration sessions, ensuring that no unitary data was twice-weighted in the sampling.
Accumbal Subterritorial Distribution
Seventy six percent (104/137) of the accumbal neurons were histologically confirmed to be in one of the three subterritories of the NAcc. Among these, the majority were placed in the shell (n = 74), while the remainder were placed primarily in the core (n = 25) but a few were found to be in the rostral pole (n = 5). All rostral pole neurons proved to be in the shell subregion (Paxinos & Watson, 1997).
Accumbal Shell Subregional Distribution
The majority of neurons recorded from the shell were localized to the ventrolateral shell (n = 49). The dorsal and ventromedial shell subregions yielded 12 and 13 of the recorded accumbal neurons, respectively.
Accumbal Border Neurons
Thirty three accumbal neurons could not be assigned a subterritorial designation due to their proximity to the boundary between shell and core. They were instead considered as a dorsal border group (n = 19) and a ventral border group (n = 14). As such, a total of 7 NAcc compartments were evaluated in the present study: dorsal shell, ventromedial shell, ventrolateral shell, rostral pole shell, core, dorsal border and ventral border (Fig. 2; Fig. 3).
Figure 2.
Mediolateral comparison of slow phasic reversal categories. Neurons that were histologically confirmed to be in either the lateral nucleus accumbens (NAcc) (i.e. core\ventrolateral shell; n = 74) or the medial NAcc (i.e. dorsal shell\rostral pole shell; n = 17) were evaluated in terms of whether they exhibited: (i) progressive or late reversal patterns (n = 32); or (ii) early reversal patterns (n = 14). To determine whether reversal categories are differentially expressed in the NAcc, a chi-square analysis was conducted. The percentage of early-reversing neurons was greater in the medial NAcc, whereas progressive/late-reversing neurons were more prevalent in the lateral NAcc [χ2(exact) = 4.70, degrees of freedom = 1, *P < 0.05). A post hoc odds ratio analysis confirmed that the differential probability of observing the two different reversal categories between regions was significant (oddsPLR/oddsER = 5.37, *P < 0.05). PLR, progressive/late-reversing; ER, early-reversing; D, dorsal; RP, rostral pole; VL, ventrolateral
Figure 3.
Examples of rapid phasic firing patterns. Each peri-event time histogram displays the firing pattern of a different neuron during the seconds before and after the lever press. The ordinate of each histogram displays average firing rate (i.e. average discharges/s calculated as a function of 0.2-s bins). Time 0 (vertical dashed line) on the abscissa marks the occurrence of the cocaine-reinforced lever press, and corresponds with the raster display above it. (A) A pre-press firing rate increase. (B) A lever press increase. (C) A post-press increase. (D) A lever press decrease. Insets depict corresponding neural waveforms. Calibrations (bars in A) of waveforms: insets A, B, and C, 0.25 ms, 0.20 mV; inset D, 0.25 ms, 0.15 mV.
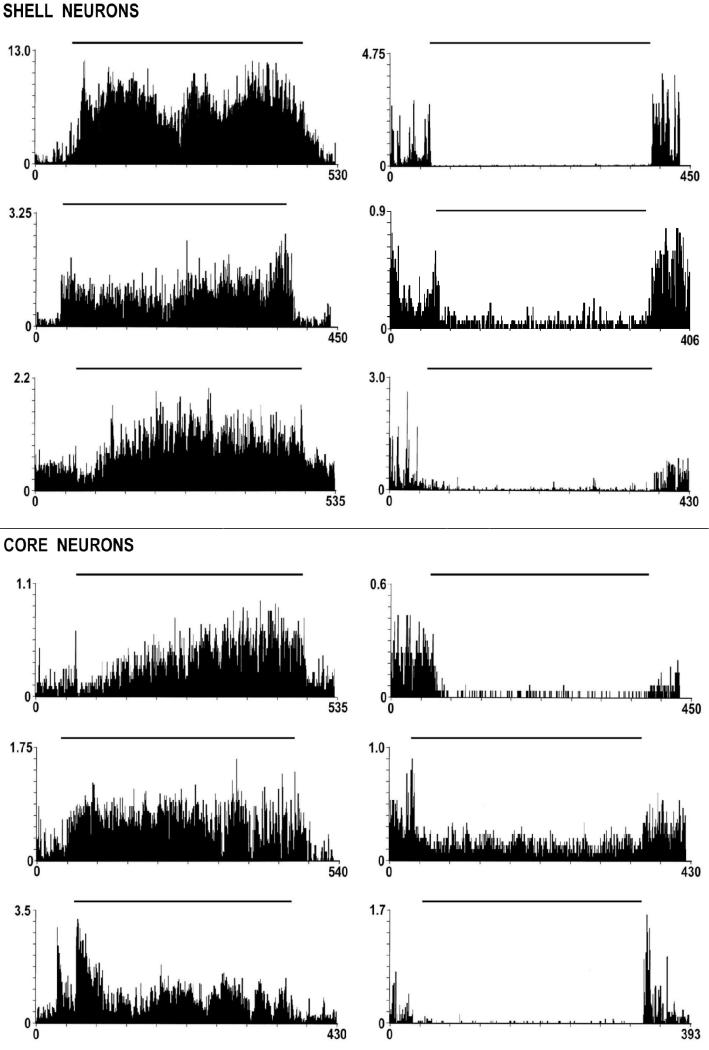
Tonic Firing Patterns
Two of the accumbal neurons from the current data pool, one from the core and one from the ventrolateral shell, were excluded from tonic analyses due to the possibility that signal decreases observed late in the experiment could not unequivocally be attributed to declined rates of firing (but perhaps electrode drift) and therefore 135 neurons are henceforth described in the present analysis. Neural recordings analyzed over the whole experiment time-frame revealed that 62% (84/135) of accumbal neurons showed either decreased rates of firing (tonic decrease) or increased rates of firing (tonic increase) during the self-administration phase. Specifically, 47% (63/135) were decreases and 16% (21/135) were increases.
Tonic category by subjects analysis
Of the 32 rats in the study, all (but one) contributed single digit quantities of unitary data to the pool (range: 1-12 neurons; average neurons/rat: 4.16 ± 0.48 [S.E.M.]). The one rat with 12 neurons yielded recordings with varying tonic firing categories from 4 different subregions. Of all subjects in the data set, 5 rats (16%) had a only a single wire which recorded a neural signal in the accumbens, with a mixture of tonic increase (one), decrease (two) and no tonic change (two) categories. Among the remaining rats with multiple accumbal recordings, only 3 rats (9%) exhibited unidirectional tonic firing changes across accumbal wires (total=7 [2 + 2 + 3]), while all other rats (24) exhibited multiple tonic categories across wires, many of which (42%) exhibited both tonic increases and tonic decreases. This observation of opposite signs in tonic firing directionality among accumbal wires implanted in the same subject reflected subregional prevalences in wire placement, consistent with the findings of the present study. The two rows of wires in the array were most often positioned to yield data from different subregions in the same rat, and often wires positioned medially exhibited tonic decreases, with lateral wires showing tonic increases. Thus, neural sampling within rats tended to reflect the characteristic neurophysiological response of the same anatomical target recorded in other rats, rather than a global trend (e.g. ubiquitous firing decrease) within a particular rat.
Subregional prevalence of tonic decreases
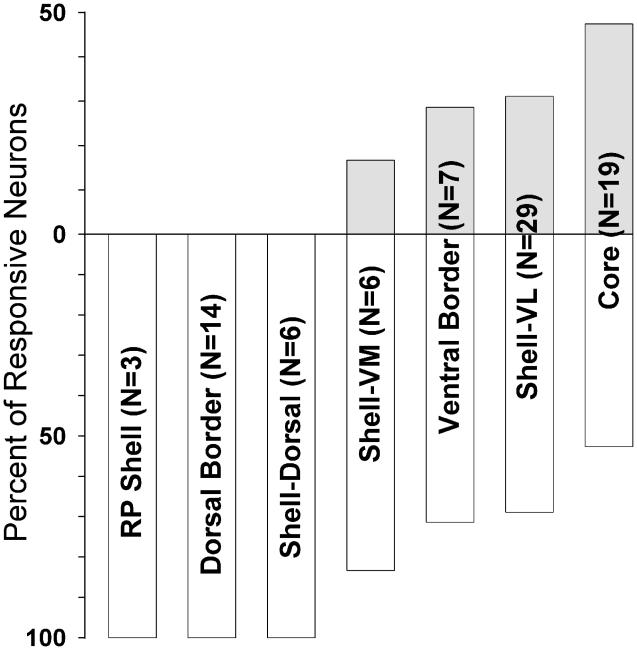
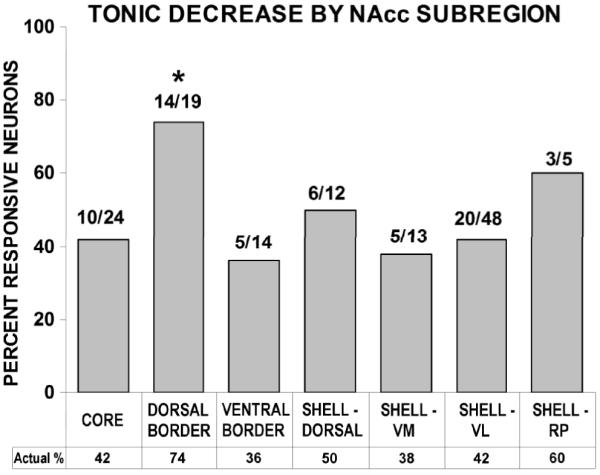
Firing pattern profiles characterized as tonic decreases were similar between shell and core subregions (Fig. 4). Using a 2 × 2 chi-square test (α = 0.05; df = 1), no differences in the prevalence of tonic decrease neurons were observed between the core and any of the shell subregions. Additionally, the core was compared to both the dorsal and ventral border neuron groups. The dorsal border region showed a greater prevalence of tonic decreases than the core (χ2 = 4.41; p < 0.05) (Fig. 5).
Figure 4.
Frequency distributions of pre-press (left panel) and post-press (right panel) rapid phasic firing rate change magnitudes in the medial nucleus accumbens (M-NAcc) vs. the lateral NAcc (L-NAcc). The magnitude of firing rate change is expressed as B/(A + B) (bottom side of x-axis). Values from 0.49 to 0 and from 0.51 to 1.00 reflect increasingly larger firing rate decreases and increases, respectively. Values of 0.50 reflect no change from baseline firing rate (vertical dashed line). The top side of the x-axis indicates twofold, fourfold, etc. increases above, or decreases below, baseline firing rate values. The ordinate scale indicates percentages of the total neurons in the medial (N = 17) and lateral (N = 74) NAcc. The distribution of pre-press B/A + B increases was greater in lateral NAcc (core/ventrolateral shell) neurons than in medial NAcc (dorsal shell/rostral pole shell) neurons [t26 = 1.71, P = 0.02).
Figure 5.
Tonic firing changes in neurons that exhibit slow phasic firing patterns are differentially expressed among nucleus accumbens (NAcc) subregions. The graph depicts tonic firing data for neurons that exhibited slow phasic firing changes. Symbols indicate subregional placement of histologically confirmed NAcc microwires (see key). B/A + B values from 0.49 to 0 and from 0.51 to 1.00 reflect increasingly larger firing rate decreases and increases, respectively. A rather broad distribution of increases and decreases of slow phasic and tonic firing magnitudes is revealed for lateral NAcc neurons (e.g. core and ventrolateral shell), including opposite signs in firing rate change between time bases (i.e. symbols in upper left and lower right quadrants). For the medial NAcc (i.e. dorsal shell and rostral pole shell) and dorsal border regions, it is notable that slow phasic changes were exclusively decreases in neurons that also exhibited tonic decreases (lower left quadrant). D, dorsal; VL, ventrolateral; RP, rostral pole; V, ventral.
Subregional prevalence of tonic increases
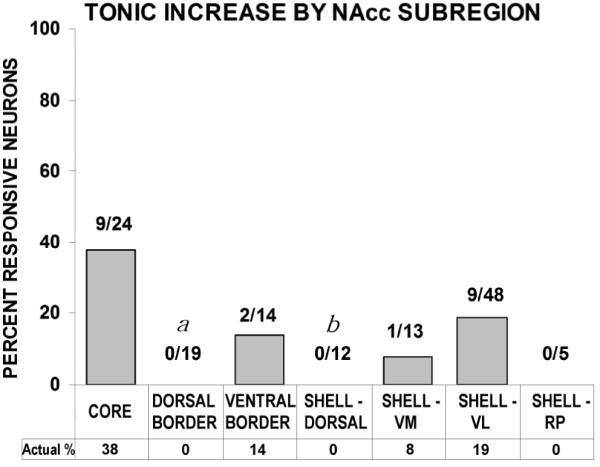
Firing pattern profiles characterized as tonic increases were similar between shell and core subregions (Fig. 4). The same analysis was conducted between subregions for tonic increase neurons as was done for tonic decrease neurons. The prevalence of tonic increases was greater in the core than in both the dorsal shell (χ2 = 6.00; p < 0.02) and dorsal border region (χ2 = 9.01; p < 0.01). (Fig. 6)
Figure 6.
Percentage of slow phasic (SP) increases and SP decreases among tonic categories. All tonic neurons (n = 135) were evaluated by category (increase, decrease, and non-responsive) for the prevalence of SP increases and decreases within them. SP decreases were observed in 33% of tonic increase neurons, 44% of tonic decrease neurons, and 43% of no tonic change neurons. SP increases were observed in 43% of tonic increase neurons, 2% of tonic decrease neurons, and 8% of no tonic change neurons. A 2 × 3 chi-square test revealed that SP increase and decrease reversal patterns were differentially expressed across tonic categories [χ2(2) = 17.3338, P < 0.001). An odds ratio analysis confirmed a differential prevalence of SP increase and decrease patterns between the tonic increase and decrease categories: odds ratio (95% confidence interval) = 0.0311 (0.0033–0.2892).
Probability of Recording Neural Signals Across Subregions
The rigorous histological analysis in the present study required the localization of all 512 wires irrespective of whether a neural recording was obtained from each one. Of the 264 wires positioned in the nucleus accumbens, nearly half (47%; 124/264) yielded neural recordings. Thirteen of these wires were determined, after conducting waveform analyses, to have recorded two neurons (separable by their distinct waveforms and confirmed using cross-correlational analysis). Histological examination of these wire tip locations provided little information about differences in cell density across subregions because the two-unit wires were located in 5 different subregions of the accumbens.
Is one more likely to record neural activity from one subregion versus another? An analysis was conducted which evaluated for each subregion the percentage of wires from which neural recordings were obtained relative to those that yielded no neural recordings. Relative to the overall accumbens recording probability of 47%, neural vs. non-neural wires differed by no more than 3 percentage points in any subregion (core 44%; dorsal shell 48%; ventromedial shell 48%; ventrolateral shell 46%; rostral pole shell 50%) with one exception: 63% of the wires in the dorsal border region yielded neural recordings.
Discussion
The majority (62%) of accumbal neurons exhibited significant firing rate changes during the self-administration phase; these changes were distributed throughout accumbal subregions: core, dorsal shell, ventromedial shell, ventrolateral shell, rostral pole shell, dorsal border and ventral border subregions. Significant shell/core differences were demonstrated among tonic increase neurons, with core exhibiting greater firing rate activation than dorsal shell. In addition, relative to core, neurons of the dorsal border region exhibited both a significantly greater percentage of tonic decrease firing and a significantly lower percentage of tonic increase firing. Thus, an overall trend towards suppression was observed in the tonic firing of medial NAcc neurons, characterized by a virtual absence of tonic increase firing throughout its anteroposterior extent. Conversely, tonic increase firing patterns were found predominantly in lateral NAcc subregions (e.g. core, VL shell) (Fig. 7).
These data, by incorporating a more refined analysis of the shell and including intra-accumbal shell/core border areas, extend the findings of our earlier report (Ghitza et al., 2006). While the 2006 paper identified subterritorial differences in firing between shell and core, the more expansive regional analysis of the present data set revealed that the prevalence of medial shell and core tonic firing patterns are not just different from one another, but that a general neurophysiological distinction exists mediolaterally in the accumbens, with shell partitioned into a functionally heterogeneous structure.
Tonic change patterns reflect the marked shift in experimental contingencies upon lever installation, as the session progresses from the first 30 min of pre-drug recording to the self-administration phase. This shift is characterized by increased behavioral activation, the onset of appetitive responding and consequent elevation of drug level. Evidence that tonic change patterns are attributable to both pharmacological factors and behavioral processing has been reported (Fabbricatore et al., 1998). The fact that the predominant response of accumbal neurons is a tonic decrease in firing rate is consistent with a pharmacologic interpretation. Data from microdialysis studies have revealed that dopamine (DA) levels increase at the start of self-administration experiments and remain elevated until the drug availability is terminated (Pettit & Justice, 1989, 1991; Pettit et al., 1990), and substantial electrophysiological literature, using in vitro and in vivo anesthetized preparations, suggests that medium spiny neurons are inhibited in the presence of both cocaine and DA. Since cocaine results in elevated DA levels, and firing rate changes associated with cocaine can be duplicated with iontophoresed DA, it is plausible that elevated DA levels mediate the inhibition of accumbal neurons (Yim & Mogenson, 1982; Yang & Mogenson, 1984, Uchimura et al., 1986; White et al., 1992; Henry & White, 1995) during drug self-administration. The minority of neurons that exhibit tonic increases may be oriented in the accumbens in such a way that they are: 1) disinhibited by dopaminoceptive GABAergic collaterals/interneurons in the surrounding area or 2) differentially influenced by dopamine neuromodulation, with elevated DA creating a more permissive environment for glutamatergic signals at these NAcc synapses (Nicola & Malenka, 1997; reviewed in: Nicola et al., 2000) and/or at dopaminergic afferents (Kalivas & Duffy, 1998; Fiorello & Williams, 2000). This may be mediated by the differential expression of D1-like and D2-like DA receptor subtypes, which exert opposite effects on adenylate cyclase-mediated signal transduction (Kebabian & Calne, 1979; Stoof & Kebabian, 1981; Sibley & Monsma, 1992) and hence the directionality of post-synaptic membrane potential. The fact that spontaneous excitation in striatal spiny neurons is not commonly observed in in vitro and in vivo anesthetized preparations (Wilson & Kawaguchi, 1996; Hernández-López et al., 1997; O’Donnell 2003) lends support to the role of cortico-limbic afferent contributions (O’Donnell & Grace, 1994; White & Kalivas, 1998, p.143) to tonic increase firing patterns.
Medial Accumbens Inhibition
While evidence is herein presented for shell/core differences in firing over the course of the self-administration experiment, the present data provide additional and unexpected evidence for distinct regional functions in the NAcc that are neither shell nor core, but in the dorsal border region between shell and core.
The dorsal border region was shown to exhibit a significantly greater prevalence of tonic decrease neurons than core. Rather than presenting itself as a transition zone between subterritories, where one might observe a gradient, the dorsal border region instead appears to be a distinct zone of inhibition. When evaluated in terms of tonic increases, not a single neuron of the dorsal border region exhibited elevated firing during the drug phase of self-administration experiments. Thus, it represents a region where firing rate suppression predominated, exhibiting the highest percentage of tonic decrease neurons and the lowest percentage of tonic increase neurons of the accumbal subregions. One factor that might contribute to this observation is differential DA expression, since tyrosine hydroxylase activity has been shown to be “very high” in the dorsal border region (Fudge & Haber, 2002).
The functional relevance of dorsal border firing rate suppression may ultimately be resolved by understanding how and whether it is uniquely connected to other brain structures. It has been known for some time that the hippocampus sends afferents to the dorsomedial shell (Kelley & Domesick, 1982) and tract tracing studies have revealed that the afferent pathway from the parvicellular basal amygdaloid nucleus and the efferent pathway to the peribrachial region of the midbrain are specific to the dorsal border region (Wright & Groenewegen, 1995; Wright et al., 1996). In addition, compared to the rest of the striatum, this area of the NAcc shows the greatest amygdalar and hippocampal afferent interaction (Groenewegen et al., 1996,1999a), putatively integrating emotional memory and limbic processing related to the self-administration context. Electrophysiological support for this came from a study by Mulder and associates (1998) that provided evidence that amygdalar and hippocampal afferents, while demonstrating differential overall accumbal topographies, not only overlap in the dorsomedial shell/medial core region, but converge and interact (Mulder et al., 1998). They found that the hippocampus may gate amygdalar afferent signals in the area where they converge, which is a rather narrow zone in the medial NAcc that includes the dorsal border region. This confirms and extends earlier findings which reported convergence of electrophysiological signals from multiple cortical and limbic afferents to this area (Finch, 1996). Subsequent investigations have revealed the dorsal border region to be the site of hippocampal monosynaptic convergence with both prefrontal cortical (French & Totterdell, 2002) and amygdalar (French & Totterdell, 2003) innervation.
Lateral Accumbens Excitation
Despite lacking readily distinguishable subregional boundaries (Zahm, 1999), marked differences in firing rate between medial and lateral areas of the shell provide evidence of functional heterogeneity within the shell subterritory. Among shell tonic increase neurons, all but one were restricted to the ventrolateral shell, while none was recorded from the dorsal shell. These differences are consistent with reports that limbic, cortical, and mesencephalic innervation of shell subregions are mediolaterally compartmentalized (Wright et al., 1996; Zahm, 1999; Groenewegen et al., 1999b; Fudge et al, 2002; Ikemoto S, 2007; Schilman et al., 2008). Subregional outputs also differ, with medial shell efferents projecting to ventromedial ventral pallidum (Zahm & Brog, 1992) while lateral shell efferents project to ventrolateral ventral pallidum (Brog et al., 1993; Usuda et al., 1998; Groenewegen et al., 1999b).
Various cell markers differ in their expression across striatal regions and some have been shown to be differentially expressed in core and shell (Zahm & Heimer, 1988; Voorn et al., 1989; Heimer et al., 1991; Voorn et al., 1994). Among them, Calbindin-D28K (CaB) immunoreactivity differs mediolaterally in accumbens; its expression is low across the anteroposterior extent of the medial accumbens, highest in core and intermediate in lateral shell (Zahm & Heimer, 1993; Jongen-Relo et al., 1994a). Interestingly, this corresponded well with the prevalence of tonic increase firing among accumbal subregions; whereas no tonic increases were found medially (rostral pole shell, dorsal shell), 38% of core and 19% of ventrolateral shell neurons exhibited tonic increases. Indeed, CaB has been shown to modulate neuronal tonic firing rhythmicity by regulating cell membrane Ca2+ channel permeability (Li et al., 1995). CaB’s capacity to influence post-synaptic electrochemical events and the extent to which its expression is activity-dependent (Gold & Bear, 1994) in the NAcc warrants further investigation into its potential role in lateral NAcc tonic increase firing.
Toward a Functional Characterization of the Accumbal Subregions
The present data reveal a distribution of accumbal firing patterns that corresponds well with anatomical predictions; medial and lateral regions exhibit differential neurophysiological signaling during drug self-administration consistent with the segregated circuits to which they are respectively linked. For example, not only does medial shell receive direct convergent amygdalar and hippocampal inputs, but several structures which innervate it (i.e. ventromedial prefrontal and entorhinal cortices; parafascicular and midline thalamic nuclei) also receive convergent input from amygdala and hippocampus (Friedman et al., 2002). Such direct limbic communication tends to be associated with circuits afferent to the medial, but not lateral, NAcc.
The lateral accumbens, on the other hand, exhibits its own discrete circuitry: core and lateral shell receive dopaminergic inputs from lateral ventral tegmental area (VTA), whereas medial shell dopaminergic input arises from posteromedial VTA (Ikemoto, 2007). Furthermore, unlike medial shell, both core and lateral shell receive dopaminergic projections from substantia nigra pars compacta (Brog et al., 1993). Parts of the lateral prefrontal cortex project preferentially to lateral accumbens, with core and lateral shell receiving inputs from dorsal and ventral agranular insular divisions, respectively (Berendse et al., 1992a). Core and lateral shell also share topographically restricted projections from basal amygdala and entorhinal cortex, receiving inputs from the rostral magnocellular basal nucleus, little or no input from accessory basal nuclei, and input limited to lateral, but not medial, entorhinal cortex (Brog et al., 1993; Wright et al., 1996; Totterdell & Meredith, 1997). Lateral shell efferents have also been shown to share common mesencephalic targets with those of the accumbens core, overlap which is not observed in medial shell efferents (reviewed in Pennartz et al., 1994). Consistent with similar observations throughout the dorsal and ventral striatum, a revision in the conceptual framework of striatal anatomy in favor of a mediolateral organization has been proposed (Voorn et al., 2004).
Recent studies using intracranial drug self-administration by Ikemoto and colleagues (2003; 2005) revealed functional evidence for mediolateral compartmentation in the ventral stratum. They extended earlier reports (Carlezon et al., 1995; Rodd-Henricks et al., 2002; Sellings & Clarke, 2003) implicating the medial shell in the primary reinforcing effects of psychomotor stimulant administration by demonstrating that, while infusions to regions of the medial ventral striatum (medial shell and medial tubercle) reliably maintain responding, infusions in lateral regions (core, lateral shell, lateral tubercle) do not. These data and the aforecited anatomical literature are consistent with the present findings indicating that the lateral shell is more similar to core than to medial shell. While the lateral accumbens was shown not to be involved in primary drug reinforcement, it (i.e. core) has been implicated in the expression of locomotor stimulant effects (Boye et al., 2001; Ikemoto, 2002; Sellings & Clarke, 2003) and in the expression of incentive reward value (Kelley et al., 1997; Salamone et al., 1999; Corbit et al., 2001).
How the medial and lateral accumbens process drug-related appetitive/instrumental information will ultimately be revealed by understanding the basal forebrain circuitry to which they are linked. The notion of corticostriatopallidothalamic circuitry is well established (Alexander et al., 1986; Groenewegen et al., 1991) and considerable progress has been made in identifying the paths by which information flows in the basal forebrain. Tract-tracing studies have identified the medial shell’s capacity to affect DA input to the entire striatum by virtue of its upstream position in basal forebrain circuitry (Berendse et al., 1992b; Voorn et al., 2004). Convergent contextual, emotional and cognitive information from limbic and cortical structures is processed through medial accumbens (French & Totterdell, 2002, 2003), which then accesses downstream striatal structures (i.e. lateral accumbens, dorsal striatum) via multiple routes: 1) a striatopallidothalamocorticostriatal circuit (“superior” loop) 2) a striatopallidomesencephalostriatal circuit (“inferior” loop) and 3) intra-accumbal signaling (“direct” route) (Groenewegen et al., 1991; Zahm, 1999, 2000; Otake & Nakamura, 2000; van Dongen et al., 2005). Lateral accumbal throughput is positioned to affect dorsal striatal signaling via similar loop processing and together these observations have led to a further refinement of this circuitry described as a spiral ascending loop (Haber et al., 2000). The present findings, in which tonic firing rate suppression occurred medially while rates that were elevated were found laterally, are consistent with the sequence: reduced medial NAcc inhibitory output, disinhibition of the ventromedial VP GABAergic signal to substantia nigra pars compacta (SNc), and a resultant decrease in DAergic inhibitory output to lateral NAcc. This involvement of the inferior loop may or may not be mutually exclusive to others mentioned above in accounting for the elevated firing rates observed in core and ventrolateral shell.
Of further interest will be confirmation of the pathways by which appetitive processing is transmitted more dorsally in the loop circuitry and the precise neurophysiological changes which occur over the course of chronic drug exposure. Some progress has been made in this regard by investigations which have shown that over long term drug self-administration, progressive drug-related changes in the striatal DA system correspond with the sequence of connectivity through the spiral loop circuit (Letchworth et al., 2001; Nader et al., 2002; Porrino et al., 2004, 2007). These changes, which initially occur in medial ventral striatum and over time affect increasingly more lateral and dorsal striatal targets, may correspond to shifts toward more compulsive drug responding.
Conclusions
Recent investigations using animal self-administration models have employed techniques with spatial resolution necessary to discern physiological differences within the shell subterritory. The present findings extend an emerging literature which revises earlier concepts of accumbal functional compartmentation by partitioning the ventral striatum on a mediolateral axis. This accords with a basal forebrain functional schema in which limbic-cortical information converges in the medial accumbens, feeds forward via superior and inferior loops to lateral accumbens, then dorsal striatum and ultimately motor cortex. Chronic drug use perturbations at any particular synaptic population in this complex circuitry has the potential of innumerable downstream aberrant consequences in the processing of appetitive/instrumental signaling. Among them is dysfunctional striatal dopamine signaling and, as some have hypothesized, the shift from volitional to compulsive drug use. Understanding the functional dynamics of the medial and lateral accumbens in this circuitry, and the role of dorsal border inhibition within it, will provide valuable insight into the process by which drug addiction develops, as well as potential sites for pharmaceutical intervention.
Acknowledgements
Research supported by NIDA grant DA 06886. Thanks to Jean Fugate, Don MacNeil, Linda King, Tony Pawlak and David Root for technical assistance. This article is dedicated to the memory of our friend and colleague, Volodymyr Fedorovitch Prokopenko (1947-2006). His scientific skills and insights, as well as his generous and good-natured disposition, are missed by any who had the pleasure to know him.
Supported by: NIDA DA06886
Literature Cited
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992a;316(3):314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ, Lohman AHM. Compartmental distribution of ventral striatal neurons projecting to the mesencephalon in the rat. J. Neurosci. 1992b;12(6):2079–2103. doi: 10.1523/JNEUROSCI.12-06-02079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur. J. Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Pennartz CMA, da Silva F.H. Lopes. Paired pulse facilitation in the nucleus accumbens following stimulation of subicular inputs in the rat. Neuroscience. 1990;35(2):301–311. doi: 10.1016/0306-4522(90)90084-h. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Mulder AB, Pennartz CMA, Manshanden I, da Silva F.H. Lopes. Responses of the nucleus accumbens following fornix/fimbria stimulation in the rat. Identification and long-term potentiation of mono- and polysynaptic pathways. Neuroscience. 1993;53(4):1049–1058. doi: 10.1016/0306-4522(93)90488-2. [DOI] [PubMed] [Google Scholar]
- Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and D-amphetamine in rats. Neuropharmacology. 2001;40(6):792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Weeks JR. Simple flow-thru swivel for infusions into unrestrained animals. Pharmacol. Biochem. Behav. 1976;5:363–365. doi: 10.1016/0091-3057(76)90090-3. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Carelli RM, King VC, Hampson RE, Deadwyler SA. Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res. 1993;626:14–22. doi: 10.1016/0006-8993(93)90557-4. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J. Neurosci. 2000;20(11):4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology. 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J. Neurosci. 1996;16(9):3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Sawyer SF, Lee R, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J. Neurosci. 1994;14(3):1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Janak PH, Woodward DJ. Neuronal and behavioral correlations in the medial prefrontal cortex and nucleus accumbens during cocaine self-administration by rats. Neuroscience. 2000;3:433–443. doi: 10.1016/s0306-4522(00)00218-9. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instumental conditioning: evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas P,W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000;20:RC89(1-5). doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav. Neurosci. 1992;106(1):181–191. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Marchand JF, Sikes RW, Chronister RB, Hubbard JI. Characterization of fimbria input to nucleus accumbens. J. Neurophysiol. 1985;54(6):1553–1567. doi: 10.1152/jn.1985.54.6.1553. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Sikes RW, Chronister RB. Dopamine action in the nucleus accumbens. J. Neurophysiol. 1985;54(6):1568–1577. doi: 10.1152/jn.1985.54.6.1568. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Di Chiara GT. A motivational learning hypothesis of the role of dopamine in compulsive drug use. J. Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- DiChiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fabbricatore AT, Uzwiak AJ, West MO, Peoples LL. Comparisons of firing rates of rat nucleus accumbens neurons during cocaine self-administration and extinction. Soc. Neurosci. Abstr. 1998;24:1736. [Google Scholar]
- Finch DM. Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus. 1996;6:495–512. doi: 10.1002/(SICI)1098-1063(1996)6:5<495::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. Selective inhibition by adenosine of mGluR IPSPs in dopamine neurons after cocaine treatment. J. Neurophysiol. 2000;83(3):1307–1314. doi: 10.1152/jn.2000.83.3.1307. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J. Neurosci. 2001a;21(8):2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J. Neurosci. 2001b;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J. Comp. Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J. Comp. Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Defining the caudal ventral striatum in primates: cellular and histochemical features. J. Neurosci. 2002;22(23):10078–10082. doi: 10.1523/JNEUROSCI.22-23-10078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience. 2002;110(2):257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J. Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminitive-stimulus task. J. Neurophysiol. 2004;92(3):1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience. 2006;137:1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Gold JI, Bear MF. A model of dendritic spine Ca2+ concentration exploring possible bases for a sliding synaptic modification threshold. Proc. Natl. Acad. Sci. 1994;91(9):3941–3945. doi: 10.1073/pnas.91.9.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nature Neuroscience. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of Dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gratton A, Wise RA. Drug- and behavior-associated changes in dopamine-related electrochemical signals during intravenous cocaine self-administration in rats. J. Neurosci. 1994;14(7):4130–4146. doi: 10.1523/JNEUROSCI.14-07-04130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD. A simple microelectrode for recording from the central nervous system. Nature. 1958;182:962. doi: 10.1038/182962a0. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Meredith GE, Haber SN, Voorn P, Wolters JG, Lohman AHM. Functional anatomy of the ventral, limbic system-innervated striatum. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. John Wiley and Sons; New York: 1991. pp. 19–60. [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog. Brain Res. 1996;107:85–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Mulder AB, Beijer AVJ, Wright CI, da Silva F.H. Lopes, Pennartz CM. Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology. 1999a;27(2):149–164. [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann. N.Y. Acad. Sci. 1999b;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedou G, Feldon J, Heidbreder CA. Effects of cocaine on dopamine in subregions of the rat prefrontal cortex and their efferents to subterritories of the nucleus accumbens. Eur. J. Pharmacol. 1999;372:143–155. doi: 10.1016/s0014-2999(99)00218-6. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Baumann MH. Autoregulation of dopamine synthesis in subregions of the rat nucleus accumbens. Eur. J. Pharmacol. 2001;411:107–113. doi: 10.1016/s0014-2999(00)00882-7. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. In: Napier TC, Kalivas PW, Hanin I, editors. The Basal Forebrain: Anatomy to Function. Plenum Press; New York: 1991. pp. 1–41. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41(1):89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J. Neurosci. 1995;15(9):6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol. Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J. Neurosci. 1997;17(9):3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto K, Satoh K, Maeda T, Fibiger HC. Neurochemical heterogeneity of the primate nucleus accumbens. Exp. Brain Res. 1995;104:177–190. doi: 10.1007/BF00242004. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113(4):939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J. Neurosci. 2003;23(28):9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu Z-H. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J. Neurosci. 2005;25(20):5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Relo AL, Voorn P, Groenewegen HJ. Immunohistochemical characterization of the shell and core territories of the nucleus accumbens in the rat. Eur. J. Neurosci. 1994a;6:1255–1264. doi: 10.1111/j.1460-9568.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Jongen-Relo AL, Docter GJ, Jonker AJ, Vreugdenhil E, Groenewegen HJ, Voorn P. Differential effects of dopamine depletion on the binding and mRNA levels of dopamine receptors in the shell and core of the rat nucleus accumbens. Mol. Brain Res. 1994b;25:333–343. doi: 10.1016/0169-328x(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J. Neurochem. 1998;70(4):1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc. Natl. Acad. Sci. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an in vivo electrochemical study. Neuroscience. 1995;64(3):599–617. doi: 10.1016/0306-4522(94)00436-9. [DOI] [PubMed] [Google Scholar]
- Kosobud AEK, Harris GC, Chapin JK. Behavioral associations of neuronal activity in the ventral tegmental area of the rat. J. Neurosci. 1994;14(11):7117–7129. doi: 10.1523/JNEUROSCI.14-11-07117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J. Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Decavel C, Hatton GI. Calbindin-D28k: role in determining intrinsically generated firing patterns in rat supraoptic neurones. J. Physiol. 1995;488(Pt 3):601–608. doi: 10.1113/jphysiol.1995.sp020993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X-Y, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82(3):767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann. N.Y. Acad. Sci. 1999 Jun 29;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Moore GP, Perkel DH, Segundo JP. Statistical analysis and functional interpretation of neuronal spike data. Annu. Rev. Physiol. 1966;28:493–522. doi: 10.1146/annurev.ph.28.030166.002425. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, da Silva F.H. Lopes. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation and interaction of inputs. J. Neurosci. 1998;18(13):5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Domesick VB. Crossroads of limbic and striatal circuitry: hypothalamo-nigral connections. In: Livingston KE, Hornykiewicz O, editors. Limbic Mechanisms: The Continuing Evolution of the Limbic System Concept. Plenum Press; New York: 1978. pp. 75–93. [Google Scholar]
- Nayak PK, Misra AL, Mulé SJ. Physiological disposition and biotransformation of (3H) cocaine in acutely and chronically treated rats. J. Pharmacol. Exp. Ther. 1976;196(3):556–569. [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature Neuroscience. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J. Neurosci. 1996;16(5):1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J. Neurosci. 1997;17(15):5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13:135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J. Neurosci. 1995;15(5):3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neurosci. Biobehav. Rev. 1985;9:261–282. doi: 10.1016/0149-7634(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Otake K, Nakamura Y. Possible pathways through which neurons of the shell of the nucleus accumbens influence the outflow of the core of the nucleus accumbens. Brain Dev. 2000;22(Suppl 1):S17–26. doi: 10.1016/s0387-7604(00)00142-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd edition Academy Press; New York: 1997. [Google Scholar]
- Pennartz CMA, Dolleman-Van Der Weel MJ, da Silva F.H. Lopes. Differential membrane properties and dopamine effects in the shell and core of the rat nucleus accumbens studied in vitro. Neurosci. Lett. 1992;136:109–112. doi: 10.1016/0304-3940(92)90660-y. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, da Silva F.H. Lopes. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J. Neurosci. 1996;16(10):3459–3473. doi: 10.1523/JNEUROSCI.16-10-03459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Guyette FX, West MO. Tonic inhibition of single nucleus accumbens neurons in the rat: a predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience. 1998;86(1):13–22. doi: 10.1016/s0306-4522(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. Tonic firing of rat nucleus accumbens neurons: changes during the first 2 weeks of daily cocaine self-administration sessions. Brain Res. 1999;822(1-2):231–236. doi: 10.1016/s0006-8993(98)01271-2. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharm. Biochem. Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine self-administration. J. Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB. Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J. Pharmacol. Exp. Ther. 1968;161(1):122–129. [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Colangelo V, La Riccia M, Pozzilli C, Passarelli F, Orzi F. Psychostimulant drugs increase glucose utilization in the shell of the rat nucleus accumbens. Neuroreport. 1994;5(18):2561–2564. doi: 10.1097/00001756-199412000-00039. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, DiChiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared to the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J. Neurosci. 2004;24(14):3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(8):1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci. Biobehav. Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Beh. Brain Res. 1993;55:159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li T-K, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of wistar rats. J. Pharmacol. Exp. Ther. 2002;303(3):1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Runyon RP, Haber A, Pittenger DJ, Coleman KA. In: Fundamentals of Behavioral Statistics. McKean BL, Holton T, editors. McGraw Hill; New York: 1996. pp. 592–594. [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology. 1992;107:160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Aberman JE, Sokolowski JD, Cousins MS. Nucleus accumbens dopamine and rate of responding: Neurochemical and behavioral studies. Psychobiology. 1999;27(2):236–247. [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci. Lett. 2008;432(1):40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J. Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J. Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr. Molecular biology of dopamine receptors. Trends Pharmacol. Sci. 1992;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; New York: 1956. [Google Scholar]
- Smith GP, Schneider LH. Relationships between mesolimbic dopamine function and eating behavior. Ann. N.Y. Acad. Sci. 1988;537:254–261. doi: 10.1111/j.1749-6632.1988.tb42111.x. [DOI] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J. Chem. Neuroanat. 1989;2(5):285–298. [PubMed] [Google Scholar]
- Totterdell S, Meredith GE. Topographical organization of projections from the entorhinal cortex to the striatum of the rat. Neuroscience. 1997;78:715–729. doi: 10.1016/s0306-4522(96)00592-1. [DOI] [PubMed] [Google Scholar]
- Uchimura N, Cherubini E, North RA. Inward rectification in rat nucleus accumbens neurons. J. Neurophysiol. 1989;62(6):1280–1286. doi: 10.1152/jn.1989.62.6.1280. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- van Dongen YC, Deniau J-M, Pennartz CMA, Galis-de Graff Y, Voorn P, Thierry A-M, Groenewegen HJ. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience. 2005;136:1049–1071. doi: 10.1016/j.neuroscience.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: Immunohistochemical distribution of enkephalin, substance P, dopamine and calcium-binding protein. J. Comp. Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- Voorn P, Brady LS, Schotte A, Berendse HW, Richfield EK. Evidence for two neurochemical divisions in the human nucleus accumbens. Eur. J. Neurosci. 1994;6:1913–1916. doi: 10.1111/j.1460-9568.1994.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method of automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Electrophysiological evidence for the existence of both D1 and D2 dopamine receptors in the rat nucleus accumbens. J. Neurosci. 1986;6:274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ. D1 dopamine receptor stimulation enables the inhibition of nucleus accumbens neurons by a D2 receptor agonist. Eur. J. Pharm. 1987;135:101–105. doi: 10.1016/0014-2999(87)90764-3. [DOI] [PubMed] [Google Scholar]
- White FJ. Electrophysiological basis of the reinforcing effects of cocaine. Beh. Pharm. 1990;1:303–315. doi: 10.1097/00008877-199000140-00004. [DOI] [PubMed] [Google Scholar]
- White FJ, Henry DJ, Hu X-T, Jeziorski M, Ackerman JM. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system. In: Lakowski JM, Galloway MP, White FJ, editors. Cocaine Pharmacology, Physiology, and Clinical Strategies. CRC Press; Ann Arbor: 1992. pp. 261–293. [Google Scholar]
- White FJ, Hu X-T, Henry DJ. Electrophysiological effects of cocaine in the rat nucleus accumbens: microiointophoretic studies. J. Pharmacol. Exp. Ther. 1993;266:1075–1084. [PubMed] [Google Scholar]
- White SR, Harris GC, Imel KM, Wheaton MJ. Inhibitory effects of dopamine and MDMA on glutamate-evoked firing of nucleus accumbens and caudate/putamen cells are enhanced following cocaine self-administration. Brain Res. 1995;660:167–176. doi: 10.1016/0006-8993(95)00309-e. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16(7):2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neuroleptics and operant behavior: the anhedonia hypothesis. Behav. Brain Sci. 1982;5:39–87. [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J. Comp. Neurol. 1995;361(3):383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AVJ, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J. Neurosci. 1996;16(5):1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurons in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324:69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res. 1982;239:401–415. doi: 10.1016/0006-8993(82)90518-2. [DOI] [PubMed] [Google Scholar]
- Yokel AR, Pickens R. Drug level of D- and L-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]
- Young AMJ, Joseph MH, Gray JA. Increased dopamine release in vivo in nucleus accumbens and caudate nucleus of the rat during drinking: a microdialysis study. Neuroscience. 1992;48(4):871–876. doi: 10.1016/0306-4522(92)90275-7. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14(2):427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J. Comp. Neurol. 1988;272(4):516–535. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50(4):751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. The efferent projections of the rostral pole of the nucleus accumbens in the rat: comparison with the core and shell projection patterns. J. Comp. Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann. N.Y. Acad. Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]