Abstract
Eukaryotic cells possess a remarkable diversity of lipids, which distribute among cellular membranes by well-characterized vesicle trafficking pathways. However, transport of lipids by alternate or “nonvesicular” routes is also critical for lipid synthesis, metabolism, and proper membrane partitioning. In the past few years, considerable progress has been made in characterizing the mechanisms of nonvesicular lipid transport and how it may go awry in particular diseases, but many fundamental question remain for this rising field.
A typical higher eukaryotic cell contains more than 1000 different lipid species. These lipids are not homogenously distributed among intracellular membranes, but instead each organelle has a characteristic lipid composition that is required for its proper function. For example, cholesterol and sphingolipids are highly enriched in the plasma membrane and endosomes, and indeed, many diseases, such as atherosclerosis, type II diabetes, and lysosomal storage disorders, are associated with defects in maintaining the correct distribution of intracellular lipids. How do these hydrophobic molecules shuttle between intracellular membranes inside the aqueous milieu of the cell?
Although trafficking largely determines the intracellular distribution of most lipids, we currently understand less about lipid trafficking than we do about protein trafficking. Nevertheless, protein and lipid do share similar properties. Both lipids and integral membrane proteins move between organelles in membrane-enclosed sacs, called transport vesicles, and there is growing evidence that lipids, like proteins, are sorted during the formation of transport vesicles.
However, unlike proteins, lipids can rapidly and efficiently move between cellular membranes by routes independent of transport vesicle, or “nonvesicular transport” pathways. This important difference between protein and lipid trafficking is not widely appreciated, in part, because the roles and mechanisms of nonvesicular lipid exchange have, in many cases, been obscure and difficult to characterize. In the past few years, researchers have made significant progress towards understanding how and why nonvesicular lipid trafficking occurs. This Essay summarizes the current state of the field and the major challenges for its future.
How much nonvesicular lipid trafficking occurs in cells?
The first studies suggesting the existence of nonvesicular lipid exchange pathways in the cell examined the movement of newly synthesized lipids from the endoplasmic reticulum (ER), where they are made, to the plasma membrane. Drugs that halt vesicular trafficking do not stop lipid transfer from the ER to the plasma membrane, indicating that some lipids, including phosphatidylcholine (PC), phosphoatidylethanolamine (PE), cholesterol, and glucosylceramide (GlcCer), can move between the ER and plasma membrane by nonvesicular pathways. Moreover, these pathways have substantial capacity because the rate of lipid transfer does not decrease when vesicular trafficking is blocked (Sleight and Pagano, 1983; Kaplan and Simoni, 1985b; Kaplan and Simoni, 1985a; Warnock et al., 1994). Nevertheless, it remains unclear what fraction of the lipid exchange between the ER and plasma membrane is nonvesicular when vesicular trafficking is not blocked.
More recently, studies have reported strong evidence for nonvesicular transfer of ceramides from the ER to the Golgi (Kok et al., 1998; Funato and Riezman, 2001; Hanada et al., 2003); GlcCer transfer from the Golgi complex to the ER and plasma membrane (Halter et al., 2007; D'Angelo et al., 2007); and, sterols from the plasma membrane to endocytic recycling compartment (Mesmin and Maxfield, 2009). For example, studies using dehydroergosterol, a fluorescent analogue of cholesterol, found that when this sterol is added to cells it initially incorporates into the plasma membrane but then moves to the endocytic recycling compartment by a nonvesicular, energy-independent pathway. Dehydroergosterol equilibrates between the plasma membrane and endocytic recycling compartment quite quickly - within 2–3 minutes- and astonishingly, an estimated one million dehydroergosterol molecules exchange between these compartments each second (Maxfield and Mondal, 2006).
Collectively, these and many other studies indicate that the cell possesses numerous pathways of nonvesicular lipid, and more pathways will probably be discovered in the future. However, in most cases, we still are uncertain about of how much nonvesicular pathways contribute to the total lipid exchange inside a cell. Are the nonvesicular pathways needed for exchanging a large proportion of lipids between organelles, or do only a small fraction of lipids moved by nonvesicular mechanisms? In addition, some classes of lipids, such as complex glycolipids, gangliosides, and sphingolipids may transfer by only vesicular routes (Wattenberg, 1990; Hechtberger and Daum, 1995).
Roles of nonvesicular lipid trafficking in cells
Nonvesicular lipid trafficking serves at least four important functions in cells. First, it provides lipids needed for membrane biogenesis in organelles that cannot obtain sufficient lipids from vesicular trafficking. Mitochondria, chloroplasts, and lipid droplets lack most of the enzymes needed to make certain lipids required for their biogenesis. These organelles are not connected to the rest of the cell by vesicular trafficking pathways and thus rely on nonvesicular trafficking pathways to obtain these lipids. Indeed, many studies show that lipids exchange between the ER and mitochondria or chloroplasts by nonvesicular routes (Voelker, 2009; Benning, 2009). Less is known about lipid transfer among lipid droplets or between lipid droplets and other organelles, but these pathways are almost certainly nonvesicular as well. There is also evidence for nonvesicular lipid exchange between the ER and peroxisomes (Raychaudhuri and Prinz, 2008).
Nonvesicular transport also helps maintain the proper level of a lipid in an organelle or domain of an organelle. Compared to vesicular routes, one obvious advantage of nonvesicular trafficking is that it can rapidly move lipids between specific compartments in cells without having to also transfer integral membrane proteins. This may be particularly important for lipids, such as cholesterol, which can be toxic to cells. Cells use a number of mechanisms to rapidly decrease cholesterol levels when they are too high, such as effluxing cholesterol out of cells to external lipoproteins and producing cholesteryl esters (i.e., ester linkages between the hydroxyl group of cholesterol and the carboxylate group of a fatty acid), which are stored in lipid droplets. Nonvesicular transport of cholesterol probably provides a route to move cholesterol quickly and efficiently to the enzymes that perform these reactions without disrupting vesicular trafficking.
Third, nonvesicular lipid trafficking may also regulate lipid metabolism. For example, the nonvesicular transfer of ceramides from the ER, where they are synthesized, to the Golgi complex, where they are converted into glycolipids and sphingolipids, may regulate the production of these lipids. Finally, it is possible that nonvesicular lipid transfer is required for the transmission of a lipid as part of a signaling or regulatory pathway. For example, diacylglycerol activate protein kinase C, and ceramides serve a signaling molecule to regulate differentiation, proliferation, programmed cell death, and apoptosis.
Mechanisms of nonvesicular lipid trafficking
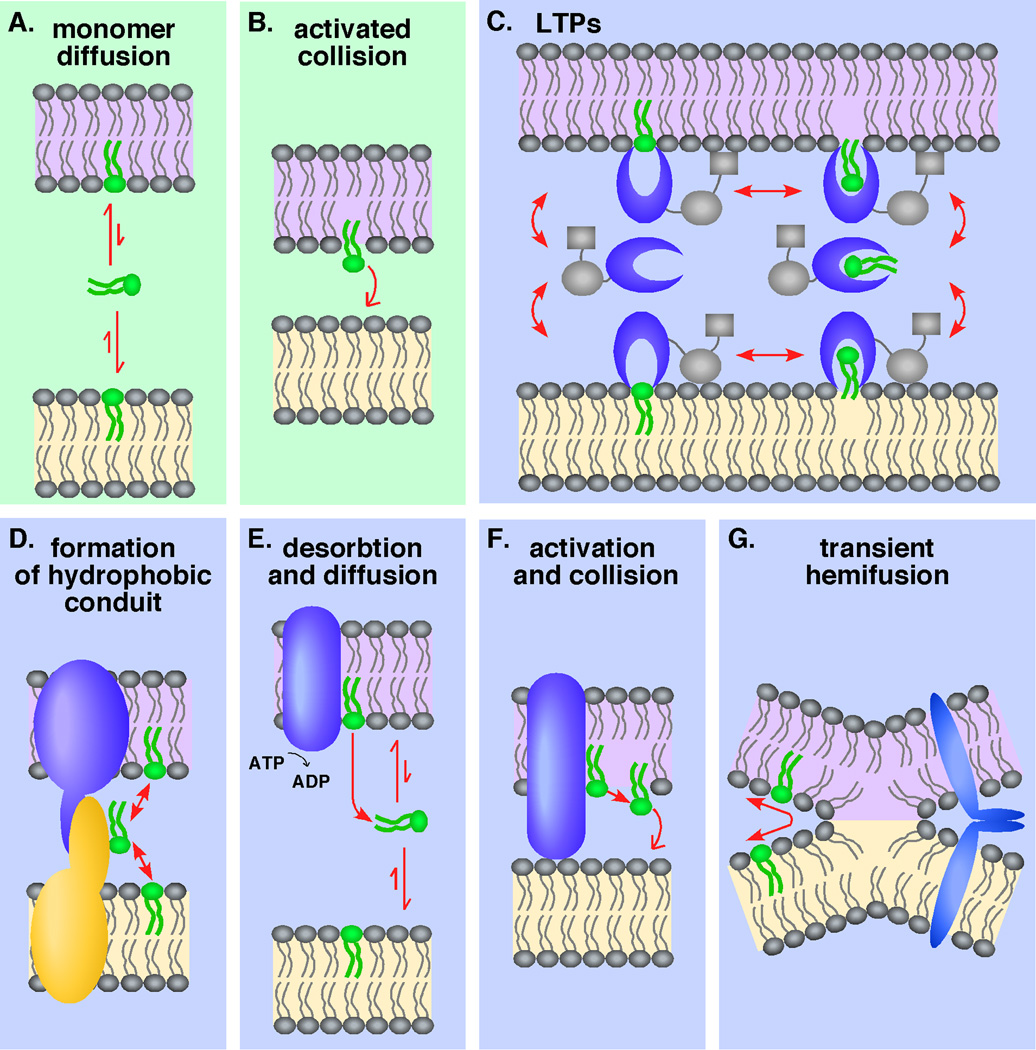
Lipid monomers can exchange spontaneously between membranes by simply diffusing through the aqueous phase (Figure 1A). However, for most classes of lipids this process occurs too slowly to be physiologically relevant; for example, most glycerolipids and sphingolipids spontaneously exchange between membranes with halftimes >40 hours. The rate-limiting step in this process is lipid desorption from a membrane, and thus, proteins that accelerate lipid transfer may increase the rate of lipid egress from the membrane.
Figure 1. Possible mechanisms of nonvesicular lipid exchange between membranes.
Lipids can spontaneously exchange between two membranes without the assistance of proteins; (A) monomers can diffuse through the aqueous phase or (B) during the collision of two membrane collision after the lipid is “activated.” (C) Lipid transport proteins (LTPs) can also exchange lipids between membranes and organelles. LTPs have a lipid-binding domain (blue) and, many times, targeting domains (purple) that may direct lipid transfer to particular membranes by binding to lipids or proteins. Lipids may exchange at membrane contact sites where two membranes come together in close proximity. Protein complexes may facilitate this process (D) by forming a tunnel that allows lipids diffuse between the membranes, (E) by promoting lipid desorption from one membrane, (F) by activating lipids prior to membrane collision, or (G) by promoting transient membrane hemifusion.
Lipid transfer between membranes may also occur when two membranes collide (Figure 1B). Although the mechanism of lipid exchange during collision is not well understood, one model is that a lipid must be "activated," or partially extended from the bilayer, prior to collision (Steck et al., 2002). This activation increases the probability of transfer to a second membrane during collision. Activation could be stochastic, resulting from the thermal motion that causes lipids to bounce or bob in a bilayer, or it could be mediated by a protein.
Proteins clearly facilitate the lipid nonvesicular transport between membranes. Although this process has been well characterized in vitro, studies are only beginning to unravel the mechanisms for these pathways inside the cell (Voelker, 2009; Benning, 2009). Nevertheless, in the three cases described below, specific details have emerged, including how defects in these lipid trafficking pathways cause disease.
CERT, a typical Lipid Transport Protein?
Ceramide is the precursor of sphingolipids, including sphingomyelin, an abundant lipid in the plasma membrane of all mammalian cells. Sphingomyelin is synthesized in the Golgi complex but ceramide is made in the ER. Therefore, to produce sphingomyelin, ceramide must be transported from the ER to the Golgi complex, and this is accomplished by CERT, the ceramide transport protein (Hanada et al., 2009).
CERT is expressed ubiquitously in higher eukaryotes, but it is not present in yeast. CERT was identified from a mutant cell line of Chinese hamster ovary cells, called LY-A, which has low levels of sphingomyelin (Hanada et al., 2003). Studies found that, although LY-A mutant cells make sphingomyelin at a reduced rate, these cells produce normal amounts of enzymes that synthesize sphingomyelin (i.e., sphingomyelin synthase) and the sphingomyelin precursors, ceramide and PC. These results suggested that LY-A cells have a defect in the nonvesicular transfer of ceramide from the ER to the Golgi complex. The gene that complemented the cell’s defect was isolated and named CERT. Disruption of the CERT gene in mice results in death at approximately embryonic day 11.5 (Wang et al., 2009), and flies lacking CERT have a dramatic decrease in ceramide phosphoethanolamine, the fly analogue of sphingomyelin (Rao et al., 2007).
CERT encodes a 68-kDa protein that has three domains, an N-terminal PH (pleckstrin homology) domain, a FFAT (two phenylalanines in an acidic tract) motif, and a C-terminal START (steroidogenic acute regulatory protein (StAR)-related) domain. The PH domain binds to phosphoinositides (PIPs) whereas the FFAT motif associates with proteins on the ER, called VAPs (vesicle-associated membrane protein-associated proteins). The START domain is the portion of the protein that transports lipids, and it binds a single molecule of ceramide in a hydrophobic cavity (Kudo et al., 2008).
CERT facilitates the movement of ceramide between liposomes in vitro (Hanada et al., 2003). The PH domain and FFAT motif in CERT target it to the ER and Golgi complex, respectively. Thus, in vivo CERT probably extracts ceramide from the ER, shuttles it through the cytoplasm, and delivers it to the Golgi complex. In general, proteins that mediate lipid transfer by this mechanism are called lipid transfer proteins (LTPs) (Figure 1C). The consumption of ceramide in the Golgi complex to produce sphingomyelin probably drives the directionality of the ceramide transport.
Ceramide transfer by CERT in vitro does not require energy. Surprisingly, however, ATP-depletion blocks ceramide transport by CERT in cells (Hanada et al., 2003), and the role that energy plays in CERT function in vivo remains an interesting, unsolved mystery. The rate-limiting step for ceramide transport by CERT is likely diffusion through the cytosol. This is probably true of other LTPs as well.
Nevertheless, it is unlikely that CERT or other LTPs diffuse long distances through the cytosol. Rather, they probably operate mostly at regions where membranes are closely apposed and come within ~20 nm of each other. Called membrane contact sites or MCSs, these junctions are present ubiquitously in all cells and are frequently found between the ER and a second organelle (Levine and Loewen, 2006).
At membrane contact sites between the ER and Golgi complex, CERT would have to diffuse only a small distance, or it may even bind both membranes simultaneously using its two targeting domains, PH and FFAT (Hanada et al., 2009). Although it is still unknown for certain if CERT localizes to membrane contact sites between the ER and Golgi complex, some LTPs are enriched at these membrane junctions, including the oxysterol-binding protein (OSBP) ORP1L in mammals and most of the OSBP-related proteins in yeast (the Osh proteins) (Levine and Munro, 2001; Loewen et al., 2003; Rocha et al., 2009; Schulz et al., 2009).
CERT is part of a large family of proteins that contain START domains, and many members of this family can facilitate lipid transfer between membranes in vitro. In addition, there are ~4 other large families of LTPs, and most cells express numerous LTPs (D'Angelo et al., 2008; Lev, 2010). Some LTPs have high specificity and bind only a few lipids whereas others can associate with a broad range of lipids. The different families of LTPs are quite diverse, with little similarities in neither sequence nor structure. However, all LTPs share the ability to bind lipid monomers with a stoichiometry of one lipid for each protein. In addition, all LTPs bind the lipid monomer in a pocket covered with a flexible "lid" domain that shields the associated lipid from the aqueous phase (Figure 1C). As with CERT, lipid exchange by LTPs does not require energy.
A major controversy in the field is whether the primary function of many LTPs in cells is to transfer lipids between membranes, as they do in vitro, or whether they serve another main purpose in cells. Aside from CERT, there is indeed compelling evidence that other LTPs, such as FAPP2 (Golgi-associated four-phosphate adaptor protein 2), NPC2 (Niemann-Pick disease, type C2), and some oxysterol-binding proteins in yeast, transfer lipids in cells (Yamaji et al., 2008; D'Angelo et al., 2008; Prinz, 2007). That said, many LTPs do not appear to transport lipids in cells but rather serve as lipid sensors or regulate lipid metabolism and signaling by presenting lipids to metabolic enzymes. For example, the Sec14 superfamily of LTPs has been proposed to present phosphoinositol to kinases that produce PIPs, and thus, these LTPs regulate many membrane trafficking and signaling events that require PIPs (Bankaitis et al., 2010).
Lipid exchange between the ER and mitochondria
Nonvesicular lipid trafficking that occurs at membrane contact sites does not always require soluble LTPs. Indeed, lipid exchange between the ER and mitochondria probably occurs independently of LTPs. Lipid transport between these organelles is critical for the synthesis of phosphatidylcholine (PC) and phosphoatidylethanolamine (PE), two of the most abundant lipids in the membranes of eukaryotes. In one of the two major pathways for producing PC, the first step is the synthesis of phosphatidylserine (PS), which occurs at the ER. PS is then transferred to the inner mitochondrial membrane, where it is decarboxylated to form PE, the precursor of PC. However, the enzymes that convert PE to PC reside back in the ER, and thus, to make PC, the PE must be return to the ER from the mitochondrial inner membrane. Consequently, producing PE and PC by this pathway requires multiple nonvesicular lipid transfer steps. Remarkably, yeast mutants that can make PE and PC solely by this pathway grow as well as wild-type cells and have similar levels of PE and PC (Trotter et al., 1995). These results indicate that nonvesicular lipid transfer between ER and mitochondria must be highly efficient.
Surprisingly, phospholipid exchange between the ER and mitochondria requires neither cytosolic factors nor energy. It is thought to occur at specialized regions of the ER, called mitochondria-associated membrane or MAM, which are closely apposed to mitochondria (Choi et al., 2006). An important question in the field is how these membrane contact sites form. In mammals, a number of proteins, such as mitofusins, GRP75 (Glucose regulated protein 75), and PACS2 (phosphofurin acidic cluster sorting protein 2), have been proposed to mediate contacts between the MAM and mitochondria, but whether any of these proteins are needed for efficient lipid exchange between these organelles is not known (Lev, 2010). In yeast, studies recently found that lipid transfer between the ER and mitochondria slows down in mutants missing a complex of four proteins called the ERMES complex, which bridges the ER and mitochondria (Kornmann et al., 2009). Thus, maintaining close contacts between the ER and mitochondria is required for efficient lipid exchange between these organelles.
There are a number of ways in which lipid transport exchange between the ER and mitochondria may occur at membrane contact sites occurs. First, protein complexes in the two organelles could interact to form a type of hydrophobic tunnel or conduit that allows lipids to passively diffuse between the two membranes with little or no contact with the aqueous phase (Figure 1D). Second, a membrane protein complex at a contact site could use energy to facilitate lipid desorption from one of the membranes. The probability that the lipid then diffuses into the adjacent membrane is comparable that of it diffusing back into original membrane (Figure 1E), leading to a net transfer of lipid from one membrane to the other. Third, if lipid transfer occurs by an activated collision mechanism, then a protein complex could also promote lipid activation and increase the chance of lipid exchange during membrane collision (Figure 1F). Membranes at contact sites may not be held a fixed distance and may frequently collide. A fourth possibility is that transmembrane proteins on two different organelles bring two membranes in close apposition so that they undergo transient hemifusion (Figure 1G). Lipids could then easily diffuse between the hemifused membranes without contacting the aqueous phase.
Defects in lipid transport to mitochondria cause multiple diseases. For example, some forms of Congenital Adrenal Hyperplasia, which is characterized by an impaired ability to produce the steroid cortisol, are caused by defects in cholesterol transport to the inner mitochondrial membranes. Steroids are synthesized from cholesterol, and the first step in this process occurs in the inner mitochondrial membranes. Transporting cholesterol to the inner mitochondrial membranes requires the LTP StAR (steroidogenic acute regulatory protein). Although StAR binds cholesterol and can transfer it between membranes in vitro (Kallen et al., 1998), its role in cholesterol transport in cells remains controversial. It is not clear if StAR moves cholesterol from the outer to the inner mitochondrial membrane, moves cholesterol from another organelle to the outer mitochondrial membrane, or regulates the proteins that are actually responsible for cholesterol transport to the inner mitochondrial membrane. Such fundamental questions need to be resolved before we can understand and begin developing treatments for many diseases caused by defects in lipid transport.
Cholesterol transfer by NPC1 and NPC2
Low-density lipoproteins (LDLs) transport cholesterol and other lipids through the bloodstream, and receptor-mediated endocytosis of LDLs serves as a major source of cholesterol in mammalian cells. When endocytosed LDL reaches late endosome/lysosome compartments, cholesteryl esters in these particles are hydrolyzed and the resulting cholesterol is subsequently trafficked to the rest of the cell. Nonvesicular mechanisms transport cholesterol from internal membranes to the outer membrane of the late endosome/lysosome and then eventually out of the organelle.
Two proteins required for this type of cholesterol transport are NPC1 and NPC2. These proteins were identified by studies on patients with Niemann-Pick type C, a rare autosomal recessive lysosomal storage disease in which cholesterol and other lipids accumulate in late endosomes/lysosomes. NPC1 is an integral membrane protein with 13 putative transmembrane domains that reside in the outer membrane of late endosomes/lysosomes. In contrast, NPC2 is a small soluble protein in the lumen of these organelles. NPC2 is an LTP that facilitates cholesterol transport between membranes in vitro (Cheruku et al., 2006). In cells, it probably transfers cholesterol between internal membranes in the late endosome/lysosome and then hands it off to NPC1 in the outer membrane (Infante et al., 2008; Kwon et al., 2009; Wang et al., 2010). NPC1 may then facilitate the egress of cholesterol from the late endosome/lysosome to other cellular compartments. However, future studies are needed to confirm this hypothesis and to characterize exactly how NPC2 transfers cholesterol to other cellular membranes.
Future
Many details of nonvesicular lipid trafficking remain open questions and are currently the focus of intense research. That said, a few concepts are clear. For one, most nonvesicular lipid transfer probably occurs at membrane contact sites, and undoubtedly, new techniques are needed to study these junctions and identify proteins that function at these key locations in the cell. In addition, a significant portion of lipid trafficking at membrane contact sites probably does not require soluble LTPs, but the mechanistic details for how transfer occurs remains an important question. Other fundamental issues in this field include the energetics of nonvesicular lipid trafficking and its regulatory mechanisms, including if and how its directionality is determined. Answering these questions is imperative for understanding how defects in nonvesicular lipid trafficking cause disease, but it is also critical for deciphering fundamental processes in eukaryotic cells, including lipid metabolism, signaling, and intracellular distribution.
Acknowledgments
I thank Ted Steck, Jim Hurley, and Tim Schulz for reading the manuscript. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bankaitis VA, Mousley CJ, Schaaf G. The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem Sci. 2010;35:150–160. doi: 10.1016/j.tibs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol. 2009;25:71–91. doi: 10.1146/annurev.cellbio.042308.113414. [DOI] [PubMed] [Google Scholar]
- Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- Choi JY, Riekhof WR, Wu WI, Voelker DR. Macromolecular assemblies regulate nonvesicular phosphatidylserine traffic in yeast. Biochem Soc Trans. 2006;34:404–408. doi: 10.1042/BST0340404. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Vicinanza M, De Matteis MA. Lipid-transfer proteins in biosynthetic pathways. Curr Opin Cell Biol. 2008;20:360–370. doi: 10.1016/j.ceb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Funato K, Riezman H. Vesicular and nonvesicular transport of ceramide from ER to the Golgi apparatus in yeast. J Cell Biol. 2001;155:949–959. doi: 10.1083/jcb.200105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter D, Neumann S, van Dijk SM, Wolthoorn J, de Maziere AM, Vieira OV, Mattjus P, Klumperman J, van Meer G, Sprong H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J Cell Biol. 2007;179:101–115. doi: 10.1083/jcb.200704091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Tomishige N, Yamaji T. CERT-mediated trafficking of ceramide. Biochim Biophys Acta. 2009;1791:684–691. doi: 10.1016/j.bbalip.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hechtberger P, Daum G. Intracellular transport of inositol-containing sphingolipids in the yeast, Saccharomyces cerevisiae. FEBS Lett. 1995;367:210–204. doi: 10.1016/0014-5793(95)00567-s. [DOI] [PubMed] [Google Scholar]
- Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen CB, Billheimer JT, Summers SA, Stayrook SE, Lewis M, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) is a sterol transfer protein. J Biol Chem. 1998;273:26285–26288. doi: 10.1074/jbc.273.41.26285. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985a;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Simoni RD. Intracellular transport of phosphatidylcholine to the plasma membrane. J Cell Biol. 1985b;101:441–445. doi: 10.1083/jcb.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok JW, Babia T, Klappe K, Egea G, Hoekstra D. Ceramide transport from endoplasmic reticulum to Golgi apparatus is not vesicle-mediated. Biochem J. 1998;333:779–786. doi: 10.1042/bj3330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci U S A. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol. 2010;11:739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Levine TP, Munro S. Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. 2001;12(6):1633–1644. doi: 10.1091/mbc.12.6.1633. 6, 1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, Mondal M. Sterol and lipid trafficking in mammalian cells. Biochem Soc Trans. 2006;34:335–339. doi: 10.1042/BST0340335. [DOI] [PubMed] [Google Scholar]
- Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA. Non-vesicular sterol transport in cells. Prog Lipid Res. 2007;46:297–314. doi: 10.1016/j.plipres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Yuan C, Allegood JC, Rawat SS, Edwards MB, Wang X, Merrill AHJ, Acharya U, Acharya JK. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc Natl Acad Sci U S A. 2007;104:11364–11369. doi: 10.1073/pnas.0705049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Prinz WA. Nonvesicular phospholipid transfer between peroxisomes and the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:15785–15790. doi: 10.1073/pnas.0808321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TA, Choi M-G, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologs. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight RG, Pagano RE. Rapid appearance of newly synthesized phosphatidylethanolamine at the plasma membrane. J Biol Chem. 1983;258:9050–9058. [PubMed] [Google Scholar]
- Steck TL, Ye J, Lange Y. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys J. 2002;83:2118–2125. doi: 10.1016/S0006-3495(02)73972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter PJ, Pedretti J, Yates R, Voelker DR. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiae. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J Biol Chem. 1995;270:6071–6080. doi: 10.1074/jbc.270.11.6071. [DOI] [PubMed] [Google Scholar]
- Tvan Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker DR. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu Rev Biochem. 2009;78:827–856. doi: 10.1146/annurev.biochem.78.081307.112144. [DOI] [PubMed] [Google Scholar]
- Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL. Identification of surface residues on niemann-pick C2 essential for hydrophobic Handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12:166–173. doi: 10.1016/j.cmet.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rao RP, Kosakowska-Cholody T, Masood MA, Southon E, Zhang H, Berthet C, Nagashim K, Veenstra TK, Tessarollo L, Acharya U, Acharya JK. Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol. 2009;184:143–158. doi: 10.1083/jcb.200807176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock DE, Lutz MS, Blackburn WA, Young WWJ, Baenziger JU. Transport of newly synthesized glucosylceramide to the plasma membrane by a non-Golgi pathway. Proc Natl Acad Sci U S A. 1994;91:2708–2712. doi: 10.1073/pnas.91.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg BW. Glycolipid and glycoprotein transport through the Golgi complex are similar biochemically and kinetically. Reconstitution of glycolipid transport in a cell free system. J Cell Biol. 1990;111:421–428. doi: 10.1083/jcb.111.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji T, Kumagai K, Tomishige N, Hanada K. Two sphingolipid transfer proteins, CERT and FAPP2: their roles in sphingolipid metabolism. IUBMB Life. 2008;60:511–518. doi: 10.1002/iub.83. [DOI] [PubMed] [Google Scholar]