Abstract
Acute ischemic stroke is a major risk for morbidity and mortality in our aging population. Currently only one drug, the thrombolytic tissue plasminogen activator, is approved by the FDA to treat stroke. Therefore, there is a need to develop novel drugs that promote neuronal survival following stroke. We have synthesized a novel neuroprotective molecule called CNB-001 that has neurotrophic activity, enhances memory, and blocks cell death in multiple toxicity assays related to ischemic stroke. In this study, we tested the efficacy of CNB-001 in a rigorous rabbit ischemic stroke model and determined the molecular basis of its in vivo activity. CNB-001 has substantial beneficial properties in an in vitro ischemia assay and improves the behavioral outcome of rabbit ischemic stroke even when administered 1 h after the insult, a therapeutic window in this model comparable to tissue plasminogen activator. In addition, we elucidated the protein kinase pathways involved in neuroprotection. CNB-001 maintains the calcium-calmodulin-dependent kinase signaling pathways associated with neurotrophic growth factors that are critical for the maintenance of neuronal function. On the basis of its in vivo efficacy and novel mode of action, we conclude that CNB-001 has a great potential for the treatment of ischemic stroke as well as other CNS pathologies.
Keywords: ischemia, trophic factor, neuroprotection, models, drugs, protein kinases
Introduction
Ischemic stroke is a major cause of adult death and disability, resulting in over 6 million deaths annually (Ingall 2004). The nerve cell death associated with cerebral ischemia is due to multiple factors resulting from the lack of oxygen, including the loss of ATP, excitotoxicity, oxidative stress, reduced neurotrophic support, and multiple other metabolic stresses (Dirnagl et al. 1999). Therefore, a drug directed against a single molecular target may not be effective in treating the nerve cell death associated with stroke. In addition, drugs that inhibit a single CNS activity with high potency are likely to be toxic because the activity is probably also required for normal brain function. Indeed, there is no effective treatment for stroke that is approved by the FDA except for recombinant tissue-type plasminogen activator (rt-PA). It follows that combinations of drugs or individual drugs that are broadly neuroprotective may be required.
One group of multi-target compounds that may meet the criteria for the treatment of stroke are plant-derived polyphenolics. Our laboratories have recently shown that two flavones, fisetin and baicalein, improve clinical function in a rigorous rabbit embolic stroke model (Lapchak et al. 2007, Maher et al. 2007). Another polyphenolic that has great potential for the treatment of neurological disorders is curcumin (Fig. 1). Curcumin prevents Alzheimer’s disease (AD) pathology in animal models (Frautschy et al. 2001) and is effective at reducing the cognitive losses associated with traumatic brain injury (Wu et al. 2006). In addition, curcumin is effective in reducing multiple pathological indices in the rodent following middle cerebral artery occlusion (Dohare et al. 2008, Thiyagarajan & Sharma 2004). At least 10 potentially neuroprotective biological activities have been attributed to curcumin (Cole et al. 2007), making this multifunctional molecule a good candidate for the treatment of stroke.
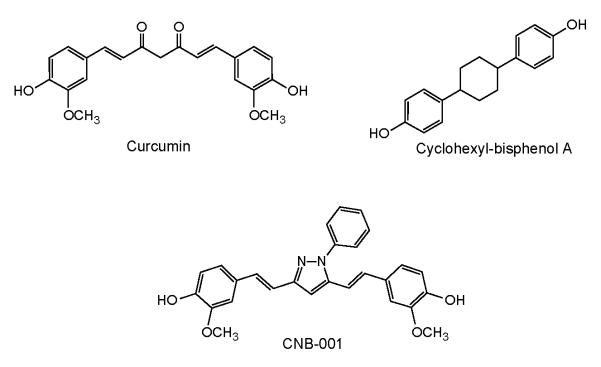
FIGURE 1.
Structure of CNB-001 and its parent compounds, curcumin and cyclohexyl-isphenol A.
There are, however, some properties of curcumin that could be improved, including its stability and its lack of neurotrophic activity and its inability to inhibit excitotoxicity. To circumvent some of these problems, we synthesized a library of hybrid molecules between cyclohexyl bisphenol A (CBA), a molecule with neurotrophic activity and curcumin (Liu et al. 2008). These compounds were then screened in a variety of assays for protection against stroke-associated nerve cell toxicity. A pyrazole derivative, called CNB-001, that lacks the labile dicarbonyl group of curcumin, was identified (Fig. 1). CNB-001 has broad neuroprotective activity in cell culture models of excitotoxicity, oxidative stress and glucose starvation, and has a unique neurotrophic activity that mimics brain derived neurotrophic factor (BDNF) (Liu et al. 2008). In addition, CNB-001 enhances long-term potentiation and memory in a rodent object recognition assay (Maher et al. 2008). Since all of these activities are relevant to the neurological problems associated with stroke (Mattson 2008), it was initially asked if CNB-001 meets the criteria for a candidate stroke treatment using a cell culture assay that mimics ischemia and may be predictive of drug efficacy in vivo (Maher et al. 2007). We then determined some of the steps in the neuroprotective pathway, and finally asked if CNB-001 is able to reduce the behavioral deficits in rabbits following an embolic stroke.
For the stroke studies, we used the rabbit small clot embolic stroke model (RSCEM), which is a possible indicator of treatments that show efficacy in human clinical trials, and was used in the development and FDA-approval of tPA (Lapchak 2010b, Zivin et al. 1985). The RSCEM uses embolization to cause multifocal cerebral ischemia, which results in graded behavioral deficits that can be quantified (Lapchak 2010a). The primary endpoint in the RSCEM is behavior based upon motor function components of the National Institutes of Health Stroke Scale (NIHSS) for stroke in humans, (Hacke et al. 2008, Lyden et al. 1999). While the RSCEM is a superior translational model to develop and evaluate the behavioral effects of novel pharmacological agents, it is not a model where infarct volume can be measured using conventional techniques due to the presence of widespread ischemic cores in multiple brain areas and the requirement of radioactive tracer to measure clot burden in brain post-mortem. Therefore, in the present study we utilized functional analysis as the primary endpoint. As secondary endpoint we measured a series of biochemical markers in ischemic cortical tissue.
It is shown that CNB-001 is effective in both cell culture and animal models of stroke. This protection is at least partially mediated via the maintenance of the MAP kinase and PI3K-Akt kinase pathways, ATP levels, and elevated calcium-calmodulin-dependent protein kinase IIα (CaMKIIα), which are all pathways whose loss or reduction have been previously implicated in stroke pathology. The unique mode of action of CNB-001 is likely a direct result of the novel paradigm used for the synthesis and selection of CNB-001.
Materials and Methods
Chemicals
Curcumin was from Sigma/Aldrich (St. Louis, MO). CNB-001 was synthesized by AQ BioPharma Co., Ltd. (Shanghai, China) according to Liu et al., 2008 (Liu et al. 2008). All other chemicals were from Sigma.
Cell Culture
Fetal calf serum (FCS) and dialyzed FCS (DFCS) were from Hyclone (Logan, UT). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Invitrogen (Carlsbad, CA). HT-22 cells (Davis & Maher 1994, Maher & Davis 1996) were grown in DMEM supplemented with 10% FCS and antibiotics. HT-22 cells expressing the BDNF receptor were generously supplied by Dr. Gerald Thiel (Homburg, Germany).
In Vitro Ischemia Assay
In vitro ischemia was done using HT-22 hippocampal neurons according to Maher et al (Maher et al. 2007). Briefly, cells were seeded onto 96-well microtiter plates at a density of 5 × 103 cells per well. The next day, the medium was replaced with DMEM supplemented with 7.5% DFCS and the cells were treated with 20 μM iodoacetic acid (IAA) alone or in the presence of the different compounds. After 2 h, the medium in each well was aspirated and replaced with fresh medium without IAA but containing the same compounds. 20 h later, the medium in each well was aspirated and replaced with fresh medium containing 5 μg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). After 4 h of incubation at 37°C, cells were solubilized with 100 μl of a solution containing 50% dimethylformamide and 20% SDS (pH 4.7). The absorbance at 570 nm was measured on the following day. Results obtained from the MTT assay correlated directly with the extent of cell death as confirmed visually. Controls included compound alone to test for toxicity and compound with no cells to test for interference with the assay chemistry.
Excitotoxicity Assay
Primary cultures of cortical neurons that die reproducibly by excitotoxicity were prepared by combining aspects of two published protocols as described (Schubert & Piasecki 2001). Briefly, BALB/c mouse embryo cortices were minced and treated with 0.1% trypsin for 20 min. After centrifugation, the cells were resuspended in B27 Neurobasal medium (Invitrogen) plus 10% fetal calf serum and were dissociated by repeated pipetting through a 1 mL blue Eppendorf pipette tip. Then the cells were plated at 1 × 105 cells per well in 96-well poly-l-lysine and laminin-coated microtiter plates (Becton Dickinson, Bedford, MA, USA) in B27 Neurobasal plus 10% fetal calf serum. Two days later the medium was aspirated and replaced by serum-free B27 Neurobasal medium plus 10 μg/mL cytosine arabinoside. The cultures were used without media change 11 days after plating and were essentially free of astrocytes. They were exposed to 10 μM glutamate followed by the test compounds. Cell viability was determined 24 h later using the fluorescent live/dead assay.
SDS-PAGE and Immunoblotting
HT-22 cells at the same density as used for the cell death assays were untreated or treated with the compounds alone or in the presence of 20 μM IAA for 2 h followed by 2 h recovery in the presence of the compounds. The cells were washed twice in phosphate buffered saline and solubilized in SDS-sample buffer containing 0.1 mM Na3VO4 and 1 mM phenylmethylsulfonyl fluoride (PMSF), boiled for 5 min and either analyzed immediately or stored frozen at −70°C. Proteins were separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose. Equal loading and transfer of the samples was confirmed by staining the nitrocellulose with Ponceau-S. Transfers were blocked for 1 h at room temperature with 5% nonfat milk in TBS/0.1% Tween 20 and then incubated overnight at 4°C in the primary antibody diluted in 5% BSA in TBS/0.05% Tween 20. The primary antibodies used were: phospho-p44/42 MAP kinase antibody (#9101 and #9106, 1/1000) and phospho-Akt ser473 (#9271, #4051, 1/1000) from Cell Signaling (Beverly, MA); ORP150 antibody (#10301, 1/100,000) from IBL (Gunma, Japan), anti-CaMKIIα (Zymed, Invitrogen #137300), actin antibody (#A5441, 1/800,000) from Sigma (St. Louis, MO), anti-Akt (#05-591, 1/1000) from Millipore (Bedford, MA.), and pan ERK antibody (#E17120, 1/1000) from Transduction Laboratories (San Diego, CA). The transfers were rinsed with TBS/0.05% Tween 20 and incubated for 1 h at room temperature in horseradish peroxidase-goat anti-rabbit or goat anti-mouse (Biorad, Hercules, CA) diluted 1/5000 in 5% nonfat milk in TBS/0.1% Tween 20. The immunoblots were developed with the Super Signal reagent (Pierce, Rockford, IL).
To assay kinase pathways in tissue, groups of animals separate from those used for behavioral analysis were embolized with identical size (100-250 μM) and weights (2 mg) of clots, followed by the drug or vehicle 1 h later. Non-embolized animals received vehicle alone (see below). Six h following embolization rabbits were euthanized with 1-1.5 ml of Beuthanasia-D via the marginal ear vein. The brain was rapidly removed from the skull within 30 s and the complete thickness of the parietal/occipital cortex was dissected on ice and then quick frozen using liquid nitrogen as previously described (Lapchak & De Taboada 2010). The identical area was taken from control animals (N=5 per group). Brain samples were sonicated in PBS containing protease (CompleteMini, Roche, Mannheim, Germany) and phosphatase inhibitors (Phosphatase Inhibitor Cocktail 2, Sigma, St. Louis, MO). Equal amounts of protein were solubilized in SDS-sample buffer and analyzed as for the cell extracts.
Total Glutathione (GSH) and ATP
Total intracellular GSH and ATP were determined by chemical and chemiluminescent assays as described (Maher et al. 2007).
Statistical Analysis
The in vitro cell death assays and the biochemical assays were repeated at least three times in triplicate each time and analyzed using Instat software. The data are presented as the mean ± SD. Statistical analysis was done by ANOVA followed by Bonferroni’s test. P < 0.05 was considered significant.
Rabbit Small Clot Embolism Model
Male New Zealand white rabbits weighing 2 to 2.5 kg were purchased from Rabbit Source Farms, Ramona, CA and were supplied food (alfalfa cubes) and water ad libitum while under quarantine in an enriched environment for at least 5 days prior to experimental use. Surgery was done in a sterile controlled environment with a room temperature between 22.8–23.2°C. Institutional Animal Care and Use Committee (IACUC) approved the surgical and treatment procedures used in this study. Care was used throughout the study to minimize pain and discomfort. Per the IACUC-approved protocol, rabbits were euthanized if they were in pain, showed extreme discomfort, or if they were unable to reach food or water.
Surgical procedures were done as described previously (Lapchak et al. 2007, Lapchak et al. 2004). Briefly, rabbits were anesthetized with isoflurane via a face-mask, 5% in 3 l/min at induction, and 2-3% in 3 l/min as a maintenance dose and placed on a QuantumHeat™ heatpack. The right internal carotid artery was exposed, and the external carotid artery and the common carotid artery were ligated. A Becton, Dickinson and Company (B-D) plastic catheter oriented toward the brain was inserted into the common carotid artery positioned toward the brain and secured with ligatures. The incision was closed around the catheter so that the distal end was accessible outside. The catheter was filled with 0.2 ml of heparinized saline (33 units/ml) and plugged with injection caps. The animals were allowed to recover from anesthesia for at least 2-3 h before embolization.
Blood was drawn from one or more donor rabbits and allowed to clot for 3 h at 37°C. The large blood clots were then suspended in phosphate-buffered saline pH 7.4 (PBS) with 0.1% bovine serum albumin (BSA) and Polytron-generated fragments were sequentially passed through sizing screens to result in a suspension of small-sized blood clots (100-250 μM), which were suspended in PBS containing 1% bovine serum albumin. The blood clot suspension was then spiked with 57Co-labeled microspheres (Perkin-Elmer Inc.) to allow for tracking into the brain. An aliquot of the solution was removed for the determination of specific activity. For embolization, rabbits were placed in a restrainer and 1 ml of a clot particle suspension was injected through the carotid catheter, which was then flushed with 5 ml of sterile saline. Rabbits are fully awake during the embolization procedure and they are self-maintaining (i.e. they do not require artificial respiration or other external support). This allows for immediate observation of the effects of embolization on behavior at the time of embolus injection and thereafter. After the embolization process was completed, the catheter was heat ligated close to the neck leaving a small portion protruding from the skin.
Clinical Rating Score Behavioral and Quantal Dose-Response Analysis
The use of clinical rating scores is a desirable primary endpoint to use when a novel therapeutic is being tested for further development and to support a clinical trial (Lapchak 2010b). Clinical scores in combination with quantal analysis is a sophisticated statistical analysis method to determine how a large population of stroke “patients”, in this case, rabbits, will respond to a treatment. To evaluate the quantitative relationship between clot dose lodged in the brain and behavioral deficits or clinical scores, logistic sigmoidal (S-shaped) quantal analysis curves were fit to dose-response data as originally described by Waud (Waud 1972). To construct a quantal analysis curve, a wide range of clot doses (3-7 mg) were injected via the indwelling carotid catheter in order to produce a spectrum of behaviorally normal to abnormal animals, which included death on the continuum of embolization-induced effects (Lapchak 2010b). In the absence of a neuroprotective treatment regimen, small numbers of microclots lodged in the brain vasculature cause no grossly apparent neurologic dysfunction. However, when large numbers of microclots become lodged in the vasculature, they invariably caused encephalopathy due to ischemia, neuronal degeneration, depletion of ATP and cell death (Lapchak & De Taboada 2010). In this study, less than 5% of animals died due to embolization, but in the event that embolization did result in a rapid death, there was a positive correlation with a high clot dose measured in brain and the animal was represented as an abnormal (or dead) on the quantal analysis curve if the rabbit received treatment, either vehicle or drug. A 3-tiered clinical scoring scale is used for analysis of embolized rabbits: normal, abnormal or dead. Embolized rabbits are scored as abnormal if they have one or more of the following symptoms: ataxia, leaning, circling, lethargy, nystagmus, loss of balance, loss of limb/facial sensation and occasionally, hind-limb paraplegia. Using a simple dichotomous rating system, with a reproducible composite result and low inter-rater variability (<5%), each surviving animal is rated as either behaviorally normal or abnormal by an observer naïve to the study design and/or treatment groups. For construction of quantal analysis curves, clot burden is plotted against behavior in order to define the P50 value. A separate quantal curve was generated for each treatment condition and a statistically significant increase in the P50 value (or the amount of blood clots in brain that produce neurologic dysfunction in 50% of a group of animals) compared to a control curve is indicative of neuroprotection or a behavioral improvement in the study population.
Drug Treatment
For test substance administration, rabbits were placed in a Plexiglas restrainer (Plaslabs Inc.) for the duration of the treatment. For all experiments in this study, rabbits were randomly allocated into treatment groups before the embolization procedure, with concealment of the randomization guaranteed by using an independent party. The randomization sequence was not revealed until all postmortem analyses were complete.
Power analysis of quantal dose-response curves indicates that a sample size of approximately 20 animals are required per group (Lapchak & Araujo 2007, Lapchak et al. 2004). The behavioral data are presented as P50 (Mean ± SEM) in mg clot for the number of rabbits in each group (n). P50 values were calculated using an iterative curve fitting algorithm as described previously (Lapchak et al. 2004). They were analyzed for significance using the t-test with SigmaStat 3.5 using the Bonferonni correction when required.
Results
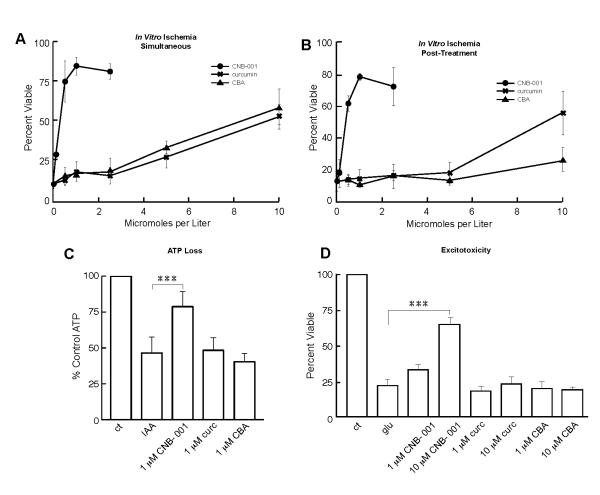
CNB-001 was both designed and selected for its ability to have multiple biological activities (Liu et al. 2008). Selection was based upon a variety of neuroprotection assays representing distinct aspects of stress and toxicity that occur in stroke. More importantly, CNB-001 has a unique neurotrophic activity similar to brain-derived neurotrophic factor (BDNF) (Liu et al. 2008). In addition, we have recently described a cell culture chemical ischemia assay that may have predictive power for screening drugs for stroke (Lapchak et al. 2007, Maher et al. 2007). This in vitro model mimics the loss of energy metabolism and ATP found in stroke, and specifically the rabbit model that was used in this study (Lapchak & De Taboada 2010). In our hands the chemical ischemia assay is much more reproducible than the commonly used oxygen-glucose deprivation assay and therefore more appropriate to study protein kinase signaling pathways. In this assay, iodoacetic acid (IAA), an irreversible inhibitor of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), is used to deplete ATP levels in hippocampal HT-22 neurons. Using this in vitro model, it was asked if CNB-001 and/or its precursors, curcumin and CBA, had neuroprotective properties. Figures 2A and B show that CNB-001 is significantly better than either curcumin or CBA in protecting cells from in vitro ischemia. When the three test compounds were added both during and at the end of a 2 h exposure to 20 μM IAA, there was a significant increase in viability with all three compounds, but the EC50 for CNB-001 was approximately 10-fold lower than that for the other compounds and the maximal level of protection was significantly greater (Fig. 2A). If the compounds were added only after the 2 h exposure to IAA, there was still significant protection by CNB-001 at 0.5-1 μM (Fig. 2B) and by curcumin at 10 μM, but CBA was no longer effective (Fig. 2B).
FIGURE 2. CNB-001 protects against IAA toxicity and ATP loss in HT-22 cells.
(A) In vitro ischemia – Simultaneous. HT-22 cells were treated with 20 μM IAA for 2 h alone or in the presence of increasing doses of CNB-001, curcumin or bisphenol A (CBA). The compounds were also included in the fresh medium added after the 2 h treatment with IAA. (B) In Vitro Ischemia - Post-treatment. HT-22 cells were treated with 20 μM IAA alone for 2 h. Increasing doses of CNB-001, curcumin or cyclohexyl-bisphenol A (CBA) were only included in the fresh medium added after the 2 h IAA treatment. In all experiments, percent survival was confirmed by microscopy and measured after 24 h by the MTT assay. Similar results were obtained in 3 independent experiments. The data are presented as the mean plus or minus the standard deviation, n=3. (C) CNB-001 maintains ATP in response to in vitro ischemia. HT-22 cells were treated with 20 μM IAA for 2 h followed by 2 h in fresh medium alone (IAA) or in the presence of 1 μM CNB-001, or 1 μM curcumin (curc), or CBA. Total ATP levels were measured by a chemiluminescent assay. Similar results were obtained in 3-5 independent experiments. (D) CNB-001 prevents excitotoxicity in mouse primary cultures of cortical neurons. After 11 days of culture, embryonic day 14 neurons were exposed to 10 μM glutamate for 10 min, followed by the addition of the indicated compounds. Ten micromolar MK801 completely blocked glutamate toxicity (not shown). Cell viability was determined 24 h later with the MTT assay and verified using a fluorescent live-dead assay. *** indicates a significant difference between IAA-treated cells and cells treated with IAA + compounds (p<0.001; ANOVA followed by Bonferroni’s test).
Previously, it was shown that some compounds that protect from in vitro ischemia do so by preventing the loss of GSH and/or ATP (Maher et al. 2007). While CNB-001 had no effect on intracellular GSH levels (data not shown), it did partially maintain ATP levels at 1 μM, its optimal dose in the in vitro ischemia assay (Fig. 2C). Neither curcumin or CBA maintained ATP levels at this concentration. Finally, excitotoxicity is an aspect of stroke pathology and a well studied drug target (Lapchak et al. 2007). The excitotoxicity assay depends upon the opening by low concentrations of glutamate of ionotropic glutamate receptors on E14 cortical neurons that have been in culture for 11 days resulting in rapid cell death. CNB-001 is effective at preventing excitotoxicity at 10 μM , but not at 1 μM , while curcumin is ineffective at both concentrations (Fig. 2D). These in vitro criteria for stroke efficacy suggest that CNB-001 may be neuroprotective in animal stroke.
Motor Function is Improved by CNB-001 Following Embolic Strokes
To determine if CNB-001 can improve behavioral function or reduce the clinical deficits associated with embolic stroke, we used a rabbit model that has been described and reviewed in detail (Lapchak 2010b). A clinical rating system and quantal analysis technique were used to determine how rabbits subjected to embolic strokes respond to CNB-001 treatment. A clinical endpoint of motor function is a valid assay to use when drugs are being developed for clinical trials (Zivin & Waud 1992). Based upon previous studies with polyphenolic natural products, CNB-001 was administered subcutaneously at a dose of 100 mg/kg (Lapchak et al. 2007, Maher et al. 2007). In this study, we only administered the drug post-embolization to parallel clinical treatment regimens. An initial exploratory study used a 5 min post-embolization treatment time, which in itself has little opportunity to be useful clinically, but is useful during early stages of drug development to ensure that the dose and route of administration of the drug can produce a neuroprotective signal. There was a significant improvement in behavior following stroke when CNB-001 was administered at this early time-point. The P50 value for the CNB-001-treated group was increased by 157% compared with control (not shown). At this dose there was no overt behavioral response or toxicity with the drug within the time frame of the study. Behavioral analysis was done 24 h after treatment, and was done by an observer naïve of the study design who simply rated the rabbits as completely normal by all criteria or abnormal (see methods), an all or nothing ‘quantal’ scale.
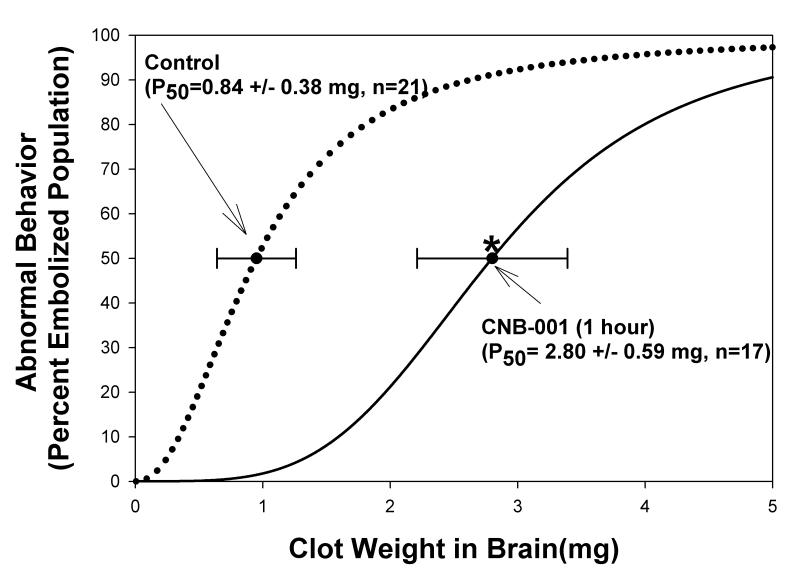
In the follow-up study, we used a 1 h post-embolization treatment time to determine if CNB-001 would improve clinical function. As shown in Figure 3, CNB-001 improved behavioral function following embolization measured by a significant increase in the P50 values defined as the amount of blood clots in the brain that produce neurologic dysfunction in 50% of a group of animals and a shift in the curve to the right. Table 1 presents the raw data for the 1 h administration study that was used to construct the sigmoidal quantal analysis curve. CNB-001 significantly (P<0.05) reduced stroke-induced behavioral deficits and increased the P50 value by 233% compared with the control group. The CNB-001-induced improvement in behavior is directly correlated with an increase in the population of rabbits that are behaviorally “normal” following embolization. This is shown in columnar fashion in Table 1. The graph and table show that a larger population of rabbits with higher levels of clot deposited in the brain (clot burdens) are behaviorally “normal” with CNB-001 treatment compared to vehicle-treated control animals with similar levels of clot burden. It should be noted that clot burden is measured post-mortem in both groups. As shown in Table 1, there are some instances where there was no equivalent clot burden in the 2 groups. Nevertheless, using the statistical quantal analysis technique, the drug effect for the overall rabbit population can be calculated and represented by a P50 value.
FIGURE 3. Motor function is improved by CNB-001 following embolic strokes. Behavioral improvement following CNB-001 treatment 1 hour post-embolization (100 mg/kg s.c.).
The DMSO control curve (dotted line) has a P50 value of 0.84 ± 0.38 mg (n=21). A shift to the right is indicative of behavioral improvement. CNB-001 treatment initiated 1 hour following embolization increased the P50 value to 2.8 ± 0.59 mg (n=17), an increase of 233%, (*P<0.0065) (dark solid line).
Table 1. Raw Quantal Analysis Data.
Data are presented as Clot weight in brain (mg), which is clot burden measured following behavioral analysis. The data in the columns for both treatment groups have been aligned to show that CNB-001-treated rabbits with higher clot doses are “normal”, whereas vehicle-treated rabbits are abnormal. Using a simple dichotomous rating system, behaviors are noted as (N) for a behaviorally normal rabbit following embolization or (A) for a behaviorally abnormal rabbit following embolization. P50 values were calculated using logistics regression analysis. . The quantal analysis curves are presented in Figure 3, where a shift to the right or an increase in P50 demonstrates CNB-001-induced behavioral improvement.
| Control | CNB-001 | ||
|---|---|---|---|
| Clot Weight | Rating Score | Clot Weight | Rating Score |
| 0.027 | N | 0.100 | N |
| 0.180 | N | 0.150 | N |
| 0.700 | A | 0.700 | N |
| 0.800 | A | --- | --- |
| 0.810 | N | 0.850 | N |
| 0.920 | N | 0.920 | N |
| 0.970 | A | --- | --- |
| 1.050 | N | --- | --- |
| 1.150 | A | --- | --- |
| 1.220 | N | --- | --- |
| 1.260 | A | --- | --- |
| 1.400 | A | --- | --- |
| 1.660 | A | --- | --- |
| 1.780 | N | --- | --- |
| 1.880 | A | --- | --- |
| 2.420 | A | --- | --- |
| 2.590 | A | --- | --- |
| 2.670 | N | 2.710 | A |
| 2.810 | A | 3.130 | N |
| --- | --- | 3.350 | A |
| 3.690 | A | 3.710 | N |
| --- | --- | 3.790 | N |
| --- | --- | 3.810 | A (2) |
| --- | --- | 3.830 | A |
| --- | --- | 3.840 | A |
| --- | --- | 4.030 | A |
| 4.200 | A | 4.370 | A |
| 4.420 | A | ||
| P50 = 0.84 ± 0.38 mg | P50 = 2.80 ± 0.59 m g | ||
There were no statistical differences (p>0.05) between the P50 value measured in the 5 min and 1 h CNB-001 treatment groups, but both were significantly different from control (p<0.05). Very few compounds work in the rabbit ischemia model 1 h post-trauma. An exception is rt-PA which has a similar therapeutic window in this assay (Lapchak 2010b).
Mode of Action
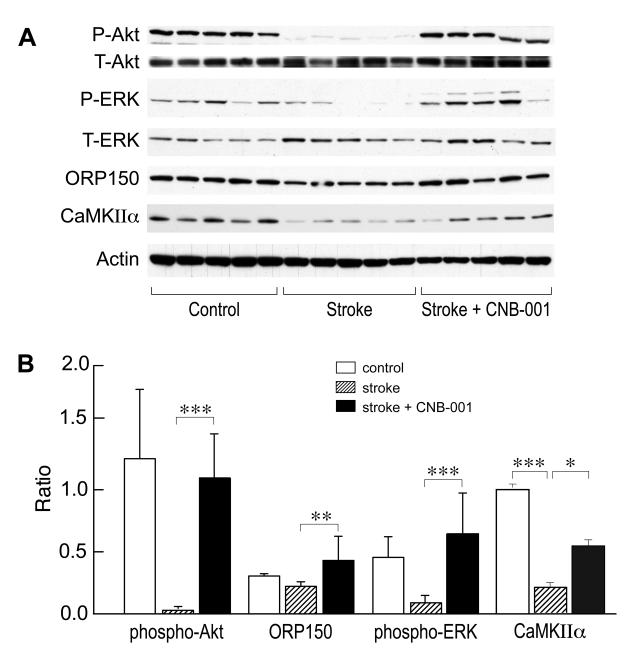
To obtain an initial understanding of how CNB-001 functions in vivo, we examined a variety of signaling pathways previously implicated in stroke. Brain extracts were taken at 6 h post-embolism from control, stroked and stroked + CNB-001 treated animals. Since the Ras-ERK cascade has been implicated in nerve cell survival in ischemia (Mehta et al. 2007), we initially examined this pathway. A significant decrease in ERK phosphorylation in the stroked animals relative to control animals was observed, and this decrease was largely prevented in the presence of CNB-001 (Fig. 4). PI3K-Akt signaling is also implicated in nerve cell survival both in vitro and in vivo (Zhao et al. 2006, Liu et al. 2010). Consistent with these observations, phosphorylation of Akt on Ser473 was largely eliminated in the stroked animals but was maintained in the presence of CNB-001 (Fig. 4). CNB-001 also has BDNF-like activity (Liu et al. 2008), and BDNF causes the activation of the ERK and Akt pathways (Rossler et al. 2004). Since the expression of CaMKIIα is also increased by BDNF (Schratt et al. 2004), we assayed CaMKIIα expression in CNB-001-treated animals. Figure 4 shows that CNB-001 increased CaMKIIα expression. Finally, the ER-associated chaperone ORP150 has been linked to the PI3K/Akt and BDNF signaling pathways (Tamatani et al. 2001), and the overexpression of ORP150 reduces infarct volume in a mouse stroke model (Tamatani et al. 2001). ORP150 expression was decreased in the stroked rabbit brains and this decrease was again prevented by CNB-001 (Fig. 4).
FIGURE 4. Effects of CNB-001 in vivo.
CNB-001 maintains ERK and Akt phosphorylation and ORP150 and CaMKIIα expression in stroked rabbits. Areas of damaged tissue were removed from the cortex of animals 6 h after embolization with identical clot weights and the tissue analyzed for proteins and phosphoproteins by western blotting. Representative western blots are shown above (A) the quantitative representation of the data (B), presented as ratio of the phosphoblots to total protein (Akt and ERK) or ORP150 and CaMKIIα protein to actin. The data for each protein of interest were analyzed independently by ANOVA followed by Bonferroni’s test, *, p<0.05; **, p<0.01; ***, p<0.001; N=5.
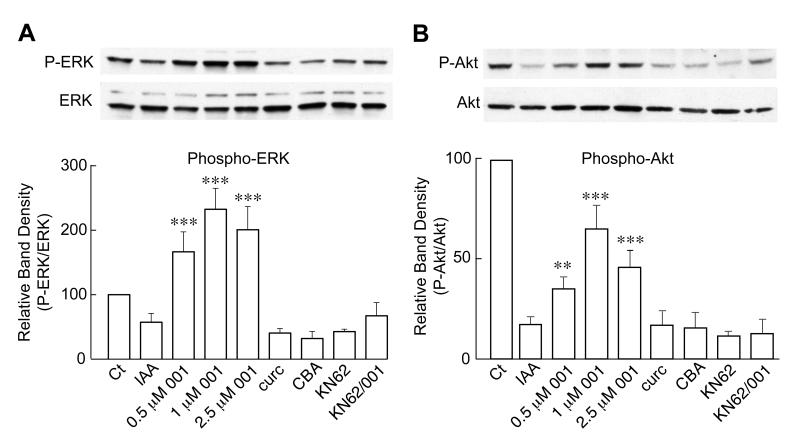
In order to study the mechanisms underlying these in vivo effects of CNB-001, we turned to the in vitro model of ischemia. Similar to the in vivo results, cells treated for 2 h with 20 μM IAA and allowed to recover for 2 h also showed decreases in both ERK and Akt phosphorylation (Fig. 5A & B). Importantly, treatment with CNB-001 at concentrations ranging from 0.5-2.5 μM prevented the decreases in ERK and Akt phosphorylation in a dose-dependent manner. In contrast, a similar concentration of CBA or curcumin (2.5 μM) was ineffective. In the HT-22 cell line used for in vitro ischemia, however, ORP150 is expressed at an extremely high level and there was no consistent change in ORP150 levels (not shown).
FIGURE 5. Effects of CNB-001 in vitro.
CNB-001 promotes ERK and Akt phosphorylation in in vitro ischemia. HT-22 cells were treated with IAA as described in methods and materials in the presence of the indicated concentrations of CNB-001 (001) and 2.5 μM curcumin (curc), 2.5 μM cyclohexyl bisphenol-A (CBA), 10 μM KN62, or 10 μM KN62 plus 1 μM CNB-001. Two h later, cells were harvested and examined by western blotting. In both cases, the CaMKII inhibitor, KN62, blocked the maintenance of phosphorylation by CNB-001, showing that CaMKII regulates the ERK (A) and Akt (B) pathways in this system. **, p<0.01; ***, p<0.001 by ANOVA followed by Bonferroni’s test. N=4, significantly different from IAA alone treated cells.
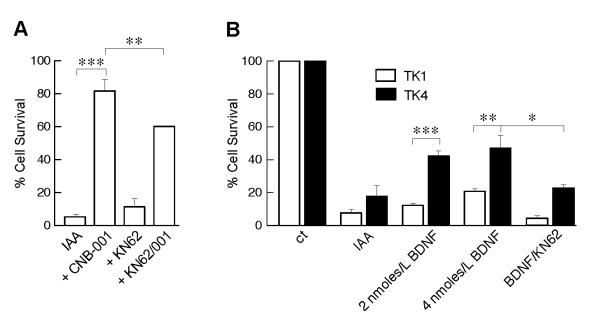
Previously, we showed that CaMKII is activated by CNB-001 (Maher et al. 2008). Thus, we asked what role CaMKII activity plays in the neuroprotective effects of CNB-001. Not only did KN62, a CaMKII inhibitor, reduce the protection by CNB-001 in the in vitro ischemia model (Fig. 6A) but it also blocked the effects of CNB-001 on both ERK and Akt phosphorylation (Fig. 5). These data show that CaMKII regulates the ERK and Akt pathways in this model and is a more proximal target of CNB-001.
FIGURE 6. CaMKII and BDNF pathways are involved in neuroprotection by CNB-001.
(A) HT-22 cells were treated with IAA in the presence or absence of 1 μM CNB-001 and 10 μM KN62 and cell viability measured 24 h later by the MTT assay. (B) HT-22 cells either expressing the active BDNF receptor (TK4) or an inactive receptor (TK1) were exposed to IAA in the presence or absence of 25 ng/ml BDNF or 50 ng/ml BDNF plus or minus 10 μM KN62 and cell viability measured 24 h later by the MTT assay. *, p<0.05; **, p<0.01; ***p<0.001 by ANOVA followed by Bonferroni’s test. N=4.
Finally, CNB-001 was selected from a library of curcumin derivatives because of its neurotrophic activity (Liu et al. 2008). Since CNB-001 rescues HT-22 cells from ischemia, and both ERK and Akt activation and CaMKIIα expression are downstream pathways activated by neurotrophic molecules such as BDNF, it was asked if BDNF is able to rescue HT-22 cells expressing the BDNF receptor, TrkA, in the in vitro ischemia model. Figure 6B shows that BDNF rescues cells transfected with wild type TrkA (TK4), but not cells containing a mutated nonfunctional receptor (TK1) and this rescue is blocked by KN62. The rescue with BDNF is not, however, as complete as with CNB-001, suggesting that CNB-001 also functions by other mechanisms that remain to be defined.
Discussion
The above data show that CNB-001, a synthetic derivative of curcumin with neurotrophic activity, inhibits nerve cell death in a stroke-related cell culture model of ischemia and can also improve clinical function in rabbits following small clot embolic strokes. Importantly, CNB-001 is equally effective when administered either immediately after embolization or 1 h later, traits shared with only a few potential stroke therapeutics, including the single FDA approved drug, rt-PA (Lapchak 2010b).
CNB-001 is a broadly neuroprotective compound as defined by cell culture assays for excitotoxicity, glucose starvation, loss of trophic factors, and oxidative stress (Liu et al. 2008). CNB-001 does not have any significant antioxidant activity itself (Liu et al. 2008), ruling out a direct antioxidant effect of the molecule. In addition, CNB-001 activates CaMKII and enhances LTP and memory in rodents (Maher et al. 2008), and increases the expression of CaMKIIα in animals (Fig. 4). Here we show both in vivo and in vitro that the ERK and Akt phosphorylation pathways as well as ORP150 expression are also involved in stroke pathology and the CNB-001 mediated protection.
Oxidative glutamate toxicity (oxytosis) and in vitro ischemia are two cell culture assays employing the hippocampal nerve cell line HT-22 that were used to determine if CNB-001 had the potential to be effective in the embolic rabbit stroke model; the effect of CNB-001 on oxytosis has been published (Liu et al. 2008). Both assays have been used to screen a large number of flavones to predict potential efficacy for stroke in animals (Maher et al. 2007); both utilize neurotoxic conditions that are similar to those observed in ischemic stroke (Sigalov et al. 2000, Tan et al. 2001). In the oxytosis assay, glutamate blocks cystine uptake, leading to glutathione (GSH) depletion, lipoxygenase activation, ROS production, and cGMP dependent Ca++ influx resulting in cell death. We have previously shown that two compounds that block this pathway by either maintaining GSH levels (fisetin) or inhibiting lipoxygenases (baicalein) are effective in the rabbit stroke model (Maher et al. 2007, Lapchak et al. 2007). CNB-001 inhibits the oxytosis pathway at a step between the depletion of GSH and mitochondrial ROS production (not shown).
In the chemical ischemia assay, IAA inhibits the glycolytic enzyme GAPDH, resulting in a loss of Akt and ERK phosphorylation, ATP depletion and oxidative stress as determined by increased ROS and lipid peroxidation (Taylor et al. 1996, Maher et al. 2007). The loss of ATP in ischemia results in cell death by necrosis (Vanlangenakker et al. 2008). This assay results in a toxicity profile very similar to the oxygen-glucose deprivation (Taylor et al. 1996), but is much more reproducible for pathway analysis and drug screening. Although CNB-001 is not a direct antioxidant, it is protective in both chemical ischemia in vitro and embolic stroke. What is the molecular basis for this protection?
Our results show that the activity of several signaling pathways implicated in neuroprotection are decreased in the cerebral cortex of stroked rabbits and that this decrease is partially attenuated by CNB-001. Specifically, CNB-001 prevents decreases in both ERK and Akt phosphorylation and maintains the levels of CaMKIIα and the pro-survival chaperone ORP150. Using our in vitro ischemia model, we were able to show that at least part of the neuroprotective action of CNB-001 is mediated by CaMKII, consistent with our previous observation that CNB-001 activates this kinase (Maher et al. 2008). CaMKII activates both ERK (Li et al. 2009) and Akt (Lahair et al. 2006) in nerve cell lines and elevates CaMKIIα protein expression (Schratt et al. 2004); ORP150 protein expression is also associated with elevated BDNF levels in the brain (Tamatani et al. 2001). Our results are in agreement with these observations because we show that the CaMKII inhibitor KN62 prevents both the maintenance of ERK and Akt phosphorylation by CNB-001 in the in vitro ischemia model as well as cell rescue by CNB-001. However, since KN62 only partially blocks the neuroprotective effects of CNB-001, it is likely that other pathways are involved. Like CNB-001, the neurotrophic molecule BDNF is also able to activate both the ERK and Akt pathways and prevent cell death in the in vitro ischemia assay (Fig. 6B).
One of the other pathways may involve ORP150, an ER resident chaperone whose overexpression was implicated in neuroprotection by a BDNF-mediated mechanism in a mouse stroke model (Tamatani et al. 2001). ORP150 is decreased at 6 h following embolic stroke in the rabbits and this decrease is prevented by CNB-001. There is evidence that ORP150 plays a role in Akt phosphorylation since in endothelial cells ORP150 silencing results in inhibition of Akt phosphorylation (Sanson et al. 2009) and phosphorylation of Akt is altered in the liver of mice expressing an antisense version of ORP150 (Nakatani et al. 2005). Thus, it is possible that the decrease in ORP150 levels contributes to the decrease in Akt phosphorylation in the rabbits. Although unlikely, we have not ruled out the possibility that CNB-001 has vascular effects that that may contribute to stroke outcome.
In conclusion, we have shown that the neurotrophic compound CNB-001 is neuroprotective in both in vitro and can significantly reduce motor function deficits in vivo, with a therapeutic window similar to that of rt-PA. The beneficial effects of CNB-001 correlates with its ability to maintain levels of ATP, and phosphorylated ERK and Akt, which are dramatically reduced following ischemia. Since CNB-001 activates CaMKII and a CaMKII inhibitor reduces protection by CNB-001 as well as ERK and Akt phosphorylation, it is likely that CaMKII is a more proximal target for CNB-001. Because of its reasonable pharmacological properties (Liu et al. 2008), and its unique ability to mimic some activities of neurotrophic growth factors such as BDNF, CNB-001 is a viable lead compound for the development of drugs to treat ischemic stroke and other neurological conditions. Based upon a correlative analysis hypothesis described by Lapchak (Lapchak 2010b), a 1 hour effective therapeutic window in the RSCEM was calculated to represent approximately 2.4-3 hours in stroke patients, a time frame in which the majority of patients arrive at the hospital. We recognize that there are potential obstacles to the widespread use of a drug with a short therapeutic window. However, with an estimated therapeutic window of approximately 3 hours, it will be possible to conduct clinical trials with the drug within the hyperacute phase following a stroke as defined by Saver and colleagues [see (Ferguson et al. 2004)]. Moreover, as we further advance the development of CNB-001 toward a clinical trial, we envision using of state-of the-art trial design with a logistic regression model adjusting for baseline NIHSS (stroke severity), age, and presence or absence of diabetes. The use of shift analysis clinical trial design will allow us to gauge change in outcome distributions over the full range of measured outcomes, incorporating both benefit and harm (Saver & Gornbein 2009).
Acknowledgements
This work was supported by National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 1 R01 NS060864-01] (DS, PAL, PM) and National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 1 U01 NS60685-01] (PAL).
1 Commonly Used Abbreviations
- BDNF
brain derived neurotrophic factor
- BSA
bovine serum albumin
- CaMKIIα
calcium-calmodulin-dependent protein kinase IIα
- CBA
cyclohexyl bisphenol A
- cGMP
cyclic guanosine monophosphate
- CNB-001
a pyrazole derivative of curcumin
- DMEM
Dulbecco’s Modified Eagle’s Medium
- ERK
extracellular receptor kinase
- FCS
fetal calf serum
- FDA
Federal Drug Administration
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HT-22
hippocampal cell line
- IAA
iodoacetic acid
- IACUC
Institutional Animal Care and Use Committee
- LTP
long-term potentiation
- MAP
mitogen-activated protein
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol-3-kinase
- PMSF
phenylmethylsulfonyl fluoride
- ROS
reactive oxygen species
- RSCEM
rabbit small clot embolic stroke model
- SDS
sodium dodecyl sulfate
- TBS
tri-buffered saline
- tPA
tissue plasminogen activator.
Footnotes
The authors state that there are no actual or potential conflicts of interest.
References
- Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Maher P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994;652:169–173. doi: 10.1016/0006-8993(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dohare P, Garg P, Jain V, Nath C, Ray M. Dose dependence and therapeutic window for the neuroprotective effects of curcumin in thromboembolic model of rat. Behav Brain Res. 2008;193:289–297. doi: 10.1016/j.bbr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Ferguson KN, Kidwell CS, Starkman S, Saver JL. Hyperacute treatment initiation in neuroprotective agent stroke trials. J Stroke Cerebrovasc Dis. 2004;13:109–112. doi: 10.1016/j.jstrokecerebrovasdis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiology of Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Ingall T. Stroke--incidence, mortality, morbidity and risk. J Insur Med. 2004;36:143–152. [PubMed] [Google Scholar]
- Lahair MM, Howe CJ, Rodriguez-Mora O, McCubrey JA, Franklin RA. Molecular pathways leading to oxidative stress-induced phosphorylation of Akt. Antioxid Redox Signal. 2006;8:1749–1756. doi: 10.1089/ars.2006.8.1749. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Efficacy and safety profile of the carotenoid trans sodium crocetinate administered to rabbits following multiple infarct ischemic strokes: a combination therapy study with tissue plasminogen activator. Brain Res. 2010a;1309:136–145. doi: 10.1016/j.brainres.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Translational stroke research using a rabbit embolic stroke model: a correlative analysis hypothesis for novel therapy development. Trans Stroke Res. 2010b;1:96–107. doi: 10.1007/s12975-010-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs. 2007;12:97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15 lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Wei J, Zivin JA. Transcranial infrared laser therapy improves clinical rating scores after embolic strokes in rabbits. Stroke. 2004;35:1985–1988. doi: 10.1161/01.STR.0000131808.69640.b7. [DOI] [PubMed] [Google Scholar]
- Li N, Wang C, Wu Y, Liu X, Cao X. Ca(2+)/calmodulin-dependent protein kinase II promotes cell cycle progression by directly activating MEK1 and subsequently modulating p27 phosphorylation. J Biol Chem. 2009;284:3021–3027. doi: 10.1074/jbc.M805483200. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. Journal of Neurochemistry. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dargusch R, Maher P, Schubert D. A broadly neuroprotective derivative of curcumin. J Neurochem. 2008;105:1336–1345. doi: 10.1111/j.1471-4159.2008.05236.x. [Accepted for publication prior to April 1337, 2008] [DOI] [PubMed] [Google Scholar]
- Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- Maher P, Akaishi T, Schubert D, Abe K. A pyrazole derivative of curcumin enhances memory. Neurobiology of Aging. 2008;31:706–709. doi: 10.1016/j.neurobiolaging.2008.05.020. Not funded by NIH. [DOI] [PubMed] [Google Scholar]
- Maher P, Davis J. The role of monoamine metabolism in oxidative glutamate toxicity. J. Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. PMCID: PMC2111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. Epub 2004 Oct.2027. [DOI] [PubMed] [Google Scholar]; J Biol Chem. 2005 Aug;2280(2034):30648. Erratum in: 2026. [Google Scholar]
- Rossler OG, Giehl KM, Thiel G. Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and Raf-1 protein kinase: role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J Neurochem. 2004;88:1240–1252. doi: 10.1046/j.1471-4159.2003.02255.x. [DOI] [PubMed] [Google Scholar]
- Sanson M, Auge N, Vindis C, et al. Oxidized low-density lipoproteins trigger endoplasmic reticulum stress in vascular cells: prevention by oxygen-regulated protein 150 expression. Circ Res. 2009;104:328–336. doi: 10.1161/CIRCRESAHA.108.183749. [DOI] [PubMed] [Google Scholar]
- Saver JL, Gornbein J. Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology. 2009;72:1310–1315. doi: 10.1212/01.wnl.0000341308.73506.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. J. Neurosci. 2001;21:7455–7462. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalov E, Fridkin M, Brenneman DE, Gozes I. VIP-Related protection against lodoacetate toxicity in pheochromocytoma (PC12) cells: a model for ischemic/hypoxic injury. J Mol Neurosci. 2000;15:147–154. doi: 10.1385/JMN:15:3:147. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Matsuyama T, Yamaguchi A, et al. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med. 2001;7:317–323. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Current Topics in Medicine and Chemistry. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- Taylor BM, Fleming WE, Benjamin CW, Wu Y, Mathews WR, Sun FF. The mechanism of cytoprotective action of lazaroids I: Inhibition of reactive oxygen species formation and lethal cell injury during periods of energy depletion. J Pharmacol Exp Ther. 1996;276:1224–1231. [PubMed] [Google Scholar]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Waud DR. Quantal bioassay and stroke. Stroke. 1992;23:767–773. doi: 10.1161/01.str.23.5.767. [DOI] [PubMed] [Google Scholar]