Current neurobiology has evolved to accept an active role of non-neuronal components of the nervous system, glial cells in particular, in neuronal activities including synaptic transmission. The two major glial cell subtypes microglia and astrocytes express some neurotransmitter receptors and can secrete a variety of chemical mediators that either facilitate or suppress neuronal activity. Of particular interest is that glial cells convert their functional phenotype from a relatively quiescent to a hyperactive or activated state in response to central or peripheral injury, so called glial activation. Cumulating evidence indicates that activated glial cells contribute to neurological disorders including the development of chronic pain conditions [1–3].
Elucidating cellular mechanisms underlying the involvement of glia in pain hypersensitivity presents new challenges to pain research. Apparently, there are reciprocal relationships between neurons and glial cells (Fig. 1). Nociceptive inputs related to injury are carried by primary afferent nerves and excite postsynaptic neurons. The same nociceptive inputs may also be read by glial cells and trigger cellular events that lead to neuronal sensitization and increased pain. Chemical mediators such as ATP and chemokines fractalkine and monocyte chemoattractant protein-1 (MCP-1/CCL2) can be released from neurons and bind to respective receptors on microglia and astrocytes. Reciprocally, glial cells release brain-derived neurotrophic factors and cytokines such as IL-1β and TNF-α to act on their receptors on neurons. There are also interactions between glial cells. Microglia may influence astrocytes by secrete cytokine IL-18. These events alter the function of the pain processing circuitry and may have fundamental significance in our understanding of chronic pain mechanisms.
Fig. 1.
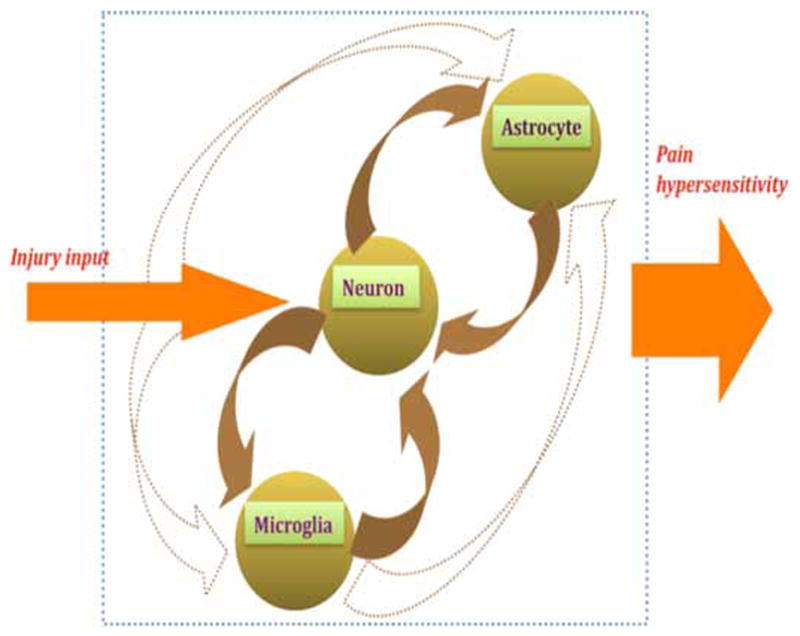
Schematic diagram illustrating interactions between neuron, microglia and astrocytes. Arrows indicate action of one cell type on other through release of chemical mediators and their binding with respective receptors. Dashed arrows show pathways to be substantiated. Note large block arrow on right illustrating increased pain as a result of neuron-glial interactions within pain processing circuitry.
Three laboratories have contributed to this special issue of The Open Pain Journal on neuron-glial interactions in the mechanisms of persistent pain. These mini-reviews are timely and offer creative thoughts for future research in this area. Durham and Garrett utilize trigeminal models to emphasize that neuron-glial interactions also occur in peripheral sensory ganglia. Satellite glial cells in trigeminal ganglion affect excitability of nociceptors through paracrine signaling as well as hemichannels formed by gap junction proteins. Davies, Kim and Oh conduct in depth discussion on microglia biology and pain processing. They analyze a variety of chemicals involved in reciprocal neuron-glial signaling and review function of potassium channels in microglia. Luongo, Palazzo, de Novellis and Maione focus on the role of endocannabinoids, which are present in microglia at very high levels, in neuron-glial crosstalk. They also correctly point out the potential protective role of glial activation in the event of injury [also see 2]. It is hoped that the focus discussion presented here will provoke studies towards a better understanding of the mechanisms of chronic pain.
Footnotes
This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/-licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
References
- 1.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–9. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]


