Abstract
Purpose
This study evaluated the clinical manifestations of and risk factors for pituitary insufficiency in children and adolescents with Rathke's cleft cysts.
Methods
Forty-four patients with Rathke's cleft cysts younger than 19 years who visited Seoul National University Children's Hospital between January 1995 and September 2009 were enrolled. Rathke's cleft cysts were confirmed histologically through an operation in 15 patients and by brain magnetic resonance imaging (MRI) in 29 patients. The clinical, hormonal, and imaging features were reviewed retrospectively.
Results
The clinical presentation of symptomatic patients was as follows: headache (65%), endocrinopathy (61%), and visual disturbance (19%). Endocrinopathy included central precocious puberty (18%), diabetes insipidus (14%), general weakness (11%), and decreased growth velocity (7%). After surgery, hyperprolactinemia resolved in all patients, but growth hormone insufficiency, hypothyroidism, and diabetes insipidus did not improve. Pituitary insufficiency except gonadotropin abnormality correlated significantly with severe headache, visual disturbance, general weakness, and cystic size. Suprasellar extension of cysts and high signals in the T2-weighted image on brain MRI were related to hypothyroidism, hypocortisolism, and diabetes insipidus. Multivariable linear regression analysis showed that only general weakness was a risk factor for pituitary insufficiency (R2=0.549).
Conclusion
General weakness is a risk factor for pituitary insufficiency in patients with Rathke's cleft cysts. When a patient with a Rathke's cleft cyst complains of general weakness, the clinician should evaluate pituitary function and consider surgical treatment.
Keywords: Rathke's cleft cysts, Pituitary insufficiency
Introdction
Rathke's cleft cyst (RCC) is a non-neoplastic lesion lined by a single layer of epithelium in the sellar and suprasellar regions and is often discovered incidentally1-3). RCC has been reported rarely in children and adolescents, and may not be discovered clinically until the age of 50-60 years4). Rathke's pouch is a component of the developmental craniopharyngeal duct that gives rise to the anterior lobe, pars intermedia, and pars tuberalis of the pituitary gland, and it normally closes in early fetal development. However, failure of this pouch to regress upon formation of the adenohypophysis and neurohypophysis can produce an RCC2, 3, 5). The incidence of RCC in children and adolescents is unknown, but Teramoto et al6) reported that an RCC was discovered in 1.7% of routine autopsies of people aged 10-29 years and in 13-22% of autopsies of older people. Although it is generally believed that a symptomatic RCC occurs rarely, the most common clinical manifestations include headache, visual disturbance, and variable endocrine insufficiencies. Less frequent presentations include pituitary apoplexy, abscess formation, and meningitis. A few cases have been reported of children and adolescents with a symptomatic RCC who showed delayed puberty, central precocious puberty, growth retardation, and menstrual abnormalities1, 2, 4, 7-11). Treatment of an RCC generally includes regular brain magnetic resonance imaging (MRI). If the RCC becomes symptomatic or its size increases, surgical treatment is recommended. The transsphenoidal approach remains the preferred method for surgical drainage of such lesions, because cystic aspiration or partial resection is usually enough to treat a symptomatic RCC and the the postoperative recurrence is rare3, 7-9).
With the increased use of brain MRI, more RCCs are being discovered. However, it is difficult to devise a strategy for treatment because a symptomatic RCC is rare in children and adolescents, and there are no reports of the clinical manifestations related to RCC, particularly endocrine dysfunction, in children and adolescents. In this study, we aimed to devise a strategy for the treatment of children and adolescents with an RCC by comparing the clinical manifestations, radiological features, hormonal changes, and risk factors of endocrine dysfunction in symptomatic patients treated surgically with asymptomatic patients whose RCC was discovered by chance.
Materials and methods
1. Patients
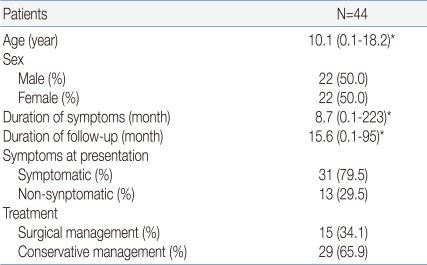
Forty-four patients (22 males and 22 females) aged from 0 to 18 who had visited Seoul National University Children's Hospital from January 1995 to September 2009 and who were treated for an RCC were included. In 15 patients (8 males and 7 females), the RCC was confirmed histologically in a sample taken during surgery, and 29 patients (14 males and 15 females) were diagnosed by brain MRI examination. Thirty-one patients (16 males and 15 females) showed symptoms, and 13 subclinical patients (6 males and 7 females) were found by chance. The median age for the entire group was 10.1 years, and the youngest patient was 1 month old. The median duration from the appearance of symptoms to diagnosis was 8.7 months, and one patient was diagnosed 223 months after the appearance of symptoms. The median follow-up period was 15.6 months; patients were included who could be traced and examined for a minimum of 1 month to a maximum of 95 months (Table 1).
Table 1.
Characteristics of Patients with Rathke's Cleft Cysts
*median (range)
2. Methods
Using retrospective examination of the patients' medical records, age, sex, height, weight, bone age, sexual maturity rating, growth velocity, date of symptom appearance, and follow-up interval were recorded.
In patients who showed symptoms, the symptoms were classified into endocrine, neurological, ophthalmic, and other fields. The diagnosis through brain MRI examination was limited to RCC diagnosed by two or more diagnostic imaging specialists from two or more times of imaging sessions. The cyst's location, size, shape, presence of calcification, hemorrhage, and peripheral rim contrast enhancement were evaluated. The signal intensity of T1- and T2-weighted images was classified into high, equal or low.
Peripheral blood samples were collected and the following basal hormone levels were measured in the plasma at time of diagnosis or before and after the operation using immunoradiometric assay methods. The concentration of growth hormone (GH), insulin like growth factor-1 (IGF-1), free thyroxine (free T4), triiodothyronine, thyroid stimulating hormone (TSH), cortisol and adrenocorticotropic hormone (ACTH) (blood sampled in the morning), luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol or testosterone, and prolactin were measured. A pituitary function test was performed after surgery. Other tests were performed; GH stimulation test using L-Dopa and clonidine or insulin tolerance test, thyrotropin releasing hormone (TRH) stimulation test, gonadotropin releasing hormone (GnRH) stimulation test, low-dose ACTH stimulation test. To assess the deficiency in antidiuretic hormone (ADH), urine output per hour, serum and urine osmotic pressure, urine specific gravity, and electrolytes were measured, or some patents were confirmed with water deprivation test. The medicines to treat the endocrinological disorders such as GH, thyroid hormone, cortisol, sex hormone, and ADH, were recorded in the follow-up examination.
Hypopituitarism was diagnosed as follows. GH deficiency was defined as a maximum GH concentration of less than 10 ng/mL in two or more GH stimulation tests using agents such as clonidine, L-Dopa, and glucagon, or a maximum GH concentration in the insulin tolerance test of less than 3 ng/mL12). A standard deviation score of basal IGF-1 concentration less than -2.013) and a growth velocity of less than 4 cm/year was separately classified as suspected GH deficiency. The combination of a decrease in both of free T4 and TSH was classified as suspected TSH deficiency, and the absence of an increase in TSH concentration in the TRH stimulation test was classified as TSH deficiency14). A morning cortisol level of less than 5 µg/dL and no increase in ACTH level was classified as suspected ACTH deficiency, In the ACTH stimulation test performed 2 weeks or more after surgery, an increase in cortisol level of less than 7 µg/dL or a maximum of less than 18 µg/dL was defined as ACTH deficiency15). Hypogonadotropic hypogonadism was defined as an LH level in GnRH stimulation test for females with bone age of more than 13 years and males with bone age of more than 15 years of less than 5 mIU/mL. In patients with sexual maturity rating of more than Tanner II in the physical examination, an LH level more than the maximum 5 mIU/mL in GnRH stimulation test for girls younger than 8 years and boys younger than 9 years was diagnosed as central precocious puberty16). Hyperprolactinemia was defined a serum prolactin level more than 25 ng/mL.
3. Statistics
The patients' age, sex and clinical symptoms at diagnosis were represented as the median, minimum, and maximum, and these were analyzed using frequency analysis. Fisher's exact test was used to analyze the results of brain MRI examination according to symptoms and treatment. Comparison of the size of the cysts was made using the Mann-Whitney U test. Fisher's exact test was used to evaluate dysfunction of each pituitary hormone according to clinical symptoms and MRI findings. The extent of hormone dysfunction in relation to the size of the cyst was compared by one-way ANOVA. Spearman's correlation analysis and univariate and multivariate linear regression analysis were used to identify relationship between clinical symptoms, MRI findings, and hormone dysfunction. The data were analyzed using SPSS version 17.0 for Windows (SPSS Inc, Chicago, IL, USA), and a P value less than 0.05 was considered significant.
Results
1. Clinical symptoms at diagnosis
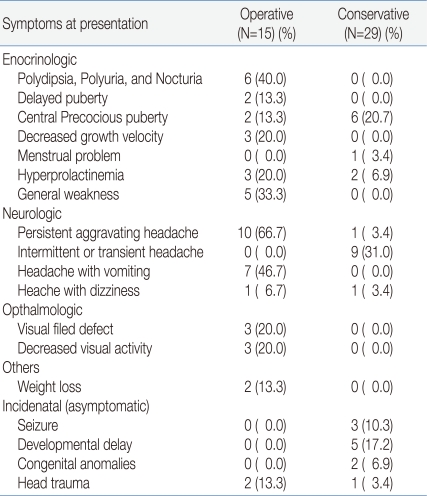
The symptoms of RCC included 20 patients with headache, 19 patients with endocrine dysfunction, and six patients with decreased visual acuity and visual field defects. Headache was accompanied mainly by vomiting. Headache was persistent and worsened in the operation group, while it was temporarily continued in most cases. The non-surgical group reported less frequent headaches but these were not accompanied by vomiting. The patients in the operation group showed most endocrine symptoms except for precocious puberty. In the operation group, eight patients had precocious puberty, six patients had diabetes insipidus, five patients had general weakness, and three patients had decreased growth velocity of less than 4 cm/year. All patients with ophthalmic problems were operated. Three patients had bitemporal hemianopsia and three patients had decreased visual acuity. 13 patients with RCC were found by chance in a brain MRI examination performed because of epilepsy, developmental disorder, congenital malformation and head injury. Two of these patients were operated operated on for asymptomatic pituitary macroadenoma (Table 2).
Table 2.
Clinical Presentations of Patients with Rathke's Cleft Cysts
2. Changes in pituitary functions before and after the operation
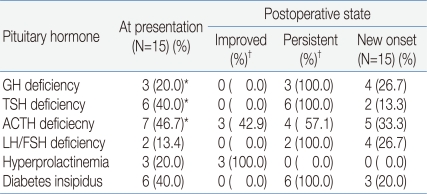
Pituitary hormone dysfunction was compared from before to after the operation. Before the operation, three patients had suspected GH deficiency, six had suspected TSH deficiency, seven had suspected ACTH deficiency, two had LH/FSH deficiency, three had hyperprolactinemia, and six had diabetes insipidus. The pituitary function test after the operation showed that seven patients had GH deficiency, eight had TSH deficiency, nine had ACTH deficiency, six had LH/FSH deficiency, and nine had diabetes insipidus. Hyperprolactinemia was resolved in all patients (Table 3).
Table 3.
Changes in Pituitary Dysfunction from Before to After Surgical Treatment
*These are suggestive hormone insufficiency, not confirmed hormone provocation test
†The results are on the basis of counts at presentation
3. Analysis of the relationship between clinical symptoms, radiological characteristics, and pituitary hormone insufficiency
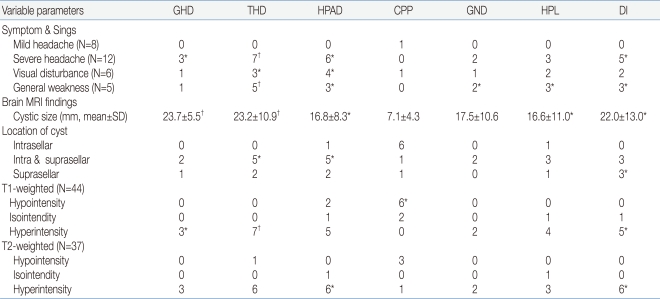
The relationships between the secretion disorder for each pituitary hormone and several factors were analyzed at the presentation of RCC. Mild headache was not significantly related to pituitary hormone dysfunction except one with precocious puberty. Severe headache correlated with TSH deficiency (P<0.001), GH deficiency (P=0.017), ACTH deficiency (P=0.003), and ADH deficiency (P=0.004). Decreased visual acuity or a visual field defect was related to ACTH deficiency (P=0.007) and TSH deficiency (P=0.047). All patients with general weakness had TSH deficiency (P<0.001) and showed other pituitary hormone disorders except for precocious puberty. The size of the cyst was related to deficiencies in all hormones except LH/FSH, and was closely related to GH deficiency (P<0.001) and TSH deficiency (P<0.001). The Cyst closer to the suprasellar area were significantly associated with TSH deficiency (P=0.011), ACTH deficiency (P=0.025), and ADH deficiency (P=0.013). Hyperintensity of the RCC in T1- and T2-weighted images correlated significantly with TSH deficiency (P<0.001) and ADH deficiency (P=0.003) (Table 4).
Table 4.
Correlation between Pituitary Hormone Insufficiency, Clinical and Radiological Findings
*P<0.05, †P<0.001
Abbreviations: GHD, growth hormone deficiency; THD, hypothyroidism; HPAD, hypocortisolism; CPP, central precocious puberty; GND, hypogonadism; HPL, hyperprolactinemia; DI, diabetes insipidus
4. Analysis of factors causing pituitary dysfunction in patients with RCCs
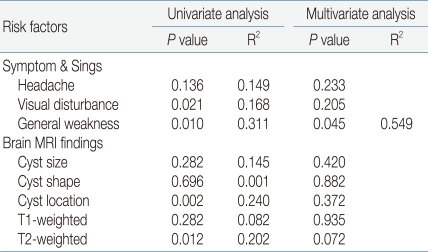
Analysis of the relationships between various clinical symptoms, radiological findings, and pituitary hormone dysfunction showed significant relationships between pituitary hormone dysfunction and visual field defect, general weakness, location of the cyst, and high signal intensity of T2-weighted image. However, after controlling for the mutual relationships between variable, only general weakness was significantly relation to pituitary hormone dysfunction (R2=0.549, P=0.045) (Table 5).
Table 5.
Factors Reflecting Pituitary Dysfunction in Patients with Rathke's Cleft Cysts
Discussion
RCC is a non-neoplastic remnant of Rathke's pouch and is mostly asymptomatic. The etiology of symptom appearance is not known precisely. In studies of adults, the cause is assumed to be the change in the cyst size because of an imbalance between secretion and absorption of the contents of the cyst, leakage, intracystic hemorrhage, or infection2, 14, 17). In this study, the clinical symptoms were headache in 20 patients (65%), endocrinopathy in 19 patients (61%), and visual disturbance in six patients (19%). Patients whose headaches were frequent and worsening or were accompanied by nausea and vomiting had pituitary insufficiency and tendency to need surgical treatments.
The interesting aspect of the endocrinopathy was the high frequency of early puberty (eight patients, 26%). All children who showed signs of early puberty were positive in the GnRH stimulation test. Of the seven girls with central precocious puberty, four were being treated with a GnRH agonist. Cases of precocious puberty in children in associated with RCC are reported rarely18-20). One case study20) reported that treatment with a GnRH agonist resolved the symptoms of early puberty without changing the cyst. The relationship between central precocious puberty and RCC is not clear. Our comparison between patients with precocious puberty and secretory dysfunction of other pituitary hormones showed several differences. The patients with precocious puberty: 1) had smaller cysts, 2) had cysts mainly in intrasellar sites, 3) showed hypointensity of the cysts in T1- and T2-weight enhanced images, 4) had no additional insufficiencies of pituitary hormones before and after the operation, 5) had improved clinical characteristics after treatment with a GnRH agonist without changes of the cyst. It is assumed that the precocious puberty associated with RCC is caused by a different mechanism than that causing dysfunction of other pituitary hormones. RCC with precocious puberty may be an independent pituitary disease or another feature of asymptomatic RCC. Karavitaki et al20) and Bader et al21) found corticotrophic pituitary adenoma in adult patients with an RCC accompanied by Cushing's syndrome; these patients showed considerable hypersecretory dysfunction associated with the RCC. It is possible that precocious puberty is caused by an RCC is association with a gonadotropic adenoma. We did not perform immunohistochemical staining to identify the hormone-secreting cells after the operation in our patients and cannot confirm whether this is true.
Several studies show that the patients with RCC may develop additional clinical symptoms and endocrinopathy after the operation2, 4, 8, 9, 22). In this study, the headache and visual disturbances experienced by all 15 patients stopped after the operation; this is a high rate of improvement compared with case reports of adults in Korea and other countries. Several studies9, 14, 23, 24) have shown improvements in visual field defects in 59-100% of patients and in headache in 40-100% of patients. Kim et al8) reported that visual acuity, visual field defects, and headache improved in 68%, 59%, and 93% of patients, respectively. However, in contrast to the clinical symptoms, the resolution of the endocrine dysfunction was less successful after the operation. Only 20% of patients showed improvement in endocrine dysfunction and some showed a worsened condition. Twenty percent of patients showed a deficiency in all pituitary hormones. The extent of changes in hormones differed between hormones. For example, hyperprolactinemia improved in all patients after the operation and no patients developed a new deficiency, whereas deficiencies in GH, TSH, and ADH continued or worsened in all patients. Usually, ACTH deficiency was evident in a high proportion of patients (seven patients, 47%) before the operation; three patients showed improvement, but five other patients developed a new deficiency after the operation. These postsurgical results are worse than those in some reports showing that 3.6-30% of patients develop a new hormone deficiency3, 8, 9, 11). However, other studies have reported similar rates to those in our study: 14-20% of patients developing a pituitary hormone dysfunction after the operation3, 8, 24, 25) and 42.9-100% recovering from hyperprolactinemia4, 8, 11, 26, 27).
In patents with a symptomatic RCC, the clinical characteristics may worsen later or improve spontaneously, and it is difficult to predict their influences on the pituitary. We compared the secretory dysfunction in our patients according to clinical symptoms and MRI findings in the diagnosis. Severe headache, decreased visual acuity, visual field defects, and a larger size and suprasellar location of the cyst were related to hypopituitarism but precocious puberty and hypogonadism were not. General weakness showed the highest correlation with secretory dysfunction of all pituitary hormones, in particularly, TSH deficiency (P<0.001), but was not related to precocious puberty. A larger cyst was related to GH deficiency and TSH deficiency (P<0.001 for each). The closer the location of the cyst to the intrasellar area, the lower the frequency of TSH deficiency and ACTH deficiency. The closer the location of the suprasellar area, the higher the frequency of ADH deficiency. Univariate linear analysis showed a higher correlation between the location of the cyst and pituitary dysfunction, including gonadotropic hormone deficiency, than the cyst size. The high signal intensity in T1- and T2-weighted images was related to TSH deficiency and ADH deficiency. These results are similar to those of Nishioka et al23) who reported the a higher frequency of dysfunction of the anterior and posterior pituitary glands in cysts with a high signal intensity in T1-weighted images. However, after excluding the correlation between several related factors in our study, multivariate analysis showed that general weakness was the only significant risk factor for pituitary dysfunction (R2=0.549, P=0.045). This suggests that a child or adolescent with an RCC who represents with general weakness should be checked carefully to determine whether active treatment is needed.
Our study has some limitation. We did not perform pituitary stimulation tests in all patients before the operation, only in those with a suspected deficiency in GH, TSH, or ACTH. Our study was also a retrospective cross-sectional design with small numbers of patients. Follow-up studies of larger groups are needed to understand the changes during adulthood.
References
- 1.Mukherjee JJ, Islam N, Kaltsas G, Lowe G, Chaelesworth M, Afshar F, et al. Clinical, radiological and pathological features of patients with rathke's cleft cysts: tumors that may recur. J Clin Endocrinol Metab. 1997;82:2357–2362. doi: 10.1210/jcem.82.7.4043. [DOI] [PubMed] [Google Scholar]
- 2.Raper D, Besser M. Clinical features, management and symptomatic rathke's cleft cyst. J Clin Neurosci. 2009;16:385–389. doi: 10.1016/j.jocn.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with rathke cleft cysts. J Neurosurg. 2005;102:189–193. doi: 10.3171/jns.2005.102.2.0189. [DOI] [PubMed] [Google Scholar]
- 4.Zada G, Ditty B, McNatt SA, McComb JG, Krieger MD. Surgical treatment of rathke cleft cysts in children. Neurosurgery. 2009;64:1132–1138. doi: 10.1227/01.NEU.0000341873.20737.56. [DOI] [PubMed] [Google Scholar]
- 5.Solov'ev GS, Bogdanov AV, Panteleev SM, Yanin VL. Embryonic morphogenesis of the human pituitary. Neurosci Behav Physiol. 2008;38:829–833. doi: 10.1007/s11055-008-9055-9. [DOI] [PubMed] [Google Scholar]
- 6.Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiolology. 1994;193:161–164. doi: 10.1148/radiology.193.1.8090885. [DOI] [PubMed] [Google Scholar]
- 7.Rhee JJ, Kim JH, Kim CJ, Lee JK, Jung HW. Radiolocal characteristics in rathke's cleft cyst. J Korean Neurosurg Soc. 2003;34:140–145. [Google Scholar]
- 8.Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG, et al. Surgical treatment of symptomatic rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004;100:33–40. doi: 10.3171/jns.2004.100.1.0033. [DOI] [PubMed] [Google Scholar]
- 9.Sade B, Albrecht S, Assimakopoulos P, Vezina JL, Mohr G. Management of rathke's cleft cysts. Surg Neurol. 2005;63:459–466. doi: 10.1016/j.surneu.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Yu SK, Kim YK, Kim HJ, Kim DJ, Chung YS, Lee KW, et al. The endocrinologic characteristics of rathke's cleft cyst-pathologically confirmed in seven cases. J Korean Endocr Soc. 2007;22:74–79. [Google Scholar]
- 11.Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, rathke's cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999;84:3972–3982. doi: 10.1210/jcem.84.11.6114. [DOI] [PubMed] [Google Scholar]
- 12.Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, et al. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 13.Lőfqvist C, Andersson E, Gelander L, Rosberg S, Blum WF, Wikland KA. Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab. 2001;86:5870–5876. doi: 10.1210/jcem.86.12.8117. [DOI] [PubMed] [Google Scholar]
- 14.Westwood ME, Butler GE, McLellan AC, Barth JH. The combined pituitary function test in children: an evaluation of the clinical usefulness of TRH and LHRH stimulation tests through a retrospective analysis of one hundred and twenty six cases. Clin Endocrinol (Oxf) 2000;52:727–733. [PubMed] [Google Scholar]
- 15.Gonzálbez J, Villabona C, Ramón J, Navarro MA, Giménez O, Ricart W, et al. Establishment of reference values for standard dose short synacthen test (250 mg), low dose short synacthen test (1 mg) and insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in normal subjects. Clin Endocrinol (Oxf) 2000;53:199–204. doi: 10.1046/j.1365-2265.2000.01028.x. [DOI] [PubMed] [Google Scholar]
- 16.Resende EAMR, Lara BHJ, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. 2007;92:1424–1429. doi: 10.1210/jc.2006-1569. [DOI] [PubMed] [Google Scholar]
- 17.Laws ER, Kanter AS. Rathke cleft cyst. J Neurosurg. 2004;101:571–572. doi: 10.3171/jns.2004.101.4.0571. [DOI] [PubMed] [Google Scholar]
- 18.Acharya SV, Gopal RA, Menon PS, Bandgar TR, Shah NS. Precocious puberty due to rathke cleft cyst in a child. Endocr Pract. 2009;15:134–137. doi: 10.4158/EP.15.2.134. [DOI] [PubMed] [Google Scholar]
- 19.Monzavi R, Kelly DF, Geffner ME. Rathke's cleft cyst in two girls with precocious puberty. J Pediatr Endocrinol Metab. 2004;17:781–785. doi: 10.1515/jpem.2004.17.5.781. [DOI] [PubMed] [Google Scholar]
- 20.Karavitaki N, Scheithauer BW, Watt J, Ansorge O, Moschopoulos M, Llaguno AV, et al. Collision lesions of the sella: co-existence of craniopharyngioma with gonadotroph adenoma and of rathke's cleft cyst with corticotroph adenoma. Pituitary. 2008;11:317–323. doi: 10.1007/s11102-007-0070-6. [DOI] [PubMed] [Google Scholar]
- 21.Bader LJ, Carter KD, Latchaw RE, Ellis WG, Wexler JA, Watson JC. Simultaneous symptomatic rathke's cleft cyst and GH secreting pituitary adenoma: a case report. Pituitary. 2004;7:39–44. doi: 10.1023/b:pitu.0000044632.15978.44. [DOI] [PubMed] [Google Scholar]
- 22.Nishioka H, Haraoka J, Izawa H, Ikeda Y. Mangnetic resonance imaging, clinical manifestations, and management of rathke's cleft cyst. Clin Endocrinol (Oxf) 2006;64:184–188. doi: 10.1111/j.1365-2265.2006.02446.x. [DOI] [PubMed] [Google Scholar]
- 23.Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic rathke's cleft cysts. Clin Neurol Neurosurg. 2001;103:96–100. doi: 10.1016/s0303-8467(01)00121-4. [DOI] [PubMed] [Google Scholar]
- 24.Kasperbauer JL, Orvidas LJ, Atkinson JL, Abboud CF. Rathke cleft cyst: diagnostic and therapeutic consideration. Laryngoscope. 2002;112:1836–1839. doi: 10.1097/00005537-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Frank G, Sciarretta V, Mazzatenta D, Farneti G, Modugno GC, Pasquini E. Transsphenoidal endoscopic approach in the treatment of rathke's cleft cyst. Neurosurgery. 2005;56:124–128. doi: 10.1227/01.neu.0000144824.80046.1f. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi K, Uozumi T, Arita K, Kurisu K, Yano T, Sumida M, et al. Pituitary function in patients with rathke's cleft cyst: significance of surgical management. Endocr J. 1994;41:535–540. doi: 10.1507/endocrj.41.535. [DOI] [PubMed] [Google Scholar]
- 27.el-Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic rathke's cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998;42:7–16. doi: 10.1097/00006123-199801000-00003. [DOI] [PubMed] [Google Scholar]