CCL22 functions to regulate EAE development through CNS macrophage chemoattraction and the balance of macrophage inflammatory and anti-inflammatory cytokine expression.
Keywords: trafficking, migration, CNS, multiple sclerosis, autoimmunity, EAE
Abstract
EAE is a demyelinating disease of the CNS and serves as a mouse model of MS. Expression of CCL22 in the draining LNs and spinal cord correlated with the onset of clinical EAE development and remained elevated. Administration of anti-CCL22 at the time of autoantigen immunization delayed the initiation of clinical disease and dampened the severity of peak initial disease and relapses. Reduced EAE severity correlated with the reduction of pathology and leukocytes in the CNS, particularly, activated CD11b+Ly6Chi macrophages. There were no differences in effector T cell-proliferative responses or effector T cell IFN-γ or IL-17 responses. However, treatment at the onset of disease did not reduce disease progression. Treatment of adoptive T cell transfer recipient mice with anti-CCL22 resulted in decreased clinical disease development accompanied by a decrease in CNS accumulation of CD11b+Ly6Chi macrophages. Neutralization of CCL22 resulted in a macrophage population whose effector cytokine expression consisted of decreased TNF and increased IL-10, a phenotype more consistent with M2 macrophages. This was corroborated by in vitro cultures of macrophages with CCL22. These results suggest that CCL22 functions to regulate development of EAE through macrophage chemoattraction and effector function.
Introduction
EAE is a CD4+ T cell-mediated, demyelinating disease of the CNS that serves as a model for MS [1]. The pathogenic mechanisms of disease development include antigen-specific T cell activation and Th1 differentiation [2], followed by T cell [3] and macrophage migration into the CNS [4, 5]. More recently, a role for IL-17-producing T cells (Th17) in disease pathogenesis has been demonstrated [6]. EAE can be induced in mice by immunization with whole myelin proteins or peptide emulsified in CFA [7] or by adoptive transfer of autoreactive T cells to normal recipient mice [8, 9]. Macrophages activated by the effector T cell response appear to be the end-stage disease effector cells, inducing damage in the CNS [10–12].
Chemokines are leukocyte chemoattractants that play a crucial role during pathogenesis of tissue-specific inflammatory diseases by directing leukocyte recruitment and/or accumulation at sites of inflammation [13, 14]. Chemokines and their receptors have been implicated as functional mediators of immunopathology in EAE [15, 16]. CCL22 (monocyte-derived chemokine) was described initially as a constitutively produced, thymus-specific chemokine, implicated in the recruitment of T cells [17–19]. One of the functions of CCL22 originally described was induction of migration of CCR4+ Th2 cells [20], but it has additionally been shown to regulate migration of Tregs [21]. CCL22 has been implicated in a number of diseases, including allergen-induced lung inflammation [22], atopic dermatitis [23, 24], and lymphoma [25]. Similarly, a presumed role for CCR4+ cells has been suggested for endotoxic shock [26], rheumatoid arthritis [27], T cell lymphoma [28], and autoimmune diabetes [29].
In EAE [30] and MS [31], high levels of CNS-derived CCL22 have been documented, but the functional significance of the chemokine expression has not been demonstrated. Therefore, the present study focused on the contribution of CCL22 expression during active EAE induced by PLP139–151 priming. Given the expression pattern of CCL22 during the development of EAE, we hypothesized that in vivo neutralization of CCL22 activity may modulate EAE development. In the present report, we describe a major role for CCL22 in the pathogenesis of EAE, which included CNS accumulation of inflammatory macrophages as well as regulation of their cytokine balance.
MATERIALS AND METHODS
Mice
Female SJL (H-2s) and C57Bl/6 mice were purchased form Harlan Sprague Dawley (Indianapolis, IN, USA) and used as described previously [32]. Animal care and use were in accordance with the Northwestern University Animal Care and Use Committee (Chicago, IL, USA) and United States Public Health Service policies.
Antigens, antibodies, and reagents
PLP139–151 (HSLGKWLGHPDKF) and myelin oligodendrocyte glycoprotein 35–55 (MEVGWYRSPFSRVVHLYRNGK) were purchased from Peptides International (Louisville, KY, USA) [32]. Fluorochrome-conjugated mAb to murine CD4 (RM4–5), CD8a (Ly-2), CD45 (Ly-5), CD11b (macrophage antigen 1), Ly6C (AL-21), and purified CD16/32 (Fc block, clone 2.4G2) were purchased from BD PharMingen (San Diego, CA, USA). Anti-mouse CCR4 (2G12) was purchased from BioLegend (San Diego, CA, USA). Custom-made rabbit anti-murine CCL22 was prepared by Invitrogen Life Technologies (Carlsbad, CA, USA) by multiple site immunization of New Zealand White rabbits with mouse rCCL22 (R&D Systems, Minneapolis, MN, USA) emulsified in CFA. Antisera was titrated by sandwich ELISA, and specificity was verified by the failure to cross-react with any other cytokine and chemokine tested (e.g., mouse CXCL10, CXCL12, CCL5, CCL3, CXCL13, CCL2, CCL20, CCL21, human TGF-β, IL-10, IL-5, and IL-12). Normal rabbit serum was used as a control Ig preparation for in vivo treatment regimens (Jackson Immunology, West Grove, PA, USA). The results obtained with the custom rabbit antisera were confirmed using neutralizing goat anti-murine CCL22 and monoclonal rat anti-murine CCL22, both of which were purchased from R&D Systems with normal goat or rat IgG as the control antibody. rCCL22 was purchased from R&D Systems, diluted in sterile PBS, and used at concentrations of 1, 10, or 100 nM in vitro. LPS from Salmonella enterica serotype typhimurium SL1181 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
EAE induction
For the active induction of EAE, female SJL/J mice were injected s.c. as described previously [32]. For the adoptive induction of EAE, female SJL/J donor mice were injected s.c. as described previously; after 7 days, the draining LN cells were harvested and restimulated in vitro with PLP139–151 for 3 days, whereupon 5 × 106 blasts were injected i.v. to normal SJL recipients [33]. Animals were graded according to their clinical severity using the following scale: Grade 0, no abnormality; Grade 1, limp tail; Grade 2, limp tail and hind-limb weakness; Grade 3, partial hind-limb paralysis; Grade 4, complete hind paralysis; Grade 5, death. A relapse was defined as an increase in one score for at least 2 consecutive days following the period of disease remission.
Gene expression analysis
CNS CCL22 expression was determined in spinal cord lesions and areas surrounding the lesions (peri-lesion) and compared with naïve mice that were not immunized with PLP139–151. Mice were perfused with 50 ml PBS at the peak of PLP139–151/CFA-induced EAE, spinal cords were embedded in OCT and frozen, and 10 μm sections were cut and stained with anti-PLP and anti-CD4 mAb. Ten to 20 pooled, demyelinated lesions were removed by laser microdissection. Equivalent areas from the peri-lesion (nondemyelinated areas adjacent to inflammatory demyelinated lesions) and from the spinal cords of naïve mice were also collected. RNA was isolated by standard methodology and hybridized and gene expression assessed using Agilent whole mouse genome microarray (Miltenyi Biotec, Auburn, CA, USA). Half of the spinal cord was used for RNA extraction in 1 ml TRIzol (Invitrogen Life Technologies) with a linear acrylamide carrier (Ambion, Austin TX, USA). cDNA was generated using the Advantage® RT-for-PCR kit (BD Biosciences, Palo Alto, CA, USA) and used as template for real-time PCR amplification of CCL22. CNS CCL22 expression was confirmed by real-time RT-PCR at various time-points after immunization using the following primer set purchased from Integrated DNA Technologies (Coralville, IA, USA): forward, 5′-GTG GCT CTC GTC CTT CTT GC-3′; reverse, 5′-GGA CAG TTT ATG GAG TAG CTT-3′ [30].
Flow cytometry
Mononuclear cells were isolated from the CNS of mice perfused intracardially with 0.15 M saline solution. Spinal cords were dissected from the vertebral canal or removed by intrathecal hydrostatic pressure. Mononuclear cells were isolated and prepared as described previously [34, 35]. Data collection was performed on a LSR II (Becton Dickinson, San Jose, CA, USA) flow cytometer in the Interdepartmental Immunobiology Center Flow Cytometry Facility (Northwestern University) using FACSDiva software (Becton Dickinson), and analysis was performed offline using FCS Express (De Novo Software, Los Angeles, CA, USA). Cell sorting was performed using a MoFlo (Dako Cytomation, Denmark) high-speed cell sorter in the Robert H. Lurie Comprehensive Cancer Center Core Flow Cytometry Facility (Northwestern University).
Histology and immunohistochemistry
Mice were anesthetized with sodium pentobarbital (Abbott Laboratories, Abbott Park, IL, USA) and perfused intracardially through the left ventricle with ice-cold PBS. Tissues were embedded in OCT prior to cryostat sectioning. Frozen sections (8–10 μm) were blocked with 5% normal goat serum in PBS for 30 min at room temperature and incubated with anti-CCL22 (clone 158113, R&D Systems) for 2 h at room temperature. Sections were treated 3% H2O2 to quench endogenous peroxidase activity and then incubated with goat secondary antibodies directly conjugated to HRP (Vectastatin ABC kit, Vector Laboratories, Burlingame, CA, USA). Biotin-avidin binding was detected by DAB substrate (Sigma-Aldrich). The sections were counterstained with methylene blue.
Proliferation
For in vitro recall proliferation assays, 5 × 106 cells/ml were cultured for 72 h in DMEM, with or without PLP139–151, supplemented with 10% FBS, 50 μM 2-ME (Sigma-Aldrich), 100 U/ml penicillin (Invitrogen Life Technologies), and 2 mM L-glutamine (Invitrogen Life Technologies). The culture was pulsed with 1 μCi [3H]thymidine/well for 18 h (Amersham, Piscataway, NJ, USA). [3H]Thymidine uptake was measured as counts/min (Top Count NXT, Packard Instrument Co., Meriden, CT, USA).
ELISPOT
Spleen cells were obtained from mice cultured in 96-well microtiter ELISPOT plates (Whatman Polyfiltronics, Clifton, NJ, USA), which were coated overnight with capture antibodies to IFN-γ (BD PharMingen) or IL-17 (eBioscience, San Diego, CA, USA). Total cell numbers recovered were determined by use of a hemocytometer. ELISPOT assay was performed as described previously [36]. Plates were washed, and spots were counted using an ELISPOT plate reader and software (Cellular Technologies Inc., Cleveland, OH, USA). At least three wells/sample were counted and presented as a mean value ± sd.
ELISA
Assessment of CCL22 expression was quantified from homogenized tissue samples. Spleen, LN, and spinal cord were harvested and homogenized in 1 ml PBS containing 0.05% Tween-20 and then clarified by centrifugation (400 g) for 10 min. Levels of CCL22 in the homogenates were quantified by sandwich ELISA. Briefly, flat-bottom, high-absorbance plates (Nunc, Rochester, NY, USA) were coated with 200 ng/well monoclonal rat anti-mouse CCL22 (R&D Systems) in PBS overnight at 4°C. Nonspecific binding sites were blocked with 2% BSA in PBS for 1 h at 37°C, and dilutions of tissue homogenate were added in triplicate for 2 h at 37°C. Biotinylated goat anti-mouse CCL22 (5 ng/well; R&D Systems) was added, and plates were incubated for 1 h at 37°C. The wells were developed using Strepavidin-peroxidase in 1:5000 dilutions (Zymed, San Francisco, CA, USA) for 30 min at 37°C and tetramethylbenzidine substrate-chromogen, and then absorbance was read at 450 nm. CCL22 levels in homogenate were quantified by comparison with the standard curves and expressed as pg/ml. The detection limit of this ELISA was 100 pg/ml. Assessment of TNF, TGF-β, and IL-10 was performed using ELISA kits purchased from R&D Systems and used according to the manufacturer's instructions.
Cell culture
Single cell suspensions from spleens or spinal cords were isolated from mice by mechanical disruption through mesh stainless steel screens. RBCs in the samples were lysed by hypotonic shock in Tris-NH4Cl (pH 7.3), and cells were resuspended in HBSS. The cells were cultured in 96-well microtiter plates (Corning-Costar, Acton, MA, USA) at a density of 5 × 106 viable lymphocytes/ml or 1 × 106 viable macrophages/ml in DMEM (Invitrogen Life Technologies) containing 5% FCS (HyClone, Logan, UT, USA), 2 mM l-glutamine (Invitrogen Life Technologies), 100 U/ml penicillin (Invitrogen Life Technologies), 100 μg/ml streptomycin (Invitrogen Life Technologies), 0.1 M nonessential amino acids (Invitrogen Life Technologies), and 5 × 10−5 M 2-ME. Cells were incubated at 37°C in a humidified atmosphere containing 7.5% CO2.
Statistical analysis
Sample median, mean, sd, and statistical significance were calculated using SPSS 13.0 software. Clinical disease comparisons of mean disease severity over the course of time were made, and statistical significance of the comparison between two means was calculated using Student's t test. Comparisons of percent affected mice were performed using a χ2 test. Comparison between two means was made using Student's t test. For any test, P values <0.05 were considered significant.
RESULTS
CNS CCL22 expression correlates with increasing EAE severity
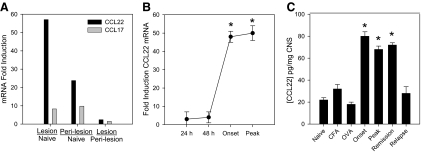
As the first step in deciphering the functional role of CCL22 in EAE, we assessed CCL22 gene expression from lumbar spinal cord of mice immunized with PLP139–151 in CFA using four different approaches in mice immunized to develop disease. The results from a gene array experiment shown in Fig. 1A indicate that there was increased expression of CCL22 and CCL17 in CNS lesions compared with unimmunized control tissue. Moreover, the peri-lesion area also expressed high levels of CCL22 and CCL17 relative to unimmunized tissue. As the levels of CCL22 expression appeared to be much higher than those of CCL17, both of which are ligands for CCR4, we decided to focus on CCL22 and used real-time RT-PCR as a confirmatory assay. The results in Fig. 1B indicate that there was significantly (P<0.05) elevated expression of CCL22 in the CNS of mice as a function of time post-disease induction, and the highest levels of mRNA expression occurred at the onset and peak of clinical disease symptoms. This quantitative result confirmed the expression pattern seen by gene array analysis. Similarly, when the CNS from immunized mice was assessed for CCL22 protein expression, there were significantly increased (P<0.05) levels detected at the onset, peak, and remission phases of disease when compared with unimmunized, CFA-immunized, and OVA-immunized control groups (Fig. 1C). To determine what cell population in the CNS was expressing CCL22, we harvested spinal cords from mice displaying peak clinical disease symptoms and performed immunohistochemistry. The majority of CCL22 was associated with the infiltrating mononuclear cells (data not shown) and confirmed the ELISA data in Fig. 1B. These data suggest that CCL22 is up-regulated as EAE develops.
Figure 1. Elevated CNS expression of CCL22 is associated with EAE development.
Mice were immunized with PLP139–151 in CFA, and CNS tissue was harvested at various time-points corresponding to milestones in disease development. (A) Gene expression analysis was performed by Agilent whole mouse genome array on laser capture microdissected tissues. The data are expressed as fold induction in immunized mice compared with naïve controls. The results indicate high CCL22 expression in CNS lesion and peri-lesion areas. (B) Real-time RT-PCR analysis of CCL22 expression was conducted on spinal cord homogenates corresponding to the indicated times after and associated with disease development using SYBR Green chemistry. The data are expressed as mean (±sd) fold induction from individual mice (n=3) calculated by the Δ comparative threshold method relative to the housekeeping gene G3PDH. (C) CCL22 protein expression in the spinal cord was determined by assessing CNS homogenates from PLP139–151-immunized mice at the indicated time-points after disease induction by ELISA. The data are expressed as mean (±sd) from individual mice (n=3) at each time-point. *P < 0.05 for all experiments where statistical analysis was performed. These results are representative of multiple experimental replicates with similar results.
Anti-CCL22 treatment ameliorates development of EAE
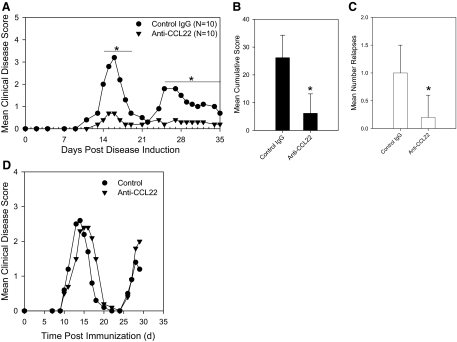
The CNS CCL22 expression data suggested an association between the chemokine and development of disease. As CCL22-deficient mice do not exist, we used a neutralizing antibody treatment approach that has been successful in the past at determining whether a chemokine has a cause or effect role in disease [15, 32, 37, 38]. To test the possibility that CCL22 is a chemokine that regulates disease, we treated mice with custom-made rabbit anti-murine CCL22 sera at Days –2 and 0 relative to immunization with PLP139–151 in CFA and compared the development and progression of disease with control antibody-treated mice. The results of this experiment are shown in Fig. 2A and indicate that there was a significant (P<0.05) amelioration of acute and relapsing EAE in the anti-CCL22-treated mice compared with the controls. This striking inhibition of disease was also evident when additional clinical disease analysis was conducted, including mean cumulative score (Fig. 2B), and also when relapsing disease was quantified (Fig. 2C). These experiments were repeated using commercial goat anti-murine CCL22 or monoclonal rat anti-mouse CCL22, and the same results were obtained (data not shown). The timing of anti-CCL22 administration was important, as mice treated with anti-CCL22 at Day 10, relative to disease induction, developed clinical symptoms similar to control-treated animals (Fig. 2D), thus suggesting a limited temporal window for CCL22 regulation of disease. To confirm that anti-CCL22 treatment resulted in a reduction of CNS CCL22 protein expression, we examined spinal cord sections from mice that had received a dose of neutralizing anti-CCL22 and performed immunohistochemistry. Using an independent polyclonal antibody, we determined that there was no detectable CNS CCL22 expression (data not shown), thereby indicating the ability of the anti-CCL22 treatment to dramatically reduce CNS CCL22 expression.
Figure 2. Anti-CCL22 treatment dampened the development and progression of EAE.
Mice were immunized with PLP139–151 in CFA at Day 0 and treated with normal goat Ig or polyclonal anti-murine CCL22 at Days –2 and 0 relative to immunization. (A) Anti-CCL22 treatment significantly (*P<0.05) inhibited acute and relapsing clinical disease. The data represent the mean clinical disease score at each specific day postimmunization. (B) In the same experiment, mean cumulative clinical score (±sd) was calculated, and there was a significant (*P<0.05) reduction in the anti-CCL22-treated group. (C) The mean number of relapses (±sd) was calculated for each group, and there was a significant (*P<0.05) reduction in the anti-CCL22-treated mice. The clinical results are representative of three independent experiments. (D) Anti-CCL22 treatment at Day +10, relative to disease induction, did not significantly inhibit acute clinical disease. The data represent the mean clinical disease score at each specific day postimmunization.
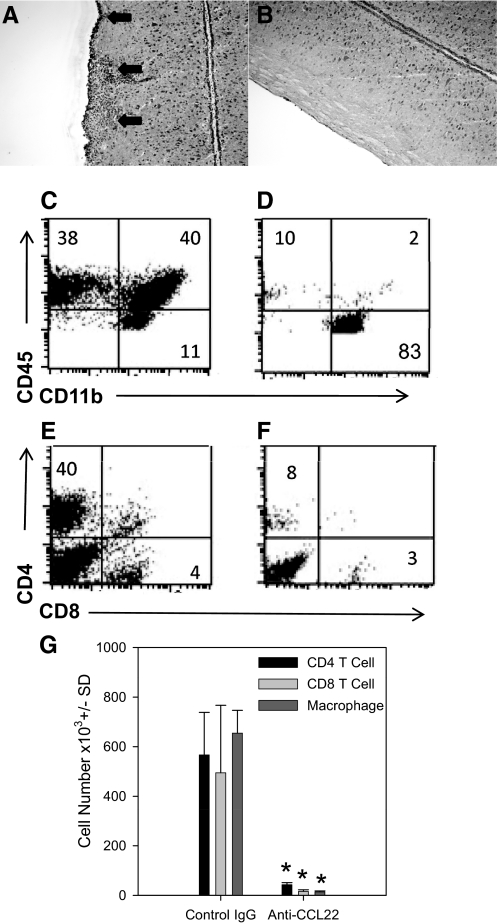
Mice treated with anti-CCL22 were examined for CNS inflammation by H&E staining of lumbar spinal cord sections. As shown in Fig. 3A, spinal cord sections from control-treated mice exhibited extensive mononuclear cell infiltrates (arrows), whereas sections from anti-CCL22-treated mice showed little, if any, mononuclear cell infiltration (Fig. 3B). Similarly, when the CNS infiltrate was further assessed and quantified by flow cytometric analysis, the results shown in Fig. 3C indicate that spinal cords from control-treated mice showed 38% lymphocytes (CD45highCD11b–), 40% macrophages/DCs (CD45highCD11b+), and 11% microglia (CD45lowCD11b+), whereas in Fig. 3D, the results from anti-CCL22-treated spinal cords indicated that there were considerably fewer CNS-infiltrating lymphocytes (10%) and macrophages/DCs (2%). When the data were gated on CD45+ events (leukocytes), the anti-CCL22-treated group showed a considerably reduced percentage of CD4 and CD8 T cells (Fig. 3F) compared with control-treated mice (Fig. 3E). The total numbers of CD4 and CD8 T cells as well as macrophages were quantified from the spinal cords of individual mice. The results shown in Fig. 3G indicate a significant (P<0.05) decrease of all three of these leukocyte subsets in the CNS of anti-CCL22-treated mice compared with controls. These results suggest that CCL22 is a potent regulator of the development and progression of clinical and histological EAE.
Figure 3. Anti-CCL22 treatment inhibited CNS inflammation.
(A) Representative photomicrograph (original magnification, 100×) of the H&E-stained lumbar spinal cord section from a control Ig-treated mouse. The data indicate extensive mononuclear cell infiltration (arrows). (B) Representative photomicrograph (original magnification, 100×) of a H&E-stained lumbar spinal cord section from an anti-CCL22-treated mouse. The data indicate a lack of mononuclear cell infiltration. (C) Representative flow cytometric analysis of CNS-infiltrating mononuclear cells from a control Ig-treated mouse. The dot plots are derived from a combined forward- and side-scatter and CD45+ gate. The percentages of cells in each quadrant are indicated: 38% lymphocytes, 40% macrophages/DCs, and 11% microglia. (D) Representative flow cytometric analysis of CNS-infiltrating mononuclear cells from an anti-CCL22-treated mouse. The dot plots are derived from a combined forward- and side-scatter and CD45+ gate. The percentages of cells in each quadrant are indicated: 10% lymphocytes, 2% macrophages/DCs, and 83% microglia. (E) Representative flow cytometric analysis of CNS-infiltrating CD4 and CD8 T cells from a control Ig-treated mouse. The dot plots are derived from a combined forward- and side-scatter and CD45+ gate. The percentages of cells in each quadrant are indicated: 40% CD4 T cells and 4% CD8 T cells. (F) Representative flow cytometric analysis of CNS-infiltrating CD4 and CD8 T cells from an anti-CCL22-treated mouse. The dot plots are derived from a combined forward- and side-scatter and CD45+ gate. The percentages of cells in each quadrant are indicated: 8% CD4 T cells and 3% CD8 T cells. (G) Quantification of the total number of CNS-infiltrating CD4 and CD8 T cells and macrophages. Individual mice (n=5) in control- and anti-CCL22-treated groups were assessed for the presence of mononuclear cells in the spinal cord at the time of peak clinical disease in the control-treated group by flow cytometry. The data are expressed as the mean cell number (±sd) and demonstrate significantly (*P<0.05) fewer CD4 and CD8 T cells and macrophages in the anti-CCL22-treated mice. The results are representative of three independent histologic experiments and two independent flow cytometric analyses.
CCL22 regulates EAE by modulating inflammatory macrophage accumulation
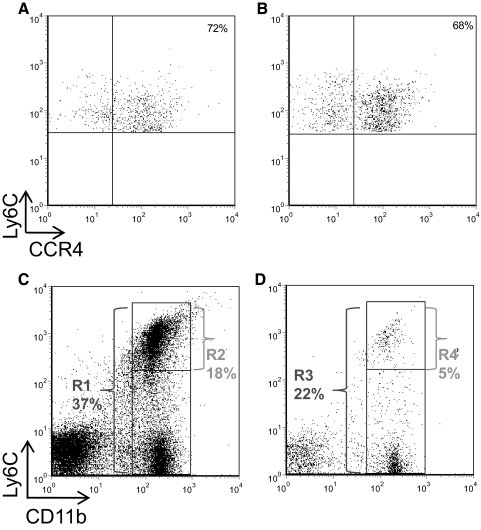
We further hypothesized that CCL22 regulates the CNS accumulation of macrophages and/or their effector functions. It was previously known that macrophages express CCR4 in mouse [39] and human [40]. To begin to address this idea, we first assessed whether CNS-infiltrating Ly6Chi inflammatory macrophages, known to be important in the development of EAE [41], expressed CCR4. CNS mononuclear cells were harvested from mice immunized with PLP139–151 to develop EAE and analyzed by flow cytometry for CCR4 cell surface expression. The results demonstrate that at peak clinical disease (Fig. 4A), ∼72% of the CD45hiCD11b+Ly6Chi macrophages in the CNS express CCR4, and that fraction is similar in relapsing disease (Fig. 4B). These data suggest that CD11b+Ly6Chi inflammatory macrophages that enter the CNS are CCR4+ and have the capacity to respond to CCL22.
Figure 4. Anti-CCL22 treatment reduced CNS accumulation of Ly6Chi macrophages.
(A) CNS-infiltrating CD11b+Ly6Chi macrophages from acute EAE express CCR4. Mice were immunized to develop EAE, and at the peak of acute clinical disease, the CNS mononuclear cells were harvested and assessed for CCR4 expression by flow cytometry. The results are shown for a representative animal (out of a total of five), the data are derived from a CD45hiCD11b+ gate, and the results show CCR4 expression as a function of Ly6Chi expression. (B) CNS-infiltrating CD11b+Ly6Chi macrophages from relapsing EAE express CCR4. Mice were immunized to develop EAE, and at the peak of relapsing clinical disease, the CNS mononuclear cells were harvested and assessed for CCR4 expression by flow cytometry. The results are shown for a representative animal (out of the remaining five), the data are derived from a CD45hiCD11b+ gate, and the results show CCR4 expression as a function of Ly6Chi expression. (C) The presence of Ly6ChiCD11b+ macrophages was assessed in the CNS of control Ig-treated mice at peak clinical disease using flow cytometry. The results are displayed from a representative animal (out of a total of three) as the percentage of total CD11b+ macrophages out of the CD45+ gate (R1=37%) and the percentage of only Ly6Chi cells from the CD45+ gate (R2=18%). (D) The presence of Ly6ChiCD11b+ macrophages was assessed in the CNS of anti-CCL22-treated mice at the time controls were showing peak clinical disease using flow cytometry. The results are displayed from a representative animal (out of a total of three) as the percentage of total CD11b+ macrophages out of the CD45+ gate (R3=22%) and the percentage of only Ly6Chi cells from the CD45+ gate (R4=5%). The results are representative of two similar experimental replicates.
We first wanted to know whether anti-CCL22 treatment resulted in alterations of numbers of Ly6Chi inflammatory macrophages in the LNs of mice during the development of the afferent autoimmune responses. The rationale for this idea was that efferent disease could be altered if there were differences in the presence of these cells during the afferent T cell priming response. Therefore, we immunized mice with PLP139–151 in CFA and treated with control antibody or anti-CCL22. At 7 days postimmunization, we assessed the numbers of peripheral CD11b+Ly6Chi cells by flow cytometry. The results indicated that 0.24% cells exhibited the CD11b+Ly6Chi inflammatory macrophage phenotype in the draining LNs (data not shown). A similar fraction of CD45+ cells in the draining LN of anti-CCL22-treated mice also exhibited the CD11b+Ly6Chi inflammatory macrophage phenotype (data not shown). The inflammatory macrophage subpopulation was quantified from individual mice in the draining LNs, and we found 0.192 ± 0.03% CD11b+Ly6Chi cells in the control-treated group compared with 0.334 ± 0.18% in the anti-CCL22-treated group (not significant; n=5). These results suggest that anti-CCL22 treatment does not alter the presence of this population of effector macrophages in the LN during the development of the afferent autoimmune response.
Therefore, to test the possibility that CCL22 regulates EAE through CNS accumulation of Ly6Chi inflammatory macrophages, mice were immunized with PLP139–151 in CFA and treated with control antibody or anti-CCL22. When the control-treated group reached peak clinical disease on average, CNS was harvested from representative mice and assessed for the presence of CD45hiCD11b+Ly6Chi cells by flow cytometry. The results shown in Fig. 4C demonstrate that in control-treated mice, 37% of the CD45+ CNS infiltrate was CD11b+ (R1), and 18% was CD11b+Ly6Chi (R2). In contrast, the results shown in Fig. 4D indicated that in anti-CCL22-treated mice, there was a reduction in CD11b+ (22%, R3) and Ly6Chi (5%, R4) cells in the CNS. In fact, in the anti-CCL22-treated mice, the ratio of Ly6Chi:total CD11b+ cells was 0.227 compared with 0.486 in control-treated mice, suggesting that there was a dramatic inhibition of the Ly6ChiCCR4+CD11b+ component of the infiltrate. When the Ly6Chi inflammatory macrophages were quantified from the two treatment groups, there was a significant reduction in the total numbers of CNS-infiltrating Ly6Chi cells in the anti-CCL22-treated group (data not shown). This result is consistent with and parallels our finding that anti-CCL22 inhibits CNS accumulation of macrophages (Fig. 3G).
Anti-CCL22 treatment does not alter peripheral T cell responses
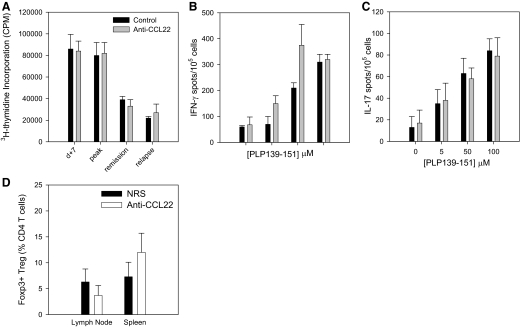
In an effort to understand the mechanism of CCL22 regulation of EAE development and progression, we assessed peripheral T cell immune responses, including antigen-specific proliferation and effector cytokine expression, from control- and anti-CCL22-treated mice. The results shown in Fig. 5A indicate that there was no difference in splenic, PLP139–151-specific T cell proliferation 7 days after immunization, at peak clinical disease, during remission, or during the relapsing phase of disease between control- and anti-CCL22-treated mice. Similarly, there was no difference in the frequency of IFN-γ (Fig. 5B)- or IL-17-producing (Fig. 5C) splenic T cells at the peak of clinical disease, as measured in the control-treated group of mice. These data strongly indicate that in vivo CCL22 neutralization does not regulate EAE through modulation of peripheral antigen-specific immune responses.
Figure 5. Anti-CCL22 does not alter peripheral antigen-specific effector T cell responses or Treg numbers.
Mice were immunized with PLP139–151 in CFA and treated with control Ig or anti-CCL22 at Days –2 and 0 relative to antigen priming. At the indicated time-points, three mice/group/time were assessed for proliferative and cytokine responses. (A) Splenic PLP139–151-specific T cell-proliferative responses were determined from control- or anti-CCL22-treated mice at Day 10 postimmunization and at times corresponding to peak clinical disease, remission, and peak-relapsing disease. The data are displayed as mean cpm (±sd), and there are no significant differences at any time-point measured. (B) Splenic PLP139–151-specific IFN-γ T cell responses were determined using ELISPOT analysis of cells from each group of mice at 10 days postimmunization. The data are displayed as mean number of cytokine-secreting cells (±sd) at each dose of peptide (0, 5, 50, and 100 nM). There are no significant differences between the two groups at any peptide dose measured. (C) Splenic, PLP139–151-specific IL-17 T cell responses were determined using ELISPOT analysis of cells from each group of mice at 10 days postimmunization. The data are displayed as mean number of cytokine-secreting cells (±sd) at each dose of peptide (0, 5, 50, and 100 μM). There are no significant differences between the two groups at any peptide dose measured. (D) The presence of Tregs in the LN and spleen was assessed by flow cytometric analysis of Foxp3 expression by CD4+CD25+-gated lymphocytes from control- or anti-CCL22-treated mice at the time when control mice showed clinical disease. The data are expressed as mean percentage of Foxp3+ cells from the CD4+ T cell population (±sd) in the LN and spleen of control (n=3)- and anti-CCL22-treated mice (n=3). There are no significant differences between the two groups in either organ analyzed. These results are representative of at least two independent experimental replicates.
CCR4 is the receptor for CCL17 and CCL22 [13] and has previously been shown to be expressed by CD4+CD25+ Tregs [21]. Therefore, to address the possibility that anti-CCL22 treatment ameliorated EAE by altering Tregs, we assessed the numbers of splenic CD4+CD25+Foxp3+ T cells from control- and anti-CCL22-treated mice by flow cytometry. When individual mice in each group were analyzed at the time that the control-treated mice were showing clinical disease, and the anti-CCL22 mice were not, we were unable to detect a significant difference in the numbers of LN or splenic Foxp3+ Tregs (Fig. 5D). Similarly, we could not detect any difference in the ability of these populations to suppress PLP139–151-specific effector T cell proliferation (data not shown). These results suggested that anti-CCL22 treatment did not inhibit clinical disease by down-regulating antigen-specific effector T cell responses or up-regulating Treg responses.
Anti-CCL22 inhibition of adoptively transferred EAE
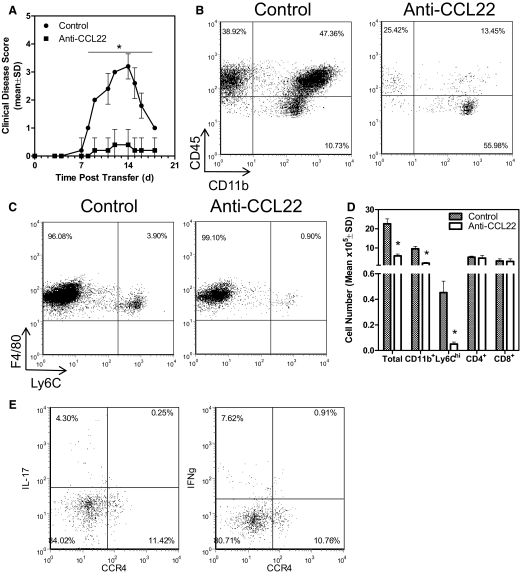
It was possible that anti-CCL22 treatment ameliorated EAE by inhibiting migration/accumulation of CD4+ T effector cells in the CNS. To address that possibility, we performed an adoptive EAE experiment, where PLP139–151-primed, in vitro, reactivated donor T cells were injected into normal SJL recipient mice that received control antibody or anti-CCL22 at Days 4 and 6 relative to T cell transfer. This time-frame was chosen based on the idea that CNS T cell migration occurs within 48 h relative to adoptive transfer [5] with macrophages presumably following. The results shown in Fig. 6A indicate that mice receiving anti-CCL22 treatment showed significantly (P<0.05) less clinical disease compared with control-treated mice. When the CNS from the control- and anti-CCL22-treated mice was examined for the presence of infiltrating leukocytes, we found a reduced presence of lymphocytes (CD45hiCD11b–) and macrophages (Fig. 6B; CD45hiCD11b+), as well as a reduced proportion of inflammatory macrophages (CD45hiF4/80+Ly6Chi; Fig. 6C). These results were quantified from individual mice in the experiment, and the results shown in Fig. 6D indicate a significant (P<0.05) decrease in the total number of CNS-infiltrating leukocytes as well as CD11b+ and Ly6Chi cells. However, the numbers of CNS CD4 and CD8 T cells were not significantly different between control- and anti-CCL22-treated mice. These results further suggest that CCL22 regulates EAE by controlling CNS inflammatory macrophage accumulation. As further evidence that anti-CCL22 did not reduce adoptive EAE by affecting disease-inducing Th1 or Th17 cell migration to the CNS, we immunized WT mice to develop EAE and isolated CNS-infiltrating CD4+ T cells at the time of disease development. Those T cells were restimulated and assessed for intracellular IFN-γ and IL-17 expression as a measurement of Th1 and Th17 cells, respectively. Additionally, the cells were assessed for the expression of CCR4 on their cell surface. The results in Fig. 6E demonstrate that neither the CNS-infiltrating CD4+ Th1 nor Th17 cells expressed CCR4. Similarly, Th1 and Th17 effector T cells isolated from the draining LNs or the spleen also did not express CCR4 (data not shown).
Figure 6. Anti-CCL22 treatment inhibits adoptively transferred EAE by reducing CNS inflammatory macrophages.
(A) Two groups of recipient mice (n=10) received i.v. injections of 5 × 106 PLP139–151-primed and reactivated lymphocyte blasts and were treated with control or polyclonal anti-murine CCL22 at Days 4 and 6 relative to T cell transfer. The data represent the mean clinical disease score at each specific day post-cell transfer and significantly demonstrate (*P<0.05) disease development in the anti-CCL22-treated group. (B) The CNS of control- or anti-CCL22-treated mice was evaluated by flow cytometry for leukocyte infiltration. The results show a decreased percentage of lymphocytes (CD45hiCD11b–) and macrophages (CD45hiCD11b+) in a representative anti-CCL22-treated animal compared with control. (C) The CNS of control- or anti-CCL22-treated mice was evaluated by flow cytometry for inflammatory macrophage infiltration. The results show a decreased percentage of inflammatory macrophages (CD45hiF4/80+Ly6Chi) in a representative anti-CCL22-treated animal compared with control. (D) The number of CNS-infiltrating leukocytes was determined in control- and anti-CCL22-treated mice (n=5, each group). The results show a significantly (*P<0.05) decreased number of total leukocytes, macrophages, and Ly6Chi inflammatory macrophages in the CNS of anti-CCL22-treated mice. (E) CNS-infiltrating Th1 and Th17 cells from mice with EAE do not express CCR4. The results shown are representative of two similar experimental replicates.
CCL22 regulates inflammatory macrophage function
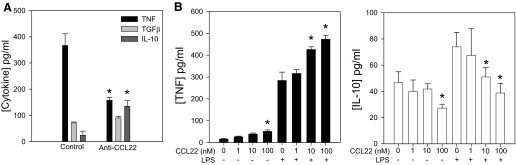
In addition to regulating cell migration, it has been hypothesized that CCR4 can regulate the inflammatory functions of macrophages leading to tissue damage [39]. Moreover, CCR4 has been suggested to modulate the signaling outcomes of TLR9 [42]. To determine whether there is an alteration of inflammatory and anti-inflammatory cytokine production by Ly6Chi inflammatory macrophages in anti-CCL22-treated mice, we immunized mice with PLP139–151 in CFA and divided the cohort into two groups. The first group received control antibody, and the second received anti-CCL22, a regimen shown to inhibit development and progression of clinical EAE (Fig. 2A). At the time of peak disease in the control-treated group, CNS-infiltrating inflammatory macrophages were isolated from both groups by flow cytometric sorting of CD45highCD11b+Ly6Chigh cells, cultured for 48 h, and assessed for TNF, TGF-β, and IL-10 by specific ELISA. The results in Fig. 7A demonstrate that inflammatory macrophages from anti-CCL22-treated mice produced significantly less (P<0.05) TNF compared with those from control-treated mice. Conversely, inflammatory macrophages from anti-CCL22-treated mice produced slightly higher levels of TGF-β and significantly (P<0.05) higher levels of IL-10 when compared with inflammatory macrophages from control-treated mice. These results suggest that an alteration in the inflammatory/anti-inflammatory cytokine ratio in macrophages from anti-CCL22-treated mice may contribute to the less-severe EAE in those animals. To address whether addition of CCL22 and thus, stimulation of CCR4 could affect macrophage effector cytokine expression, we isolated inflammatory macrophages and cultured them in vitro with CCL22. The results shown in Fig. 7B indicate that the highest dose of CCL22 (100 nM) could significantly (P<0.05) enhance TNF and reduce IL-10 expression. Additionally, when the macrophages were stimulated with LPS and CCL22, there was a more substantial, significant (P<0.05) increase in TNF and decrease in IL-10 expression with the 10- and 100-nM doses (Fig. 7B). This result suggests that CCL22 can modulate effector cytokine expression alone or in the presence of an additional activation signal.
Figure 7. Anti-CCL22 treatment alters macrophage inflammatory cytokine expression.
(A) Cytokine expression patterns from CD11b+Ly6Chi macrophages from control- or anti-CCL22-treated mice were determined by ELISA from cells sorted from control- or anti-CCL22-treated mice. The data are displayed as mean cytokine production (±sd) and indicate a significantly (*P<0.05) decreased TNF level and a significantly (P<0.05) increased IL-10 level by CD11b+Ly6Chi macrophages from anti-CCL22- compared with control-treated mice. (B) Stimulation of Ly6Chi macrophages with CCL22 and LPS together results in significantly increased TNF and significantly (*P<0.05) decreased IL-10 compared with macrophages. These results are representative of two similar experimental replicates.
DISCUSSION
Select chemokines have been shown to play a role in the development and progression of EAE [15, 43] and have been postulated to regulate MS and/or serve as biologic markers for disease progression [44]. CCL22 has long been shown to be up-regulated in the CNS of mice with EAE [30] and has also been detected in the CSF of MS patients [31]; however, the functional role of this chemokine has not been explored in CNS-demyelinating disease pathogenesis. In the present study, we tested the hypothesis that CCL22 regulated acute disease development and relapsing disease progression by modulating the CNS infiltration of inflammatory mononuclear cells. Our approach of treating mice with neutralizing anti-CCL22 resulted in decreased clinical disease (Fig. 2), as well as decreased CNS inflammation (Fig. 3). CCL22 is one of two ligands for CCR4, which has been shown to be expressed by Th1 and Th2 cells [29, 45, 46], Tregs [21, 47], invariant NK T cells [48], and macrophages [39]. Therefore, we considered the following possibilities that might explain the amelioration of clinical EAE by anti-CCL22 treatment: 1) CCL22 regulated development of antigen-specific Th1 and/or Th17 cells; 2) CCL22 mediated the accumulation of antigen-specific effector T cells in the CNS; 3) CCL22 modulated the migration of Tregs to CNS; or 4) CCL22 regulated the accumulation of inflammatory macrophages in the CNS.
We [49, 50] and others [51] previously demonstrated that CCL3 and CCL2 could alter Th1 and Th2 cytokine production. Therefore, we explored the idea that CCL22 regulated EAE by affecting the development Th1 and/or Th17 effector cytokine responses. If in vivo neutralization of CCL22 ameliorated EAE through this mechanism, we would have expected to see changes in the frequencies of antigen-specific IFN-γ- and/or IL-17-producing cells in the anti-CCL22-treated mice. We saw no alteration in either of these effector T cell phenotypes in the spleen (Fig. 5) or in the draining LNs (not shown). It is unlikely that anti-CCL22 treatment led to amelioration of clinical and histologic EAE by enhanced Treg accumulation in the draining LNs or the CNS. Although CD4+CD25+ Tregs express CCR4 [21], the receptor for CCL17 and CCL22, in vivo neutralization of CCL22 would inhibit the functionality of the ligand and eliminate its ability to induce directional migration and tissue cellular accumulation. In support of this idea, immunophenotypic analysis of LN-, spleen-, and CNS-derived lymphocytes did not detect a significant accumulation of Foxp3+ Treg in any tissue (Fig. 5). Furthermore, when we assessed the ability of CD4+CD25+ Treg from control- or anti-CCL22-treated mice for their functional ability to suppress effector T cell proliferation, we did not see any differences (not shown).
As CCR4 is expressed by T cells during EAE development [52], we considered the possibility that CCL22 regulated EAE development through recruitment of antigen-specific Th1 and/or Th17 cells to the CNS. Anti-CCL22 treatment of recipients of PLP139–151-specific T cells, which contain subsets of Th1 and Th17 phenotypes, resulted in amelioration of disease (Fig. 6A). Using this experimental approach, we determined that anti-CCL22-treated mice had no significant differences in CNS CD4 and CD8 T cell numbers, but there was a significant difference in macrophages, especially the inflammatory subpopulation (Fig. 6B–D). Moreover, neither CNS-infiltrating Th1 nor Th17 cells expressed CCR4 (Fig. 6E), thereby making it unlikely that these disease-inducing cells were the target of anti-CCL22 treatment.
We feel that the current results best support the conclusion that anti-CCL22 treatment inhibited clinical and histologic EAE through two potential, nonmutually exclusive mechanisms: reduction of CNS inflammatory macrophage accumulation and alteration of macrophage effector cytokine phenotype. Ly6Chi expression has been used to characterize inflammatory macrophages [53, 54] involved in host defense [55] and EAE development [41]. Although our data indicate that anti-CCL22 treatment reduced the overall accumulation of lymphocytes and macrophages in the CNS (Fig. 3), the Ly6ChiCCR4+ macrophage subpopulation was also greatly reduced in the CNS of treated mice (Figs. 4 and 6). Additionally, Ly6Chi macrophages isolated from the CNS of anti-CCL22-treated mice showed less of an inflammatory and more of a regulatory cytokine phenotype, as measured by decreased TNF and increased IL-10 expression (Fig. 7). This type of M2 macrophage phenotype has been previously shown to inhibit the development of EAE [56]. Our in vitro experiments also support the idea that CCL22 can stimulate Ly6Chi macrophages to produce more TNF and less IL-10. Therefore, we believe that the data support the idea that anti-CCL22 treatment inhibited the migration and the inflammatory function of a macrophage population critical for the development of disease.
The current results expand our knowledge of disease pathogenesis by providing evidence that the innate immune cells, including inflammatory macrophages, use CCL22 to achieve optimal inflammatory cytokine expression and thus, disease development. Trujillo et al. [39] showed that macrophage expression of CCR4 was essential for up-regulation of inflammatory function, and in the absence of CCR4 and therefore, the ability to respond to CCL22, macrophages assumed an M2 phenotype. Furthermore, Ishii et al. [42] demonstrated that CCR4 and TLR9 cooperate during innate immune stimulation to produce inflammatory cytokines. Our data would also extend this idea to suggest that CCR4 and TLR4 cooperate during inflammatory macrophage stimulation. Therefore, in addition to anti-CCL22 treatment inhibiting CNS accumulation, it is possible that neutralizing this chemokine prevented optimal expression of inflammatory cytokines such as TNF, thereby resulting in less EAE. In our working model of EAE disease induction, we believe that APCs become activated through TLR [57] and present autoantigens to T cells that accumulate in the CNS in response to CCL3 [32] and CXCL10 [58]. In general, macrophages use CCR2 to accumulate in the CNS [59], and specifically, the Ly6Chi fraction, known to regulate disease [41], uses CCR4 to accumulate in the CNS and achieve full inflammatory cytokine potential (present report; ref. [39]). These results suggest the possibility that small MW antagonists can be used, similar to what has been proposed for CCR2 [60] and for modulation of CCR4 biology and potential use as a modifier in human MS.
ACKNOWLEDGMENTS
The study was supported by National Institutes of Health grants NS34510 (W.J.K.) and NS030871 (S.D.M.) and a grant from the Myelin Repair Foundation (S.D.M.).
Footnotes
- EAE
- experimental autoimmune encephalomyelitis
- Foxp3+
- forkhead box p3+
- MS
- multiple sclerosis
- PLP
- proteolipid protein
- Treg
- regulatory T cell
AUTHORSHIP
R-N.E.D. designed and performed experiments, analyzed data, and wrote the manuscript. N.L. performed experiments. E.F. performed experiments and analyzed data. K.D. performed experiments and analyzed data. A.P.K. designed and performed experiments and analyzed results. S.D.M. analyzed data and provided resources for experiments. W.J.K. was the principal investigator, designed experiments, analyzed data, and wrote the manuscript.
REFERENCES
- 1. Hohlfeld R., Kerschensteiner M., Stadelmann C., Lassmann H., Wekerle H. (2000) The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. J. Neuroimmunol. 107, 161–166 [DOI] [PubMed] [Google Scholar]
- 2. Krakowski M. L., Owens T. (1997) The central nervous system environment controls effector CD4+ T cell cytokine profile in experimental allergic encephalomyelitis. Eur. J. Immunol. 27, 2840–2847 [DOI] [PubMed] [Google Scholar]
- 3. Hickey W. F., Hsu B. L., Kimura H. (1991) T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28, 254–260 [DOI] [PubMed] [Google Scholar]
- 4. Cross A. H., Cannella B., Brosnan C. F., Raine C. S. (1990) Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab. Invest. 63, 162–170 [PubMed] [Google Scholar]
- 5. Hickey W. F. (1991) Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1, 97–105 [DOI] [PubMed] [Google Scholar]
- 6. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 7. Tuohy V. K., Sobel R. A., Lu Z., Laursen R. A., Lees M. B. (1992) Myelin proteolipid protein: minimum sequence requirements for active induction of autoimmune encephalomyelitis in SWR/J and SJL/J mice. J. Neuroimmunol. 39, 67–74 [DOI] [PubMed] [Google Scholar]
- 8. Kuchroo V. K., Sobel R. A., Laning J. C., Martin C. A., Greenfield E., Dorf M. E., Lees M. B. (1992) Experimental allergic encephalomyelitis mediated by cloned T cells specific for a synthetic peptide of myelin proteolipid protein. Fine specificity and T cell receptor Vβ usage. J. Immunol. 148, 3776–3782 [PubMed] [Google Scholar]
- 9. Whitham R. H., Bourdette D. N., Hashim G. A., Herndon R. M., Ilg R. C., Vandenbark A. A., Offner H. (1991) Lymphocytes from SJL/J mice immunized with spinal cord respond selectively to a peptide of proteolipid protein and transfer relapsing demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 146, 101–107 [PubMed] [Google Scholar]
- 10. Brosnan C. F., Bornstein M. B., Bloom B. R. (1981) The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J. Immunol. 126, 614–620 [PubMed] [Google Scholar]
- 11. Huitinga I., Van Rooijen N., De Groot C. J. A., Uitdehaag B. M. J., Dijkstra C. D. (1990) Suppression of experimental allergic encephalomyelitis in Lewis rat after elimination of macrophages. J. Exp. Med. 172, 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dogan R. N., Elhofy A., Karpus W. J. (2008) Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J. Immunol. 180, 7376–7384 [DOI] [PubMed] [Google Scholar]
- 13. Murphy P. M., Baggiolini M., Charo I. F., Hebert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. (2000) International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176 [PubMed] [Google Scholar]
- 14. Zlotnik A., Yoshie O. (2000) Chemokines: a new classification system and their role in immunity. Immunity 12, 121–127 [DOI] [PubMed] [Google Scholar]
- 15. Karpus W. J., Fife B. T., Kennedy K. J. (2003) Immunoneutralization of chemokines for the prevention and treatment of central nervous system autoimmune disease. Methods 29, 362–368 [DOI] [PubMed] [Google Scholar]
- 16. Karpus W. J., Ransohoff R. M. (1998) Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 161, 2667–2671 [PubMed] [Google Scholar]
- 17. Chang M. s., McNinch J., Elias Iii C., Manthey C. L., Grosshans D., Meng T., Boone T., Andrew D. P. (1997) Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J. Biol. Chem. 272, 25229–25237 [DOI] [PubMed] [Google Scholar]
- 18. Imai T., Chantry D., Raport C. J., Wood C. L., Nishimura M., Godiska R., Yoshie O., Gray P. W. (1998) Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 273, 1764–1768 [DOI] [PubMed] [Google Scholar]
- 19. Chantry D., Romagnani P., Raport C. J., Wood C. L., Epp A., Romagnani S., Gray P. W. (1999) Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3+, CD4+, CD8low thymocytes. Blood 94, 1890–1898 [PubMed] [Google Scholar]
- 20. Andrew D. P., Chang M. S., McNinch J., Wathen S. T., Rihanek M., Tseng J., Spellberg J. P., Elias C. G. (1998) STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 161, 5027–5038 [PubMed] [Google Scholar]
- 21. Iellem A., Mariani M., Lang R., Recalde H., Panina-Bordignon P., Sinigaglia F., D'Ambrosio D. (2001) Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gonzalo J. A., Pan Y., Lloyd C. M., Jia G. Q., Yu G., Dussault B., Powers C. A., Proudfoot A. E., Coyle A. J., Gearing D., Gutierrez-Ramos J. C. (1999) Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163, 403–411 [PubMed] [Google Scholar]
- 23. Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., Torii H., Komine M., Asahina A., Tamaki K. (2002) Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin. Exp. Immunol. 127, 270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vestergaard C., Yoneyama H., Murai M., Nakamura K., Tamaki K., Terashima Y., Imai T., Yoshie O., Irimura T., Mizutani H., Matsushima K. (1999) Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Invest. 104, 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Berg A., Visser L., Poppema S. (1999) High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma. Am. J. Pathol. 154, 1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chvatchko Y., Hoogewerf A. J., Meyer A., Alouani S., Juillard P., Buser R., Conquet F., Proudfoot A. E., Wells T. N., Power C. A. (2000) A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 191, 1755–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katschke K. J., Jr., Rottman J. B., Ruth J. H., Qin S., Wu L., LaRosa G., Ponath P., Park C. C., Pope R. M., Koch A. E. (2001) Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 44, 1022–1032 [DOI] [PubMed] [Google Scholar]
- 28. Jones D., O'Hara C., Kraus M. D., Perez-Atayde A. R., Shahsafaei A., Wu L., Dorfman D. M. (2000) Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood 96, 685–690 [PubMed] [Google Scholar]
- 29. Kim S. H., Cleary M. M., Fox H. S., Chantry D., Sarvetnick N. (2002) CCR4-bearing T cells participate in autoimmune diabetes. J. Clin. Invest. 110, 1675–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Columba-Cabezas S., Serafini B., Ambrosini E., Sanchez M., Penna G., Adorini L., Aloisi F. (2002) Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J. Neuroimmunol. 130, 10–21 [DOI] [PubMed] [Google Scholar]
- 31. Galimberti D., Fenoglio C., Comi C., Scalabrini D., De Riz M., Leone M., Venturelli E., Cortini F., Piola M., Monaco F., Bresolin N., Scarpini E. (2008) MDC/CCL22 intrathecal levels in patients with multiple sclerosis. Mult. Scler. 14, 547–549 [DOI] [PubMed] [Google Scholar]
- 32. Karpus W. J., Lukacs N. W., McRae B. L., Strieter R. M., Kunkel S. L., Miller S. D. (1995) An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 155, 5003–5010 [PubMed] [Google Scholar]
- 33. Miller S. D., Karpus W. J. (2007) Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. Chapter 15, Unit 15.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pope J. G., Karpus W. J., VanderLugt C., Miller S. D. (1996) Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler's virus- induced demyelinating disease. Evidence for a CD4 + T cell-mediated pathology. J. Immunol. 156, 4050–4058 [PubMed] [Google Scholar]
- 35. Hendrzak J. A., Wallace P. D., Morahan P. S. (1994) Optimizing the detection of cell surface antigens on elicited or activated mouse peritoneal macrophages. Cytometry 17, 349–356 [DOI] [PubMed] [Google Scholar]
- 36. Karpus W. J., Kennedy K. J., Smith W. S., Miller S. D. (1996) Inhibition of relapsing experimental autoimmune encephalomyelitis in SJL mice by feeding the immunodominant PLP139–151 peptide. J. Neurosci. Res. 45, 410–423 [DOI] [PubMed] [Google Scholar]
- 37. Karpus W. J., Kennedy K. J., Kunkel S. L., Lukacs N. W. (1998) Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J. Exp. Med. 187, 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kennedy K. J., Strieter R. M., Kunkel S. L., Lukacs N. W., Karpus W. J. (1998) Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1α and monocyte chemotactic protein-1. J. Neuroimmunol. 92, 98–108 [DOI] [PubMed] [Google Scholar]
- 39. Trujillo G., O'Connor E. C., Kunkel S. L., Hogaboam C. M. (2008) A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 172, 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yogo Y., Fujishima S., Inoue T., Saito F., Shiomi T., Yamaguchi K., Ishizaka A. (2009) Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir. Res. 10, 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King I. L., Dickendesher T. L., Segal B. M. (2009) Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113, 3190–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ishii M., Hogaboam C. M., Joshi A., Ito T., Fong D. J., Kunkel S. L. (2008) CC chemokine receptor 4 modulates Toll-like receptor 9-mediated innate immunity and signaling. Eur. J. Immunol. 38, 2290–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardona A. E., Li M., Liu L., Savarin C., Ransohoff. R. M. (2008) Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J. Leukoc. Biol. 84, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Savarin-Vuaillat C., Ransohoff R. M. (2007) Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics 4, 590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andrew D. P., Ruffing N., Kim C. H., Miao W., Heath H., Li Y., Murphy K., Campbell J. J., Butcher E. C., Wu L. (2001) C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 166, 103–111 [DOI] [PubMed] [Google Scholar]
- 46. Freeman C. M., Stolberg V. R., Chiu B. C., Lukacs N. W., Kunkel S. L., Chensue S. W. (2006) CCR4 participation in Th type 1 (mycobacterial) and Th type 2 (schistosomal) anamnestic pulmonary granulomatous responses. J. Immunol. 177, 4149–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee I., Wang L., Wells A. D., Dorf M. E., Ozkaynak E., Hancock W. W. (2005) Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med. 201, 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meyer E. H., Wurbel M-A., Staton T. L., Pichavant M., Kan M. J., Savage P. B., DeKruyff R. H., Butcher E. C., Campbell J. J., Umetsu D. T. (2007) iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J. Immunol. 179, 4661–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karpus W. J., Lukacs N. W., Kennedy K. J., Smith W. S., Hurst S. D., Barrett T. A. (1997) Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 158, 4129–4136 [PubMed] [Google Scholar]
- 50. Lukacs N. W., Chensue S. W., Karpus W. J., Lincoln P., Keefer C., Strieter R. M., Kunkel S. L. (1997) C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am. J. Pathol. 150, 1861–1868 [PMC free article] [PubMed] [Google Scholar]
- 51. Gu L., Tseng S., Horner R. M., Tam C., Loda M., Rollins B. J. (2000) Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404, 407–411 [DOI] [PubMed] [Google Scholar]
- 52. Fife B. T., Paniagua M. C., Lukacs N. W., Kunkel S. L., Karpus W. J. (2001) Selective CC chemokine receptor expression by central nervous system-infiltrating encephalitogenic T cells during experimental autoimmune encephalomyelitis. J. Neurosci. Res. 66, 705–714 [DOI] [PubMed] [Google Scholar]
- 53. Getts D. R., Terry R. L., Getts M. T., Muller M., Rana S., Shrestha B., Radford J., Van Rooijen N., Campbell I. L., King N. J. (2008) Ly6c+ ″inflammatory monocytes″ are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J. Exp. Med. 205, 2319–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hatakeyama S., Iwabuchi K., Ogasawara K., Good R. A., Onoe K. (1994) The murine c-fgr gene product associated with Ly6C and p70 integral membrane protein is expressed in cells of a monocyte/macrophage lineage. Proc. Natl. Acad. Sci. USA 91, 3458–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Serbina N. V., Hohl T. M., Cherny M., Pamer E. G. (2009) Selective expansion of the monocytic lineage directed by bacterial infection. J. Immunol. 183, 1900–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tierney J. B., Kharkrang M., La Flamme A. C. (2009) Type II-activated macrophages suppress the development of experimental autoimmune encephalomyelitis. Immunol. Cell Biol. 87, 235–240 [DOI] [PubMed] [Google Scholar]
- 57. Prinz M., Garbe F., Schmidt H., Mildner A., Gutcher I., Wolter K., Piesche M., Schroers R., Weiss E., Kirschning C. J., Rochford C. D., Brück W., Becher B. (2006) Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116, 456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fife B. T., Kennedy K. J., Paniagua M. C., Lukacs N. W., Kunkel S. L., Luster A. D., Karpus W. J. (2001) CXCL10 (IFN-γ-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 166, 7617–7624 [DOI] [PubMed] [Google Scholar]
- 59. Fife B. T., Huffnagle G. B., Kuziel W. A., Karpus W. J. (2000) CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192, 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buntinx M., Hermans B., Goossens J., Moechars D., Gilissen R. A., Doyon J., Boeckx S., Coesemans E., Vanlommen G., Van Wauwe J. P. (2008) Pharmacological profile of JNJ-27141491, a non-competitive and orally active antagonist of the human chemokine receptor CCR2. J. Pharmacol. Exp. Ther. 327, 1–9 [DOI] [PubMed] [Google Scholar]