STIM2-dependent SOCE signaling pathway in DCs may regulate Ca2+ levels at the immunological synapse.
Keywords: CRAC, SOCE, STIM2, Orai2
Abstract
SOCE via CRAC channels is a critical signaling event in immune cells. Recent studies have identified key proteins underlying this process; STIM is an ER Ca2+ sensor that interacts with Orai, an intrinsic, pore-forming protein of the CRAC channel. In heterologous expression systems, STIM1 regulates SOCE by interacting with Orai1, -2, and -3. In native tissues, however, the precise roles of STIM and Orai proteins are not well defined. Here, we have investigated the molecular components of SOCE signaling in mouse DCs. We show that DCs predominantly express STIM2 and only very low levels of STIM1 compared with T lymphocytes. Upon store depletion with Tg, STIM2 aggregates and interacts selectively with Orai2. In contrast, Tg fails to aggregate STIM1 or enhance STIM1-mediated interactions with Orai proteins. Consistent with this biochemical characterization, stimulation of DCs with the adhesion molecule ICAM-1 selectively recruits STIM2 and Orai2 to the IS. Together, these data demonstrate a novel, STIM2-dependent SOCE signaling pathway in DCs.
Introduction
[Ca2+]i signaling is critical to the diverse functions of immune cells. Activation of cell surface tyrosine kinase receptors and GPCRs elevates Ca2+ via two major mechanisms. The generation of IP3 mobilizes Ca2+ from ER Ca2+ stores. In turn, depletion of ER Ca2+ stores triggers a SOCE through plasma membrane CRAC channels. In lymphocytes, mast cells, and other immune cell types, SOCE constitutes the major pathway for increasing [Ca2+]i. Previously, we have described SOCE and ICRAC in DCs [1], primary APCs that can trigger adaptive immune responses as well as induce tolerance. Although DCs are terminally differentiated, Ca2+ signaling plays an important role in their function, influencing DC maturation, migration, antigen uptake, and presentation [1–3]. Our data show that SOCE, through CRAC channels, is a major pathway for Ca2+ entry in these cells [1]. However, the molecular composition of CRAC channels in DCs is unknown.
Recently, the results of large-scale RNA interference screens have revealed two key families of proteins participating in SOCE: STIM, an ER Ca2+ sensor [4, 5], and Orai, a pore-forming subunit of CRAC channels [6, 7]. Mammalian cells express two isoforms of STIM: STIM1 and STIM2 [8], with ∼50% aa identity. Both contain paired EF-hands in the N-terminal domain, which project into the ER lumen. Upon a drop in ER Ca2+, STIM molecules aggregate into a cluster [9] and interact with Orai [10–12]. In T cells and fibroblasts, STIM1 is essential for activation of CRAC channels and NFAT; deletion of the STIM1 gene impairs these processes [13]. Furthermore, in DC-pulsed T cells, STIM1 and Orai1 assemble at the IS [14] and in close proximity with the TCR machinery. In contrast to STIM1, STIM2 appears to regulate basal [Ca2+]i levels [15], rather than directly influencing SOCE. Although mutational analysis has revealed that Orai1 is a key pore-forming subunit of the CRAC channel [10, 16, 17], all three isoforms of Orai (Orai1–3 [16, 18]) are known to interact with STIM1 in response to store depletion [7]. Accordingly, aggregation of STIM1 has shown to be sufficient to induce the ICRAC. However, most studies of STIM and Orai to date have used heterologously expressed proteins, and the precise roles of STIM/Orai in native tissues remain to be determined.
Here, we have identified and characterized the molecular components that mediate SOCE in BM-derived DCs. Surprisingly, and in contrast to the predominant role of STIM1 in lymphocytes and mast cells, our data show that STIM2 mediates SOCE signaling in DCs. Moreover, we show that STIM2 moves to the DC IS upon adhesion-mediated activation (ICAM-1).
MATERIALS AND METHODS
Full Materials and Methods are available in Supplemental Materials. All experimental procedures involving animals were approved by the Georgetown University Animal Care and Use Committee (Washington, DC, USA) and conform to National Institutes of Health guidelines.
Cell culture and flow cytometry
BM cells were isolated from the femurs and tibias of normal C57Bl/6 mice (7–12 weeks old) and cultured for 5 days at 3 × 105 cells/ml in the presence of GM-CSF and IL-4 [1]. Splenic DCs were isolated as described previously [19]. DCs were enriched from spleen or BM cultures to >90% by centrifugation over histodenz (140 mg/ml, 20 min, 500 g). CD3+ and CD8+ T cells were purified from mouse spleen using negative isolation kits (R&D Systems, Minneapolis, MN, USA). Enriched DCs and T cells were analyzed for purity using flow cytometry by staining for CD11c, CD3, or CD8.
Western blotting and immunoprecipitation
DCs were treated with Tg (5 μM) for 2 min in HBSS. Solubilization, immunoprecipitation, SDS-PAGE, Western blotting, and ECL detection were performed as described earlier [20, 21] using anti-STIM1 (1:1000, BD Biosciences, San Jose, CA, USA), anti-STIM2 (1:500, ProSci Inc., Poway, CA, USA), anti-Orai1 (1:500, ProSci Inc.), anti-Orai2 (1:400, ProSci Inc.), and anti-Orai3 (1:300, ProSci Inc.). We also used anti-Orai1 (1:1000, Sigma Chemical Co., St. Louis, MO, USA) and anti-Orai2 (1:500, Alomone Labs, Israel) and observed similar results (data not shown).
Immunofluorescence and confocal microscopy
DCs plated on poly-D-lysine-coated coverslips were incubated with 2 μM Tg for 10 min at room temperature to examine STIM1 and STIM2 aggregation. Cells were washed with PBS and fixed with 3% paraformaldehyde overnight at 4°C and stained with anti-STIM1 (1:200 dilution, BD Biosciences) and STIM2 (1:100 dilution, ProSci Inc.) as described previously [20, 21].
ICAM-1 and CD3/CD28 stimulation
Polystyrene latex beads were coated with ICAM-1 Fc chimeric protein (R&D Systems) or anti-CD3 (eBioscience, San Diego, CA, USA) and anti-CD28 (BioLegend, San Diego, CA, USA) antibodies.
Ca2+ imaging and electrophysiology
DCs were plated on coverslips in culture medium and loaded with Fluo-4 AM (3–5 μM) for 20 min at 25°C. Cells were washed with several volumes of bathing solution and left for 20 min before recording. The dye was excited at 480 ± 15 nm. Emitted fluorescence was filtered with a 535 ± 25 bandpass filter captured by a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) and read into a computer. Analysis was performed offline using Simple PCI software (Compix Inc., Sewickley, PA, USA). Standard bathing solution was (in mM) 140 NaCl, 4 KCl, 10 HEPES, 10 glucose, 2 CaCl2, and 1 MgCl2, pH 7.3.
Whole cell patch-clamp recordings were performed using an EPC8 amplifier (HEKA Elektronik, Lambrecht, Germany). The current signal was low-pass-filtered at 3 kHz and sampled at 10 kHz. For ICRAC recording, the bathing solution was (in mM) 135 NaCl, 10 tetraethylammonium chloride, 1 MgCl2, 10 glucose, 10 HEPES, 10 sucrose, and 2 or 10 Ca2+/Mg2+ (300 mOsm/L). The pipette solution contained (in mM) 120 cesium methanesulfonate, 20 CsBAPTA, 3 MgCl2, 0.1 CaCl2, 10 mM HEPES, and 0.02 IP3, pH 7.4 (300 mOsm/L). ICRAC was recorded with 200 ms voltage ramps from –120 to +120 from a holding potential of 0 mV.
RESULTS AND DISCUSSION
DCs predominantly express STIM2 not STIM1
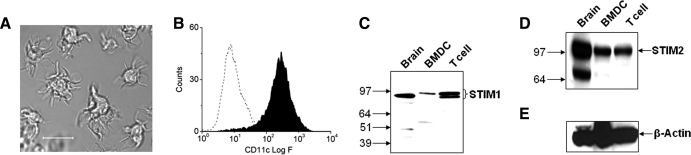
Previously, we have demonstrated SOCE through CRAC channels in mature and immature DCs [1]. To determine the molecular components of ICRAC in these cells, we analyzed purified DCs (Fig. 1A and B) for expression of STIM and Orai proteins. Using RT-PCR, we detected transcripts for STIM1, STIM2, and Orai1–3 (data not shown). To confirm protein expression, we used DC lysates to perform Western blot analysis (Figs. 1 and 2). Surprisingly, we found that compared with splenic T cells, DCs express low levels of STIM1 (detected as a single band in DCs and brain and a doublet in T cells at ∼85 kDa; Fig. 1C). In contrast, we found robust expression of STIM2 (detected as a single band at ∼100 kDa; Fig. 1C) in these cells.
Figure 1. Expression of STIM proteins in murine DCs.
(A) Bright-field image of BM-derived DCs with typical dendritic processes (original scale bar, 20 μm). (B) Flow cytometric analysis shows that enriched cultures were >90% positive for the DC marker, CD11c. (C) Western blots showing the expression of STIM1 and (D) STIM2 in DCs. Whole cell lysates (30 μg) were used for each sample. Whole mouse brain and mouse T cells were used as positive controls. (E) The blots were stripped and reprobed with β-actin as a loading control. BMDC, BM-derived DC.
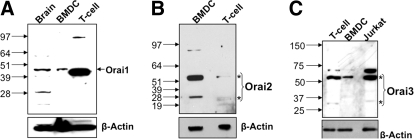
Figure 2. Expression of Orai proteins in DCs.
Western blots showing the expression of (A) Orai1, (B) Orai2, and (C) Orai3 in DCs and T cells. Whole cell lysates (30 μg) were used for each sample. Whole mouse brain, T cells, and Jurkat T cells were used as positive controls. The blots were stripped and reprobed with β-actin as a loading control. *Monomer and dimer of Orai proteins.
Similarly, we found differential protein expression of the Orai isoforms (Fig. 2A–C). Notably, DCs express significantly lower levels of Orai1 and Orai3 compared with T cells. For Orai1, a single immunoreactive band was detected at ∼45 kDa (Fig. 2A), which is likely a glycosylated form of the monomeric Orai1 protein, as demonstrated by Gwack and co-workers [18]. To confirm this result, we performed immunoblotting on lysates from myc-Orai-1-expressing HEK293 cells (Supplemental Fig. 1A). This analysis revealed a prominent 45-kDa band. Orai3 was detected as a monomer at ∼30 kDa and a more prominent band at ∼60 kDa, which likely represents a dimer (Fig. 2C). This interpretation is supported by our analysis of Orai3-expressing HEK293 cells (Supplemental Fig. 1C), in which treatment with DTT produced a marked increase in the ∼30-kDa monomeric band.
In contrast to the other Orai proteins, DCs express relatively high levels of Orai2 (Fig. 2B), detected as a monomer at ∼28 kDa and a more prominent band at ∼56 kDa representing a dimer. Analysis of Orai2-expressing HEK293 cells revealed a similar immunoblot pattern (Supplemental Fig. 1C). Further, we saw similar bands with a different commercial anti-Orai2 antibody (Alomone Labs; data not shown). We also confirmed expression of Orai2 by immunocytochemistry (Supplemental Fig. 2B). A previous study has identified two splice variants of murine Orai2 associated with functional CRAC channels [22]. Using specific primers, we detected both of these splice variants—Orai2 small and Orai2 large—in BM-derived and splenic DCs (Supplemental Fig. 2A).
As functional support for Orai2 in DCs, we explored sensitivity of SOCE to 2-APB. Recent studies have demonstrated that 2-APB differentially blocks SOCE and ICRAC mediated by the different Orai isoforms; 2-APB (50 μM) completely inhibits Orai1 and Orai2 but does not affect Orai3 [23, 24]. Although these studies were performed with heterologously expressed channels, this differential sensitivity to 2-APB, nonetheless, may prove useful in distinguishing the role of Orai isoforms in native tissues. We found that 2-APB (50 μM) produced a near 100% inhibition of SOCE (n=250) and an ∼90% block of ICRAC (–100 mV; n=8; Supplemental Fig. 3). Furthermore, in the absence of store depletion, 2-APB produces a weak activation of Orai1 and a robust activation of Orai3 but has no effect on Orai2 [24]. Interestingly, in DCs, 2-APB application failed to produce a Ca2+ rise (Supplemental Fig. 3C; n=30). Moreover, we did not detect any whole cell currents following 2-APB application (Supplemental Fig. 3D; n=5). These data are consistent with a primary role for Orai2 in SOCE and ICRAC in DCs.
Tg-induced store depletion promotes oligomerization of STIM2
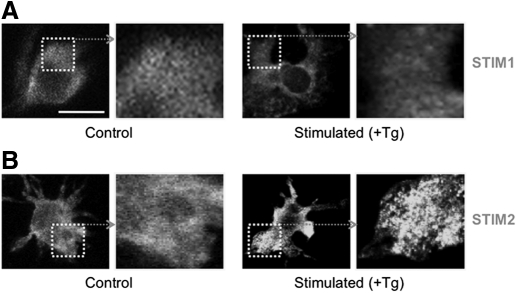
Recent evidence has shown that aggregation of STIM1 is essential for CRAC channel activation in Jurkat T cells [9]. We therefore tested whether there is an aggregation of STIM1 or alternatively, STIM2 in DCs in response to store depletion. Fig. 3A shows that DCs exhibit diffuse immunostaining for STIM1 (consistent with the low expression of STIM1 in these cells), and this was unaltered by treatment with Tg (2 μM for 10 min). We found that same anti-STIM1 antibody produced marked immunostaining of T cells, confirming the functionality of the antibody (Supplemental Fig. 4A). In contrast to STIM1, Tg produced a punctate pattern of STIM2 immunostaining (Fig. 4B). Thus, STIM2 rather than STIM1 aggregates upon store depletion in DCs.
Figure 3. Tg induces aggregation of STIM2.
(A) Immunofluorescence labeling of DCs for (A) STIM1 and (B) STIM2 under control conditions (unstimulated) and after Tg treatment (Stimulated; 2 μM Tg for 10 min). Fixed cells were permeabilized and stained with STIM1 and STIM2 antibody followed by a secondary antibody (FITC-conjugated), which alone, produced negligible staining (see Supplemental Fig. 2B).
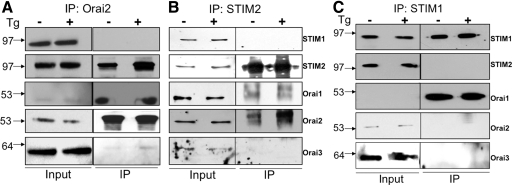
Figure 4. Tg induces the association of STIM2 and Orai2 but not STIM1.
(A and B) Coimmunoprecipitation showing the enhanced association between STIM2 with Orai2 upon Tg treatment. Although detected in the immunoprecipitation (IP) complex, Orai1 levels are not increased by Tg treatment. (C) STIM2 and Orai2 are not present in the coimmunoprecipitation using a STIM1 antibody. Moreover, Tg treatment does not alter levels of Orai1 in this immunoprecipitation complex.
Stim2 and Orai2 interact upon store depletion
Store depletion is known to produce physical interactions between native and heterologously expressed STIM1 and Orai1 [10–12]. Our results showing STIM2 oligomerization and the predominant expression of Orai2 in DCs led us to examine further the possibility of interactions between STIM2 and Orai2 in response to store depletion. We found that under basal conditions, STIM2 coimmunoprecipitated with Orai2, and this association increased markedly following treatment with Tg (Fig. 4A and B). Surprisingly, we did not detect any STIM1 when we immunoprecipitated with an anti-Orai2 (Fig. 4A) or anti-STIM2 antibody (Fig. 4B). Significantly, STIM2 and Orai2 did not coimmunoprecipitate with STIM1 (using anti-STIM1 antibody; Fig. 4C). Although Orai1 was present in the immunoprecipitation complex, its levels were not altered by Tg treatment (Fig. 4C). These findings thus rule out the possibility of STIM1 participating in the robust increase of STIM2 and Orai2 association following store depletion. Furthermore, little Orai1 or Orai3 was found to associate with Orai2 in control and Tg-treated DCs. Taken together, these data support the hypothesis that store depletion in DCs triggers oligomerization of STIM2, which in turn, interacts with Orai2.
STIM2 and Orai2 in DCs are recruited to the IS
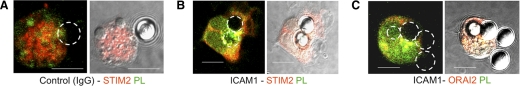
Antigen presentation, the major function of DCs, occurs at a specialized junction between DCs and T cells–the IS–which facilitates the aggregation of peptide-MHC class II complexes and cognate TCRs, as well as costimulatory molecules, to enhance T cell stimulation [25]. [Ca2+]i signaling, which plays an important role in antigen presentation and T cell activation, may also be facilitated at the IS. Recently, Lioudyno et al. [14] described the movement of Orai1 and STIM1 to the IS in T cells upon stimulation with superantigen (staphylococcal enterotoxin B)-pulsed, autologous DCs. We therefore asked whether STIM2 and Orai2 are similarly recruited to the IS in DCs. Fig. 5 shows that pulsing DCs with ICAM1-coated beads triggered actin polymerization (green phalloidin staining), and aggregation of STIM2 (red, Fig. 5B) and Orai2 (red, Fig. 5C) directed to the site of contact. The coaggregation of F-actin and STIM2/Orai2 appears as intense yellow staining and was evident in the majority of cells studied (n>30). In contrast, actin and STIM2 did not polarize to beads coated with IgG alone (Fig. 5A, n>30), demonstrating the specificity for bone fide synaptic contact. We also observed some weaker Orai-actin costaining distant from the IS (Fig. 5C). The underlying mechanism for this aggregation pattern is unclear, but interestingly, MHC class II in DCs similarly aggregates at the IS and at the opposite pole [26, 27]. For comparison, we explored trafficking of STIM1 in T cells. As reported previously, pulsing T cells with anti-CD3/CD28-coated beads produced marked STIM1 immunostaining and F-actin aggregation at the contact zone (Supplemental Fig. 3B). Taken together, our data show that STIM2 and Orai2 are recruited to the IS of DCs.
Figure 5. Orai2 and STIM2 are localized at the IS.
Representative confocal images of STIM2 and ORAI2 immunostaining in DCs stimulated with IgG (A)- or ICAM-1 (B and C)-coated beads. Circles indicate positions of beads clustered with DCs (original scale bars, 15 μm). Cells were colabeled with F-actin [phalloidin (PL), green] to reveal actin polarization toward contact sites. STIM2 (red) and ORAI2 (red) are polarized along with F-actin to the ICAM1-coated bead, as evident by the merged (yellow) staining. Immunostaining was assessed by a blinded scoring of >40 random conjugates from three independent experiments.
Significance of STIM2 and Orai2 signaling in DCs
Ca2+ signaling is critical for DC maturation and function [2, 3]. Previously, we have shown that Ca2+ entry in DCs is predominantly mediated by SOCE [1]. Here, we identify the molecular components for SOCE signaling in DCs: STIM2 and Orai2. Significantly, this represents the first report of STIM1-independent SOCE signaling in immune cells. Previous studies using primary T lymphocytes, Jurkat T cells, RBL-2H3 mast cells, and expression systems have reported STIM1 to be necessary and sufficient to signal ER store depletion leading to SOCE [4, 5, 9, 13]. In contrast, we present several lines of evidence arguing against a role for STIM1 in SOCE DCs. Notably, we found that compared with T cells, DCs express high levels of STIM2 and only low levels of STIM1. One could argue that even low levels of STIM1 might be sufficient to trigger SOCE. However, our functional and biochemical data do not support this position. First, we found that depletion of the ER Ca2+ store triggers the aggregation of STIM2 and not STIM1. Second, we find that store depletion fails to increase physical associations between STIM1 and Orai proteins; moreover, STIM1 does not associate with Orai2 under control or stimulated conditions. Instead, we find that store depletion markedly increases the association between STIM2 and Orai2. Thus, our data indicate that SOCE in DCs is likely triggered by STIM2, which in turn, interacts with Orai2.
Recent studies in expression systems [15, 24, 28] indicate that STIM2 possesses the capacity to trigger SOCE. Further, overexpression of STIM2 can restore SOCE in T cells [29] or fibroblasts [13], which are deficient in STIM1. The biological relevance of STIM2-triggered SOCE in immune cells, however, is unclear, as knockdown of STIM2 produces only a minor reduction of SOCE measured in T cells and fibroblasts [13]. In other tissues, STIM2 appears to play a more prominent role in SOCE. For example, STIM2 appears to be the dominant trigger for SOCE in the brain; genetic deletion of STIM2 virtually abolishes SOCE in neurons [30]. Further, STIM2 contributes ∼50% of SOCE in human myoblasts and myotubes [31]. These data and our present findings indicate that STIM1 and STIM2 likely function in a tissue-specific manner. Whether these proteins have functionally distinct properties is unclear. Darbellay et al. [31] show that STIM1 and STIM2 are functionally equivalent (at least for SOCE activation); the effects of knockdown of either isoform can be rescued by overexpression of the other. On the other hand, Brandman and colleagues [15] report that STIM2 has an overall reduced affinity for ER Ca2+ compared with that of STIM1. The implication of this finding is that a smaller decrease in ER Ca2+ could be sufficient to activate STIM2 and trigger SOCE. In DCs, this could lead to enhanced Ca2+ entry, even in response to weak, external stimuli, a property that could assist the innate immune function of DCs. In addition to triggering SOCE, STIM1 can bind and activate various TRPC channels [32]. Whether STIM2 shares this property is unknown, although STIM1 and STIM2 contain a polylysine C terminus implicated in electrostatic activation of TRPC channels. Finally, native STIM1, but not STIM2, can insert into the plasma membrane [33, 34], which although not essential for SOCE, may contribute to other cellular functions such as cell adhesion. Thus, the selective expression of STIM2 in DCs may lead to altered cell properties independently of SOCE.
Several studies have shown Orai1 to be the essential subunit of the CRAC channel [6, 10, 17]. In contrast, we show that Orai2 constitutes the major Orai isoform in DCs, and this is based on Orai2 expression, physical interactions with STIM2, and characteristic 2-APB sensitivity. The significance of Orai2-mediated SOCE in DCs is unclear. Although, Orai1–3 possess similar channel properties, including conductance and ion selectivity, they do exhibit different gating properties. Orai1-mediated ICRAC shows a rapid Ca2+-induced inactivation [35], not seen in Orai2 [22]. Assuming these properties are reproduced in native tissues, then Orai2-mediated SOCE in DCs may be able to maintain a more sustained increase in [Ca2+]i. Indeed, DCs exhibit prolonged Ca2+ transients in response to stimulation of GPCRs [1, 36], rather than the oscillatory pattern, which expresses Orai1, commonly observed in T cells. In addition, Orai1 and Orai3 subunits mediate the arachidonate-gated current observed in many nonexcitable cells [37]. The paucity of these proteins in DCs, therefore, suggests a reduced sensitivity to inflammatory arachidonic acid.
Significantly, we show that STIM2 and Orai2 are recruited to the DC immune synapse upon stimulation with ICAM-1. The IS supports clustering of adhesion molecules, peptide-MHC class II, and costimulatory molecules and facilitates productive encounters with T cells [26, 38–40]. The formation of the IS in DCs is accompanied by an increase in [Ca2+]i [41], as well as actin polarization toward the T cell contact site [26, 39]. Ca2+ is likely important for this cytoskeletal rearrangement. Indeed, surface expression of MHC class II and CD86 in DCs is inhibited by Ca2+ chelation [3]. Our data, showing that key components of SOCE cluster at the IS, suggest that SOCE may participate in the initiation or maintenance of IS and/or IS signaling. Future studies, will hopefully address these questions.
In conclusion, our data indicate that STIM2 and Orai2 are key molecules underlying SOCE in DCs. Moreover, we show that STIM2 and Orai2 cluster at the IS, suggesting that focal Ca2+ entry regulates properties of DC synapses.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institutes of Health (G.P.A.). We thank members of the Ahern lab for assistance with cell culture and animals.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 2-APB
- 2-aminoethyldiphenyl borate
- BM
- bone marrow
- Ca2+
- calcium
- [Ca2+]i
- intracellular calcium concentration
- CRAC
- calcium release-activated calcium
- HEK
- human embyronic kidney
- ICRAC
- calcium release-activated calcium current
- IP3
- inositol trisphosphate
- IS
- immunological synapse
- SOCE
- store-operated calcium entry
- STIM
- stromal-interacting molecule
- Tg
- thapsigargin
- TRPC
- transient receptor potential canonica
AUTHORSHIP
B.C.B. and S.C.P. designed and performed research, analyzed data, and wrote the paper. G.P.A. designed research, analyzed data, and wrote the paper.
REFERENCES
- 1. Hsu S.f., O'Connell P. J., Klyachko V. A., Badminton M. N., Thomson A. W., Jackson M. B., Clapham D. E., Ahern G. P. (2001) Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J. Immunol. 166, 6126–6133 [DOI] [PubMed] [Google Scholar]
- 2. Czerniecki B. J., Carter C., Rivoltini L., Koski G. K., Kim H. I., Weng D. E., Roros J. G., Hijazi Y. M., Xu S., Rosenberg S. A., Cohen P. A. (1997) Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J. Immunol. 159, 3823–3837 [PubMed] [Google Scholar]
- 3. Koski G. K., Schwartz G. N., Weng D. E., Czerniecki B. J., Carter C., Gress R. E., Cohen P. A. (1999) Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J. Immunol. 163, 82–92 [PubMed] [Google Scholar]
- 4. Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Velicelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 8. Williams R. T., Manji S. S., Parker N. J., Hancock M. S., Van Stekelenburg L., Eid J. P., Senior P. V., Kazenwadel J. S., Shandala T., Saint R., Smith P. J., Dziadek M. A. (2001) Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem. J. 357, 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu M. M., Buchanan J., Luik R. M., Lewis R. S. (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong H. L., Cheng K. T., Liu X., Bandyopadhyay B. C., Paria B. C., Soboloff J., Pani B., Gwack Y., Srikanth S., Singh B. B., Gill D. L., Ambudkar I. S. (2007) Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 282, 9105–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., Kahr H., Madl J., Gruber H., Groschner K., Romanin C. (2008) Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 283, 8014–8022 [DOI] [PubMed] [Google Scholar]
- 13. Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A. (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 9, 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lioudyno M. I., Kozak J. A., Penna A., Safrina O., Zhang S. L., Sen D., Roos J., Stauderman K. A., Cahalan M. D. (2008) Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Natl. Acad. Sci. USA 105, 2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandman O., Liou J., Park W. S., Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vig M., Beck A., Billingsley J. M., Lis A., Parvez S., Peinelt C., Koomoa D. L., Soboloff J., Gill D. L., Fleig A., Kinet J. P., Penner R. (2006) CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 16, 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 [DOI] [PubMed] [Google Scholar]
- 18. Gwack Y., Srikanth S., Feske S., Cruz-Guilloty F., Oh-hora M., Neems D. S., Hogan P. G., Rao A. (2007) Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 282, 16232–16243 [DOI] [PubMed] [Google Scholar]
- 19. O'Connell P. J., Wang X., Leon-Ponte M., Griffiths C., Pingle S. C., Ahern G. P. (2006) A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 107, 1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bandyopadhyay B. C., Swaim W. D., Liu X., Redman R. S., Patterson R. L., Ambudkar I. S. (2005) Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J. Biol. Chem. 280, 12908–12916 [DOI] [PubMed] [Google Scholar]
- 21. Bandyopadhyay B. C., Ong H. L., Lockwich T. P., Liu X., Paria B. C., Singh B. B., Ambudkar I. S. (2008) TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J. Biol. Chem. 283, 32821–32830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gross S. A., Wissenbach U., Philipp S. E., Freichel M., Cavalie A., Flockerzi V. (2007) Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 282, 19375–19384 [DOI] [PubMed] [Google Scholar]
- 23. DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr. (2008) Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 283, 19265–19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peinelt C., Lis A., Beck A., Fleig A., Penner R. (2008) 2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J. Physiol. 586, 3061–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krummel M. F., Cahalan M. D. (2010) The immunological synapse: a dynamic platform for local signaling. J. Clin. Immunol. 30, 364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De la Fuente H., Mittelbrunn M., Sanchez-Martin L., Vicente-Manzanares M., Lamana A., Pardi R., Cabanas C., Sanchez-Madrid F. (2005) Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol. Biol. Cell 16, 3314–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloom O., Unternaehrer J. J., Jiang A., Shin J. S., Delamarre L., Allen P., Mellman I. (2008) Spinophilin participates in information transfer at immunological synapses. J. Cell Biol. 181, 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parvez S., Beck A., Peinelt C., Soboloff J., Lis A., Monteilh-Zoller M., Gill D. L., Fleig A., Penner R. (2008) STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J. 22, 752–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picard C., McCarl C. A., Papolos A., Khalil S., Luthy K., Hivroz C., LeDeist F., Rieux-Laucat F., Rechavi G., Rao A., Fischer A., Feske S. (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 360, 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berna-Erro A., Braun A., Kraft R., Kleinschnitz C., Schuhmann M. K., Stegner D., Wultsch T., Eilers J., Meuth S. G., Stoll G., Nieswandt B. (2009) STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci. Signal. 2, ra67 [DOI] [PubMed] [Google Scholar]
- 31. Darbellay B., Arnaudeau S., Ceroni D., Bader C. R., Konig S., Bernheim L. (2010) Human muscle economy: myoblast differentiation and excitation/contraction coupling use the same molecular partners, STIM1 and STIM2. J. Biol. Chem. 285, 22437–22447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cahalan M. D. (2009) STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 11, 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manji S. S., Parker N. J., Williams R. T., van Stekelenburg L., Pearson R. B., Dziadek M., Smith P. J. (2000) STIM1: a novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta 1481, 147–155 [DOI] [PubMed] [Google Scholar]
- 34. Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoth M., Penner R. (1993) Calcium release-activated calcium current in rat mast cells. J. Physiol. 465, 359–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stolk M., Leon-Ponte M., Merrill M., Ahern G. P., O'Connell P. J. (2006) IP3Rs are sufficient for dendritic cell Ca2+ signaling in the absence of RyR1. J. Leukoc. Biol. 80, 651–658 [DOI] [PubMed] [Google Scholar]
- 37. Mignen O., Thompson J. L., Shuttleworth T. J. (2009) The molecular architecture of the arachidonate-regulated Ca2+-selective ARC channel is a pentameric assembly of Orai1 and Orai3 subunits. J. Physiol. 587, 4181–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benvenuti F., Hugues S., Walmsley M., Ruf S., Fetler L., Popoff M., Tybulewicz V. L., Amigorena S. (2004) Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153 [DOI] [PubMed] [Google Scholar]
- 39. Al-Alwan M. M., Liwski R. S., Haeryfar S. M., Baldridge W. H., Hoskin D. W., Rowden G., West K. A. (2003) Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J. Immunol. 171, 4479–4483 [DOI] [PubMed] [Google Scholar]
- 40. Brossard C., Feuillet V., Schmitt A., Randriamampita C., Romao M., Raposo G., Trautmann A. (2005) Multifocal structure of the T cell–dendritic cell synapse. Eur. J. Immunol. 35, 1741–1753 [DOI] [PubMed] [Google Scholar]
- 41. Montes M., McIlroy D., Hosmalin A., Trautmann A. (1999) Calcium responses elicited in human T cells and dendritic cells by cell-cell interaction and soluble ligands. Int. Immunol. 11, 561–568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.