Targeted inhibition of δ-PKC exerted a lung-protective effect and significantly attenuated pulmonary inflammatory cell infiltration, disruption of lung architecture, and pulmonary edema associated with sepsis.
Keywords: lung inflammation, δ-PKC TAT peptide inhibitor, cecal ligation and double puncture (2CLP), chemokines, ARDS
Abstract
Inflammation and proinflammatory mediators are activators of δ-PKC. In vitro, δ-PKC regulates proinflammatory signaling in neutrophils and endothelial and epithelial cells, cells that can contribute to lung tissue damage associated with inflammation. In this study, a specific δ-PKC TAT peptide inhibitor was used to test the hypothesis that inhibition of δ-PKC would attenuate lung injury in an animal model of ARDS. Experimental ARDS was induced in rats via 2CLP, a model of polymicrobial sepsis. Following 2CLP surgery, the δ-PKC TAT inhibitory peptide (2CLP+δ-PKC TAT in PBS) or PBS (2CLP+PBS) was administered intratracheally. Controls consisted of SO, where animals underwent a laparotomy without 2CLP. Twenty-four hours after SO or 2CLP, blood, BALF, and lung tissue were collected. 2CLP induced δ-PKC phosphorylation in the lung within 24 h. Treatment with the δ-PKC TAT inhibitory peptide significantly decreased pulmonary δ-PKC phosphorylation, indicating effective inhibition of δ-PKC activation. Plasma and BALF levels of the chemokines CINC-1 and MIP-2 were elevated in 2CLP + PBS rats as compared with SO rats. Treatment with δ-PKC TAT reduced 2CLP-induced elevations in chemokine levels in BALF and plasma, suggesting that δ-PKC modulated chemokine expression. Most importantly, intratracheal administration of δ-PKC TAT peptide significantly attenuated inflammatory cell infiltration, disruption of lung architecture, and pulmonary edema associated with 2CLP. Thus, δ-PKC is an important regulator of proinflammatory events in the lung. Targeted inhibition of δ-PKC exerted a lung-protective effect 24 h after 2CLP.
Introduction
Sepsis and the related systemic inflammatory response are the leading causes of death in surgical intensive care units. More than 200,000 people die each year in the United States from sepsis and associated complications [1]. The lung is the organ most often affected with pulmonary dysfunction, resulting in acute lung injury or the more severe ARDS [2–5]. Mortality in patients with ARDS ranges from 30% to 60%, and recovery is often associated with long-term pulmonary and nonpulmonary morbidity [6–8]. ARDS is characterized by an intense inflammatory response. This activates a cascade of proinflammatory events that leads to leukocyte infiltration into the lung [6, 9–12]. Damage to the alveolar capillary barrier increases permeability, precipitating an influx of protein-rich edema fluid that impairs gas exchange and arterial oxygenation. As the disease evolves, interstitial inflammation progresses to fibrosis, further compromising gas exchange and impeding pulmonary mechanics [2]. Therapy for ARDS is largely supportive, as the mechanisms underlying these pathological changes are poorly understood [3–5].
Leukocyte migration and sequestration into lung tissue have been implicated in the pathogenesis of ARDS [6, 9, 11–14]. This influx of leukocytes, such as neutrophils and macrophages into the lung, is mediated by local chemokine production in response to systemic inflammation. CXC chemokines such as IL-8 are elevated significantly in the BALF of patients with ARDS, and increased IL-8 levels are associated with increased neutrophil infiltration [15–18]. In rodents, the chemokines CINC-1 and MIP-2 regulate leukocyte recruitment. Both are elevated in rat models of sepsis [6, 14, 19–21]. These chemokines may be particularly important in sepsis-induced ARDS, as in contrast to injury in other organ systems, free radicals and proteolytic enzymes derived from leukocytes are believed to contribute substantially to the pulmonary damage.
δ-PKC has been identified as a critical inflammatory regulator and is instrumental in neutrophil recruitment, sequestration, and activation in the lung. In neutrophils, δ-PKC controls antiapoptotic signaling and proinflammatory events [22–27]. δ-PKC regulates cytokine-elicited oxygen radical production, degranulation, and activation of the transcription factor NF-κB. δ-PKC also regulates adhesion molecule expression in endothelial and epithelial cells; δ-PKC inhibition prevented neutrophil adherence and migration [21, 28–30]. In δ-PKC null mice, neutrophil adhesion, migration, oxygen radical generation, and degranulation are limited [31]. Thus, δ-PKC may have an important regulatory role in the inflammatory response.
A highly specific, isotype-selective inhibitory peptide derived from the first unique region (V1) of δ-PKC can inhibit δ-PKC activity effectively [32]. Coupling this inhibitor to a membrane-permeant TAT peptide sequence permits effective intracellular delivery into target cells [32, 33]. Extensive in vitro and in vivo studies demonstrated that when taken up by cells, the δ-PKC TAT peptide produces a unique dominant-negative phenotype that effectively inhibits activation of δ-PKC but not of other PKC isotypes [25, 32–34]. Studies in neutrophils and endothelial cells revealed that this δ-PKC TAT inhibitory peptide mediated blockade of proinflammatory signaling [24–26, 35–37].
δ-PKC is activated by multiple proinflammatory stimuli, including cytokines, such as TNF and IL-1, or PAMPS, such as LPS [21, 22, 38, 39]. δ-PKC is an important component of proinflammatory signaling pathways that regulate activation of the transcription factor NF-κB, which regulates gene expression of chemokines, adhesion molecules, and cytokines, including proinflammatory mediators (TNF, IL-1, etc.), which can initiate and perpetuate inflammation and thus, function in a positive-feedback loop. Therefore, we hypothesize that sepsis and the systemic inflammatory response activate δ-PKC, and this kinase plays an important role in the initiation and perpetuation of inflammation and the development of tissue damage in sepsis-associated lung injury. Using a well-characterized rodent model of ARDS secondary to intra-abdominal sepsis (2CLP in rats), we tested the hypothesis that targeted delivery of the dominant-negative, cell-permeant δ-PKC TAT inhibitory peptide into the lung attenuates inflammation and acute lung injury.
MATERIALS AND METHODS
Animal protocol
Animal procedures and handling adhered to National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at Children's Hospital of Philadelphia (Philadelphia, PA, USA), the University of Pennsylvania School of Medicine (Philadelphia, PA, USA), and Temple University School of Medicine (Philadelphia, PA, USA). Male Sprague-Dawley rats (225–250 g; Charles River, Boston, MA, USA) were used in all experiments. Rats were housed in a climate-controlled facility and given free access to food and water. 2CLP was performed on isoflurane-anesthetized rats as described previously [20, 40–42]. Controls consisted of SO, where animals underwent a laparotomy without cecal ligation or puncture. After the procedure but prior to emergence, SO and 2CLP animals were fluid-resuscitated with 40 ml/kg sterile saline injected s.c.
In the 2CLP animal groups, closure of the abdominal incision was followed by open tracheotomy with a 24-gauge i.v. cannula [40]. Animals were randomized to receive the δ-PKC TAT inhibitory peptide (200 ug/kg in 200 ul PBS) or a like volume of PBS (vehicle). These were administered in two divided doses via the tracheal cannula. After the procedure, the cannula was removed, the incision closed, and animals were fluid-resuscitated as above, awakened, and allowed free access to food and water. This dose of the δ-PKC TAT peptide inhibitor was selected based on in vitro and in vivo studies with this inhibitory peptide [24–26, 32–34, 43].
At 24 h postsurgery, animals were re-anesthetized with 50 mg/kg i.p. pentobarbital. Blood was obtained for plasma chemokine measurements, and the animals were killed by exsanguination. The lungs were fixed for staining or frozen immediately in liquid nitrogen for δ-PKC analysis. In one group of animals, BALF was obtained by inserting a cannula into the trachea and instilling 1.5 ml room temperature sterile PBS until the lungs were fully distended as described previously [44]. The fluid was withdrawn and saved. This process was repeated until a total of 4.5 ml PBS had been instilled. The samples were pooled for each animal and the volumes recorded. The percent BALF recovered was calculated and BALF volumes normalized. Lung permeability was assessed by determining protein concentrations in BALF as described previously [44, 45]. The BALF was centrifuged to remove cells, and supernatants were aliquoted and frozen at –70°C.
δ-PKC inhibitor peptide synthesis
The PKC TAT peptide antagonist consisted of a peptide derived from the first unique region (V1) of δ-PKC (SFNSYELGSL: aa 8–17 of δ-PKC) coupled via an N-terminal Cys-Cys bond to a membrane-permeant peptide sequence in the HIV TAT gene product (YGRKKRRQRRR: aa 47–57 of TAT) [32]. The peptides were synthesized by Mimotopes (Melbourne, Australia) and purified to >95% by preparative reverse-phase HPLC. The use of a Cys-Cys disulfide bond limits directed intracellular delivery of the δ-PKC TAT inhibitor to the cytoplasm [33, 46]. Extensive in vitro and in vivo animal studies have demonstrated that the TAT peptide alone is nontoxic and does not alter δ-PKC activity [24–26, 33, 46, 47].
Determination of δ-PKC phosphorylation
An aliquot (0.15 g) of frozen lung tissue was homogenized in 3 ml buffer containing 10 mM Hepes (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1 mM Na-orthovanadate, 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 1% Triton X-100, 5 μg/ml leupeptin, and phosphatase inhibitor cocktail and protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). Protein concentrations of the cell lysates were determined by the bicinchoninic acid protein assay kit, according to the manufacturer's instructions (Thermo Scientific, Rockford, IL, USA). Proteins (30 μg/lane) were separated on 4–12% SDS-PAGE gels and transferred to nitrocellulose membranes. δ-PKC (Thr505) phosphorylation was determined by immunoblotting using a phospho-specific δ-PKC (Thr505) antibody (Cell Signaling Technology, Beverly, MA, USA) as described previously [26, 33, 48]. Equal protein loading was confirmed by reprobing membranes using a δ-PKC antibody that recognizes phosphorylated and nonphosphorylated forms of δ-PKC (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Chemokine measurements
Levels of the chemokines CINC-1 (R&D Systems, Minneapolis, MN, USA) and MIP-2 (BioSource, Camarillo, CA, USA) in BALF and plasma were measured by ELISA, according to the manufacturer's instructions. The lower limit of detection was 1 pg/ml for rat CINC-1 and MIP-2.
Lung histology, morphology, and morphometry
Lungs were inflated and fixed overnight in 10% neutral-buffered formalin. The lungs were then coded and lung sectioning performed in a blinded manner by the Pathology Core at Children's Hospital of Philadelphia. The lung sections were paraffin-embedded, cut into 5 μm sections, and stained with H&E. Morphometric analysis was then performed by a second independent, blinded observer. Sections were evaluated for alterations consistent with ARDS (inflammatory cell infiltration, septal thickening, and protein and fluid accumulation in the interstitial and alveolar spaces). Quantitative histomorphometry was performed using computer-assisted image analyses (Image-Pro Plus, Media Cybernetics, Bethesda, MD, USA). Random fields (n=6) from each section (n=3 sections/animal) were digitally imaged at 100× to morphometrically differentiate the proportion of VG from the proportion of VP. These data were used to calculate the lung expansion index (VG/VP) [49–52]. Analysis of sections for degree of cellularity, defined as the number of nuclei/fixed area-digitized field, was performed by digitally imaging (400×) the same number of random fields from each section. Computer-assisted image analysis was adjusted to detect only blue-stained nuclei. Using this technique, we were able to count the number of blue-stained nuclei/unit area in each of the fixed, area-digitized fields.
Statistical analysis
Results are expressed as means ± se for number (n) of studies performed. Data were analyzed by Student's t test for two group comparisons or ANOVA, followed for multiple comparisons with the Bonferroni test for post hoc significance. Differences were considered significant when P < 0.05.
RESULTS
Intratracheal administration of the δ-PKC TAT peptide inhibitor blocks 2CLP-mediated phosphorylation of δ-PKC (Thr505) in the lung
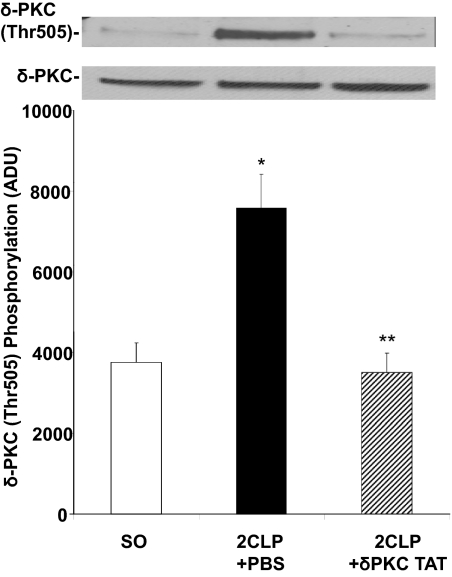
Activation of δ-PKC is a multistep process that can be assessed by quantifying phosphorylation of Thr505 in the δ-PKC activation loop [26, 48, 53]. As shown in Fig. 1, there is little phosphorylation of δ-PKC in lung tissue homogenates obtained from rats 24 h post-SO. In contrast, 24 h after 2CLP and treatment with PBS vehicle (2CLP+PBS), we observed significant increases in δ-PKC phosphorylation. Relative to 2CLP + PBS-treated rats, there was significantly less δ-PKC (Thr505) phosphorylation in rats subjected to 2CLP and treated with the δ-PKC TAT inhibitory peptide (2CLP+δ-PKC TAT). Thus, intratracheal administration of the δ-PKC TAT inhibitory peptide was able to limit 2CLP-induced phosphorylation of δ-PKC in the lung.
Figure 1. Intratracheal administration of the δ-PKC TAT peptide inhibitor blocks 2CLP-mediated phosphorylation of δ-PKC (Thr505) in the lung.
Lung tissue was harvested 24 h post-SO or 2CLP. δ-PKC (Thr505) phosphorylation was determined by Western blot analysis using a phospho-specific δ-PKC (Thr505) antibody. Equal protein loading was determined by Western blot analysis for total δ-PKC. (Upper) Representative Western blots. (Lower) Densitometry analysis of δ-PKC (Thr505) phosphorylation. Values are expressed in arbitrary densitometry units (ADU); n = 8 (mean±se); *P < 0.01 SO versus 2CLP + PBS and **P < 0.01 2CLP + PBS versus 2CLP + δ-PKC TAT.
Intratracheal administration of the δ-PKC TAT peptide inhibitor decreases 2CLP-mediated elevation of the chemokines CINC-1 and MIP-2
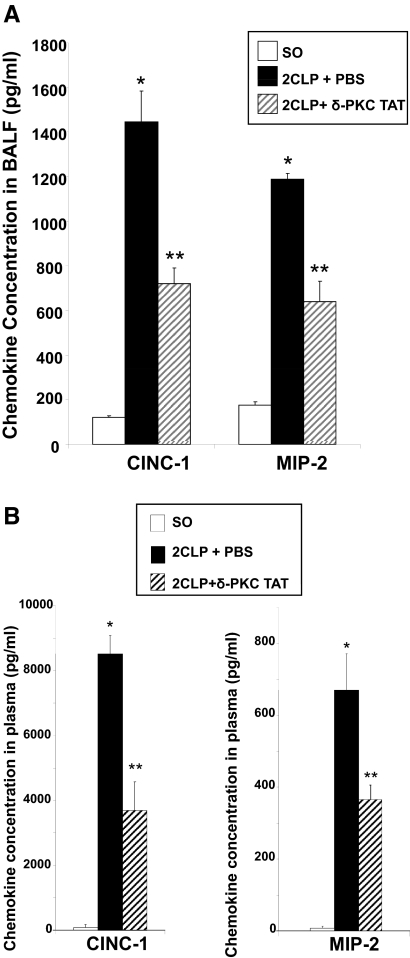
Migration of leukocytes into the lung and subsequent activation are dependent on proinflammatory cytokines and chemokines. To investigate the effects of targeted δ-PKC inhibition, we determined chemokine levels in BALF and plasma. Data comparing chemokine levels obtained 24 h after SO, 2CLP + PBS, and 2CLP + δ-PKC TAT are presented in Fig. 2. In rats treated with PBS vehicle following 2CLP surgery (2CLP+PBS), the BALF concentration of CINC-1 was significantly (tenfold) higher than that for SO rats (Fig. 2A). A similar, statistically significant (fivefold) difference in BALF MIP-2 levels was noted (Fig. 2A). There were no significant differences in BALF CINC-1 and MIP-2 levels in SO rats as compared with unoperated control (T0) rats (CINC-1: 119±9 pg/ml SO vs. 128±23 pg/ml T0; MIP-2: 187±14 pg/ml SO vs. 200±30 pg/ml T0; P=NS; n=8). These results are consistent with our previous studies demonstrating that relative to controls, 2CLP increased CINC-1 expression in the rat lung [20]. In 2CLP rats treated with the δ-PKC TAT inhibitory peptide (2CLP+δ-PKC TAT), BALF levels of CINC-1 and MIP-2 were ∼50% lower than the PBS-treated 2CLP cohort, indicating that the δ-PKC TAT inhibitor produced significant reductions in the levels of these chemokine levels in the lung (Fig. 2A).
Figure 2. Intratracheal administration of the δ-PKC TAT peptide inhibitor decreases 2CLP-mediated elevations of the chemokines CINC-1 and MIP-2 in the lung and plasma.
(A) BALF was collected by instilling and withdrawing 1.5 ml sterile PBS three times from the lungs via an intratracheal cannula (24 h post-2CLP or SO). CINC-1 and MIP-2 levels are expressed as mean ± se (pg/ml; n=8–14). *P < 0.01 SO versus 2CLP + PBS; **P < 0.01 2CLP + PBS versus 2CLP + δ-PKC TAT. (B) Plasma CINC-1 and MIP-2 levels are expressed as mean ± se (n=8–14). *P < 0.01 SO versus 2CLP + PBS; **P < 0.01 2CLP + PBS versus 2CLP + δ-PKC TAT.
We found a similar pattern when measuring CINC-1 and MIP-2 in plasma. Levels of CINC-1 and MIP-2 were barely detectable 24 h after SO (Fig. 2B). By comparison, plasma levels of CINC-1 and MIP-2 were significantly higher in 2CLP rats treated with PBS vehicle than those following SO. As was the case with BALF, circulating levels of CINC-1 and MIP-2 in 2CLP rats treated with the δ-PKC TAT inhibitor were ∼50% lower than those observed in the PBS-treated 2CLP cohort (Fig. 2B). Thus, targeted delivery of the δ-PKC TAT peptide inhibitor to the lung decreased 2CLP-induced local (BALF) and systemic (plasma) expression and/or release of CINC-1 and MIP-2.
Intratracheal administration of the δ-PKC TAT peptide inhibitor attenuates 2CLP-induced lung injury 24 h following 2CLP
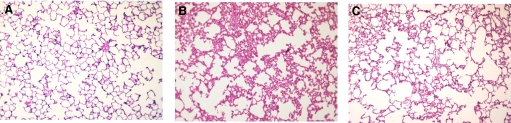
Representative lung micrographs for each animal group are shown in Fig. 3. H&E staining demonstrated normal lung architecture in rats following SO (Fig. 3A). In contrast, there are significant alterations in lung histology 24 h post-2CLP (Fig. 3B). H&E staining revealed septal thickening, increased cellularity, a proteinacious exudate, and an inflammatory infiltrate, all features that are present in clinical ARDS [20, 40, 44]. Each of these changes was attenuated in septic rats treated with the δ-PKC TAT inhibitory peptide (Fig. 3C). Thus, treatment of 2CLP with the δ-PKC TAT inhibitory peptide limited the development of histologic changes consistent with ARDS.
Figure 3. Intratracheal administration of δ-PKC TAT peptide inhibitor decreases lung injury 24 h following 2CLP.
H&E staining of lung sections obtained from rats 24 h post-SO or 2CLP. Representative histomicrographs of lung sections of (A) SO; (B) 2CLP + intratracheal administration of PBS (vehicle); and (C) 2CLP + intratracheal administration of δ-PKC TAT peptide inhibitor. Original magnification, ×100.
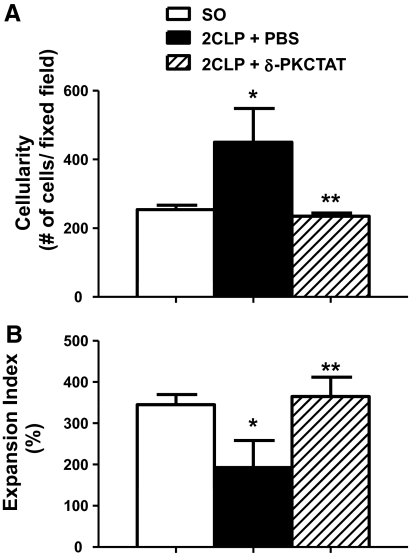
Morphometric analysis was used to quantify the effects of 2CLP + PBS and 2CLP + δ-PKC TAT on the cellular composition of lung tissue samples. Use of this technique revealed that relative to SO, 2CLP rats treated with PBS vehicle had substantially increased numbers of neutrophils and monocyte/macrophages in the alveolar and interstitial spaces (Fig. 4A). Treatment with the δ-PKC TAT inhibitory peptide (2CLP+δ-PKC TAT) significantly reduced the influx of inflammatory leukocytes into the lungs of 2CLP rats (Fig. 4A). 2CLP + PBS also significantly decreased the lung expansion index VG/VP relative to SO (Fig. 4B). This change was attenuated significantly by treatment with the δ-PKC TAT inhibitory peptide. These results demonstrate improved lung stability and area for gas exchange. Thus, treatment of 2CLP animals with the δ-PKC TAT inhibitory peptide limited the development of morphometric changes that characterize ARDS.
Figure 4. Quantitative image analysis of lung sections.
(A) Intratracheal administration of δ-PKC TAT peptide inhibitor decreases sepsis-induced lung cellularity as a reflection of inflammatory cell influx into the lung 24 h following 2CLP; n = 6/group (mean±se); *P < 0.01 SO versus 2CLP + PBS and **P < 0.01 2CLP + PBS versus 2CLP + δ-PKC TAT. (B) Intratracheal administration of δ-PKC TAT peptide inhibitor increases the lung expansion index in septic rats 24 h following 2CLP; n = 6/group; mean ± se; *P < 0.01 SO versus 2CLP + PBS and **P < 0.01 2CLP + PBS versus 2CLP + δ-PKC TAT.
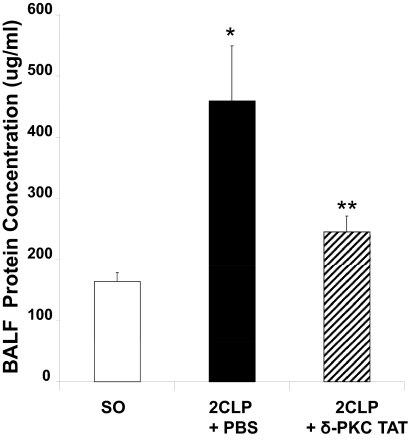
Total protein concentration in BALF was used as an index of lung permeability and lung injury. We found that 24 h following 2CLP (2CLP+PBS), total BALF protein content increased over 2.5-fold as compared with SO rats (Fig. 5). Treatment with δ-PKC TAT inhibitory peptide significantly reduced the protein levels in BALF as compared with 2CLP rats treated with PBS vehicle. Thus, treatment of 2CLP with the δ-PKC TAT inhibitory peptide limited the development of lung injury.
Figure 5. Intratracheal administration of δ-PKC TAT peptide inhibitor decreases total protein concentrations in BALF 24 h following 2CLP.
BALFs were collected 24 h post-2CLP and total protein concentrations determined. Values are mean ± se (μg/ml); n = 8; *P < 0.01 SO versus 2CLP + PBS; **P < 0.02 2CLP + PBS versus 2CLP + δ-PKC TAT.
DISCUSSION
The results of the present study demonstrate that δ-PKC plays an important regulatory role in sepsis-induced lung injury in a rat model of ARDS. We found that 2CLP, which has been shown to produce histological and functional changes in rats that are consistent with ARDS [40, 44], also activated pulmonary δ-PKC. Intratracheal delivery of a single dose of a δ-PKC TAT inhibitory peptide had a dramatic effect in this model of ARDS. The inhibitory peptide decreased δ-PKC phosphorylation (a step in δ-PKC activation), reduced pulmonary and circulating chemokine levels, limited inflammatory cell influx, attenuated capillary leak and the development of pulmonary edema, and overall, exerted a protective effect on the lung. To our knowledge, no prior studies have examined the effect of directed pulmonary inhibition of δ-PKC in a preclinical model of ARDS. These findings provide important insight into the molecular mechanisms involved in the pathogenesis of lung injury associated with sepsis.
Infiltration of leukocytes into the lung is a key characteristic of ARDS and is believed to contribute to the pathophysiology. The mechanisms that trigger this infiltration are poorly understood. Knowledge is particularly limited regarding lung injury secondary to intra-abdominal sepsis. It is generally accepted that pulmonary endothelial cells and resident macrophages produce chemokines such as CINC-1 and MIP-2 in rats or IL-8 in humans. These are essential for the recruitment of neutrophils into the pulmonary vascular, interstitial, and alveolar spaces, locations where neutrophils are seldom found [7, 54]. CINC-1 and MIP-2 [6, 19, 21] are critical regulators of neutrophil recruitment and activation following the polymicrobial insult induced by CLP [14, 20]. This role may be particularly important in sepsis-induced ARDS, as in contrast to injury in other organ systems, free radicals and proteolytic enzymes derived from neutrophils are believed to contribute substantially to the pulmonary damage. In the present study, targeted delivery of the δ-PKC TAT inhibitory peptide significantly decreased the levels of CINC-1 and MIP-2 in the lung, and this inhibition limited the characteristic pulmonary, inflammatory infiltrate. This change, in turn, attenuated a pulmonary capillary leak, interstitial edema, and atelectasis. Thus, the δ-PKC TAT inhibitory peptide appears to have interfered with a chain of events that culminates in experimental lung injury and ARDS.
The mechanism by which δ-PKC inhibition attenuates pulmonary CINC-1 and MIP-2 levels may be at the level of chemokine synthesis. Chemokine expression in the lungs is controlled largely by NF-κB activation in alveolar macrophages, pulmonary endothelial cells, and perhaps pneumocytes themselves. Previous studies demonstrated that inhibition of NF-κB activation by the heat shock protein 70 protected against 2CLP-mediated lung injury [20]. δ-PKC regulates NF-κB activity in pulmonary epithelial, endothelial, and other cell types [21, 22, 24, 28, 29, 55, 56]. NF-κB activation is controlled by IKK. Once activated, IKK phosphorylates the NF-κB inhibitor IκB, leading to its ubiquitination and rapid degradation [57]. The degradation of IκB releases NF-κB for translocation to the nucleus and activation of transcription of target genes. δ-PKC is an important regulator of IKK activation, and inhibition of δ-PKC blocked phosphorylation of IκB, translocation of the p65 NF-κB subunit to the nucleus, and formation of a DNA-binding complex [22, 24, 28, 29, 38, 55]. In human neutrophils, δ-PKC was a positive regulator of TNF-mediated assembly of TNFR1–TNFR type 1-associated death domain–receptor-interacting protein–TRAF2, a signaling complex required for IKK activation [24]. Moreover, a recent study by Puneet et al. [38] demonstrated a role for δ-PKC in the regulation of NF-κB in macrophages from septic patients. This study also identified δ-PKC as an important downstream component of the SphK1 pathway activated by TLR4/TLR2. Inhibition of SphK1 (and subsequently, δ-PKC) decreased lethality in a mouse model of sepsis (CLP). δ-PKC also regulates expression of the adhesion molecules ICAM-1 and VCAM-1, and δ-PKC inhibition prevents neutrophil adherence and migration in cell culture and animal models of lung injury [21, 28–30]. PKC-δ−/− mice had reduced cytokine expression and decreased infiltration of neutrophils and macrophages in BAL and lung tissue in response to pulmonary inflammation triggered by asbestos [58]. Thus, inhibition of δ-PKC could prevent neutrophil influx into the lungs by attenuating activation of proinflammatory cellular signaling pathways that regulate chemokine, cytokine, and adhesion molecule expression.
Intratracheal administration of the δ-PKC TAT peptide inhibitor also decreased circulating levels of CINC-1 and MIP-2, suggesting a reduction in systemic inflammation. Polymicrobial sepsis induces a severe systemic inflammatory response that leads to activation of immune cells in multiple organ compartments and the synthesis and release of pro- and anti-inflammatory cytokines. Plasma chemokine levels are a reflection of chemokine synthesis from multiple sources. The lung and liver are considered major contributors to the circulating levels of cytokines and chemokines during the development of systemic inflammation [59, 60]. In the lung, it has been hypothesized that inflammatory mediators generated by the lung cross the injured lung barrier, enter the circulation, and exacerbate the systemic inflammatory response. The decrease in systemic chemokines could reflect local pulmonary action of the δ-PKC TAT inhibitor, resulting in preservation of the alveolar capillary barrier and decreased release of pulmonary-derived proinflammatory mediators into the circulation. The histological profiles are consistent with preserved parenchymal and alveolar-capillary interface. Nonpharmacologic methods, such as mechanical lung-protective ventilation strategies, have produced similar results [61, 62]. Alternatively, the 2CLP-induced disruption of the alveolar capillary barrier may promote the release of the δ-PKC TAT inhibitor into the circulation, where it acts on different organ systems. Biodistribution studies of the δ-PKC TAT peptide by other investigators demonstrated that i.p. injection of this inhibitor resulted in significant inhibition of δ-PKC in multiple organs, including lung, liver, kidney, and heart [33]. Future studies will evaluate the biodistribution of this δ-PKC TAT inhibitory peptide outside of the lung compartment following intratracheal administration. These studies will also establish whether higher doses of the δ-PKC TAT inhibitor will decrease pulmonary and systemic cytokine/chemokine levels further and determine whether there are redundant or overlapping signaling pathways involved in this inflammatory response. Based on our experience with adenoviral-targeted gene delivery to the lung and on other investigators′ experience with intratracheal delivery of small interfering RNA, oligonucleotides, and antibodies, we would expect that organ distribution would be dose-dependent with selective targeting to the lungs at the lower peptide doses [44, 63, 64].
Targeting δ-PKC activity in the lung offers a unique, therapeutic strategy. There is a lag time between nonpulmonary injury and the influx of neutrophils into the lung compartment and the initiation of lung damage. This lag period of between 6 and 48 h, depending on type of injury and severity of injury, offers a therapeutic window where treatment could be initiated, which would stop the progression to neutrophil-mediated lung injury. The δ-PKC TAT peptide inhibitor is currently being tested for the treatment of acute myocardial infarction. A phase I/II clinical trial to test safety and efficacy was completed successfully, and no adverse events were reported following intracoronary injections of the δ-PKC TAT peptide inhibitor in patients undergoing percutaneous coronary intervention [65]. Thus, clinical administration of this peptide appears to be safe. Although our study suggests that inhibition of δ-PKC may offer an attractive therapeutic option for the treatment of ARDS, further details are required. The present study demonstrated a significant, δ-PKC TAT inhibitory peptide-mediated lung-protective effect at 24 h following 2CLP. This time-point was selected based on our previous studies with this animal model, where there were significant alterations in pulmonary histologic and functional changes consistent with ARDS [20, 40, 44]. Further studies are required to establish dose-response curves, to determine the effects of δ-PKC inhibition on the response to 2CLP at time-points later than 24 h, and most importantly, to establish the therapeutic window by determining the effect of targeted delivery of the δ-PKC TAT inhibitory peptide when administered at different times following 2CLP. Nonetheless, the results of the present study indicate that targeted delivery of the δ-PKC TAT inhibitory peptide limits leukocyte-mediated lung injury and may offer a novel, therapeutic approach to ARDS.
ACKNOWLEDGMENTS
This work was supported in part by National Institute of General Medical Sciences grants 2R56GM064552 (L.E.K.) and 2R01GM059930 (C.S.D.), University of Pennsylvania Research Foundation grant (L.E.K., H.M.K., and C.S.D.), and the Stavropoulos Sepsis Research Program (C.S.D.).
Footnotes
- 2CLP
- cecal ligation and double puncture
- ARDS
- acute respiratory distress syndrome
- BALF
- BAL fluid
- SO
- SHAM operation
- SphK1
- sphingosine kinase 1
- Thr505
- threonine 505
- VG
- gas exchange space per unit area
- VP
- lung parenchyma per unit area
AUTHORSHIP
S.W.S., H.L., and N.R.R. performed the experiments and analyzed and assembled data. M.R.W conceived of, performed, and interpreted the morphometric studies. L.E.K., H.M.K., and C.S.D. contributed to study conception, experimental design, and data analysis. L.E.K., H.M.K., M.R.W., and C.S.D. wrote the manuscript.
REFERENCES
- 1. Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 2. Fein A. M., Calalang-Colucci M. G. (2000) Acute lung injury and acute respiratory distress syndrome in sepsis and septic shock. Crit. Care Clin. 16, 289–317 [DOI] [PubMed] [Google Scholar]
- 3. Wheeler A. P., Bernard G. R. (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369, 1553–1564 [DOI] [PubMed] [Google Scholar]
- 4. Steinberg K. P., Hudson L. D., Goodman R. B., Hough C. L., Lanken P. N., Hyzy R., Thompson B. T., Ancukiewicz M. National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 354, 1671–1684 [DOI] [PubMed] [Google Scholar]
- 5. The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 342, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 6. Reutershan J., Ley K. (2004) Bench-to-bedside review: acute respiratory distress syndrome—how neutrophils migrate into the lung. Crit. Care 8, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ware L. B., Matthay M. A. (2000) The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 8. Phua J., Badia J. R., Adhikari N. K. J., Friedrich J. O., Fowler R. A., Singh J. M., Scales D. C., Stather D. R., Li A., Jones A., Gattas D. J., Hallett D., Tomlinson G., Stewart T. E., Ferguson N. D. (2009) Has mortality from acute respiratory distress syndrome decreased over time?: a systematic review. Am. J. Respir. Crit. Care Med. 179, 220–227 [DOI] [PubMed] [Google Scholar]
- 9. Lee W. L., Downey G. P. (2001) Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care 7, 1–7 [DOI] [PubMed] [Google Scholar]
- 10. Goodman R. B., Pugin J., Lee J. S., Matthay M. A. (2003) Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 14, 523–535 [DOI] [PubMed] [Google Scholar]
- 11. Aldridge A. J. (2002) Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur. J. Surg. 168, 204–214 [DOI] [PubMed] [Google Scholar]
- 12. Abraham E. (2003) Neutrophils and acute lung injury. Crit. Care Med. 31, S195–S199 [DOI] [PubMed] [Google Scholar]
- 13. Coldren C. D., Nick J. A., Poch K. R., Woolum M. D., Fouty B. W., O'Brien J. M., Gruber M. P., Zamora M. R., Svetkauskaite D., Richter D. A., He Q., Park J. S., Overdier K. H., Abraham E., Geraci M. W. (2006) Functional and genomic changes induced by alveolar transmigration in human neutrophils. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L1267–L1276 [DOI] [PubMed] [Google Scholar]
- 14. Guo R. F., Riedemann N. C., Sun L., Gao H., Shi K. X., Reuben J. S., Sarma V. J., Zetoune F. S., Ward P. A. (2006) Divergent signaling pathways in phagocytic cells during sepsis. J. Immunol. 177, 1306–1313 [DOI] [PubMed] [Google Scholar]
- 15. Donnelly S. C., Haslett C., Strieter R. M., Kunkel S. L., Walz A., Robertson C. R., Carter D. C., Pollok A. J., Grant I. S. (1993) Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341, 643–647 [DOI] [PubMed] [Google Scholar]
- 16. Miller E. J., Cohen A. B., Nagao S., Griffith D., Maunder R. J., Martin T. R., Weiner-Kronish J. P., Sticherling M., Christophers E., Matthay M. A. (1992) Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am. Rev. Respir. Dis. 146, 427–432 [DOI] [PubMed] [Google Scholar]
- 17. Goodman R. B., Strieter R. M., Martin D. P., Steinberg K. P., Milberg J. A., Maunder R. J., Kunkel S. L., Walz A., Hudson L. D., Martin T. R. (1996) Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 154, 602–611 [DOI] [PubMed] [Google Scholar]
- 18. Villard J., Dayer-Pastore F., Hamacher J., Aubert J. D., Schlegel-Haueter S., Nicod L. P. (1995) GRO α and interleukin-8 in Pneumocystis carinii or bacterial pneumonia and adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 152, 1549–1554 [DOI] [PubMed] [Google Scholar]
- 19. Guo R. F., Ward P. A. (2002) Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic. Biol. Med. 33, 303–310 [DOI] [PubMed] [Google Scholar]
- 20. Weiss Y. G., Bromberg Z., Raj N., Raphael J., Goloubinoff P., Ben-Neriah Y., Deutschman C. S. (2007) Enhanced Hsp70 expression alters IκB kinase proteasomal degradation in experimental ARDS. Crit. Care Med. 35, 2128–2138 [DOI] [PubMed] [Google Scholar]
- 21. Page K., Li J., Zhou L., Iasvovskaia S., Corbit K. C., Soh J. W., Weinstein I. B., Brasier A. R., Lin A., Hershenson M. B. (2003) Regulation of airway epithelial cell NF-κ B-dependent gene expression by protein kinase C δ. J. Immunol. 170, 5681–5689 [DOI] [PubMed] [Google Scholar]
- 22. Kilpatrick L. E., Lee J. Y., Haines K. M., Campbell D. E., Sullivan K. E., Korchak H. M. (2002) A role for PKC-δ and PI 3-kinase in TNF-α-mediated antiapoptotic signaling in the human neutrophil. Am. J. Physiol. Cell Physiol. 283, C48–C57 [DOI] [PubMed] [Google Scholar]
- 23. Wang K., Scheel-Toellner D., Wong S. H., Craddock R., Caamano J., Akbar A. N., Salmon M., Lord J. M. (2003) Inhibition of neutrophil apoptosis by type 1 IFN depends on cross-talk between phosphoinositol 3-kinase, protein kinase C-δ, and NF-κ B signaling pathways. J. Immunol. 171, 1035–1041 [DOI] [PubMed] [Google Scholar]
- 24. Kilpatrick L. E., Sun S., Korchak H. M. (2004) Selective regulation by δ-PKC and PI 3-kinase in the assembly of the antiapoptotic TNFR-1 signaling complex in neutrophils. Am. J. Physiol. Cell Physiol. 287, C633–C642 [DOI] [PubMed] [Google Scholar]
- 25. Kilpatrick L. E., Sun S., Mackie D., Baik F., Li H., Korchak H. M. (2006) Regulation of TNF mediated antiapoptotic signaling in human neutrophils: role of {δ}-PKC and ERK1/2. J. Leukoc. Biol. 80, 1512–1521 [DOI] [PubMed] [Google Scholar]
- 26. Kilpatrick L. E., Sun S., Li H., Vary T. C., Korchak H. M. (2010) Regulation of TNF-induced oxygen radical production in human neutrophils: role of {δ}-PKC. J. Leukoc. Biol. 87, 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chakrabarti S., Zee J. M., Patel K. D. (2006) Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J. Leukoc. Biol. 79, 214–222 [DOI] [PubMed] [Google Scholar]
- 28. Cummings R., Zhao Y., Jacoby D., Spannhake E. W., Ohba M., Garcia J. G., Watkins T., He D., Saatian B., Natarajan V. (2004) Protein kinase Cδ mediates lysophosphatidic acid-induced NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. J. Biol. Chem. 279, 41085–41094 [DOI] [PubMed] [Google Scholar]
- 29. Rahman A., Anwar K. N., Uddin S., Xu N., Ye R. D., Platanias L. C., Malik A. B. (2001) Protein kinase C-δ regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen-activated protein kinase. Mol. Cell. Biol. 21, 5554–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo C. H., Lim J. H., Kim J. H. (2005) VCAM-1 upregulation via PKCδ-p38 kinase-linked cascade mediates the TNF-α-induced leukocyte adhesion and emigration in the lung airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L307–L316 [DOI] [PubMed] [Google Scholar]
- 31. Chou W. H., Choi D. S., Zhang H., Mu D., McMahon T., Kharazia V. N., Lowell C. A., Ferriero D. M., Messing R. O. (2004) Neutrophil protein kinase Cδ as a mediator of stroke-reperfusion injury. J. Clin. Invest. 114, 49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L., Hahn H., Wu G., Chen C. H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G. W., II, Mochly-Rosen D. (2001) Opposing cardioprotective actions and parallel hypertrophic effects of δ PKC and ε PKC. Proc. Natl. Acad. Sci. USA 98, 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Begley R., Liron T., Baryza J., Mochly-Rosen D. (2004) Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem. Biophys. Res. Commun. 318, 949–954 [DOI] [PubMed] [Google Scholar]
- 34. Bright R., Steinberg G. K., Mochly-Rosen D. (2007) δPKC mediates microcerebrovascular dysfunction in acute ischemia and in chronic hypertensive stress in vivo. Brain Res. 1144, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ikeno F., Inagaki K., Rezaee M., Mochly-Rosen D. (2007) Impaired perfusion after myocardial infarction is due to reperfusion-induced δPKC-mediated myocardial damage. Cardiovasc. Res. 73, 699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monti M., Donnini S., Giachetti A., Mochly-Rosen D., Ziche M. (2010) δPKC inhibition or εPKC activation repairs endothelial vascular dysfunction by regulating eNOS post-translational modification. J. Mol. Cell. Cardiol. 48, 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kilpatrick L., Standage S., Li H., Raj N., Korchak H. M., Deutschman C. S. (2009) A novel molecular therapeutic target for the treatment of ARDS. Shock 31, 62 [Google Scholar]
- 38. Puneet P., Yap C. T., Wong L., Yulin L., Koh D. R., Moochhala S., Pfeilschifter J., Huwiler A., Melendez A. J. (2010) SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328, 1290–1294 [DOI] [PubMed] [Google Scholar]
- 39. Vancurova I., Miskolci V., Davidson D. (2001) NF-κ B activation in tumor necrosis factor α-stimulated neutrophils is mediated by protein kinase Cδ. Correlation to nuclear Iκ Bα. J. Biol. Chem. 276, 19746–19752 [DOI] [PubMed] [Google Scholar]
- 40. Weiss Y. G., Maloyan A., Tazelaar J., Raj N., Deutschman C. S. (2002) Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J. Clin. Invest. 110, 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bromberg Z., Raj N., Goloubinoff P., Deutschman C. S., Weiss Y. G. (2008) Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome. Crit. Care Med. 36, 246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abcejo A. S., Andrejko K. M., Raj N. R., Deutschman C. S. (2009) Failed interleukin-6 signal transduction in murine sepsis: attenuation of hepatic glycoprotein 130 phosphorylation. Crit. Care Med. 37, 1729–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qi X., Inagaki K., Sobel R. A., Mochly-Rosen D. (2008) Sustained pharmacological inhibition of δPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J. Clin. Invest. 118, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiss Y. G., Tazelaar J., Gehan B. A., Bouwman A., Christofidou-Solomidou M., Yu Q. C., Raj N., Deutschman C. S. (2001) Adenoviral vector transfection into the pulmonary epithelium after cecal ligation and puncture in rats. Anesthesiology 95, 974–982 [DOI] [PubMed] [Google Scholar]
- 45. Venet F., Huang X., Chung C-S., Chen Y., Ayala A. (2010) Plasmacytoid dendritic cells control lung inflammation and monocyte recruitment in indirect acute lung injury in mice. Am. J. Pathol. 176, 764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bright R., Raval A. P., Dembner J. M., Perez-Pinzon M. A., Steinberg G. K., Yenari M. A., Mochly-Rosen D. (2004) Protein kinase C δ mediates cerebral reperfusion injury in vivo. J. Neurosci. 24, 6880–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Inagaki K., Hahn H. S., Dorn G. W., II, Mochly-Rosen D. (2003) Additive protection of the ischemic heart ex vivo by combined treatment with δ-protein kinase C inhibitor and ε-protein kinase C activator. Circulation 108, 869–875 [DOI] [PubMed] [Google Scholar]
- 48. Vary T. C., Goodman S., Kilpatrick L. E., Lynch C. J. (2005) Nutrient regulation of PKCε is mediated by leucine, not insulin, in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 289, E684–E694 [DOI] [PubMed] [Google Scholar]
- 49. Deoras K. S., Wolfson M. R., Searls R. L., Hilfer S. R., Sheffield J. B., Shaffer T. H. (1990) Use of a touch sensitive screen and computer assisted image analysis for quantitation of developmental changes in pulmonary structure. Pediatr. Pulmonol. 9, 109–118 [DOI] [PubMed] [Google Scholar]
- 50. Miller T. L., Shashikant B. N., Pilon A. L., Pierce R. A., Shaffer T. H., Wolfson M. R. (2006) Effects of an intratracheally delivered anti-inflammatory protein (rhCC10) on physiological and lung structural indices in a juvenile model of acute lung injury. Biol. Neonate 89, 159–170 [DOI] [PubMed] [Google Scholar]
- 51. Wolfson M. R., Greenspan J. S., Deoras K. S., Rubenstein S. D., Shaffer T. H. (1992) Comparison of gas and liquid ventilation: clinical, physiological, and histological correlates. J. Appl. Physiol. 72, 1024–1031 [DOI] [PubMed] [Google Scholar]
- 52. Wolfson M. R., Kechner N. E., Roache R. F., Dechadarevian J-P., Friss H. E., Rubenstein S. D., Shaffer T. H. (1998) Perfluorochemical rescue after surfactant treatment: effect of perflubron dose and ventilatory frequency. J. Appl. Physiol. 84, 624–640 [DOI] [PubMed] [Google Scholar]
- 53. Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 54. Downey G. P., Dong Q., Kruger J., Dedhar S., Cherapanov V. (1999) Regulation of neutrophil activation in acute lung injury. Chest 116, 46S–54S [PubMed] [Google Scholar]
- 55. Minami T., Abid M. R., Zhang J., King G., Kodama T., Aird W. C. (2003) Thrombin stimulation of vascular adhesion molecule-1 in endothelial cells is mediated by protein kinase C (PKC)-δ-NF-κB and PKC-ζ-GATA signaling pathways. J. Biol. Chem. 278, 6976–6984 [DOI] [PubMed] [Google Scholar]
- 56. Lu Z-G., Liu H., Yamaguchi T., Miki Y., Yoshida K. (2009) Protein kinase Cδ activates RelA/p65 and nuclear factor-κB signaling in response to tumor necrosis factor-α. Cancer Res. 69, 5927–5935 [DOI] [PubMed] [Google Scholar]
- 57. Karin M., Ben-Neriah Y. (2000) Phosphorylation meets ubiquitination: the control of NF-[κ]B activity. Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 58. Shukla A., Lounsbury K. M., Barrett T. F., Gell J., Rincon M., Butnor K. J., Taatjes D. J., Davis G. S., Vacek P., Nakayama K. I., Steele C., Mossman B. T. (2007) Asbestos-induced peribronchiolar cell proliferation and cytokine production are attenuated in lungs of protein kinase C-δ knockout mice. Am. J. Pathol. 170, 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puneet P., Moochhala S., Bhatia M. (2005) Chemokines in acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L3–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mercer-Jones M. A., Shrotri M. S., Peyton J. C., Remick D. G., Cheadle W. G. (1999) Neutrophil sequestration in liver and lung is differentially regulated by C-X-C chemokines during experimental peritonitis. Inflammation 23, 305–319 [DOI] [PubMed] [Google Scholar]
- 61. Wolfson M. R., Hirschl R. B., Jackson J. C., Gauvin F., Foley D. S., Lamm W. J. E., Gaughan J., Shaffer T. H. (2008) Multicenter comparative study of conventional mechanical gas ventilation to tidal liquid ventilation in oleic acid injured sheep. ASAIO J. 54, 256–269 [DOI] [PubMed] [Google Scholar]
- 62. Stüber F., Wrigge H., Schroeder S., Wetegrove S., Zinserling J., Hoeft A., Putensen C. (2002) Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med. 28, 834–841 [DOI] [PubMed] [Google Scholar]
- 63. Perl M., Chung C-S., Lomas-Neira J., Rachel T-M., Biffl W. L., Cioffi W. G., Ayala A. (2005) Silencing of Fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am. J. Pathol. 167, 1545–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Danahay H., Giddings J., Christian R. A., Moser H. E., Phillips J. A. (1999) Distribution of a 20-mer phosphorothioate oligonucleotide, CGP69846A (ISIS 5132), into airway leukocytes and epithelial cells following intratracheal delivery to Brown-Norway rats. Pharm. Res. 16, 1542–1549 [DOI] [PubMed] [Google Scholar]
- 65. Direct Inhibition of δ-Protein Kinase C Enzyme to Limit Total Infarct Size in Acute Myocardial Infarction (δ MI) Investigators, Bates E., Bode C., Costa M., Gibson C.M., Granger C., Green C., Grimes K., Harrington R., Huber K., Kleiman N., Mochly-Rosen D., Roe M., Sadowski Z., Solomon S., Widimsky P. (2008) Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation 117, 886–896 [DOI] [PubMed] [Google Scholar]