Review expands the argument that LAB is a multi-faceted protein, which can regulate signaling in nearly all leukocytes.
Keywords: LAT, PLCγ, Grb2, PI3K
Abstract
LAB/NTAL/Lat2 is a transmembrane adaptor protein closely related to LAT. It is expressed in various myeloid and lymphoid cells, many of which also express LAT. Phosphorylation of LAB occurs following engagement of various ITAM- and non-ITAM-linked receptors and can play positive and negative roles following receptor engagement. LAT binds PLCγ directly, resulting in efficient Ca2+ flux and degranulation. However, LAB does not contain a PLCγ-binding motif and only binds PLCγ indirectly, possibly via Grb2, thereby resulting in suboptimal signaling. As LAT can signal more efficiently than LAB, competition between the 2 for space/substrates in the lipid rafts can attenuate signaling. This competition model requires coexpression of LAT; however, LAB is repressive, even in cells lacking substantial LAT expression such as macrophages and mature B cells. The reported interaction between LAB and the ubiquitin E3-ligase c-Cbl suggests 1 possible mechanism for LAT-independent inhibition by LAB, but such a model requires further investigation. Given the wide-reaching expression pattern of LAB, LAB has the ability to modulate signaling in virtually every type of leukocyte. Regardless of its ultimate mode of action, the potent regulatory capability of LAB proves this protein to be a complex adaptor that warrants continued, substantial scrutiny by biochemists and immunologists alike.
Introduction
Tyrosine phosphorylation is a critical, receptor-proximal event in the signaling cascades of many receptors involved in the regulation of immune cell development and function. Many key regulatory receptors in immunity are armed with signaling chains possessing ITAMs [1]. Upon engagement of the associated receptor, ITAMs are tyrosine-phosphorylated and recruit SH2 containing protein tyrosine kinases, including those of the Syk/Zap70 family. Recruitment and activation of Syk or Zap70 by ITAMs facilitate the tyrosine phosphorylation of additional downstream substrates amplifying and diversifying the signal. The effective coordination, amplification, and ultimately, translation of ITAM-initiated, tyrosine-based signaling to a variety of other enzymatic pathways, including serine/threonine kinases, phospholipases, and transcriptional pathways, require adaptor proteins [2, 3]. Nonenzymatic adaptor proteins are often composed of a variety of protein–protein interaction domains, including SH2, SH3, PH, and postsynaptic density protein-Drosophila disc large tumor suppressor-zonula occludens-1 protein (PDZ), interspersed with motifs bound by these domains, such as tyrosine-phosphorylation sites or proline-rich segments [4]. Using these various interaction mechanisms, adaptors facilitate signaling by bringing enzymes into proximity with cofactors and/or their substrates. The LAT and LAB/NTAL/Lat2, hereafter referred to as LAB, are 2 closely related transmembrane adaptor proteins [5], which are found in lipid rafts and are tyrosine-phosphorylated rapidly upon engagement of various receptors providing critical recruitment points for the development of multicomponent signaling complexes [4]. The role of LAT in TCR- and FcεRI-mediated signaling has been studied extensively and reviewed elsewhere [6]. Here, we aim to highlight the emerging role of LAB as an immune cell regulator in a variety of cells. Our overview suggests that this protein may constitute a critical regulatory checkpoint in virtually every leukocyte involved in innate and adaptive immunity. Given the broad expanse of the influence of LAB on immune regulation, greater scrutiny of this protein is warranted during our quest to understand and control immune responses during infection or malignancy. Here, we discuss the role of LAB-mediated negative regulation in a competition model when expressed in the same cells as LAT (Fig. 1), how LAB mediates positive signaling (Fig. 2), and how LAB may negatively regulate signaling in the absence of LAT (Fig. 3).
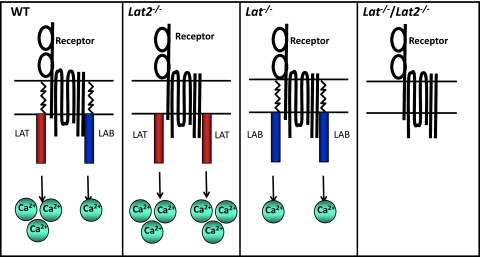
Figure 1. LAB/LAT competition model.
Engagement of receptors, such as FcεRI, FcγRI, BCR, and TREM-1, results in significant Ca2+ mobilization in WT cells, mainly through activation of LAT, as LAB can only activate Ca2+ mobilization weakly. Lat2−/− mice display enhanced Ca2+ mobilization as a result of increased accumulation of highly active LAT in lipid rafts in the absence of the relatively poorly activating LAB. In the absence of LAT, LAB can only mobilize Ca2+ weakly. In the absence of both adaptors, Ca2+ mobilization is largely ablated. Absence of one of the adaptors (LAB or LAT) or both adaptors affects degranulation in a similar manner to Ca2+ mobilization.
Figure 2. Hypothetical roles for LAB-dependent positive signaling.
Engagement of FcεRI (shown) or other receptors, such as FcγRI, BCR, and TREM-1/2, results in activation of various tyrosine kinases, such as Fyn, Lyn, and/or Syk, leading to phosphorylation of LAB, which can then recruit the Grb2-Sos complex, resulting in Ras-MAPK activation and IL-3 production, which promotes survival. Phosphorylated LAB may also recruit a Grb2-PLCγ complex, resulting in partial Ca2+ responses. Lastly, phospho-LAB may be involved in the LAT-independent Fyn-Gab2-PI3K pathway described previously, resulting in PKC activation and degranulation. It is through this pathway that the direct phosphorylation of LAB by KIT may potentiate FcεRI-derived signals. ?, Unknowns in these pathways.
Figure 3. c-Cbl may negatively regulate proximal signaling.
Engagement of the BCR activates Syk, resulting in the recruitment of LAB, which recruits c-Cbl to the complex, likely via an interaction with Grb2. In the absence of LAB, proximal signaling events are enhanced, suggesting that c-Cbl may negatively regulate proximal signaling. c-Cbl has been shown to negatively regulate Syk activation, and BCR and LAB have been shown to be ubiquitinated (Ub). However, it is unknown whether degradation, recycling, or just negative regulation of the proteins in this signaling complex occurs and whether this is c-Cbl-mediated.
LAT AND LAB STRUCTURE AND INTERACTIONS
LAB is the product of the Lat2 gene. Human Lat2 resides at 7q11.23 and encodes a 243-aa protein, and murine Lat2 is on chromosome 5 and encodes a 203-aa protein [7, 8]. Human Lat on chromosome 16p11.2 encodes a 233-aa protein, and murine Lat on chromosome 7 encodes a 242-aa protein [9, 10]. Alternatively spliced isoforms of LAT and LAB have been reported at the cDNA level, but their expression at the protein level, and therefore, their functional significance are unknown [5, 10]. The Lat2 gene (also known as Williams-Beuren syndrome chromosome region 5) is 1 of 24 loci in the section of chromosome 7 that is absent in Williams-Beuren syndrome, a condition characterized by cardiovascular disease, unique personality characteristics, cognitive impairment, and distinct facial characteristics [11]. LAB is reportedly expressed in most leukocyte lineages, including B cells, mast cells, NK cells, and myeloid cells but not resting T cells [7, 8]. LAT is expressed in T cells, mast cells, NK cells, monocytes, megakaryocytes, and platelets [12]. Thus, multiple cell types express LAT and LAB. In SDS-PAGE analysis, murine and human LAB resolve as doublets of 25–30 kDa [7, 8, 13]. Human and murine LAT resolve as doublets of 36–38 kDa [9, 10]. LAT and LAB are type III integral membrane proteins that carry a CxxC palmitoylation site responsible for targeting them to membrane microdomains (glycolipid-enriched membranes/lipid rafts), a characteristic critical for their scaffolding function [7]. Current data suggest that like LAT, LAB nucleates signaling complexes primarily through phosphotyrosine-based protein–protein interactions. Accordingly, 9 cytoplasmic tyrosine residues are conserved between mouse and human LAB, suggesting conservation of function. Similar to LAT, there are 5 putative binding sites (YxN) for the SH2 domain of the cytosolic adaptor protein Grb2 [7, 8], 1 of which is homologous to a tyrosine residue in LAT known to bind the related adaptor GADS SH2 domain [5]. However, there are significant differences between LAB and LAT. and perhaps most importantly, LAB does not have a direct PLCγ binding site like that found in LAT (Y132LVV) [14]. The specific residues of LAB and their functions have been reviewed elsewhere [5]. Therefore, although many similarities between the LAT and LAB structures and expression pattern exist, there are significant differences that could affect their function in various cell types. Indeed, although initial examination suggested LAB as a B cell version of LAT, more recent work has unveiled significant functional differences between LAT and LAB, which will be discussed further below.
MYELOID CELLS
Mast cells
Despite its discovery in B cells, LAB has been characterized most extensively in FcεRI-mediated signaling in mast cells [8, 13, 15, 16], which express LAT and LAB, and multiple laboratories have worked to compare and contrast the roles of LAB and LAT in the survival and degranulation of these cells [15–18]. Degranulation, arachidonic acid metabolism, and cytokine production result from mast cell activation via FcεRI engagement, and sensitization of mast cells with IgE in the absence of antigen promotes their survival [16]. FcεRI-induced degranulation and cytokine production are enhanced by SCF-induced activation of KIT, and KIT activation is also necessary for mast cell growth, differentiation, and survival [16]. LAT and LAB are phosphorylated following engagement of FcεRI on mast cells [16]. Interestingly, antigen-mediated degranulation is inhibited partially in Lat−/− BMMCs, further decreased in Lat−/−/Lat2− /− BMMCs, and surprisingly, increased in Lat2−/− BMMCs [15, 17]. In fact, Lat2−/− BMMCs display enhanced FcεRI-mediated tyrosine phosphorylation of Syk, LAT, and PLCγ1/2, as well as increased calcium mobilization, Erk activation, and cytokine production. Lastly, Lat2−/− mice show enhanced, passive cutaneous anaphylactic responses, suggesting their enhanced FcεRI signaling is physiologically relevant [15, 17]. Based on these findings, it has been suggested that LAB acts as a negative regulator of LAT signaling in WT cells but may also deliver positive signals when expressed in Lat−/− cells [15, 17]. Indeed, increased LAT was found in the lipid rafts of Lat2−/− BMMCs and increased LAB in the rafts of Lat−/− BMMCs, leading to the suggestion that competition between LAT and LAB for localization to lipid rafts and availability of binding proteins may explain the divergent roles for LAB (Fig. 1) [15, 17]. However, Volna et al. [17] demonstrated that LAT and LAB cluster in lipid rafts independently. Rivera [19] attempted to reconcile these disparate findings by suggesting that following FcεRI engagement, the independent lipid raft compartments containing LAT or LAB are brought together, allowing phosphorylation of the LAT and LAB molecules in proximity to the engaged FcεRI. Loss of either adaptor would then allow increased phosphorylation of the other adaptor, as the concentration of LAT- or LAB-specific rafts would increase in the absence of the competing adaptor [19]. Although this competition model (Fig. 1) is an attractive one that is consistent with many observations, evidence from other cell types suggests that interference with LAT signaling is only 1 of the mechanisms to explain the inhibitory function of LAB.
Although this “no room at the inn” model of competition for the localization of LAT or LAB to the lipid rafts may explain increased signaling via LAT in the absence of LAB, questions remain about which signaling events are controlled specifically by LAT or LAB. It would appear that murine Y136 or human Y132 of LAT is key. This tyrosine is a consensus-binding site for the SH2 domains of PLCγ (Y136LVV) but is not conserved in LAB, and attempts to demonstrate direct PLCγ association with LAB have failed [8]. It is well documented that LAT uses Y136 to bind PLCγ1 directly or PLCγ2 and via this tyrosine, coordinates a trimeric complex of PLCγ and the cytosolic adaptors GADS and SLP76, necessary for full PLCγ activation, Ca2+ mobilization, and degranulation [18, 20]. Thus, in Lat2−/− cells, which lack competition for lipid rafts or substrates, it appears that signaling via the GADS-SLP76-PLCγ axis is enhanced as a result of more efficient use of LAT, resulting in greater activation of PLCγ [15]. The residual calcium mobilization of Lat−/− BMMCs and the complete absence of this response in Lat−/−/Lat2− /− cells suggest that in the absence of LAT, LAB can, to some extent, mediate signals on its own [15]. How then, in the absence of LAT, can LAB initiate PLCγ signaling? Although LAB cannot bind PLCγ directly, indirect binding of PLCγ to LAB via Grb2/GADS may occur, resulting in some, but significantly decreased, Ca2+ mobilization and degranulation in Lat−/− BMMCs. Indeed, fusion proteins containing the carboxy-terminal SH2 domains of PLCγ1 and PLCγ2 have been shown to pull down LAB [8]. In addition, Syk-phosphorylated LAB was shown to pull down PLCγ from the HuMC-1, and this interaction required the Grb2-binding sites in LAB, suggesting that Grb2 mediates this interaction (Fig. 2) [8, 21]. Perhaps the level of Ca2+ mobilization may be controlled by a preference of LAT or LAB for the PLCγ1 or PLCγ2 isoform in mast cells. Barker et al. [22] showed that PLCγ2 is the more abundant isoform in the RBL-2H3 mast cell line, but PLCγ1 appears to be the main isoform involved in the antigen response; therefore, it would be interesting to determine whether LAT/LAB have a particular preference for either of these PLCγ isoforms in mast cells during a response to antigen. However, the affinities and significance of potential indirect interactions between LAB and PLCγ1 and/or -2 have yet to be tested.
Degranulation downstream of FcεRI activation uses 2 distinct pathways, 1 of which involves an increase in intracellular calcium levels and activation of the calcium-dependent PKCβ, and the other pathway uses PI3K to activate the calcium-independent PKCδ isoform [23]. As mentioned previously, degranulation is decreased significantly in Lat−/− BMMCs and increased in Lat2−/− BMMCs [15]. In WT BMMCs, LAT and LAB may coordinate Ca2+ responses as discussed above, and an additional FcεRI-induced, LAT-independent Fyn-Gab2-PI3K complex results in PKCδ activation and Ca2+ mobilization [23]. The Fyn-Gab2-PI3K complex catalyzes conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) to PIP3, which creates a membrane-docking site for PH domain-containing molecules, including PLCγ, Vav, and Bruton's tyrosine kinase, resulting in phosphorylation of downstream signaling components [23, 24]. LAB has not been shown to be involved in the Fyn/Gab2/PI3K signaling axis; however, Fyn can phosphorylate LAB directly, suggesting a possible role for LAB in the regulation of this pathway [14, 17, 25]. The efficient LAT-mediated Ca2+ responses may account for the degranulation in Lat2−/− cells, and the inefficient LAB-mediated Ca2+ responses or the alternative Fyn-Gab2-PI3K signaling axis (possibly mediated by LAB; Fig. 2) may account for the partial response in Lat−/− cells. Whether LAB is involved directly in this pathway, however, remains to be determined.
In addition to its role in FcεRI-mediated degranulation, LAB regulates the expansion and survival of mast cells, and mast cell survival is mediated by the SCF receptor (KIT) or IL-3 signaling [18]. IgE stimulation of the FcεRI pathway also facilities mast cell survival via autocrine production of IL-3 [18]. Moreover, activation of the antiapoptotic factor Akt in the absence of IL-3 can help to maintain limited survival of mast cells [25]. SCF signaling is absolutely required for mast cell development and survival in vivo, such that mice with mutations in the Steel locus encoding SCF or the Dominant White Spotting locus encoding KIT lack mast cells [26]. Notably, the SCF and FcεRI survival pathways involve LAB phosphorylation, whereas LAT is phosphorylated following FcεRI engagement but not following stimulation with SCF [16]. Engagement of FcεRI results in LAB phosphorylation via the protein tyrosine kinases Lyn and Syk recruited to the ITAM of the FcεRI γ-chain. In contrast, KIT, a receptor tyrosine kinase, phosphorylates LAB directly [21]. Interestingly, whereas Syk phosphorylates primarily the predicted Grb2-binding sites (Y193 and Y233) of LAB in vitro, perhaps facilitating the indirect recruitment of PLCγ to LAB, Lyn and KIT phosphorylate different tyrosines in LAB [21]. This may allow some flexibility in LAB-mediated signaling, depending on the initial stimulus.
Following sensitization with IgE, LAT and LAB promote prolonged Erk activation and mast cell survival through membrane retention of the Ras-activating complex, Grb2-Sos, recruited to LAT/LAB upon phosphorylation (Fig. 2) [18]. The fact that disruption of LAT-mediated pathways blocks degranulation significantly suggests that the LAT-GADS-SLP76-PLCγ pathway mediates mast cell degranulation. However, the absence of LAT does not affect mast cell survival, suggesting that LAT or LAB can use the LAT/LAB-Grb2-Sos pathway for mast cell survival [25]. Engagement of FcεRI in BMMCs results in sustained Erk phosphorylation followed by the production of IL-3, which is required for their survival [18]. Following IL-3 stimulation, Lat2−/− and Lat−/− BMMCs display normal proliferation; however, following deprivation of IL-3, Lat2−/− BMMCs have increased rates of apoptosis compared with WT, Lat−/−, and Lat−/−/Lat2−/− cells [18, 25]. A variation of the competition model can explain this complex finding. In this particular instance, LAB may positively regulate survival by competing with LAT for space in the lipid rafts, whereas LAT negatively regulates mast cell survival by recruiting the lipid phosphatase SHIP1 [25], which dephosphorylates the PI3K-induced product, PIP3, at the 5′ position; thus, increased recruitment of SHIP1 by LAT results in decreased Akt phosphorylation, resulting in decreased survival signals. LAB can counteract the recruitment of SHIP1 to LAT, thereby enhancing cell survival during IL-3 deprivation [25]. Although it is possible that LAB can increase Akt phosphorylation and mast cell survival by blocking the recruitment of SHIP1 to LAT, it is also possible that LAB may mediate the Fyn/Gab2/PI3K axis, as mentioned previously, thereby increasing Akt phosphorylation directly. Engagement of FcεRI with IgE following IL-3 deprivation can rescue Lat2−/− BMMC survival and partially rescue WT and Lat−/− BMMC survival; however, engagement of FcεRI is unable to rescue Lat−/−/Lat2−/− BMMCs. Therefore, survival following FcεRI engagement correlates with the level of IL-3 produced by the various cells, suggesting that LAT and/or LAB are required for BMMC IL-3 production and ultimately survival following FcεRI stimulation [18]. These data suggest that LAT and LAB can display positive and negative signaling characteristics, perhaps as a result of their differential phosphorylation by various kinases. However, it appears that through positive or negative regulation, LAB may play the predominant role in mediating mast cell survival in the absence of IL-3. Perhaps LAB is architecturally suited to deliver optimal survival signals, and the more robust signals transmitted by LAT are best fitted to cell activation. Thus, the network of interactions between these proteins can provide for intricate control of Ca2+ responses, degranulation, and survival signaling in BMMCs.
Most of the aforementioned studies were performed using murine BMMCs, and studies about human cells actually add confusion to the story, as they don't recapitulate fully what has been found in the mouse studies. As mentioned previously, degranulation is inhibited partially in Lat−/− BMMCs, further decreased in Lat−/−/Lat2−/− BMMCs, and increased in Lat2−/− BMMCs [15, 17]. In contrast, LAB does not appear to have any inhibitory effect in HuMCs. In these studies, short hairpin RNA/siRNA knockdown of LAB, LAT, or both molecules results in decreased degranulation and calcium mobilization following antigen or antigen and SCF stimulation [16, 21]. These results are supported by the finding that siRNA knockdown of LAB in RBLs also results in reduced, rather than enhanced, calcium mobilization and secretory responses [27]. The fact that siRNA studies in rat and HuMCs yield findings contrary to the analysis of knockout murine BMMC could be a result of technical issues associated with transfection or incomplete knockdown but more likely suggests that the effects of LAB may differ depending on whether the cells develop in the absence of LAB or lose the protein at a later point. Given the noted effects of LAB and LAT on BMMC development and survival, a biological explanation certainly seems plausible. Alternatively, rat and HuMCs may have fundamental signaling differences when compared with murine BMMCs. Indeed, Gilfillan et al. [28] have suggested that HuMC activation, like that of BMMC, is mediated primarily through the recruitment and activation of PLCγ by LAT but that LAT-mediated PLCγ activation is enhanced further by LAB and PI3K [24, 28, 29]. Whether the PI3K pathway described in HuMC is similar to the Fyn/Gab2/PI3K pathway described previously in BMMCs and/or dependent on LAB remains unknown (Fig. 2) [23].
A second finding from the study of LAT and LAB in HuMC is elucidation of their role in KIT signaling, which enhances FcεRI-mediated activation of HuMCs. Although engagement of FcεRI on HuMCs leads to phosphorylation of LAT and LAB, SCF-KIT signaling induces phosphorylation of LAB but not LAT. Indeed, SCF potentiates phosphorylation of LAB following engagement of FcεRI, and synergy between KIT and FcεRI is lost when LAT or LAB is knocked down, providing additional evidence for enhancement of an initial LAT-mediated pathway by LAB [16, 21, 28].
Monocytes, macrophages, and neutrophils
Dissection of the role of LAB in myeloid cells other than mast cells is limited at present, and further study is required in this area. However, LAT and low levels of LAB are expressed in monocytes, and we have shown recently that LAB expression is up-regulated following differentiation into macrophages in vitro, and LAT expression is lost [30–32]. Consistent with this finding, the human monocytic cell lines U937 and THP-1 express LAB and readily detectable LAT, whereas murine and human macrophages express abundant LAB with only barely detectable LAT. LAB is also expressed in primary human granulocytes [30, 31].
In various cell types, such as mast, B, and NK cells, LAB phosphorylation has been demonstrated previously downstream of multiple ITAM-coupled receptors, including TREM-1 and -2 [30, 32]. Activation of LAB has been demonstrated following engagement of TREM-1 on a monocytic cell line (U937) and primary human granulocytes, resulting in a LAB-Grb2 complex (Fig. 2) [30]. LAB phosphorylation is also induced following TREM-2 engagement in murine macrophage cell lines and primary mouse macrophages [32]. Interestingly, although TREM-1 and TREM-2 lead to LAB phosphorylation, the function of LAB in these pathways differs. On one hand, siRNA knockdown of LAB results in enhanced TREM-1-mediated Erk1/2 phosphorylation and decreased but prolonged calcium mobilization in U937 cells. Consistent with its negative regulation of ITAM-mediated proximal signaling, LAB suppresses the synergistic production of TNF-α and IL-8 production induced by costimulation of these cells with LPS and anti-TREM-1 [30]. In contrast, loss of LAB expression in macrophages results in an absence of TREM-2-mediated Erk activation, although phosphorylation of multiple proximal substrates is increased, including (Fig. 3) Syk, c-Cbl, and p85, similar to Lat2−/− BMMCs [17, 32]. An important difference in these studies may be the expression of LAT in U937 cells compared with the near-absence of LAT in macrophages [30, 32]. Thus, the activity of LAB in monocytes appears consistent with the competition model. The accentuated use of LAT by monocytes with knocked-down LAB would be expected to result in enhanced Erk signaling and cytokine production. It should be noted, however, that the direct role of LAT here remains unknown. In contrast, macrophages lacking LAB do not up-regulate LAT expression, and no phosphorylation of the residual LAT expressed in macrophages can be detected upon TREM-2 signaling, even in Lat2−/− macrophages, suggesting that LAT does not play a significant role here. Clearly, the competition model (Fig. 1) does not fully explain the suppressive role of LAB in myeloid cells, but the recruitment of c-Cbl may provide a second model for suppression (Fig. 3).
Using THP-1 cells, Brdicka et al. [7] demonstrated that in addition to Grb2, Sos1, and Gab1, c-Cbl can interact with phosphorylated LAB. c-Cbl is an E3-ubiquitin ligase, well known for its ability to target proteins for degradation [33]. Brdicka et al. [7] also found that LAB is ubiqitinated following activation in a B cell line, suggesting c-Cbl-mediated degradation. Consistent with a role for c-Cbl in LAB-mediated repression of proximal ITAM signals, TREM-1 and -2 signaling is dependent on Src and Syk kinases, both known targets of c-Cbl degradation [34, 35]. Accordingly, in murine macrophages, we have found that LAB binds Grb2, c-Cbl, and indirectly, the p85 subunit of PI3K downstream of TREM-2 engagement. It is tempting to speculate that c-Cbl recruitment to TREM-2/LAB receptor complexes may facilitate degradation of proximal kinases, resulting in lower signaling although the E3-ligase capacity of c-Cbl, and ubiquitination of LAB complexes has yet to be tested in this system (Fig. 3). Notably, we detect not only higher levels of TREM-2-induced phospho-Syk but also higher overall levels of Syk in Lat2−/− macrophage lines, suggesting that regulation of signaling protein degradation may be an important facet of LAB-mediated inhibition [32]. Together, these observations make it clear that LAB can negatively regulate proximal signaling, independently of competition with LAT, and that perhaps c-Cbl plays a role in this process (Fig. 3) [32]. Clearly, further work is required to test this model and to determine whether this type of LAB-mediated negative regulation occurs in other cell types lacking LAT expression.
LYMPHOID CELLS
NK cells
Similar to mast cells, NK cells express LAT and LAB, and they each play a role in NK cell development [36]. LAT was described initially in NK cells as a 36-kDa peptide phosphorylated following engagement of the low-affinity receptor for IgG (FcγRIIIA, CD16) [37]. Leibson and colleagues [38] subsequently suggested involvement of LAT in NK cell signaling associated with cytotoxicity. Multiple laboratories have now assessed the functional and biochemical ramifications of disruptions of Lat and/or Lat2 in NK cells. Increased NK cell numbers are found in Lat−/− and Lat−/−/Lat2−/− mice, likely as a result of the lack of T cells in these mice. Loss of LAT, LAB, or both adaptors also results in alterations in the Ly49 repertoire of inhibitory receptors on NK cells, indicating a role for these adaptors in the later stages of NK cell development [36, 39]. PLCγ2−/− NK cells display alterations in the Ly49 repertoire, more closely resembling Lat−/− and Lat−/−/Lat2−/− cells than Lat2−/− cells [39, 40], and PLCγ2 plays a more prominent role than PLCγ1 in NK cells [40]. Together, these observations suggest that in NK cells, LAT may efficiently signal directly through PLCγ2, and LAB may signal indirectly through PLCγ2 or PLCγ1. However, as in mast cells, preferential use of PLCγ isoforms by LAT and LAB in NK cells has yet to be examined. As predicted by the initial study of CD16 in human NK cells, LAT and LAB are dispensable for non-ITAM-mediated cellular cytotoxicity and IFN-γ production; however, DAP12 ITAM-mediated responses are ablated by the loss of LAT and LAB. DAP12-mediated cytotoxicity and IFN-γ production are abolished almost completely in IL-2-activated Lat−/−/Lat2−/− NK cells [36]. Detailed analysis of NK cells lacking LAT or LAB by our laboratory revealed a more complicated scenario. In resting NK cells, ITAM-mediated, NK1.1-induced IFN-γ production was normal in Lat−/−, increased in Lat2−/−, and abolished almost completely in Lat−/−/Lat2−/− NK cells [39]. These functional phenotypes are in agreement with the data from BMMCs, suggesting competitive interference of LAT signaling by LAB. However, in IL-2-activated cells, NK1.1-induced IFN-γ production was decreased in Lat−/− cells, intact in Lat2−/−, and again, severely diminished in Lat−/−/Lat2−/− NK cells [39]. The divergent findings in resting versus IL-2-activated NK cells may be explained, in part, by the apparent existence of 2 signaling cassettes in NK cells: ITAM-Syk-LAB/LAT and ITAM-Zap70-LAT. ITAM-mediated signaling is ablated in a NK-like cell line with limited expression of LAT and Syk, as Zap70 apparently cannot couple efficiently to LAB-dependent processes, effectively blocking both ITAM signaling cassettes. However, introduction of sufficient Syk or LAT expression in these cells reconstitutes the ITAM-Syk-LAB/LAT or ITAM-Zap70-LAT signaling cassette, respectively, circumventing any functional deficit [39]. Indeed, NK cells cultured in IL-2 have reduced levels of LAT and increased LAB expression and signaling. However, these cells also up-regulate the mast cell immunoreceptor signal transducer, mast cell immuno-receptor signal transducer/cytokine-dependent hematopoietic cell linker, a protein that suppresses Syk activity. Thus, IL-2-activated NK cells are more dependent on LAB but starved of Syk activation, resulting in reduced ITAM signaling [39]. As mentioned above, in mast cells, KIT has been shown to phosphorylate different tyrosine residues of LAB than Syk, making it tempting to speculate that IL-2 may also phosphorylate LAB, possibly on unique tyrosine residues, leading to the formation of different complexes that could affect the function of IL-2-activated NK cells compared with resting NK cells. To our knowledge, however, this possibility has not yet been addressed. Examples such as these highlight the multiplicity and plasticity of NK signaling pathways as an explanation for the resiliency of NK cell signaling to mutations in 1 particular signaling cassette or another [36]. As with mast cells, where LAT or LAB is required for ITAM signaling, the exact mechanism whereby LAB substitutes effectively for LAT in coupling to PLCγ remains unknown.
B cells
As the name implies, LAB was described initially as a B cell adaptor [8]. Although LAB expression outside of B cells is now well appreciated, a great deal of work has been performed to understand the function of LAB in B cells. Like NK cells cultured in IL-2 or monocytes treated with M-CSF or GM-CSF, B cells undergo dynamic regulation of LAT and LAB levels during development. LAT is expressed in pre-B cells and subsequently, down-regulated during development [13]. Thus, a LAT-SLP76 complex or SLP65/Blnk can link activated pre-BCR to downstream events in pre-B cells by inducing pre-BCR down-regulation, calcium flux, or pre-B cell differentiation [41]. Accordingly, Blnk−/− mice display a partial block at the pre-B cell stage of development, and this block is increased in severity in Blnk−/−/Lat−/− mice [41]. Lat−/− mice display a slight increase in CD43+B220+ pre-B cells, indicating a partial block at this stage of development. However, this is not as severe as in Blnk−/− or Blnk−/−Lat−/− mice, indicating that Blnk transmits pre-BCR signaling efficiently in the absence of LAT, thereby facilitating B cell development [41]. In contrast to LAT, LAB is expressed during all stages of B cell development with the highest expression in mature B cells [13]. Here, again, the ITAM signaling cassette migrates from a LAT-based cassette to one dominated by LAB as the lineage develops. Indeed, it was thought originally that LAB might recruit Blnk directly to the membrane and form a similar complex to LAT-SLP76. However, LAB does not bind Blnk, and B cell development is affected minimally in Lat−/−/Lat2−/− mice or mice lacking either adaptor alone, indicating that LAB is not required for appropriate use of Blnk and is not involved intimately in pre-B cell differentiation [13, 41, 42]. Introduction of LAB into a Blnk−/−/Lat−/− pre-B cell line resulted in minor down-regulation of the pre-BCR, no calcium flux, and no B cell differentiation [42]. However, insertion of the PLCγ-binding motif from LAT (Y136 of LAT) into LAB rendered LAB capable of facilitating pre-BCR down-regulation and calcium flux; however, this was still insufficient to promote pre-B cell differentiation in the absence of Blnk and LAT. The N terminus of LAB was identified by chimeric swap mutants as an inhibitory domain that prevents pre-B cell differentiation [42]. Therefore, it appears that Blnk and a LAT-SLP76 complex are involved in B cell differentiation, and although introduction of a PLCγ-binding site into LAB can reconstitute some of LAT functions, the N terminus of LAB has an inhibitory domain that is responsible for preventing pre-B cell differentiation [42].
LAT is not expressed in mature B cells, and LAB becomes tyrosine-phosphorylated following BCR engagement and associates with Grb2, Sos1 (Ras-MAPK pathway), and Gab1 (PI3K pathway), suggesting an ability to activate multiple signaling pathways in mature B cells (Fig. 2) [7, 8]. However, mature Lat2−/− B cells have slightly elevated Ca2+ mobilization and increased levels of proliferation following BCR cross-linking. Lat2−/− mice also have increased levels of natural antibodies, implying a negative role for LAB in B cell signaling [8, 13]. In fact, in contrast to pre-B cells, LAB colocalizes with and internalizes with the BCR in mature B cells (Fig. 3), and Lat2−/− B cells have partial defects in ligand-induced BCR internalization [43]. This may be a result of differential signaling mechanisms in pre- and mature B cells or of different techniques and should be examined further. Preventing BCR internalization has been shown previously to enhance or prolong calcium mobilization and Erk phosphorylation, suggesting that BCR internalization decreases signaling [43]. Mutch et al. [43] mapped the ability to facilitate BCR internalization to the cytoplasmic region of LAB, and Malhotra et al. [44] showed recently that LAB recruits a Grb2-dynamin complex and Vav, which activate Rac1 and Rac2, resulting in BCR internalization. It has been shown previously that BCR signaling and internalization/endocytosis of receptor-bound antigen for processing and presentation to T cells are mutually exclusive events [45]. Therefore, whether LAB-mediated internalization of the BCR is involved in down-regulating signaling events or in the antigen-presentation pathway is unknown at present. LAB can be ubiquitinated following BCR stimulation, suggesting that E3 ligases may control LAB-mediated internalization of the BCR and/or the turnover of LAB [7, 43]. However, Mutch et al. [43] did not observe any loss of LAB protein for at least 1 h after the initiation of the BCR signal, suggesting that LAB-mediated internalization of the BCR may truncate signaling without direct turnover of LAB or LAB-associated proteins. Regardless of the exact mechanism, the enhanced BCR signaling in Lat2−/− B cells, which do not express LAT, again demonstrates the ability of LAB to negatively regulate signaling through mechanisms other than interference with LAT, perhaps by affecting receptor or signaling complex turnover and/or recycling (Fig. 3).
As LAB appears to play a significant role in B cells, does it have any role in B cell-associated disease states? Lat2−/− mice or mice with a LAB transgene expressed in Lat−/− T cells develop autoimmune disease characterized by increased levels of natural antibodies [13, 14, 46]. The development of autoimmune disease in these 2 seemingly opposite mouse models is discussed further in the following T cell section. Therefore, it may be important to determine the levels of LAB or the LAB:LAT ratio in cells from patients with autoimmune disease, such as systemic lupus erythematosus. It would be interesting to see if the levels of LAB are perturbed in these cells and to examine the possibility of LAB as a therapeutic target in the event of misregulated expression in autoimmune situations.
T cells
An extensive body of literature has documented the role of LAT in T cell development and function [47]. Naturally, given the suggested functional homology of LAT and LAB, the 2 proteins were compared immediately for their ability to facilitate T cell development and function. Lat−/− mice display a block in thymocyte development, as they are unable to differentiate past the double-negative (CD4–CD8–) CD25+CD44– stage as a result of a profound block in pre-TCR signaling [47]. Lat−/− T cells expressing a LAB transgene or LAT with a mutation in the PLCγ-binding site (LAT-Y136F) restored T cell development partially, suggesting the ability of LAB to facilitate critical aspects of pre-TCR signaling [14, 48, 49]. As LAB and LAT-Y136F lack a canonical PLCγ-binding motif, these data were interpreted as indicating an absolute requirement for PLCγ signaling in normal T cell development and the ability of LAB to direct this activation, at least partially, in an indirect manner [14, 50]. Consistent with the ability of LAB to restore T cell development partially, LAB expression in Lat−/− Jurkat cells rescues TCR-mediated Ca2+ mobilization and Erk activation partially [7]. Similar to B cells and macrophages, LAB binds Grb2, Sos, and c-Cbl in these reconstituted cells (Fig. 2), and mutagenesis confirmed the requirement for the 3 distal tyrosines (Y136, Y193, and Y233) of LAB for reconstitution of function. These tyrosines are known Grb2-binding sites, indicating that in T cell development, LAB functions primarily via recruitment of Grb2 [7, 50]. Interestingly, mice with Lat−/− T cells expressing a LAB or LAT-Y136F transgene developed a severe autoimmune disease characterized by high numbers of hyperactivated T cells that produced large amounts of type II cytokines [14, 48, 49]. Recent data suggest that although LAT is required for normal T cell development, it may also mediate a negative-feedback loop required to keep CD4+ T cell responses in check [51]. The type II autoimmunity observed in mice with Lat−/− T cells expressing a LAB or LAT-Y136F transgene is, therefore, likely a result of a defect in this negative-feedback loop mediated by LAT, again suggesting that similar to LAB, LAT can also play some positive and negative roles.
Although these studies show that ectopically expressed LAB can substitute for some LAT-mediated responses during T cell development, little LAB is expressed in naïve, resting T cells, suggesting that it does not participate in T cell development or homeostasis under normal circumstances. However, activated T cells do express LAB, and it appears that LAB is involved in the negative regulation of T cell signaling, as aged (>6 months) Lat2−/− mice develop an autoimmune syndrome [52]. Lat2−/− T cells of 6-month-old mice were hyperactivated, produced more cytokine, and displayed enhanced, LAT-mediated signaling events as compared with WT T cells [52].
As Lat2−/− mice and LAT-Y136F mice demonstrated autoimmune disease, Zhu et al. [52] crossed these mice to examine the severity of the disease. LAB deficiency aggravated the autoimmune disease in LAT-Y136F mice, resulting in even greater numbers of T cells and autoantibody production, again demonstrating a negative role for LAB. This autoimmune syndrome was also found in T cell-specific Lat2−/− mice, suggesting that LAB negatively regulates T cell signaling to prevent autoimmunity. However, more recent data by Gregoire et al. [46] challenge this claim by documenting antinuclear antibodies, even in 6-month-old Lat−/−/Lat2−/− mice, which lack T cells. Regardless of the exact mechanism of the autoimmune syndrome in Lat2−/− mice, it is clear that the limited recruitment capacity of LAB is sufficient to facilitate partial signaling, thereby permitting some T cell development. Once T cells develop, however, LAB-mediated regulation of signaling is critical in the maintenance of T cell homeostasis and prevention of autoimmunity.
CONCLUDING REMARKS
Although some aspects of LAB signaling have been elucidated in various cell types, it is clear that further study is required in all of the cells listed above to clarify further the role/roles of LAB in development and signaling. However, it seems clear that LAB does not just have 1 universal role, and some of this may depend on the presence or absence of LAT. A competition model has been suggested previously for the negative role of LAB when LAT and LAB are expressed in the same cells (Fig. 1). LAT successfully mediates many signaling events, such as Ca2+ mobilization and degranulation, as a result of the presence of a PLCγ-binding motif, allowing the formation of a LAT-PLCγ-GADS-SLP76 complex. It appears that LAB can perform some of the same functions as adequately as LAT, as a result of their structural similarities; however, the lack of a PLCγ-binding motif in LAB accounts for some of its less efficient signaling than LAT. When LAT and LAB are present, they compete for space/substrates in the lipid rafts at activation sites, thereby resulting in regulated signaling, and in the absence of LAB, increased levels of raft-associated LAT can accumulate and produce enhanced signaling. In the absence of LAT, dampened signaling occurs, but it is not ablated completely, suggesting that LAB can perform some positive signaling, possibly via an indirect manner (LAB-Grb2-PLCγ) or by alternative pathways, such as the LAT-independent and possibly, LAB-dependent, Fyn-Gab2-PI3K pathway (Fig. 2). The competition model of LAB-mediated suppression obviously does not hold true in cells where only LAB is expressed, such as macrophages and mature B cells. One possibility for an alternative mechanism is the recruitment of the E3-ubiquitin ligase c-Cbl to the signaling complex, perhaps to dampen/regulate signals, possibly via internalization/degradation of the receptor and other members of the signaling complex, but clearly more work is needed in this area (Fig. 3). Therefore, it appears that the role of LAB varies according to the presence or absence of LAT and that their relative regulation of various signaling pathways is complex, and further study is required to fully understand the role of this adaptor protein. LAB appears to be involved intimately in regulating signaling, which when uncontrolled, can result in various disease states, such as autoimmune diseases, inflammatory diseases, septic shock, and allergic conditions such as asthma. This suggests that greater understanding of the role of LAB in these conditions is required, and this may lead to the identification of a therapeutic target to control some of these disease states.
ACKNOWLEDGMENTS
We thank Drs. Kerry Campbell, Alasdair Gilfillan, and Geraldine O'Connor for critically reading and editing the manuscript.
Footnotes
- Blnk
- B cell linker
- BMMC
- bone marrow-derived mast cell
- c-Cbl
- c homologue of Casitas B-lineage lymphoma
- DAP12
- DNAX-activating protein of molecular mass 12 kDa
- Gab
- Grb2-associated binding protein
- GADS
- Grb2-related adaptor downstream of Shc
- Grb2
- growth factor receptor-bound protein 2
- HuMC
- human mast cell
- KIT
- c-kit signaling pathway
- LAB
- linker for activation of B cells
- LAT
- linker for activation of T cells
- NTAL
- non-T cell activation linker
- PH
- Pleckstrin homology
- PIP3
- phosphatidylinositol 3,4,5 trisphosphate
- RBL
- rat basophilic leukemia cell
- SCF
- stem cell factor
- SH
- Src homology domain
- SHIP1
- Src homology domain 2-containing inositol-5′-phosphatase 1
- siRNA
- small interfering RNA
- SLP76
- Src homology 2 domain-containing leukocyte protein of 76 kDa
- Sos
- son of sevenless homolog
- TREM
- triggering receptor expressed on myeloid cells
AUTHORSHIP
S.J.O. and D.W.M. wrote and edited the manuscript.
DISCLOSURE
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
REFERENCES
- 1. McVicar D. W., Burshtyn D. N. (2001) Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci. STKE 2001, re1 [DOI] [PubMed] [Google Scholar]
- 2. Ford J. W., McVicar D. W. (2009) TREM and TREM-like receptors in inflammation and disease. Curr. Opin. Immunol. 21, 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vely F., Vivier E. (2005) Natural killer cell receptor signaling pathway. Sci. STKE 2005, cm6 [DOI] [PubMed] [Google Scholar]
- 4. Horejsi V. (2004) Transmembrane adaptor proteins in membrane microdomains: important regulators of immunoreceptor signaling. Immunol. Lett. 92, 43–49 [DOI] [PubMed] [Google Scholar]
- 5. Iwaki S., Jensen B. M., Gilfillan A. M. (2007) Ntal/Lab/Lat2. Int. J. Biochem. Cell Biol. 39, 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wange R. L. (2000) LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci. STKE 2000, re1 [DOI] [PubMed] [Google Scholar]
- 7. Brdicka T., Imrich M., Angelisova P., Brdickova N., Horvath O., Spicka J., Hilgert I., Luskova P., Draber P., Novak P., Engels N., Wienands J., Simeoni L., Osterreicher J., Aguado E., Malissen M., Schraven B., Horejsi V. (2002) Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196, 1617–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen E., Zhu M., Zhang W., Koonpaew S. (2003) LAB: a new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 4, 117–123 [DOI] [PubMed] [Google Scholar]
- 9. Weber J. R., Orstavik S., Torgersen K. M., Danbolt N. C., Berg S. F., Ryan J. C., Tasken K., Imboden J. B., Vaage J. T. (1998) Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J. Exp. Med. 187, 1157–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W., Sloan-Lancaster J., Kitchen J., Trible R. P., Samelson L. E. (1998) LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92, 83–92 [DOI] [PubMed] [Google Scholar]
- 11. Stevens R. D., Tipney H. J., Wroe C. J., Oinn T. M., Senger M., Lord P. W., Goble C. A., Brass A., Tassabehji M. (2004) Exploring Williams-Beuren syndrome using myGrid. Bioinformatics 20 (Suppl. 1), i303–i310 [DOI] [PubMed] [Google Scholar]
- 12. Facchetti F., Chan J. K., Zhang W., Tironi A., Chilosi M., Parolini S., Notarangelo L. D., Samelson L. E. (1999) Linker for activation of T cells (LAT), a novel immunohistochemical marker for T cells, NK cells, mast cells, and megakaryocytes: evaluation in normal and pathological conditions. Am. J. Pathol. 154, 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y., Horvath O., Hamm-Baarke A., Richelme M., Gregoire C., Guinamard R., Horejsi V., Angelisova P., Spicka J., Schraven B., Malissen B., Malissen M. (2005) Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol. Cell. Biol. 25, 4455–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janssen E., Zhu M., Craven B., Zhang W. (2004) Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-γ1 binding. J. Immunol. 172, 6810–6819 [DOI] [PubMed] [Google Scholar]
- 15. Zhu M., Liu Y., Koonpaew S., Granillo O., Zhang W. (2004) Positive and negative regulation of FcεRI-mediated signaling by the adaptor protein LAB/NTAL. J. Exp. Med. 200, 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tkaczyk C., Horejsi V., Iwaki S., Draber P., Samelson L. E., Satterthwaite A. B., Nahm D. H., Metcalfe D. D., Gilfillan A. M. (2004) NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc ε RI aggregation. Blood 104, 207–214 [DOI] [PubMed] [Google Scholar]
- 17. Volna P., Lebduska P., Draberova L., Simova S., Heneberg P., Boubelik M., Bugajev V., Malissen B., Wilson B. S., Horejsi V., Malissen M., Draber P. (2004) Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J. Exp. Med. 200, 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamasaki S., Ishikawa E., Sakuma M., Kanagawa O., Cheng A. M., Malissen B., Saito T. (2007) LAT and NTAL mediate immunoglobulin E-induced sustained extracellular signal-regulated kinase activation critical for mast cell survival. Mol. Cell. Biol. 27, 4406–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivera J. (2005) NTAL/LAB and LAT: a balancing act in mast-cell activation and function. Trends Immunol. 26, 119–122 [DOI] [PubMed] [Google Scholar]
- 20. Gross B. S., Melford S. K., Watson S. P. (1999) Evidence that phospholipase C-γ2 interacts with SLP-76, Syk, Lyn, LAT and the Fc receptor γ-chain after stimulation of the collagen receptor glycoprotein VI in human platelets. Eur. J. Biochem. 263, 612–623 [DOI] [PubMed] [Google Scholar]
- 21. Iwaki S., Spicka J., Tkaczyk C., Jensen B. M., Furumoto Y., Charles N., Kovarova M., Rivera J., Horejsi V., Metcalfe D. D., Gilfillan A. M. (2008) Kit- and Fc εRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell. Signal. 20, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barker S. A., Caldwell K. K., Pfeiffer J. R., Wilson B. S. (1998) Wortmannin-sensitive phosphorylation, translocation, and activation of PLCγ1, but not PLCγ2, in antigen-stimulated RBL-2H3 mast cells. Mol. Biol. Cell 9, 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parravicini V., Gadina M., Kovarova M., Odom S., Gonzalez-Espinosa C., Furumoto Y., Saitoh S., Samelson L. E., O'Shea J. J., Rivera J. (2002) Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3, 741–748 [DOI] [PubMed] [Google Scholar]
- 24. Gilfillan A. M., Tkaczyk C. (2006) Integrated signaling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 [DOI] [PubMed] [Google Scholar]
- 25. Roget K., Malissen M., Malbec O., Malissen B., Daeron M. (2008) Non-T cell activation linker promotes mast cell survival by dampening the recruitment of SHIP1 by linker for activation of T cells. J. Immunol. 180, 3689–3698 [DOI] [PubMed] [Google Scholar]
- 26. Besmer P., Manova K., Duttlinger R., Huang E. J., Packer A., Gyssler C., Bachvarova R. F. (1993) The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. Suppl., 125–137 [PubMed] [Google Scholar]
- 27. Draberova L., Shaik G. M., Volna P., Heneberg P., Tumova M., Lebduska P., Korb J., Draber P. (2007) Regulation of Ca2+ signaling in mast cells by tyrosine-phosphorylated and unphosphorylated non-T cell activation linker. J. Immunol. 179, 5169–5180 [DOI] [PubMed] [Google Scholar]
- 28. Gilfillan A. M., Peavy R. D., Metcalfe D. D. (2009) Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 43, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tkaczyk C., Beaven M. A., Brachman S. M., Metcalfe D. D., Gilfillan A. M. (2003) The phospholipase C γ 1-dependent pathway of Fc ε RI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J. Biol. Chem. 278, 48474–48484 [DOI] [PubMed] [Google Scholar]
- 30. Tessarz A. S., Weiler S., Zanzinger K., Angelisova P., Horejsi V., Cerwenka A. (2007) Non-T cell activation linker (NTAL) negatively regulates TREM-1/DAP12-induced inflammatory cytokine production in myeloid cells. J. Immunol. 178, 1991–1999 [DOI] [PubMed] [Google Scholar]
- 31. Tridandapani S., Lyden T. W., Smith J. L., Carter J. E., Coggeshall K. M., Anderson C. L. (2000) The adapter protein LAT enhances Fcγ receptor-mediated signal transduction in myeloid cells. J. Biol. Chem. 275, 20480–20487 [DOI] [PubMed] [Google Scholar]
- 32. Whittaker G. C., Orr S. J., Quigley L., Hughes L., Francischetti I. M., Zhang W., McVicar D. W. (2010) The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells. J. Biol. Chem. 285, 2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidt M. H., Dikic I. (2005) The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6, 907–918 [DOI] [PubMed] [Google Scholar]
- 34. Lupher M. L., Jr., Rao N., Lill N. L., Andoniou C. E., Miyake S., Clark E. A., Druker B., Band H. (1998) Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem. 273, 35273–35281 [DOI] [PubMed] [Google Scholar]
- 35. Yokouchi M., Kondo T., Sanjay A., Houghton A., Yoshimura A., Komiya S., Zhang H., Baron R. (2001) Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J. Biol. Chem. 276, 35185–35193 [DOI] [PubMed] [Google Scholar]
- 36. Chiesa S., Mingueneau M., Fuseri N., Malissen B., Raulet D. H., Malissen M., Vivier E., Tomasello E. (2006) Multiplicity and plasticity of natural killer cell signaling pathways. Blood 107, 2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerboni C., Gismondi A., Palmieri G., Piccoli M., Frati L., Santoni A. (1998) CD16-mediated activation of phosphatidylinositol-3 kinase (PI-3K) in human NK cells involves tyrosine phosphorylation of Cbl and its association with Grb2, Shc, pp36 and p85 PI-3K subunit. Eur. J. Immunol. 28, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 38. Jevremovic D., Billadeau D. D., Schoon R. A., Dick C. J., Irvin B. J., Zhang W., Samelson L. E., Abraham R. T., Leibson P. J. (1999) Cutting edge: a role for the adaptor protein LAT in human NK cell-mediated cytotoxicity. J. Immunol. 162, 2453–2456 [PubMed] [Google Scholar]
- 39. Whittaker G. C., Burshtyn D. N., Orr S. J., Quigley L., Hodge D. L., Pascal V., Zhang W., McVicar D. W. (2008) Analysis of the linker for activation of T cells and the linker for activation of B cells in natural killer cells reveals a novel signaling cassette, dual usage in ITAM signaling, and influence on development of the Ly49 repertoire. Blood 112, 2869–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tassi I., Presti R., Kim S., Yokoyama W. M., Gilfillan S., Colonna M. (2005) Phospholipase C-γ 2 is a critical signaling mediator for murine NK cell activating receptors. J. Immunol. 175, 749–754 [DOI] [PubMed] [Google Scholar]
- 41. Su Y. W., Jumaa H. (2003) LAT links the pre-BCR to calcium signaling. Immunity 19, 295–305 [DOI] [PubMed] [Google Scholar]
- 42. Herzog S., Jumaa H. (2007) The N terminus of the non-T cell activation linker (NTAL) confers inhibitory effects on pre-B cell differentiation. J. Immunol. 178, 2336–2343 [DOI] [PubMed] [Google Scholar]
- 43. Mutch C. M., Sanyal R., Unruh T. L., Grigoriou L., Zhu M., Zhang W., Deans J. P. (2007) Activation-induced endocytosis of the raft-associated transmembrane adaptor protein LAB/NTAL in B lymphocytes: evidence for a role in internalization of the B cell receptor. Int. Immunol. 19, 19–30 [DOI] [PubMed] [Google Scholar]
- 44. Malhotra S., Kovats S., Zhang W., Coggeshall K. M. (2009) Vav and Rac activation in B cell antigen receptor endocytosis involves Vav recruitment to the adapter protein LAB. J. Biol. Chem. 284, 36202–36212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hou P., Araujo E., Zhao T., Zhang M., Massenburg D., Veselits M., Doyle C., Dinner A. R., Clark M. R. (2006) B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 4, e200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gregoire C., Simova S., Wang Y., Sansoni A., Richelme S., Schmidt-Giese A., Simeoni L., Angelisova P., Reinhold D., Schraven B., Horejsi V., Malissen B., Malissen M. (2007) Deletion of the LIME adaptor protein minimally affects T and B cell development and function. Eur. J. Immunol. 37, 3259–3269 [DOI] [PubMed] [Google Scholar]
- 47. Zhang W., Sommers C. L., Burshtyn D. N., Stebbins C. C., DeJarnette J. B., Trible R. P., Grinberg A., Tsay H. C., Jacobs H. M., Kessler C. M., Long E. O., Love P. E., Samelson L. E. (1999) Essential role of LAT in T cell development. Immunity 10, 323–332 [DOI] [PubMed] [Google Scholar]
- 48. Aguado E., Richelme S., Nunez-Cruz S., Miazek A., Mura A. M., Richelme M., Guo X. J., Sainty D., He H. T., Malissen B., Malissen M. (2002) Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296, 2036–2040 [DOI] [PubMed] [Google Scholar]
- 49. Sommers C. L., Park C. S., Lee J., Feng C., Fuller C. L., Grinberg A., Hildebrand J. A., Lacana E., Menon R. K., Shores E. W., Samelson L. E., Love P. E. (2002) A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296, 2040–2043 [DOI] [PubMed] [Google Scholar]
- 50. Koonpaew S., Janssen E., Zhu M., Zhang W. (2004) The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J. Biol. Chem. 279, 11229–11235 [DOI] [PubMed] [Google Scholar]
- 51. Mingueneau M., Roncagalli R., Gregoire C., Kissenpfennig A., Miazek A., Archambaud C., Wang Y., Perrin P., Bertosio E., Sansoni A., Richelme S., Locksley R. M., Aguado E., Malissen M., Malissen B. (2009) Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 31, 197–208 [DOI] [PubMed] [Google Scholar]
- 52. Zhu M., Koonpaew S., Liu Y., Shen S., Denning T., Dzhagalov I., Rhee I., Zhang W. (2006) Negative regulation of T cell activation and autoimmunity by the transmembrane adaptor protein LAB. Immunity 25, 757–768 [DOI] [PubMed] [Google Scholar]