Abstract
An improved understanding of renal cell carcinoma (RCC) biology has translated into major advances in the treatment of patients with metastatic RCC in recent years. Clinical and pathologic criteria can be used to identify RCC patients with poor prognoses. Such patients, however, are often excluded from the cancer clinical trials that guide treatment recommendations. This article reviews available information on the management of patients with metastatic RCC and poor risk features, focusing on the role of vascular endothelial growth factor (VEGF) pathway and mammalian target of rapamycin (mTOR) inhibitors. While patients with poor risk features have a more guarded outcome, treatment with temsirolimus has produced meaningful improvements in overall survival for this population. Definitive phase III trial data are lacking for the VEGF pathway inhibitors in patients with poor prognostic features. However, available data suggest that such patients tolerate VEGF pathway blockade reasonably well and are likely to achieve some benefit relative to treatment with interferon. Ongoing translational research efforts may help to define novel treatment approaches specific for patients with metastatic RCC and poor prognostic features.
Keywords: renal cell carcinoma, prognostic criteria, vascular endothelial growth factor (VEGF)-pathway inhibitors, mammalian target of rapamycin (mTOR) inhibitors
Introduction
Renal cell carcinoma (RCC) is predicted to account for 58,000 new cases and nearly 13,000 deaths in 2009.1 For patients who present with early stage disease the 5-year survival is estimated at 66%. However, up to 40% of those who present with localized disease will develop metastases,2,3 and the 5-year survival in metastatic disease is still less than 20%.4,5 For patients with poor prognostic features, as defined by the Memorial Sloan Kettering Cancer Center (MSKCC) staging system, the outlook can be even more grim with median survival of 4 months and few patients surviving 1 year.6
In recent years an improved understanding of RCC tumor biology has translated into major advancements in the treatment of patients with metastatic RCC. Several new molecularly targeted agents have been identified that have led to significant improvements in progression-free survival and a general increase in overall survival. These include inhibitors of the vascular endothelial growth factor (VEGF) pathway (eg, sunitinib, sorafenib, and bevacizumab), and inhibitors of the mammalian target of rapamycin (mTor) pathway (eg, temsirolimus and everolimus).
Cancer clinical trials are often conducted in well selected populations with strict inclusion criteria that exclude patients with poor performance status or significant co-morbidities. For example, only one of the pivotal trials that resulted in Food and Drug Administration (FDA) approval for the agents discussed above included a substantial number of patients with poor prognostic features (Table 1). For this reason, the optimal approach to the management of patients with metastatic RCC and poor risk features may be difficult to determine from trial reports. In this article, we review the available information on management of patients with RCC and poor risk features, focusing on the role of VEGF-pathway and mTOR inhibitors in this population.
Table 1.
Phase III trials of targeted therapy in advanced renal cell carcinoma
| Agent | Trial | Size | Percent poor risk | Overall PFS (95% CI) | Overall response rate (95% CI) | Overall disease control rate |
|---|---|---|---|---|---|---|
| Sunitinib | Motzer 200737 | N = 750 | 6.4% | 11 months (10–12) | 31% (26–36) | 79% |
| Sorafenib | Escudier 200757 | N = 903 | 0% | 5.5 months | 10% (7–13) | 62% (57–66) |
| Temsirolimus | Hudes 200735 | N = 626 | 74% | 5.5 months (3.9–7.0) | 8.6% (4.8–12.4) | 32.1% (25.7–38.4) |
| Everolimus | Motzer 200836 | N = 410 | 15% | 4.0 months (3.7–5.5) | 1% | 64% |
| Bevacizumab/IFN | Escudier 200738 | N = 649 | 9% | 10.2 months | 31% | 77% |
| Bevacizumab/IFN | Rini 200839 | N = 732 | 10% | 8.5 months (7.5–9.7) | 25.5% (20.9–30.6) |
Defining prognostic criteria
Clinical prognostic factors
Prognostic criteria can be used in designing and stratifying participants in clinical trials, counseling patients, and directing therapy. Several investigators have attempted to define prognostic criteria for patients with metastatic RCC in order to guide clinical decision making. Table 2 summarizes the most commonly applied prognostic models.
Table 2.
Clinical prognostic criteria
| Memorial Sloan Kettering Cancer Center Prognostic Criteria – version 1 |
Motzer 19996 | Corrected serum calcium Hemoglobin Karnofsky performance status Lactate dehydrogenase Prior nephrectomy |
| Memorial Sloan Kettering Cancer Center Prognostic Criteria – version 2 |
Motzer 20024 | Corrected serum calcium Hemoglobin Karnofsky performance status Lactate dehydrogenase Time from initial diagnosis to treatment with IFN-α |
| Cleveland Clinic | Mekhail 20059 | Corrected serum calcium Hemoglobin Lactate dehydrogenase Presence of liver, lung, or retroperitoneal nodal metastasis Prior radiotherapy Time from initial diagnosis to treatment with IFN-α |
| French Prognostic Criteria | Escudier 200219 | Alkaline phosphatase Corrected serum calcium Lactate dehydrogenase Number of metastatic sites Time from nephrectomy to metastatic disease |
The Memorial Sloan Kettering Cancer Center (MSKCC) criteria, first published in 1999, defined pretreatment clinical features that were predictive of survival in patients with metastatic RCC who had not received prior therapy. Five prognostic factors, including hemoglobin, lactate dehydrogenase, corrected serum calcium, prior nephrectomy, and Karnofsky performance status, were identified by multivariate analysis as having independent prognostic implications with regard to overall survival.6
Based on these criteria, patients were stratified into 3 risk categories, favorable (no risk factors), intermediate (1 or 2 risk factors), and poor (3 or more risk factors), according to the number of high-risk features present. The prognostic criteria were validated using an internal bootstrap technique in which subpopulations of the parent sample group were tested against the prognostic model.6 Median survival in the favorable-risk group was 20 months, in the intermediate-risk group 10 months, and in the poor-risk group 4 months. These prognostic criteria became a standard by which participants with RCC are assessed and stratified prior to enrollment in clinical trials.
Following results of 2 phase III trials demonstrating a survival benefit for cytoreductive nephrectomy prior to interferon-α (IFN-α),7,8 debulking nephrectomy followed by IFN became a standard approach for patients presenting with stage IV disease. Concurrent with this, the MSKCC criteria were modified based on a new prognostic model in which time to treatment with IFN replaced the presence or absence of prior nephrectomy.4 This prognostic model included Karnofsky performance status, hemoglobin, corrected serum calcium, lactate dehydrogenase, and time from initial diagnosis to treatment with IFN within or more than one year. The MSKCC version 2 criteria were also established by multivariate analysis and validated by internal bootstrap technique. Median survival in the favorable risk group was 30 months, in the intermediate risk group 14 months, and in the poor risk group 5 months.4 The MSKCC prognostic criteria version 2 have been recognized as an appropriate model for risk stratification in phase III trials utilizing IFN as the control arm and in single-arm phase II trials evaluating progression-free survival.
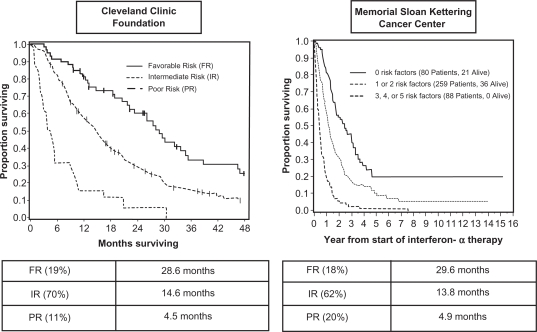
The MSKCC prognostic criteria version 2 were externally validated in an independent sample population with survival as the primary endpoint by investigators from the Cleveland Clinic.9 Median survival times were 28.6, 14.6 and 4.5 months in the favorable, intermediate, and poor risk groups, respectively (P < 0.0001) (Figure 1). Four of the five previously identified risk factors, hemoglobin, corrected serum calcium, lactate dehydrogenase, and time from initial diagnosis to treatment with IFN, were found to be independently predictive of survival. Performance status was not found to be a significant predictive factor; however, all participants involved in this assessment had been subjects of clinical trials that required Easter Cooperative Oncology Group (ECOG) performance status of 0 or 1 for enrollment. Therefore, all participants would have had favorable performance status by MSKCC criteria. This study also identified prior radiotherapy and the presence of liver, lung, or retroperitoneal nodal metastasis as independent poor risk factors.9
Figure 1.
Survival curves from MSKCC and Cleveland Clinic criteria by prognostic category. Adapted with permission from Bukowski RM. Prognostic factors for survival in metastatic renal cell carcinoma: update 2008. Cancer. 2009;115(10 Suppl):2273–2281.59 Copyright © 2009 John Wiley & Sons, Inc.
Negrier et al developed a prognostic model for patients being treated with interleukin-2 (IL-2) and IFN cytokine therapies. Four independent factors, including presence of liver metastases, duration from primary tumor to metastasis less than 1 year, more than 1 metastatic site, and neutrophilia, were predictive of rapid progression on cytokine therapy. Patients who demonstrated 3 or more of these factors had an 80% probability of rapid progression despite therapy.10
It should be noted that the clinical prognostic models described above have focused on survival as the primary endpoint following IFN and/or low-dose IL-2 as the therapy. While this has aided in trial design and balancing treatment arms, the extent to which these models are valid in trials that look at progression-free survival as the primary endpoint and involve agents other than low dose cytokines is not fully established.
Newer prognostic models may be needed in the era of anti-angiogenic and targeted therapy. Choueiri et al identified 5 clinical factors by multivariate analysis that predicted for progression-free survival in patients treated with the antiangiogenesis agents sunitinib, sorafenib, axitinib, or bevacizumab.11 These included time from diagnosis to treatment less than 2 years, neutrophil count, platelet count, ECOG performance status, and corrected serum calcium. When stratified into 3 groups, zero or 1 factor, 2 factors, versus 3 or more factors, the median progression-free survivals were 20.1, 13.0, and 3.9 months, respectively.
Recently Heng and colleagues described a more extensive prognostic risk model for patients treated with VEGF-targeted therapy.12 They studied 645 patients who had not received prior anti-VEGF therapy and were treated with sunitinib, sorafenib, or bevacizumab plus IFN. By Cox proportional hazard model, 6 independent predictors of poor survival were identified including anemia, hypercalcemia, Karnofsky performance status less than 80%, time from initial diagnosis to initiation of therapy less than 1 year, neutrophilia, and thrombocytosis. Patients were categorized as favorable risk (no adverse factors), intermediate risk (1 to 2 adverse factors), or poor risk (3 to 6 adverse factors). At the time of publication, median overall survival was not reached in the favorable-risk group, 27 months in the intermediate-risk group, and 8.8 months in the poor-risk group.12 The model was internally assessed by bootstrap validation, and external validation is ongoing. Nonclear cell and sarcomatoid histologies as well elevated lactate dehydrogenase (LDH) and nephrectomy status were also shown to be indicators of poor prognosis but were not identified as independent prognostic features and thus were not included in the model. Similar analyses restricted to patients treated with mTOR inhibitors have yet to be performed.
Pathologic and molecular prognostic factors
Pathologic features that have been proposed as prognostic in RCC include nuclear grade, histologic subtype, and molecular biomarkers (Table 3). While nuclear grade has been recognized as an independent predictor of survival in early stage disease,13–15 it has not been shown to correlate with survival in the metastatic setting.9,16 Patard et al presented an analysis of 4000 patients with stage I-III RCC showing differences in survival based on histologic subtype.14 In this study estimated 5-year survival rates for those with clear cell, papillary, and chromophobe tumors were 64%, 70%, and 84% respectively (P < 0.001).14 Sarcomatoid features within the tumor have also been associated with poor prognosis.16,17 Sarcomatoid features can be present in all histologic subtypes, and median survival for patients presenting with such features ranges from 2 to 9 months.17–20
Table 3.
Biomarkers of prognosis in renal cell carcinoma
| Histology |
| Clear cell |
| Chromophobe |
| Papillary |
| Sarcomatoid |
| Molecular biomarkers |
| CAIX tumor expression |
| p53 tumor expression |
| PTEN tumor expression |
| Vimentin tumor expression |
| pAKT tumor expression |
| IMP3 tumor expression |
| B7H1/B7H4 tumor expression |
| Genetic biomarkers |
| VHL mutation, deletion, and/or hypermethylation |
Prognostic molecular biomarkers have been identified by both DNA microarray and tissue array techniques. Using tissue array data from 150 metastatic clear cell renal cell tumors, Kim et al isolated CAIX, p53, PTEN, and vimentin as independent prognostic factors for survival in metastatic RCC.21 Increased immunohistochemical staining of p53 and vimentin predicted for poor survival, while increased staining with CAIX and PTEN were associated with more favorable outcomes. Leibovich et al found that while low CAIX expression in nephrectomy specimens correlated with worse survival, this observation was not seen after adjusting for nuclear grade or tumor necrosis, and concluded that CAIX expression as a predictive marker required additional investigation.22 Increased CAIX tumor expression has also been found to be an independent predictor of prolonged survival in patients treated with IL-2.23–25
Mutations, deletions, and/or hypermethylation in the VHL gene have been identified in 60% of patients with clear cell RCC, but results from studies assessing whether VHL loss correlates with survival have been variable.26–28 PTEN loss has been associated with AKT activation, and studies have shown that pAKT tumor expression correlates with worse survival.29 Tumor expression of the insulin-like growth factor-II mRNA binding protein, IMP3, has been linked to poor outcome perhaps due to its association with poor prognostic features including tumor necrosis and sarcomatoid differentiation.30 Hoffmann et al found a 42% increased risk of death from RCC in patients whose tumor IMP3 expression was positive.30 Finally, expression of B7H1 and B7H4 in renal cancers (molecules that are associated with tumor induced immune suppression) has been associated with poor survival.31,32 While research focused on tissue based prognostic biomarkers in RCC potentially provides a means of identifying novel therapeutic targets and predictors of therapeutic response, the clinical parameters outlined above remain the standard approach for risk stratification in ongoing clinical trials and treatment selection in clinical practice.
Treatment of RCC in patients with poor prognostic features
IFN-α in the treatment of patients with poor prognostic features
The MSKCC prognostic criteria were developed in patients receiving IFN in the first-line setting (Table 2).4 Outcome of patients in the poor-risk group was disappointing. Median overall survival following treatment with IFN was 4.9 months, 1-year survival was 20%, and 12 months progression-free survival was only 10%. Other studies have suggested that even intermediate-risk patients (as defined by the French criteria [Table 2]) do not benefit from cytokine-based treatments.33 This work has led to the recommendation by many that angiogenesis inhibitors and targeted therapies should be the preferred treatment approach in patients with intermediate-and poor-risk RCC.
mTor inhibitors in the treatment of patients with poor prognostic features
Temsirolimus, a mammalian target of rapamycin (mTor) kinase inhibitor, was first evaluated in patients with metastatic RCC in a randomized phase II study.34 The primary endpoint was objective tumor response. In this study participants were retrospectively classified according to MSKCC prognostic criteria. When compared to treatment with IFN,4 median survival in temsirolimus-treated patients was 1.6- to 1.7-fold longer for populations with intermediate and poor prognosis. This advantage was not observed in the favorable prognosis population, raising the suggestion that mTor inhibition was particularly active in patients with poor prognostic features or that mTor activity was associated with more aggressive disease.34
Recognizing the limitations of treatment with IFN in the poor risk setting, Hudes et al examined the effect of treating with temsirolimus or combination temsirolimus plus IFN compared to standard IFN in poor prognosis patients.35 Inclusion criteria consisted of at least 3 of 6 predictors of short-term survival as defined by the Cleveland Clinic.9 Seventy-four percent of patients were classified as poor risk by the more restrictive MSKCC model with the remainder being considered of intermediate risk. Eligibility criteria also included Karnofsky performance status of 60 or more, no prior systemic therapy, and adequate bone marrow, renal, and hepatic function.
Results demonstrated improved overall survival and progression-free survival in participants who received temsirolimus alone, median overall survival 10.9 months versus 7.3 months with IFN alone (P < 0.0001). Median progression-free survivals were 1.9 months for IFN, 5.5 months for temsirolimus, and 4.7 months for the combination. There were no significant differences in objective response rates: 4.8% for IFN, 8.6% for temsirolimus, and 8.1% for the combination. However, disease control rate, including stable disease, was significantly higher in the temsirolimus arm (32.1%) compared to the IFN arm (15.5%, P < 0.001). There were also fewer patients with grade 3 and 4 toxicities in the temsirolimus arm (P = 0.02).35 According to subgroup analyses, the benefit of temsirolimus relative to IFN was more pronounced in patients with factors that have been identified as indicators of poor prognosis, eg, high LDH, low Karnofsky performance status, non-clear cell histology, and no prior nephrectomy. Based on these results, temsirolimus was granted FDA approval for treatment of patients with advanced RCC in 2007 and is now considered a standard therapy for patients with poor risk features.
Everolimus was approved for treatment of metastatic RCC in the second-line setting based on results of a phase III placebo-controlled trial published in 2008. All participants in this study had disease that progressed following sorafenib and/or sunitinib therapy.36 This study was stopped early after results of the second interim analysis showed a significant delay in progression-free survival from 1.9 months in the placebo arm to 3.9 months in the everolimus arm. Although this trial enrolled patients who had failed prior VEGF targeted therapy (a group for which no prognostic modeling exists) the majority of patients were deemed to be of favorable or intermediate MSKCC risk based on pre-treatment variables. Only 15% of patients were considered poor risk.36 Thus the value of everolimus in previously untreated patients with poor risk features remains to be established.
VEGF pathway inhibitors in the treatment of patients with poor prognostic features
Although no trials with vascular endothelial growth factor (VEGF) pathway inhibitors have been restricted to patients with predominantly poor prognostic features, some information regarding the activity of these agents in this population can be gleaned from looking at subsets of patients with poor prognostic features. The phase III trials that confirmed the benefit of sunitinib, bevacizumab + IFN, and pazaponib each enrolled a small proportion of patients with poor prognostic features.37–40
Sunitinib was compared to IFN in the first-line setting in a randomized phase III trial in which the overall response rate was 31% and median progression-free survival 11 months for sunitinib compared to a response rate of 6% and median progression-free survival of 5 months with IFN, P < 0.001.37 Participants in the phase III sunitinib trial were stratified according to LDH level, ECOG performance status, and whether or not they had previously undergone nephrectomy.37 Sunitinib treatment was assessed by Cox proportional-hazard model across a series of factors previously identified as portending poor prognosis including previous nephrectomy, age, sex, ECOG performance status, LDH level, time since diagnosis, hemoglobin level, and corrected serum calcium level, and demonstrated improved progression-free survival across each subgroup. Overall survival assessed in a follow-up of this study similarly showed significant benefit from sunitinib treatment among patients with poor prognostic features (Table 4).41
Table 4.
Results of an analysis of OS by individual baseline factors
| Factor |
OS |
P | |
|---|---|---|---|
| HR | 95% CI | ||
| Treatment (sunitinib vs IFN-α) | 0.764 | 0.623–0.936 | 0.0096 |
| ECOG PS (1 vs 1) | 0.515 | 0.417–0.636 | <0.0001 |
| Hemoglobin (≥ vs < LLN) | 0.504 | 0.401–0.634 | <0.0001 |
| Time from diagnosis to treatment (≥ vs < 1 year) | 0.574 | 0.461–0.715 | <0.0001 |
| Corrected calcium (≤ vs > 10 mg/dL) | 0.466 | 0.327–0.664 | <0.0001 |
| Alkaline phosphatase (≤ vs > ULN) | 0.676 | 0.542–0.844 | 0.0005 |
| Lactate dehydrogenase (≤ vs > 1.5 × ULN) | 0.500 | 0.337–0.742 | 0.0006 |
| No of metastastic sites (1 vs ≥ 2) | 0.664 | 0.503–0.876 | 0.0037 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ULN, upper limits of normal; ECOG, Eastern Cooperative Oncology Group.
Reprinted from with permission. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with etastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. Copyright © 2009 American Society of Clinical Oncology. All Rights Reserved.
Participants in the phase III sunitinib trial were also assessed according to MSKCC prognostic criteria version 2,4 and in all risk groups, favorable, intermediate, and poor, sunitinib showed improved progression-free survival as compared to IFN. Only 48 of the 750 (6.4%) participants enrolled in the trial, however, met criteria for poor risk characterization. Median progression-free survival for poor-risk patients in the sunitinib group was 4 months compared to 1 month in the IFN group. In the follow-up analysis median overall survival was 5.3 months in poor risk patients treated with sunitinib (95% confidence interval [CI], 4.2 to 10.0) compared to 4.0 months in those treated with IFN (95% CI, 2.7 to 7.2).41 Taken together, these data indicate that sunitinib has efficacy in patients with poor prognostic features comparable to that observed for the trial as a whole.
In an effort to expand the available data and apply it to an unselected “real-world” population that would be more consistent with community practice Heng et al performed a retrospective analysis comparing sunitinib with IFN that included a large proportion of patients with poor risk features.12 For patients with poor risk features median overall survival on sunitinib was 10.7 months compared to 4.1 months on IFN, P = 0.0329. Median progression-free survival in the patients included in this analysis was 8.9 months on sunitinib, 2 months less than that seen in the phase III trial, a difference that was attributed at least in part to the inclusion of patients with poorer risk profiles.
More recently combination therapy with bevacizumab and IFN has been evaluated in two randomized phase III trials in advanced RCC.38,39 In the first, 649 previously untreated patients were randomized to receive either IFN-α-2a in combination with bevacizumab or placebo, and in the second 732 previously untreated patients were randomized to receive either bevacizumab plus IFN or IFN alone. The primary endpoint, overall survival, was not met in either trial. However, both studies reported significant improvement in progression-free survival, from 5.2 months to 8.5 months (P < 0.0001),39 and from 5.2 months to 10.2 months (P = 0.0001).38
Only 10% of participants in the trials evaluating bevacizumab were classified as having poor prognostic features. When assessed by subgroup analysis, Escudier et al did not find a statistically significant improvement in progression-free survival in poor risk patients treated with bevacizumab plus IFN compared to placebo plus IFN (hazard ratio, 0.81, 95% CI, 0.46 to 1.42, P = 0.5083).38 In contrast, Rini et al did find a statistically significant improvement in progression-free survival in patients with 3 or more MSKCC risk factors treated with bevacizumab plus IFN compared to IFN alone.39 Rini et al also noted increased toxicities in the combination arm. Given the small numbers of participants classified as poor risk in these trials and the reliance on subgroup analysis, it is difficult to make definitive conclusions on the efficacy of bevacizumab in this subpopulation.
Treatment of metastatic RCC according to histologic subtype
Clear cell is the dominant histology in RCC and the focus of most clinical trials. While papillary and chromophobe tumors generally portend more favorable prognoses than clear cell histology, sarcomatoid tumors portend a worse prognosis. Patients identified in a phase II study of sunitinib comprised 6% papillary and 2% sarcomatoid variants.42 Given the small numbers, subgroup analyses assessing sunitinib’s efficacy in these populations was not possible. A larger expanded access trial evaluating sunitinib in 4564 participants reported by Gore et al included 13% of patients with tumors displaying nonclear cell histology. Objective response rate in the overall study population was 17% with stable disease seen in 59%. Among those with nonclear cell histology the objective response rate was 11% with stable disease seen in 57%,43 suggesting that while sunitinib may preferentially benefit those with clear cell histology, it is likely also active in those with tumors of nonclear cell histologies.
Management of sarcomatoid RCC is particularly challenging due to its typically aggressive behavior. While RCC is considered highly resistant to chemotherapy, gemcitabine-containing regimens have shown some efficacy in patients with tumors containing sarcomatoid features with response rates of 5% to 17%.44
Nanus and colleagues reported on 18 patients, 56% with sarcomatoid advanced RCC, treated with combination doxorubicin 50 mg/m2 and gemcitabine 1500 or 2000 mg/m2 every 2 to 3 weeks with granulocyte-stimulating factor support.45 Four of the 11 patients with sarcomatoid disease experienced a tumor response, and 2 additional patients experienced disease stability. The two patients in this study who experienced a complete remission both had sarcomatoid histology.
This combination was further studied in ECOG 8802, a phase II trial involving patients with tumors containing greater than 25% sarcomatoid features.46 Of 38 patients treated, there were 7 documented responses, 1 undocumented, and 9 patients with stable disease. Median overall survival was 8.8 months. Taken together, these studies suggest that gemcitabine-containing regimens may offer some benefit in treating patients with sarcomatoid variant RCC.
VEGF pathway inhibitors have been tested in combination with chemotherapy agents in other solid tumors with regimens containing bevacizumab showing efficacy in patients with breast, colon, lung and brain cancers.47–52 Michaelson et al studied the combination of sunitinib plus gemcitabine in 34 patients with advanced RCC and noted antitumor activity in 19.53 Of the 9 patients with poor risk or high grade RCC, 5 experienced a partial response. Grade 4 adverse events, including 1 myocardial infarction, 1 pulmonary embolism, and 2 patients with severe neutropenia, were observed in 4 patients. Results of this phase I study suggest that sunitinib in combination with gemcitabine may be active in patients with poor risk profiles and/or sarcomatoid histology, and a phase II study is underway to more clearly assess the efficacy of this combination.
Expanded access trials in RCC
Expanded access trials of sunitinib and sorafenib have included large number of patients, many of whom would be ineligible for participation in the more restrictive pivotal trials geared toward drug approval. Although these studies are not structured randomized controlled trials, they do provide valuable data regarding drug efficacy and tolerability in a patient population that is more reflective of community practice. Additionally expanded access trials are often large enough to provide efficacy data in subpopulations, such as those with poor prognostic features. Results, however, must be interpreted with caution given the study design. There are fewer requirements on study data parameters than in phase II and III clinical trials and thus data collected on response to therapy or toxicity may be incomplete.
The safety and efficacy of sunitinib was studied in an expanded access trial reported by Gore and colleagues in 2009.43 This study enrolled 4564 patients and included both previously treated and untreated participants. Only those who the investigator judged would not derive clinical benefit from sunitinib were excluded, and all participants had confirmed metastatic RCC, adequate organ function, and resolution of acute toxic effects of prior therapy. Tumor measurements and clinical assessments were not specified in the protocol but were performed according to local standards. Seven percent of study participants had brain metastases, 13% with ECOG performance status 2 or higher, 13% with nonclear cell RCC, 32% were aged 65 years or older, and 9.8% met criteria for poor prognosis by MSKCC criteria.
Of the 3464 patients evaluated, the overall response rate was 17%. Among those with brain metastases the overall response rate was 12%, among those with ECOG performance status 2 or more 9%, among those with nonclear cell RCC 11%, and among those aged 65 years or older 17%.43 The incidence of grade 3 and 4 toxicities was comparable in the poor prognosis subgroups and overall population. Additionally, stable disease for at least 3 months was seen in 52% of patients with brain metastases, 52% of those with ECOG performance status 2 or more, 57% of those with tumors displaying nonclear cell histology, and 60% of those aged 65 years or older. Of the 373 patients meeting poor risk criteria by MSKCC criteria, median progression-free survival was 4.1 months (95% CI, 3.1 to 5.0) and median overall survival 5.3 months (95% CI, 4.6 to 6.4). This result is comparable to that seen in the phase III study comparing sunitinib to IFN37,41 in which final analysis showed a median overall survival of 5.3 months in patients with poor risk features.41 Although sufficiently large randomized data is lacking, this expanded access study data suggest that sunitinib is a reasonable treatment option for patients with poor prognostic features. Patient survival, however, was still considerably shorter than in the general study population as a whole.
Brain metastases develop in about 10% of patients with metastatic RCC.54 Sunitinib has been shown to penetrate the central nervous systemic in animal studies.55 Seven percent of participants in the expanded access trial had brain metastases, and the toxicity profiles were similar among them as compared to the overall study population.43 However, it is notable that as of the data cutoff date, patients with brain metastases had received fewer treatment cycles than the overall study population. While antitumor activity of sunitinib and other VEGF inhibitors against brain metastases has been reported, a definitive role for VEGF-receptor inhibition in the treatment or prophylaxis of brain metastases has not been established.
An expanded access trial of sorafenib reported by Riechelmann et al enrolled 58 patients, including 14% poor risk according to the MSKCC prognostic index.56 Median progression-free survival was 7.5 months (95% CI, 5.4 to 11.3 months). Among the 54 patients reported on, partial responses were seen in 20%, stable disease for ≥6 months in 28%, and progressive disease at 8 weeks in 18%. Although results were similar to those reported in the phase III trial of sorafenib,57 toxicities were greater with 64% of participants experiencing a grade 3 or 4 event.56 By univariate analysis, poor prognostic factors, including abnormal creatinine clearance, age, performance status, line of treatment, and presence of significant comorbidities, were not associated with increased likelihood of grade 3 or 4 adverse events.56 These results imply that sorafenib may be effective in patients with poor risk features and that toxicities of treatment are not greater in this patient population.
The Advanced Renal Cell Carcinoma Sorafenib (ARCCS) trial in North America included 2488 participants.58 Inclusion criteria included adequate treatment of brain metastases and ECOG performance status 0 to 2, although waivers to participate were granted for patients with ECOG performance status 3 or 4. Exclusion criteria included treatment within the prior 4 weeks, life expectancy less than 2 months, uncontrolled hypertension, or renal failure requiring dialysis. Of 2488 enrolled, 1850 were evaluated for response. Unconfirmed results reported 17.5% partial response, and confirmed results showed 0.1% complete response, 3.6% partial response, 79.9% stable disease, and 16.4% progressive disease. Specific information has yet to be reported on sorafenib activity in the subset of patients with poor risk features.
Future directions
Based on an improved understanding of RCC tumor biology, targeted therapies are being developed and outcomes are improving substantially for patients with advanced RCC. Clinical and pathologic criteria can be used to identify patients with poor prognosis. While patients with poor risk features have a more limited outcome, treatment with temsirolimus has led to meaningful improvements in overall survival in this population making this the standard of care. Definitive phase III trial data are lacking for the VEGF pathway inhibitors in this patient population. Nonetheless, current available data from subsets of phase III trials, retrospective analyses, and expanded access studies, suggest that patients with poor prognostic features tolerate VEGF pathway blockade reasonably well and likely achieve some benefit relative to patients treated with interferon. Nonetheless progression-free survival and overall survival for this population remain poor on VEGF pathway inhibitors relative to those with more favorable disease prognostic criteria. Therefore, more clinical investigation in this patient population is clearly necessary. Recent correlative science studies have begun to identify molecular features associated with poor prognosis RCC that may help to define novel treatment approaches specific for this patient population. In the meantime, VEGF pathway inhibitors, including sunitunib, can be considered as alternatives to temsirolimus in untreated and treatment refractory patients with poor risk features.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lam JS, Leppert JT, Belldegrun AS, Figlin RA. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23(3):202–212. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 3.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 7.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 8.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 9.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 10.Negrier S, Gomez F, Douillard JY, et al. Prognostic factors of response or failure of treatment in patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. World J Urol. 2005;23(3):161–165. doi: 10.1007/s00345-004-0467-z. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110(3):543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 12.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 13.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19(6):1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 14.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23(12):2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 15.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 16.Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;98(12):2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 17.Mani S, Todd MB, Katz K, Poo WJ. Prognostic factors for survival in patients with metastatic renal cancer treated with biological response modifiers. J Urol. 1995;154(1):35–40. [PubMed] [Google Scholar]
- 18.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol. 2001;25(3):275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Escudier B, Droz JP, Rolland F, et al. Doxorubicin and ifosfamide in patients with metastatic sarcomatoid renal cell carcinoma: a phase II study of the Genitourinary Group of the French Federation of Cancer Centers. J Urol. 2002;168(3):959–961. doi: 10.1016/S0022-5347(05)64551-X. [DOI] [PubMed] [Google Scholar]
- 20.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27(5):612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173(5):1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 22.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25(30):4757–4764. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 23.Bui MH, Visapaa H, Seligson D, et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171(6 Pt 1):2461–2466. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 24.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9(2):802–811. [PubMed] [Google Scholar]
- 25.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11(10):3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Vaziri SA, Jaeger E, et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180(3):860–865. doi: 10.1016/j.juro.2008.05.015. discussion 865–866. [DOI] [PubMed] [Google Scholar]
- 27.Smits KM, Schouten LJ, van Dijk BA, et al. Genetic and epigenetic alterations in the von hippel-lindau gene: the influence on renal cancer prognosis. Clin Cancer Res. 2008;14(3):782–787. doi: 10.1158/1078-0432.CCR-07-1753. [DOI] [PubMed] [Google Scholar]
- 28.Patard JJ, Fergelot P, Karakiewicz PI, et al. Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer. 2008;123(2):395–400. doi: 10.1002/ijc.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horiguchi A, Oya M, Uchida A, Marumo K, Murai M. Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol. 2003;169(2):710–713. doi: 10.1097/01.ju.0000038952.59355.b2. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann NE, Sheinin Y, Lohse CM, et al. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma. Cancer. 2008;112(7):1471–1479. doi: 10.1002/cncr.23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson RZX, Hohse CM, et al. Serum soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. 2008 Genitourinary Cancers Symposium; 2008. abstr 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson RH ZX, Lohse CM, et al. Evaluation of soluble B7x as a serum marker in patients with clear cell renal cell carcinoma. J Clin Oncol; 2008 ASCO Annual Meeting; 2008. May, abstr 5052. [Google Scholar]
- 33.Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer. 2007;110(11):2468–2477. doi: 10.1002/cncr.23056. [DOI] [PubMed] [Google Scholar]
- 34.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 35.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 38.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 39.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26(33):5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sternberg CN, Szczylik C, Lee E, et al. A randomized, double-blind, phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC) J Clin Oncol. 2009;27(15s) Abstr 5021. [Google Scholar]
- 41.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 43.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 44.Rini BI, Vogelzang NJ, Dumas MC, Wade JL, 3rd, Taber DA, Stadler WM. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18(12):2419–2426. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 45.Nanus DM, Garino A, Milowsky MI, Larkin M, Dutcher JP. Active chemotherapy for sarcomatoid and rapidly progressing renal cell carcinoma. Cancer. 2004;101(7):1545–1551. doi: 10.1002/cncr.20541. [DOI] [PubMed] [Google Scholar]
- 46.N Haas JM, Pins M, Liu G, et al. ECOG 8802: Phase II trial of doxorubicin (Dox) and gemcitabine (Gem) in metastatic renal cell carcinoma (RCC) with sarcomatoid features. Genitourinary Cancers Symposium; 2009. abstr 285. [Google Scholar]
- 47.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 48.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 49.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 50.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 51.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 52.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaelson AS, Ryan D, McDermott D, et al. A phase I study of sunitinib in combination with gemcitabine in advanced renal cell carcinoma and other solid tumors. Genitourinary Cancers Symposium 2008; abstr 362. [Google Scholar]
- 54.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 55.Patyna S, Laird AD, Mendel DB, et al. SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol Cancer Ther. 2006;5(7):1774–1782. doi: 10.1158/1535-7163.MCT-05-0333. [DOI] [PubMed] [Google Scholar]
- 56.Riechelmann RP, Chin S, Wang L, et al. Sorafenib for metastatic renal cancer: the Princess Margaret experience. Am J Clin Oncol. 2008;31(2):182–187. doi: 10.1097/COC.0b013e3181574084. [DOI] [PubMed] [Google Scholar]
- 57.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 58.JJ Knox RAF, Stadler WM, McDermott DF, et al. The Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial in North America: Safety and efficacy. J Clin Oncol; 2007 ASCO Annual Meeting Proceedings; 2007. Jun, 18S. [Google Scholar]
- 59.Bukowski RM. Prognostic factors for survival in metastatic renal cell carcinoma: update 2008. Cancer. 2009;115(10 Suppl):2273–2281. doi: 10.1002/cncr.24226. [DOI] [PubMed] [Google Scholar]