Abstract
Chronic pain is mainly a result of two processes: peripheral and central sensitization, which can result in neuroplastic changes. Previous psychophysical studies suggested a decrease of the so-called pain-inhibiting-pain effect (DNIC) in chronic pain patients. We aimed to study the DNIC effect on the neuronal level using magnetoencephalography and electroencephalography in 12 patients suffering from advanced unilateral knee osteoarthritis (OA). DNIC was induced in patients by provoking the typical OA pain by a slightly hyperextended joint position, while they received short electrical pain stimuli. Although the patients did not report a reduction of electrical pain perception, the cingulate gyrus showed a decrease of activation during provoked OA pain, while activity in the secondary somatosensory cortex did not change. Based on much stronger DNIC induction at comparable intensities of an acute counterirritant pain in healthy subjects this result suggests a deficit of DNIC in OA patients. We suggest that the strength of DNIC is subject to neuronal plasticity of descending inhibitory pain systems and diminishes during the development of a chronic pain condition.
Keywords: counterirritation, DNIC, osteoarthritis, chronic pain, EEG, MEG
Introduction
The role of the brain in chronic pain conditions remains speculative. Although chronic pain of the musculosceletal system such as osteoarthritis (OA) is one of the most frequent reasons for permanent impairment in elderly people, the neurobiological mechanisms of chronification remain vague. Evidence suggests that peripheral sensitization and cortical reorganization, so-called functional plasticity, may play a role in chronic pain patients. In OA patients, spontaneous pain and stiffness as well as pain provoked by movements are caused by recurrent stages of activated inflammation in OA (Benito et al 2005; Bonnet and Walsh 2005). The provoked types of pain, ie, enhanced painfulness of nociceptive stimuli (hyperalgesia) and painfulness of non-nociceptive stimuli (allodynia) are signs of both peripheral sensitization of afferent fibers of the joint capsule and central sensitization of projecting neurons in the spinal cord. The mechanisms of chronic pain are, however, largely unknown and are believed to depend on spinal (Sandkuhler and Liu 1998) and cortical processes (Ramachandran 1993; Bromm et al 2000; Flor 2000, 2003; Melzack et al 2001).
Chronic pain has also been suggested to be caused by a deficit of endogenous antinociceptive systems. In particular persistent noxious stimulation is known to trigger endogenous antinociceptive systems via descending pathways. In experiments exploring this phenomenon, two painful stimuli are usually applied concomitantly at different sites, one being tonic, sometimes referred to as heterotopic conditioning noxious stimulus (HCNS), the other being phasic and used to probe the inhibition effect mediated by tonic pain. Psychophysical studies in healthy volunteers used various types of experimental HCNS (eg, thermal, mechanical, electrical) to reduce the perception of pain due to a phasic test stimulus at remote stimulus sites (Pertovaara et al 1982; Chen et al 1985; Talbot et al 1987; Price and McHaffie 1988; Reinert et al 2000). Le Bars and colleagues (1979a, 1979b) suggested a spinal and supraspinal circuitry of ascending and descending pathways as anatomical correlates of the “pain-inhibits-pain” phenomenon and labeled it “diffuse noxious inhibitory controls” (DNIC).
Animals subjected to acute inflammatory knee arthritis also exhibit DNIC effectively (Danziger et al 2001; Danziger et al 1999; Le Bars et al 1979a; Le Bars et al 1979b; Schaible et al 1991; Villanueva et al 1984; Witting et al 1998). In addition several authors implicated a time dependant attenuation of DNIC-like phenomena as cause of chronic pain in low back pain, temporomandibular disorder, fibromyalgia, and osteoarthritis (Kosek and Ordeberg 2000a, 2000b; Leffler et al 2002a, 2002b). Danziger and colleagues (1999) used C-fiber-evoked spinal responses to demonstrate a lack of DNIC in chronic but not acute experimental monoarthritis in the rat. Kosek and Orderberg (2000b) observed absence of pressure pain inhibition but normal thermal pain inhibition in presence of counterirritation in OA patients.
The aim of the present study was to investigate the magnitude of counterirritation effects (DNIC function) in OA patients at the subjective level (pain ratings) and at the central nervous level using multichannel electroencephalogram (EEG) and magnetencephalogram (MEG). OA pain was provoked for a definite time as heterotopic counterirritant against intracutaneous electrical pain stimuli that were also recorded under control conditions.
Experimental procedure
Patients
A total number of 12 patients (3 male, 9 female) with a mean age of 61.4 ± 10.5 years, a mean weight of 83.4 ± 12.4 kg and a mean length of 167.2 ± 9.0 cm suffering from painful OA of one knee were recruited for this study. The following clinical parameters were defined as inclusion criteria: i) patients with unilateral OA of the knee, ii) radiological grade IV OA based on X-ray, iii) pain for a minimum of 6 months limiting walking distance, and iv) presence of pain phases at rest. In order to have a maximally homogeneous group regarding the severity of complaints we included only patients who were scheduled for an intraarticular infiltration on behalf of their conservative treatment. In addition to these criteria patients were selected for the study when instantaneous relief from OA pain could be obtained by a comfortable, slightly flexed knee position. This allowed us to examine the patient with and without ongoing OA pain, an important feature of our study design regarding the internal control condition (see below). To exclude other influences than osteoarthitis on pain processing the following exclusion criteria were defined: i) Any previous spine surgery, ii) any surgery or diseases of the peripheral and central nervous system; iii) infectious, inflammatory or neoplastic diseases; iv) epidural injections within one week prior to investigation; v) bilateral osteoarthrosis or pain from other causes (migraine, neuropathy etc).
Participation in the study was voluntary and patients were assured in writing that refusal to participate would not affect their care in any way. The patients were free to withdraw from the study at any time. Ethical approval of the protocol was obtained by the local ethics committee. This study was conducted in accordance with the Declaration of Helsinki.
Clinical assessment
To prove absence of sensory deficits all subjects were clinically investigated in detail before starting the experimental procedure. Mechanosensibility was tested using a tuning folk (128 Hz) for vibration threshold and calibrated Semmes Weinstein nylon filaments for pressure sensibility. Thermosensibility was tested by repeated short (0.5 s) and long (3 s) contacts with cold (22 °C) and warm (43 °C) test tubes. Quantitative testing of knee function was performed by the Aichroth score which comprises 11 parameters each ranging between 0 (none) and 5 (maximum severity) thus yielding a maximum sum score of 55.
Phasic test stimulus
Pain-relevant evoked potentials and magnetic fields were induced by intracutaneous application of brief (20 ms), slightly painful electrical pulses at the fingertip (middle finger) according to the method described by Bromm and Meier (1984) (Scharein and Bromm 1998). Stimuli were presented at the middle finger contralateral to the OA side. The total session included four blocks of a standardized protocol consisting of 60 single stimuli delivered at randomized interstimulus intervals (8 to 12 s) and intensities at 2-fold individual pain threshold. Thus, each block lasted for 10 min, interblock intervals were 10 (blocks 1–3) and 20 min (blocks 3–4), the latter to allow intraarticular injection in 6 patients (see below). Randomization of interstimulus intervals served to stabilize a constant vigilance level of the subjects during the session (Bromm and Lorenz 1998). Sensation (ST) and pain threshold intensity (PT) were determined before the first block by three series of stimuli each ascending and descending in steps of 0.02 mA between below ST and 0.1 mA above PT. Thus, thresholds were calculated by averaging six values at which the stimulus was either first noted at all (NRS = 1; ST) or as a beginning pinprick-like pain (NRS = 4; PT) during ascending series or as no longer noticeable (NRS = 0; ST) or no longer painful (NRS < 4; PT) during descending series.
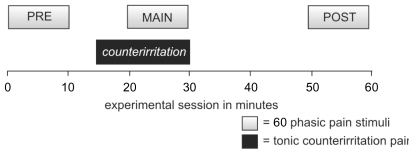
Experimental sessions followed an identical time schedule to minimize influences of circadian rhythm. The first block of the session was made to familiarize patients with the experimental protocol. Data of this block were discarded. The second block (Pre) was used to obtain baseline values. The next block (MAIN) was recorded in the presence of a painful counterirritating knee pain, for duration of 10 min. Finally a last block (POST) was performed after complete relief of pain. The sequence of events and procedures is illustrated in Figure 1. Pain ratings of each single stimulus were obtained by a standardized procedure. Three seconds after each stimulus a tone (1000 Hz, 80 dB SPL, 0.5 s) prompted subjects to rate their sensations on a numerical ranking scale (0–10) with values of 4 and more denoting increasing pain.
Figure 1.
Four blocks of a standardized protocol were applied. The first block was only used to allow familiarization and habituation and was discarded. Then this habituation block was followed by block PRE. During the MAIN block a tonic counterirritation-pain (knee-pain induced by knee stress) was applied concurrently with the 60 phasic pain stimuli. This counterirritation was released and pain had disappeared before the POST block started.
Counterirritation stimulus
A target feature of our design was to study patients during PRE and POST without (counterirritation off) and during MAIN with tonic pain (counterirritation on). Therefore, during “counterirritation off ”, patients were examined in a relaxed supine position with the affected knee rested slightly flexed as favored to completely avoid pain. Before the POST block (“counterirritation off ”) half of the patients received an additional injection of a local anesthetic (10 ml of Bupivacaine® 0.5% plus triamcinolon 40 mg) into the joint to evaluate any putative contribution of ongoing peripheral nociceptive input. These injections were anyhow part of the planned treatment and performed also if the patients declined study participation. During “counterirritation on”, the patients knee was rested into a position of slight hyperextension by loading it with a sandbag (2 kg), which provoked the typical OA pain contralateral to the phasic pain. This procedure established an aching unpleasant type of pain during the MAIN block.
Evoked brain activity
The combined application of EEG and MEG is favorable because MEG is much more sensitive than EEG to measure activity of the secondary somatosensory cortex (SII) after pain stimuli due to the tangential orientation of its equivalent current dipole (ECD) located in the parietal operculum (Bromm 2001; Timmermann et al 2001). In contrast, the radial orientation of the ECD after pain stimuli renders the EEG more sensitive than MEG to locate pain-relevant cingulate activity (Bromm et al 2000). Thus, the combined analysis of MEG and EEG allowed us to examine whether “pain inhibits pain” effects occur differentially among sensory and limbic projection systems of nociceptive processing.
EEG measurements
The EEG was recorded at 64 positions of the extended 10–20 system using two 32 channel amplifier (Synamp, Neuroscan, bandpass 0.3–300 Hz), referenced to linked earlobes and digitized online with a sampling rate of 200 Hz. The impedance of all electrodes was kept below 5 kΩ. A total number of 60 peri-stimulus segments (duration 2500 ms) per block were averaged off-line according to the onset of the painful electrical stimulus. Vertical and horizontal electrooculograms were used to reject epochs contaminated by artifacts due to blinks and eye movements (max. 10%).
We focused our analysis on the N1 and P2 components of the evoked potentials in the EEG and the magnetic counterpart of N1, subsequently referred to as M1, in the MEG (see below). The P2 amplitude at the vertex (Cz) electrode has been demonstrated as a very sensitive parameter for the objective quantification of analgesic drug efficacy in a number of studies (Scharein and Bromm 1998).
MEG measurements
Pain-related magnetic brain activity was measured as stimulus induced changes in the MEG. The MEG was recorded by a 31 sensor dewar system (Phillips, Hamburg), centered over the C3 or C4 position of the temporo-parietal cortex of the hemisphere contralateral to the body site of intracutaneous electical stimulation. Data were recorded with a neuroscan synamp amplifier with a bandpass filtering of 0.3–70 Hz (24 dB/octave), digitized online with a sampling rate of 200 Hz and 12-bit resolution. Pre- and poststimulus segments of 2500 ms were controlled for artifacts and averaged over the 60 stimuli per block. A mean global field curve (MGF) was calculated by averaging all rectified data. For each subject the peak value was estimated in a defined time window from 80 to 160 ms reflecting the M1 value.
Brain source localization
Equivalent current dipole modeling
After visual artifact control (on the average less than 10% of all recordings were discarded) a common mode rejection for MEG and a common average reference for EEG were performed, which has to be done to suppress backround noise especially in MEG data and to calculate the global field/potential power also. Then we focused the analysis of EEG potentials on a time range from 0–500 ms post stimulus when the N2-P2 components of pain-evoked potentials are generated in the mid cingulate gyrus (Bromm and Lorenz 1998; Kakigi et al 2000; Bentley et al 2003; Vogt 2005). A time-window ranging from 0 to 500 ms was used for MEG data to reconstruct dipolar activity within SII (Kakigi et al 1999; Timmermann et al 2001). The averaged responses were subjected to a single fixed dipole approximation, which is sufficient for SII and cingulate gyrus evaluations as known from earlier studies (Scharein and Bromm 1998). In a relative maximum of the mean global field power (GFP) between 80 and 160 ms for MEG and between 140 and 340 ms for EEG with a goodness of fit (GOF) better than 90%. The only anatomical restrictions for the sources were that their location had to be in the brain morphology, defined by a binary overlay, which was calculated from the individual MRI scans. The boundary element method (BEM) and a 3-shell-sphere model served as volume conductors for EEG and MEG data, respectively using the CURRY© software (Philips® Research, Hamburg, Germany). For the estimation of the mean coordinates for all subjects the x coordinate was flipped to calculate mean coordinates over subjects. In a last step all dipole coordinates were transformed into the Talairach coordinate space. In addition a grandmean dataset was also calculated to visualize grandmean waveforms.
Statistical analysis
All statistical analyses were made using SPSS version 9.0 (SPSS inc., Chicago, USA). All psychophysical and physiological parameters were first subjected to a one-sample Kolmogorov-Smirnov test for normal distribution and then analyzed with paired t tests to compare the effect of counterirriation on the painratings, EEG and MEG electrode/sensor data and source reconstruction results.
Results
Patients receiving intraarticular injections of local anesthetics before the POST block did not differ in pain ratings and neurophysiological parameters from patients not receiving this treatment. We regard this result as evidence that there was no significant contribution of ongoing peripheral nociceptive input that could have influenced the results in the POST block. We therefore performed all subsequent analysis pooling these subgroups together.
The patients had a mean Aichroth score value of 36.5 ± 3.7 points indicating that we recruited a homogenous collective presenting functional disability by OA.
Pain ratings
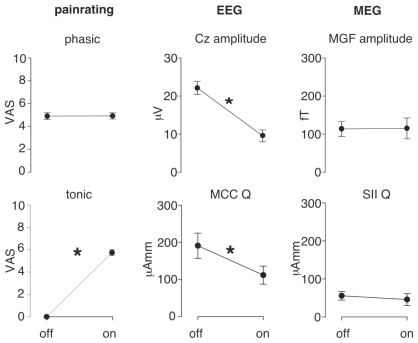
Patients rated the intensity of tonic pain induced at the knee with a mean value of 5.83 ± 0.94. The mean intensity of phasic pain stimuli was (VAS) 4.91 ± 1.01 for “counterirrtation off ” and 4.93 ± 0.93 during counterirriation. This difference was not significant (T11 = 0.093; p = 0.93) (Figure 4).
Figure 4.
Statistical results for all subjective and objective parameters. Phasic painratings were not affected during counterirritation. EEG Cz amplitude and the mid-cingulate cortext (MCC) dipole strength (Q) showed significant reduction during counterirritation (“on”). On the other side MEG mean global field peaks (MGF) amplitude and secondary somatosensory cortex (SII) dipolestrength (Q) were not affected by the counterirritation.
EEG and MEG measures
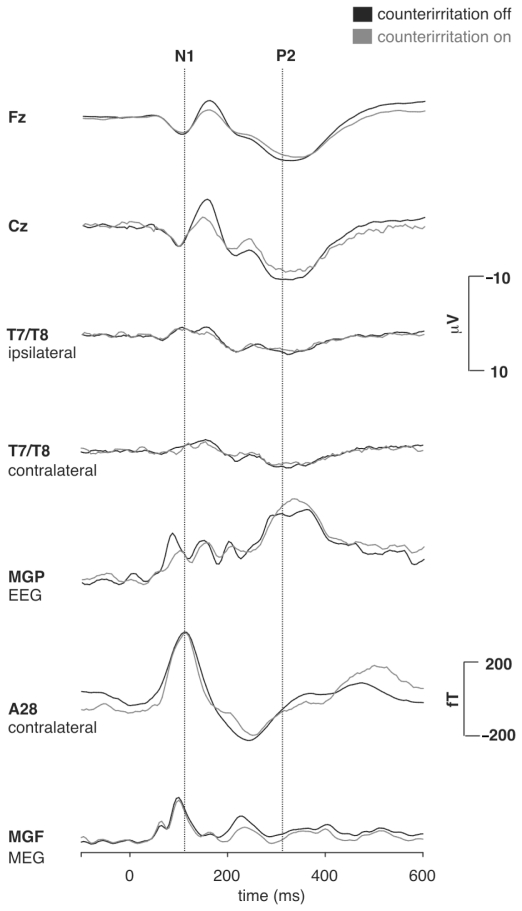
The intracutaneous electric stimuli elicited a major N2-P2 component in the EEG with maximum amplitude over the vertex electrode (Cz). An earlier N1 component of smaller amplitude than the vertex N2-P2 occurred maximally over the temporo-parietal cortex contralaterally to the stimulated hand (T7 or T8 for respective right or left hand stimulation). It exhibited a phase reversal over frontal sites (Fz). The magnetic counterpart of N1, ie, M1, represented the major component of the average MEG waveform as it coincided with the peak of the mean global field power (MGF) curve at approximately 105 ms post-stimulus. There was no equivalent magnetic counterpart of the electrical N2-P2 component (Figure 2).
Figure 2.
Grand mean average waveforms for the EEG channels (upper 5 traces: Fz, Cz, T7, T8, and MGP; = mean global potential curve) and MEG (lower 2 traces: SQUID sensor A28 and MGF; = mean global field curve). Patients showed a decrease of the EEG N2/P2-component during counterirritation, whereas no change occurred in the EEG N1 and the MEG M1 during counterirritation.
Figure 4 summarizes the effect of counterirritation on the electric N2/P2 components and the effects on the magnetic M1 component and on dipoles and psychophysics as well. It demonstrates a significant amplitude reduction of the N2/P2-Amplitude (from 22.2 ± 6.0 μV to 9.6 ± 5.4 μV, T11 = −6.0; p < 0.001) from counterirritation-off compared to counterirritation-on. No counterirritation effect occurred in the electric N1 (from −2.24 ± 1.92 mV to −2.46 ± 1.93 mV, T11 = −0.89; p < 0.39) or magnetic M1 components (from 115.0 ± 82.3 fT to 113.7 ± 60.0 fT, T11 = 0.062; p < 0.95) in either measurement.
Source reconstruction results confirmed the results already made on the sensor level (Figure 3). The differential sensitivity of MEG and EEG towards respective N1 and P2 components is confirmed by results of the dipole reconstruction analysis (Figure 3). It yielded a tangentially oriented generator of N1/M1, ideally reconstructed from the magnetic field maps, within the parietal operculum compatible with the SII, and a radially oriented generator of P2, ideally reconstructed from the electric potential map, within the caudal portion of the mid cingulate cortex (MCC). The EEG P2 could be modeled by a fixed dipole located in the right MCC with the coordinates (x = 16.9 ± 21.8; y = −17.8 ± 28.9; z = 41.2 ± 38.5) and a goodness of fit higher than 90%. Dipole strength of the MCC activity measured with the EEG was significantly influenced by counterirritation (from 190.8 ± 117.3 μAmm to 111.6 ± 84.1 μAmm, T11 = −2.47; p < 0.05). This effect was due to significantly smaller MCC activation during MAIN (“counterirritation on”) compared to the mean of PRE and POST (“counterirritation off ”). The MEG M1 could be modeled with a fixed dipole in the contralateral SII cortex with the coordinates (x = 56.5 ± 22.9; y = −24.0 ± 23.4; z = 24.8 ± 21.6) and a goodness of fit higher than 90%. Furthermore, the SII dipole strength measured with the MEG were not significantly different due to the counterirritation (55.5 ± 33.8 μAmm to 45.7 ± 47.1 μAmm, T11 = −0.72; p < 0.49).
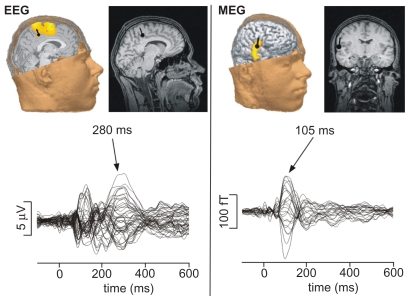
Figure 3.
Dipole reconstruction with current density of a single patient (EM) with the corresponding butterfly plots of all channels after painful electrical finger stimulation (60 stimuli) without counterirritation. Maximal EEG activity (P2; 280 ms) could be explained with a GOF > 95% by a single fixed dipole localized in the mid cingulate gyrus with the coordinates x = 2.5; y = −12.9; z = 40.5. MEG activity (N1-correlate) after 105 ms yielded in a single dipole in the contralateral SII-cortex (GOF > 95%) with the coordinates x = 59.7; y = −30.1; z = 15.6.
Discussion
The present study examined the modulatory effect of tonic knee pain stimulation, referred to as noxious counterirritation, upon the painfulness of a concurrent phasic stimulus in OA patients. We analyzed the effects of a transient provocation of their typical pain due to OA through forcing the joint into a hyperextended position by loading it with a sandbag. After this manipulation, knee pain was eliminated by either removing the sandbag or additional injection of an anesthetic into the knee. Phasic pain sensation was probed by repetitive intracutaneous electrical stimuli applied to the tip of the middlefinger of the contralateral hand. In different blocks with and without noxious counterirritation pain ratings and evoked EEG potentials and MEG fields following the painful electrical stimuli were evaluated.
The major result was that patients did not experience a reduction of phasic pain during the presence of the noxious counterirritant. However, they yielded a significant reduction of EEG activity in MCC activity that generates the pain-evoked P2 component at the vertex (Cz electrode) following the electrical stimuli. In contrast, the pain induced SII activity generating the electric N1 and magnetic M1 component remained unaffected during counterirritation. At comparable levels of tonic pain intensity (visual analogue scale 5 to 6) as in our study healthy subjects generally perceive concurrent phasic pain significantly less intense and additionally show a much stronger “pain-inhibits-pain” effect upon pain-evoked potentials, such as the P2 elicited by intracutaneous electrical stimulation (Chen and Treede 1985; Reinert et al 2000). The relatively small effect of counterirritation in our OA patients is consistent with findings of Kosek and Orderberg (2000a, 2000b) who observed a lack of counterirritation effects in OA patients that normalized after surgical pain relief. Similar deficits in DNIC were observed in other chronic conditions of musculo-skeletal pains (Peters et al 1992; Leffler et al 2002a, 2002b).
Several factors might influence the strength by which a painful phasic stimulus is attenuated by a concurrent tonic pain: tonic pain is typically more unpleasant than phasic pain and might distract the subjects’ attention away from the phasic pain stimulus. This assumption is supported by the finding that later components in the pain-evoked potentials that are generated by limbic brain structures such as the cingulate cortex processing cognitive and emotion aspects of pain yield greater attenuation by tonic pain than middle-latency components indicating sensory-discriminative aspects of pain processed in the somatosensory cortex (Lorenz and Garcia-Larrea 2003). It is, therefore, possible that painful counterirritation in experimental studies with normal subjects exerted a stronger cognitive-emotional reaction and distraction from phasic electrical stimuli than did the provoked knee pain in our patients. However, inhibition of phasic pain by noxious counterirritation as explained in the theory of DNIC involves a distinct spino-bulbo-spinal feedback-loop that goes beyond a pure distraction effect (Le Bars et al 1979a, 1979b; Willer et al 1984; Lautenbacher et al 2007). Furthermore, nociceptive input is modulated by multiple endogenous mechanisms coordinated by the periaqueductal grey (PAG) and rostroventral medulla (RVM) network which receives extensive input from the limbic forebrain such as the prefrontal cortex, insular cortex and rostral cingulate gyrus (Basbaum and Fields 1978; Dubner and Ren 1999). It is currently unclear whether these pathways involving supra-spinal sites contribute to DNIC or whether it comprises a more specific pathway as originally suggested. Therefore, we use the term DNIC here more generally as neurophysiologic correlate of the pain inhibiting pain phenomenon.
Previous studies suggest that the functional difference between acute and chronic monoarthritis may impact on DNIC, attributed to the fact that the populations of activated neurons receiving inputs from the inflamed joint and the centrally projecting neurons might not be the same during the acute and chronic stages of arthritis (Danziger et al 1999, 2001; Ren and Dubner 2002). Chronic pain and inflammation induce a reorganization of the spinal transmission of nociceptive signals, which could modify the triggering of inhibitory controls (Ren and Dubner 1996; Danziger et al 1999, 2001; Hunt and Manthy 2001; Guan et al 2002). Reorganization within the dorsal horn of the spinal cord is generally regarded as important for eliciting pain by nonnoxious stimuli, eg, touch, called mechanical hyperalgesia or allodynia (Ren and Dubner 2002; Dubner 2004). Unlike nociceptive pain that is peripherally conducted by nociceptive Aδ- and C-fibers, mechanoreceptive Aß-fibers conduct allodynia. It is therefore possible that the type and origin of pain according to nociceptive vs allodynic pain projected by different pathways contribute to differences in the recruitment of DNIC. Notably, wide-dynamic-range (WDR-) neurons of lamina V of the spinal dorsal horn that are robustly sensitized by tissue inflammation and injury, and targeted by Aß-input causing allodynia, mainly activate the primary and secondary somatosensory cortex and posterior insula via lateral thalamic nuclei. In contrast, nociceptive-specific (NS) neurons of lamina I and II, targeted by Aδ- and C-fibers, mainly activate the brainstem and cingulate cortex and anterior insula via multisynaptic pathways involving medial thalamic nuclei (Hunt and Manthy 2001). Input to this multisynaptic system from superficial dorsal horn neurons appears to be much more important for the triggering of DNIC than deeper dorsal horn neurons (Le Bars et al 1979a, 1979b; Villanueva et al 1984). It is therefore possible, that the transient exaggeration of OA pain activated the superficial dorsal horn neurons and brainstem-medial thalamic pathway to the MCC significantly less than other forms of counterirritant pain typically applied in healthy subjects, eg, muscle ischemia, heat pain or experimental skin inflammation. This assumption would also explain less recruitment of descending inhibition by chronic than acute pain states.
Dipole analysis of EEG and MEG activity following the phasic intracutaneous electrical stimuli provides further support for a pathway-specific manifestation of DNIC. Our dipole reconstruction results with N1 and P2 components generated in SII and MCC, respectively, is consistent with numerous studies examining pain evoked brain potentials and fields (Bromm and Lorenz 1998; Treede et al 1999; Hauck et al 2006). We observed a selective inhibition at the level of the MCC which receives major input from superficial dorsal horn neurons and medial thalamic pathways that are presumed to be predominantly activated by strong types of counterirritant pains. The SII cortex, which mainly receives input from deeper dorsal horn and lateral thalamic neurons (Hunt and Manthy 2001) accordingly failed to show an association with DNIC.
In addition to the quality of the pain applied as counterirritant, there are constitutional factors that are relevant to explain the weakness or lack of DNIC in chronic OA. Edwards and colleagues (2003) documented a significant attenuation of counterirritation effects in older compared to younger healthy subjects. According to Larivière and colleagues (2007) DNIC deficits appear to start beyond the age of 50. Using multivariate analysis of life quality, health status, and psychological and physiological measures in healthy subjects Edward and colleagues (2003) identified DNIC as a robust predictor of clinical pain and physical health with greater DNIC in people with less pain. Notably, age-dependent differences in DNIC appeared to partially mediate age differences in physical functioning. The effect of sex on DNIC is inconsistent. Some authors failed to find differences of DNIC between female and male healthy subjects (Baad-Hansen et al 2005; Bud et al 2005). Staud and colleagues (2003) observed a gender-specific lack of DNIC upon thermal “wind-up”, ie, women yielded less reduction of temporal summation of repeated heat pain stimuli when the contralateral hand was immersed into hot water. Ge and colleagues (2004) used repeated injections of hypertonic saline into the trapezoid muscle as counterirritant and found that the temporal development of inhibition of phasic pressure pain differed between men and women. In both sexes DNIC diminished over time, but men exhibited longer-lasting DNIC than women. This study is particularly interesting as it shows that repeated ongoing pain states appear to reduce the recruitment of descending inhibition, especially in women. Based on these evidences it is possible that age and sex impact on neuronal plasticity of inhibitory pain systems leading to a generally greater risk for chronic pain in older women. The sample size of our study was, however, too small to statistically estimate any variance of DNIC due to age or sex.
Conclusion
We documented a DNIC dysfunction in chronic pain patients suffering from OA. It is of interest to study comparable DNIC effects in patients with acute knee pain and after prolonged relieve of knee pain for example after knee replacement. The robustness of DNIC as laboratory tool appears promising to study the temporal development of chronic pain with further experiments to finally better understand which patients might benefit at an appropriate time point from surgical interventions.
Acknowledgments
This study is supported by a grant of the Deutsche Forschungsgemeinschaft (Qu 156/1-1). The authors report no conflicts of interest.
References
- Baad-Hansen L, Poulsen HF, Jensen HM, et al. Lack of sex differences in modulation of experimental intraoral pain by diffuse noxious inhibitory controls (DNIC) Pain. 2005;116:359–65. doi: 10.1016/j.pain.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–62. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- Benito MJ, Veale DJ, Fitzgerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DE, Derbyshire SW, Youell PD, et al. Caudal cingulate cortex involvement in pain processing: an inter-individual laser evoked potential source localisation study using realistic head models. Pain. 2003;102:265–71. doi: 10.1016/S0304-3959(02)00405-0. [DOI] [PubMed] [Google Scholar]
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- Bromm B. Brain images of pain. News Physiol Sci. 2001;16:244–9. doi: 10.1152/physiologyonline.2001.16.5.244. [DOI] [PubMed] [Google Scholar]
- Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. 1998;107:227–53. doi: 10.1016/s0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol. 1984;6:405–10. [PubMed] [Google Scholar]
- Bromm B, Scharein E, Vahle-Hinz C. Cortex areas involved in the processing of normal and altered pain. Prog Brain Res. 2000;129:289–302. doi: 10.1016/S0079-6123(00)29021-3. [DOI] [PubMed] [Google Scholar]
- Chen AC, Treede RD, Bromm B. Tonic pain inhibits phasic pain: evoked cerebral potential correlates in man. Psychiatry Res. 1985;14:343–51. doi: 10.1016/0165-1781(85)90102-7. [DOI] [PubMed] [Google Scholar]
- Danziger N, Gautron M, Le Bars D, et al. Activation of diffuse noxious inhibitory controls (DNIC) in rats with an experimental peripheral mononeuropathy. Pain. 2001;91:287–96. doi: 10.1016/S0304-3959(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Danziger N, Weil-Fugazza J, Le Bars D, et al. Alteration of descending modulation of nociception during the course of monoarthritis in the rat. J Neurosci. 1999;19:2394–400. doi: 10.1523/JNEUROSCI.19-06-02394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Weil-Fugazza J, Le Bars D, et al. Stage-dependent changes in the modulation of spinal nociceptive neuronal activity during the course of inflammation. Eur J Neurosci. 2001;13:230–40. doi: 10.1046/j.0953-816x.2000.01375.x. [DOI] [PubMed] [Google Scholar]
- Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Endogenous mechanisms of sensory modulation. Pain Suppl. 1999;6:S45–S53. doi: 10.1016/S0304-3959(99)00137-2. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–65. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Flor H. The functional organization of the brain in chronic pain. Prog Brain Res. 2000;129:313–22. doi: 10.1016/S0079-6123(00)29023-7. [DOI] [PubMed] [Google Scholar]
- Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, et al. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–20. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Ge HY, Madeleine P, Arendt-Nielsen L. Sex differences in temporal characteristics of descending inhibitory control: an evaluation using repeated bilateral experimental induction of muscle pain. Pain. 2004;110(1–2):72–8. doi: 10.1016/j.pain.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hauck M, Bischoff P, Schmidt G, et al. Clonidine effects on pain evoked SII activity in humans. Eur J Pain. 2006;10:757–65. doi: 10.1016/j.ejpain.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hunt S, Manthy PW. The molecular dynamics of pain control. Nat Neurosc. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Watanabe S, Yamasaki H. Pain-related somatosensory evoked potentials. J Clin Neurophysiol. 2000;17:295–308. doi: 10.1097/00004691-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Watanabe S, Yamasaki H, et al. Pain-related brain activities: magnetoencephalographic studies. Electroencephalogr Clin Neurophysiol Suppl. 1999;49:245–9. [PubMed] [Google Scholar]
- Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000a;4:229–38. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000b;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- Lariviere M, Goffaux P, Marchand S, et al. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–10. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979a;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supra-spinal involvement and theoretical implications. Pain. 1979b;6:305–27. doi: 10.1016/0304-3959(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Leffler AS, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain. 2002a;6:149–59. doi: 10.1053/eujp.2001.0312. [DOI] [PubMed] [Google Scholar]
- Leffler AS, Kosek E, Lerndal T, et al. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain. 2002b;6:161–76. doi: 10.1053/eujp.2001.0313. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Melzack R, Coderre TJ, Katz J, et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–74. doi: 10.1111/j.1749-6632.2001.tb05822.x. [DOI] [PubMed] [Google Scholar]
- Pertovaara A, Kemppainen P, Johansson G, et al. Ischemic pain nonsegmentally produces a predominant reduction of pain and thermal sensitivity in man: a selective role for endogenous opioids. Brain Res. 1982;251:83–92. doi: 10.1016/0006-8993(82)91276-8. [DOI] [PubMed] [Google Scholar]
- Peters ML, Schmidt AJ, Van den Hout MA, et al. Chronic back pain, acute postoperative pain and the activation of diffuse noxious inhibitory controls (DNIC) Pain. 1992;50:177–87. doi: 10.1016/0304-3959(92)90159-9. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, et al. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22:3206–14. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, McHaffie JG. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. Pain. 1988;34:245–52. doi: 10.1016/0304-3959(88)90119-4. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc Natl Acad Sci U S A. 1993;90:10413–20. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert A, Treede R, Bromm B. The pain inhibiting pain effect: an electrophysiological study in humans. Brain Res. 2000;862:103–10. doi: 10.1016/s0006-8993(00)02077-1. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–37. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–80. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Neugebauer V, Cervero F, et al. Changes in tonic descending inhibition of spinal neurons with articular input during the development of acute arthritis in the cat. J Neurophysiol. 1991;66:1021–32. doi: 10.1152/jn.1991.66.3.1021. [DOI] [PubMed] [Google Scholar]
- Scharein E, Bromm B. The intracutaneous pain model in the assessment of analgesic efficacy. Pain Rev. 1998;5:216–46. [Google Scholar]
- Staud R, Robinson ME, Vierck CJ, Jr, et al. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101(1–2):167–74. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Duncan GH, Bushnell MC, et al. Diffuse noxious inhibitory controls (DNICs): psychophysical evidence in man for intersegmental suppression of noxious heat perception by cold pressor pain. Pain. 1987;30:221–32. doi: 10.1016/0304-3959(87)91078-5. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Ploner M, Haucke K, et al. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86:1499–503. doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, et al. The cortical representation of pain. Pain. 1999;79:105–11. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Cadden SW, Le Bars D. Diffuse noxious inhibitory controls (DNIC): evidence for post-synaptic inhibition of trigeminal nucleus caudalis convergent neurones. Brain Res. 1984;321:165–8. doi: 10.1016/0006-8993(84)90695-4. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain. 1984;107(Pt 4):107–112. doi: 10.1093/brain/107.4.1095. [DOI] [PubMed] [Google Scholar]
- Witting N, Svensson P, Arendt-Nielsen L, et al. Differential effect of painful heterotopic stimulation on capsaicin-induced pain and allodynia. Brain Res. 1998;801:206–10. doi: 10.1016/s0006-8993(98)00440-5. [DOI] [PubMed] [Google Scholar]