Abstract
Background
Early identification of individuals at risk of developing persistent pain is important to decrease unnecessary treatment costs and disability. However there is scant comprehensive information readily available to assist clinicians to choose appropriate assessment instruments with sound psychometric and clinical properties.
Objective
A national insurer commissioned the development of a compendium of assessment instruments to identify adults with, or at-risk of developing, persistent pain. This paper reports on the instrument identification and review process.
Methods
A comprehensive systematic literature review was undertaken of assessment instruments for persistent pain of noncancer origin, and their developmental literature. Only assessment instruments which were developed for patients with pain, or tested on them, were included. A purpose-built ‘Ready Reckoner’ scored psychometric properties and clinical utility.
Results
One hundred sixteen potentially useful instruments were identified, measuring severity, psychological, functional and/or quality of life constructs of persistent pain. Forty-five instruments were short-listed, with convincing psychometric properties and clinical utility. There were no standard tests for psychometric properties, and considerable overlap of instrument purpose, item construct, wording, and scoring.
Conclusion
No one assessment instrument captured all the constructs of persistent pain. While the compendium focuses clinicians’ choices, multiple instruments are required for comprehensive assessment of adults with persistent pain.
Keywords: persistent pain, assessment, psychometric properties, evidence-base, clinical utility
Background
An evidence-based practitioner integrates the best available research evidence with clinical judgment and patient preferences, when assessing and treating.1 Standardized assessment instruments are available for many health conditions. These initially served as checklists for health practitioners to consider essential elements in history taking.2 Nowadays, they are used to predict disability, assess risk, and identify appropriate treatment strategies.2–4 A comprehensive health assessment should incorporate information from relevant standardized instruments with a sound history, clinical tests and clinical judgment.1–4
This paper deals with assessment instruments for ‘persistent’, or ‘chronic pain’. Different health care providers use different philosophies and techniques when assessing patients with extended pain histories.2–4 Siddall and Cousins5 defined ‘persistent (or extended) pain’ as “pain that exists beyond three months” (p. 511), a definition recently reiterated by Cousins.6 However Blyth, Cousins, and colleagues applied a similar definition to ‘chronic pain’.7–10 For this paper, we use the term ‘persistent pain’ to identify “pain that persisted beyond expected healing times.”
Inappropriate treatment for patients with persistent pain results in higher costs.9–11 There is convincing evidence that early identification of patients at-risk of developing persistent pain reduces disability and increases return-to-work rates.9–14 This paper reports on a systematic literature review that identified and critically appraised assessment instruments for persistent pain. The review was commissioned by the New Zealand Accident Compensation Corporation, New Zealand’s sole, 24-hour, no fault injury insurer and rehabilitation purchaser. This organization is committed to providing health care providers with evidence-based resources. The outcome of this review was a compendium of high quality assessment instruments to assist health practitioners to identify patients with, or at risk of, persistent pain.
Methods
Project purpose
The systematic literature review identified psychometrically sound, clinically-useful assessment instruments for persistent pain, for use by multidisciplinary health care teams, or individual practitioners, in face-to-face or telephone situations, and in different locations (large hospitals, rural practices, primary and secondary health care environments).
Reference group
An expert multidisciplinary reference group assisted the literature review team.
Search strategy
A comprehensive approach was taken to identify persistent pain assessment instruments (Appendix 1). The search identified 1) any published questionnaire, survey, instrument or rating scale developed primarily to assess persistent (noncancer) pain in adults, as well as instruments which had been developed for other purposes, and subsequently tested on this patient group and 2) relevant peer-reviewed published background literature for each identified instrument.
Background literature
Developmental literature reported the instrument’s psychometric properties. This usually reflected results of psychometric testing, the instrument’s performance with different patient groups, and/or validation of instrument translation in other languages. Epidemiological literature reported threshold/cut points or population norms from population-application of the instrument.
Exclusion criteria
Instruments were excluded if they dealt with specific health conditions or body areas, were unavailable in English, were not fully described for clinical application, did not specifically assess noncancer persistent pain, and/or had no background literature.
Critical appraisal
Psychometric properties are reported and interpreted differently.2–4 Terminology differs depending on health discipline, philosophy of pain mechanisms, the research paradigm used, the purpose of testing, and common usage. This review applied a published classification of psychometric tests for consistency of interpretation.4
Project-specific scoring system
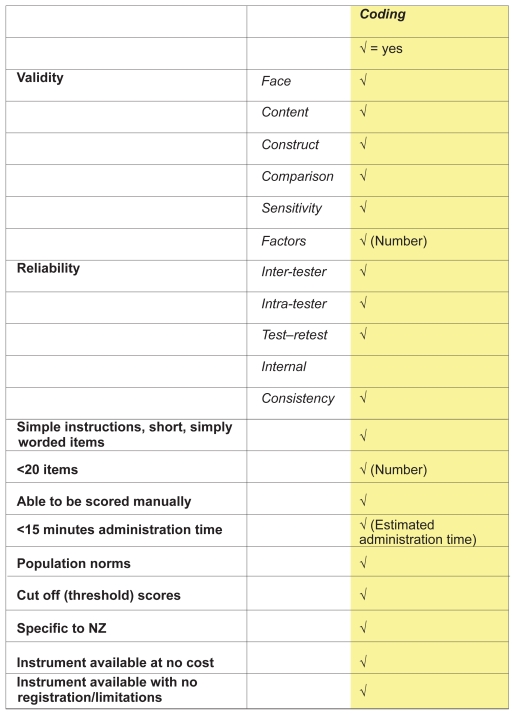
The lack of any published scoring system to rate the quality of assessment instruments for psychometric properties and clinical utility prompted the development of a purpose-built scoring system for this review (Ready Reckoner). This system gave equal weighting to 19 elements of psychometric properties and clinical utility (total score 19) (Figure 1), comprising:
Figure 1.
Ready Reckoner developed to review persistent pain assessment measures.
Evidence of validity testing (face, content and construct validity, sensitivity, factor analysis)
Evidence of reliability testing (inter-tester, intra-tester, test–retest, internal consistency)
Availability of instructions for administration, and ease of literacy
Efficiency of administration using limited number of items
Capacity of manual score
Efficient (short) instrument administration time
Published thresholds/cut points, and/or normative values, specific (or able to be extrapolated) to New Zealand (NZ) persistent pain patients
Relevance to NZ clinical settings, and
No cost, and/or no professional membership, instrument registration or training requirements.
Instrument identification, assessment, and short-listing
Step 1: Finding and collating instruments
A comprehensive list of potentially relevant assessment instruments was identified from iterative searches of the literature following discussions between the project team and expert reference group.
Step 2: Preparation for short-listing
The Step 1 instruments were reviewed and considered for short-listing (Step 3) if they met these conditions:
The instrument was primarily reported for assessment, rather than outcome measurement
At least four psychometric property measures were reported in background literature (reflecting any testing for validity, reliability, sensitivity, factor analysis or comparison with other measures)4
The instrument authors could be contacted.
Step 3: Short-listing
The purpose, psychometric properties, and clinical utility of each instrument was critically considered using the reporting framework4 and the Ready Reckoner. Instruments which survived Step 3 were included in the compendium.
Results
Overview
Instrument classification
Assessment instruments were classified into the category that best described their purpose. These classifications were largely self-reported from the background literature, and comprised pain severity, psychological, functional, or multidimensional assessment of persistent pain, and general health status/quality of life.
Instrument inclusion
Step 1 identified 116 instruments from approximately 350 publications (see row 1 of Table 1, and Appendix 2). In four instrument classification categories, sub-groups of specific instrument purpose were identified.
Table 1.
Consort diagram of instrument inclusion at each step
| Pain severity | Psychological | Functional | Multidimensional | General health/Quality of life | Totals | |
|---|---|---|---|---|---|---|
| Step 1 | CPG, DN4, IDPain, LANSS, LF-MPQ, SF-MPQ, NPQ_LF, NPQ_SF, NPS, NPSI, Unidimensional VAS, NRS, VRS | BDI, BPCI, BPP, CCSI-R, CES-D, CPAQ, CPCI_65, CPCI partner, CPCI_42, CPCI_ abbreviated, CPS-ES, CPVI, CRPP, CSQ_50, CSQ_24, CSQ_R(27), CSQ_abbrev, DAPOS, DRAM, FABQ, FAPS, FPC, HADS, INTRP, K10, MLPC, MPRCQ, MSPQ, PAIRS, PAQ-R, PASS-40, PASS-72, PASS-SF, PBC, PBPI, PBQ, PCI, PCL, PCQ, PCS, PCS-S, PCSI, PDI, P3, PSCQ, PSEQ, PaSolQ, PVAQ, SCL-27, SOPA-35, SOPA-57, SOPA-short, TSK, TSK-11, VMDPMI, ZSRD | BPI_I, COPM, FACS, FASQ, FIE, FIPS, IFS-CP, KSMBJC, OccRQ, PDI, PDQ, PGPQ, PSFS, RADL, RFQ, SRI, WL_26, WLQ | BPI, BPI_SF, DPQ, GPQ, HBQ-P, HIPSPSQ, MRFA, ORQ, ÖMPSQ, PCOQ, PCP:S, PCP:EA, POP, POQ, POQ-42, POQ-SF, POQ-DC-28, POQ-FU-34, POQ-DC-5, VDPQ, WDDI, WHYMPI, WHYMPI_S | EQ-5D, HSQ, NHP, QWBS, WHOQOL, WHOQOLBref, SF-36, SIP | |
| Total | 11 | 56 | 18 | 23 | 8 | 116 |
| Step 2 | CPG, DN4, LANSS, LF-MPQ, SF-MPQ, NPQ_LF, NPQ_SF, Unidimensional | BDI, BPP, CES-D, CPAQ, CPCI_65, CPCI partner, CPCI_42, CPCI_abbreviated, CPVI, CRPP, CSQ_50, CSQ_24, CSQ_R(27), CSQ_abbrev, FABQ, HADS, K10, MPRCQ, MSPQ, PBC, PCI, PSEQ, TSK, TSK-11, ZSRD | BPI_I, COPM, FACS, FASQ, FIPS, OccRQ, PDI, PGPQ, PSFS, RADL, SRI, WL_26, WLQ | BPI, BPI_SF, DPQ, GPQ, ORQ, ÖMPSQ, WDDI, WHYMPI, WHYMPI_S | HSQ, NHP, SF-36, SIP | |
| Total | 8 | 25 | 13 | 9 | 4 | 59 |
| Step 3 | CPG, DN4, LANSS, SF-MPQ, NPQ_LF, NPQ_SF, Unidimensional | BDI, BPP, CES-D, CPAQ, CPCI_65, CPCI partner, CPCI_42, CPCI_abbreviated, CPVI, CRPP, CSQ_24, FABQ, HADS, K10, MSPQ, PBC, PCI, PSEQ, TSK-11, | BPI_I, COPM, FACS, FIPS, OccRQ, PDI, PGPQ, PSFS, RADL, SRI, WLQ | BPI_SF, GPQ, ORQ, ÖMPSQ, WHYMPI, WHYMPI_S | NHP, SF-36 | |
| Total | 7 | 19 | 11 | 6 | 2 | 45 |
Abbreviations: BDI, Beck Depression Inventory; BPCI, Brief Pain Coping Inventory; BPI (BPI_SF), Brief Pain Inventory; BPI_I, Brief Pain Inventory-Interference; BPP, Biobehavioral Pain Profile; CCSI-R, Cognitive Coping Strategies Inventory- Revised; CES-D, Center for Epidemiologic Studies-Depression Scale; COPM, Canadian Occupational Performance Measure; CPAQ, Chronic Pain Acceptance Questionnaire; CPCI_65, CPCI_partner, CPCI_42, CPCI_ abbreviated, Chronic Pain Coping Inventory suite; CPG, Chronic Pain Grade; CPS-ES, Chronic Pain Self-efficacy Scale; CPVI, Chronic Pain Values Inventory; CRPP, Cognitive Risk Profile for Pain; CSQ_50, CSQ_R(27), CSQ24, CSQ_abbreviated, Coping Strategies Questionnaire; DAPOS, Depression, Anxiety and Positive Outlook; DN4, Douleur Neuropathique 4; DPQ, Dartmouth Pain Questionnaire; DRAM, Distress and Risk Assessment Method Pain; EQ-5D, EuroQol; FABQ, Fear-Avoidance Beliefs Questionnaire; FACS, Functional Abilities Confidence Scale; FAPS, Fear Avoidance of Pain Scale; FASQ, Functional Assessment Screening Questionnaire; FIE, Functional Interference Estimate; FIPS, Family Impact of Pain Scale; FPC, Fear of Pain Comparisons; GPQ, Glasgow Pain Questionnaire; HADS, Hospital Anxiety and Depression Scale; HBQP, Health Background Questionnaire for Pain; HIPSPSQ, Hunter Integrated Pain Service (HIPS) Patient Screening Questionnaire; HSQ, Health Status Questionnaire 2.0; IFS-CP, Inventory of Functional Status – Chronic Pain; INTRP, Inventory of Negative Thoughts in Response to Pain; K10, Kessler Psychological Distress Scale; KSMBJC, Kempsey Survey of Muscle, Joint and Bone Conditions; LANSS, Leeds Assessment of Neuropathic Symptoms and Signs; MLPC, Multidimensional Locus of Pain Control Questionnaire; MRFA, Medical Rehabilitation Follow Along measure – Musculoskeletal Form; MPQ_LF, McGill Pain Questionnaire Long Form; MPQ_SF, McGill Pain Questionnaire Short Form; MPRCQ, Multidimensional Pain Readiness to Change Questionnaire; MSPQ, Modified Somatic Perception Questionnaire; NHP, Nottingham Health Profile; NPQ_LF, Neuropathic Pain Questionnaire Long Form; NPQ_SF, Neuropathic Pain Questionnaire Short Form; NPS, Neuropathic Pain Scale; NPSI, Neuropathic Pain Symptom Inventory; NRS, Numeric Rating Scale; OccRQ, Occupational Role Questionnaire; ObsRWQ, Obstacles to Return-to-Work Questionnaire; ÖMPSQ, Örebro Musculoskeletal Pain Screening Questionnaire; PAIRS, Pain and Impairment Relationship Scale; PAQ-R, Pain Attitudes Questionnaire-Revised; PASS, PASS-SF(20), PASS- 40, PASS-72, Pain Anxiety Symptoms Scale; PBC, Pain Behavior Checklist; PBPI, Pain Beliefs and Perceptions Inventory; PBQ, Pain Beliefs Questionnaire; PCI, Pain Coping Inventory; PCL, Pain Cognition List; PCOQ, Patient Centered Outcomes Questionnaire; PCP:S, PCP:ES, Profile of Chronic Pain: Screen, and Extended Assessment Battery; PCQ, Pain Cognitions Questionnaire; PCS (PCS_S), Pain Catastrophizing Scale; PCSI, Pain Coping Style Inventory; PDI, Pain Distress Inventory (in Psychological); PDI, Pain Disability Index (in Functional); PDQ, Pain Disability Questionnaire; PGPQ, Patient Goal Priority Questionnaire; POP, Pain Outcomes Profile; POQ, POQ-42, POQ-SF, POQ-DC-28, POQ-FU-34, POQ-DC-5, Pain Outcomes Questionnaire suite; PPP, Pain Patient Profile; PSCQ, Pain Stages of Change Questionnaire; PSEQ, Pain Self Efficacy Questionnaire; PSFS, Patient-Specific Functional Scale; PaSolQ, Pain Solutions Questionnaire; PVAQ, Pain Vigilance and Awareness Questionnaire; QWBS, Quality of Well-Being Scale – Self-Administered; RADL, Resumption of Activities of Daily Living Scale; RFQ, Risk Factor Questionnaire; SCL_27, Symptoms of Chronic Pain List; SF36, Short Form-36 Health Survey; SIP, Sickness Impact Profile; SOPA_57 SOPA_35 SOPA_S, Survey of Pain Attitudes; SRI, Spouse Response Inventory; TSK (TSK-11), Tampa Scale for Kinesiophobia; VMDPMI, Vanderbilt Multidimensional Pain Management Inventory; VRS, Verbal Rating Scale; VAS, Visual Analogue Scale; VDPQ, Vermont Disability Prediction Questionnaire; WDDI, Work Disability Diagnosis Interview; WHOQOL, WHOQOL-Bref, World Health Organization Quality of Life; WHYMPI, WHYMPI_S, West Haven-Yale Multidimensional Pain Inventory; WL-26, Work Limitations-26; WLQ, Work Limitations-Questionnaire; ZSRD, Zung Self-Rating Depression Scale.
For pain severity assessment, there were three sub-groups (uni-dimensional; multidimensional; neuropathic; and nonneuropathic pain discrimination).
For psychological assessment, there were nine sub-groups (general psychological states; anxiety, depression and mood; physiological manifestations of anxiety and depression relative to persistent pain; pain cognition; catastrophizing, negative thoughts and fear; risk factor identification; pain function; coping and management; and behavioral change readiness).
For functional assessment, there were six sub-groups (occupation focus; general function; interference [disability] in activities; impact on others; patient-specific instruments; and functional performance).
For multidimensional assessment of persistent pain, there were seven subgroups (occupational issues; expectations; yellow flags; pain dimensions; prediction of future disability; pain profiling; outcome prediction instruments).
No sub-groups were identified in the general health/quality of life instruments, as all measured similar constructs.
Step 2 retained 59 instruments. Appendix 3 outlines the excluded instruments and the primary reason for exclusion. Step 3 short-listed 45 instruments. Reasons for short-listing (or excluding) instruments at this step are outlined in the ‘Short-listing decisions’ section of this paper.
Table 1 outlines the review consort diagram. Instrument abbreviations are expanded in Appendix 2.
Psychometric properties
There was no consistency regarding research methods or statistics for psychometric testing. This constrained development of a general picture of psychometric performance, and questioned whether one statistic was preferable to another, in demonstrating desirable characteristics of instrument performance. Table 2 reports the frequency of psychometric statistical tests, and their reported purpose, from Step 2 (considering 59 instruments).
Table 2.
Frequency of use, and purpose, of statistics to describe psychometric property
| Test | Purpose | % | Test | Purpose | % |
|---|---|---|---|---|---|
| 1. Cronbach’s alpha | Internal consistency | 56.4% | 13. Face validity | 7.3% | |
| 2. Student t-test/ANOVA | Responsiveness | 38.2% | 14. Kappa | Internal consistency | 5.5% |
| 3. Criterion validity | Concurrent and predictive | 32.7% | 15. Wilcoxin rank test | Responsiveness | 3.6% |
| 4. Construct validity | Convergent and divergent validity OR ability to differentiate between groups | 32.7% | 16. Spearman correlation coefficient | Inter-rater reliability | 1.8% |
| 5. Pearson r | Intra-rater reliability | 20.0% | 17. Spearman correlation coefficient | Test–retest | 1.8% |
| 6. Intra-class correlation coefficient (ICC) | Intra-rater reliability | 16.4% | 18. Percent agreement | Inter-rater reliability | 1.8% |
| 7. Cut point | Sensitivity and specificity | 16.4% | 19. Percent agreement | Test-retest | 1.8% |
| 8. Effect size | Responsiveness | 14.5% | 20. ICC | Inter-rater reliability | 1.8% |
| 9. Pearson r | Test–retest | 2.7% | 21. ICC | Internal consistency | 1.8% |
| 10. Pearson r | Internal consistency | 10.9% | 22. Pearson r | Inter-rater reliability | 0.0% |
| 11. Area under receiver operating characteristic (ROC) curve | Responsiveness | 9.1% | 23. Spearman correlation coefficient | Intra-rater reliability | 0.0% |
| 12. Kappa | Test–retest | 7.3% | 24. Percent agreement | Intra-rater reliability | 0.0% |
Short-listing decisions (Step 3)
Pain severity assessment instruments
Unidimensional scales
There is considerable detail regarding the psychometric properties of pain severity scales (Visual Analog Scale [VAS]/Verbal Rating Scale [VRS]/Numeric Rating Scale [NRS]).15,16 These instruments are useful for quick initial assessment of one pain dimension.
Multidimensional pain severity assessment instruments
The Chronic Pain Grade (CPG) scores the severity of persistent pain in three domains (characteristic pain intensity, disability, and persistence). The developmental literature17–21 provides evidence of strong psychometric properties in terms of high intra-rater reliability, internal consistency for a range of health conditions and construct validity compared to other instruments. Normative data is available.
The McGill Pain Questionnaire (Long Form) (LF-MPQ) has strong psychometric properties and normative data.22 It includes a univariate pain scale (NRS). The instructions for completing the pain adjectives section are complex and it is recommended that they should be read to patients. There are several methods of scoring, and little information as to which is most appropriate. The McGill Pain Questionnaire (Short Form) (SF-MPQ)23 does not contain the long-form body chart, and includes a VAS rather than an NRS. The short form version is less complex, quicker to administer and easier to score and interpret than the LF-MPQ. It covers the main elements of the LF-MPQ, and thus would appear to be preferable.
Distinguishing between neuropathic and nonneuropathic pain
Self-report and interview administration
The Douleur Neuropathique 4 Questions (DN4) is a 10-item instrument with high inter-rater reliability, high Kappa on retest, strong face validity and moderate correlation with health practitioners’ diagnosis.24–26 An interview contributes three items, using Yes/No responses, the remainder using a 0–100 scale. This instrument is appropriate for telephone administration.
Self-report and consultation administration
The Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale is a seven-item instrument which includes five self-report items and two sensory tests.27 The self-report questions use Yes/No responses, and the sensory testing requires the health practitioner to be with the patient for testing. Literature reports moderate Kappa test-retest scores and moderate Cronbach’s alpha for internal consistency.27–31 Its cut-point of 12 is sensitive (83%), and specific (87%) for differentiating between neuropathic and nonneuropathic pain.27 It assesses five types of pain (thermal, dysesthesia, paroxysmal, evoked and autonomic dysfunction), and is appropriate to primary health care settings.
Self-report administration
The Neuropathic Questionnaire, Long Form (NPQ_LF) is a 12-item self-report instrument.32 The NPQ_LF has sound discriminant capacity between different pain-type groups.26 Its cut-point of 0 is reported to be moderately sensitive (66.6%) and specific (74.4%).26 There is a short form of three items (NPQ_SF), reported to have similar psychometric properties and discriminant capacity.32
Table 3 reports the consistently high Ready Reckoner scores for the short-listed pain severity instruments with respect to psychometric properties and clinical utility.
Table 3.
Ready Reckoner for short-listed pain severity assessment measures
| CPG | DN4 | LANSS | MPQ_SF | NPQ_LF | NPQ_SF | Unidimensional | ||
|---|---|---|---|---|---|---|---|---|
| Validity | Face | ✓ | ✓ | ✓ | ||||
| Content | ✓ | ✓ | ||||||
| Construct | ✓ | |||||||
| Comparison | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Sensitivity | ✓ | ✓ | ✓ | ✓ | ||||
| Factors | 2 | 9 | 1 | 0 | 6 | 6 | 1 | |
| Reliability | Inter-tester | ✓ | ||||||
| Intra-tester | ✓ | ✓ | ||||||
| Test–retest | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Internal | ✓ | |||||||
| Consistency | ||||||||
| Simple instructions, short, simply worded items | ✓ | ✓ | ✓ | ✓ | ||||
| <20 items | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| • Number of items | 7 | 10 | 7 | 17 | 12 | 12 | 1 | |
| Able to be scored manually | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| <15 minutes administration time | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| • Estimated average time to administer | 15 | 10 | 10 | 15 | 10 | 10 | 2 | |
| Norms | ✓ | ✓ | ✓ | ✓ | ||||
| Cut off scores | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Specific to NZ | ||||||||
| No cost | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| No registration/limitations | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Total ✓ | 12 | 10 | 8 | 9 | 10 | 10 | 13 |
Abbreviations: CPG, Chronic Pain Grade; DN4, Douleur Neuropathique 4; LANSS, Leeds Assessment of Neuropathic Symptoms and Signs; MPQ_SF, McGill Pain Questionnaire Short Form; NPQ_LF, Neuropathic Pain Questionnaire Long Form; NPQ_SF, Neuropathic Pain Questionnaire Short Form.
Category 2: Psychological pain assessment instruments
The number and range of instruments in this category highlights the importance of considering the purpose, strengths and limitations of psychological assessment instruments prior to choosing the most appropriate one to use.
Anxiety, depression, and mood
The review found five relevant instruments measuring anxiety, depression and/or mood (Beck Depression Inventory [BDI], Kessler Psychological Distress Scale [K10], Hospital Anxiety and Depression Scale [HADS], Center for Epidemiologic Studies Depression Scale [CES-D], Zung Self-Rating Depression Scale [ZSRD]). Preference was given to clinically flexible, multi-construct psychological instruments relevant to persistent pain, thus ZSRD (a generic measure of depression) was excluded from further consideration.
The BDI is a well established 21-item self-report rating inventory measuring characteristic attitudes and symptoms of depression and mood. It has high internal consistency for both psychiatric and nonpsychiatric patients.33 The BDI has comparable sensitivity and specificity with CES-D for persistent pain patients.
The K10 is a widely reported two-domain, 10-item measure of nonspecific psychological distress, primarily intended as a measure of mood, anxiety, and depression.34 Its developmental literature reported high sensitivity and specificity, internal consistency and intra-rater reliability. It has been used extensively in population surveys in Australia.35 The K10 is appropriate for primary health care settings, and for telephone delivery.
The CES-D is a 20-item measure of anxiety, depression, and depressed mood symptoms, with demonstrated sensitivity and responsiveness to change.36 It has been validated in different languages. It has shorter forms (CES-D-10 (10 item), Revised Form (eight item), Iowa Form (11 item) and Boston Form (10 item), however the original CES-D is the best researched.37,38
The HADS is a two-domain, 14-item measure designed to detect anxiety and depression, independent of somatic symptoms.39 The instrument has high internal consistency and strong correlations with quality of life measures (eg, Life Satisfaction Questionnaire). It has limited background literature for persistent pain patients. It has been validated in different languages. The HADS can only be used by psychologists with appropriate training.40
Physiological manifestations of anxiety and depression relative to persistent pain
The Modified Somatic Perception Questionnaire (MSPQ) is a 13-item measure of heightened somatic and autonomic awareness related to anxiety and depression.41 It was developed to identify clinically significant psychological distress in patients with persistent back pain. It has strong internal consistency, evidence of convergent and divergent validity with measures of anxiety and depression (eg, Minnesota Multiphasic Personality Inventory [MMPI], ZSRD, LF-MPQ) and significant discriminant validity within different groups of pain sufferers.41,42 It is one of the few instruments linking physical and psychological symptoms and thus adds important information to persistent pain assessments. It uses short wording which is appropriate for primary health care settings and telephone delivery.
Pain cognition and risk factor identification
No instrument was retained for short-listing.
Catastrophizing, negative thoughts, fear
Three instruments were retained for short-listing.
The Fear Avoidance Beliefs Questionnaire (FABQ) is a 16-item, short-worded, work-focused measure of patients’ beliefs about how physical activity and work affect their pain.43,44 The FABQ developmental literature reports high intra-tester reliability and test–retest, high internal consistency, and sound criterion and construct validity, tested against work time lost in the last 12 months, self-reported disability and poor behavioral performance. The FABQ is moderately correlated with the MSPQ, and highly correlated with the Tampa Scale for Kinesiophobia (TSK-11).43 It is appropriate for primary health care settings and telephone delivery.
The TSK is available in long (17 items) and short (11 items) versions. Both instruments have similar high intra-rater reliability and internal consistency, and are sensitive to differences between patients, and interventions.45 The shorter version (TSK-11) is more relevant for primary health care assessment, and for telephone administration. The TSK has been validated in other languages.
The Pain Distress Inventory (PDI) measures pain-related interference with role functioning, using domains of family/home responsibilities, recreation, social activity, occupation, sexual behavior, self-care and life-support activity.46 It has sound internal consistency and content validity, and is sensitive to responses from different patient sub-groups. It correlates strongly with the Oswestry Disability Index and moderately with the BDI. The PDI is relevant to primary and secondary settings.
Behavioral change readiness
Four instruments were retained in this sub-group.
The Pain Behavior Checklist (PBC) is a 49-item, short-worded self-report about behaviors related to pain, assessing avoidance, complaint, and help-seeking.47 The PBC has moderate evidence of intra-rater reliability, low correlations with measures of depression, good discriminant validity between different types of headache sufferers, and low correlations with measures of noise sensitivity.47 Population norms reflect 1. 5 standard deviations around the mean.47,48
The Chronic Pain Values Inventory (CPVI) is a patient-centered six-domain instrument assessing family, intimate relations, friends, work, health, and growth or learning.49 The CPVI is based on a values-based cognitive-behavioral approach to assessing persistent pain. The developmental literature reports strong correlations with other pain measures (Pain Anxiety Symptoms Scale [PASS], Chronic Pain Acceptance Questionnaire [CPAQ], work capacity, and medications), and significant discriminant abilities.49 This instrument evaluates the discrepancy between importance and success in patient values related to functioning in different environments (family, intimate relations, friends, work, health and growth and learning).
The Multidimensional Pain Readiness to Change Questionnaire (MPRCQ) scored highly on psychometric properties (convergent and discriminant validity, intra-rater reliability and internal consistency). However, the authors advised that at present, this instrument was available only for research purposes.50 It was thus excluded from further consideration for the compendium.
The Pain Self-Efficacy Questionnaire (PSEQ) is a 10-item short-worded instrument which measures self-confidence in performing functional and social activities, despite the presence of pain.51 The PSEQ is relevant to assess pain cognition in primary health care settings and can be administered by telephone. It has high intra-rater reliability, internal consistency and stability over retest.
Pain function, coping and management
Twelve instruments (six sets of instruments) were short-listed. Three measured persistent pain coping (Chronic Pain Coping Inventory [CPCI] suite of four instruments, Coping Strategies Questionnaire [CSQ] suite of four instruments, and the Pain Coping Inventory [PCI]). The other instruments comprised the CPAQ which measures acceptance of persistent pain, the Cognitive Risk Profile for Pain (CRPP) which considers persistent pain management beliefs, and the Biobehavioral Pain Profile (BPP) which measures reactions to persistent pain.
The CPCI suite (CPCI_65 [original], CPCI_partner, CPCI_42, CPCI_abbreviated) measures persistent pain coping strategies.52–54 The original CPCI includes domains of guarding, resting, asking for assistance, relaxation, task persistence, exercise/stretch, seek support, and coping self-statements. All versions of the CPCI demonstrate moderate to high internal consistency, and strong evidence of convergent, divergent and criterion validity tested within-instrument domains, and with other instruments (eg, CES-D and Numerical Pain Rating Scale [NRS]). An advantage of this instrument is its ‘partner’ version, and the shorter versions which may be used for primary care assessment, or for retest. The instruments have relatively complex wording which may not be appropriate for people of low literacy. The instrument has been validated in other languages.
The CSQ suite assesses persistent pain coping.55,56 It contains a long form (CSQ_LF of 50 items), a revised form (CSQ_R of 27 items) and an abbreviated 24 item form (CSQ-24). All forms have similarly strong internal consistency, and strong concurrent and divergent validity with VAS and HADS. The CSQ_LF and the CSQ_R are wordy, thus they may not be suitable for some persistent pain patients with low literacy. Scoring instructions are published for the abbreviated version, which appeared most appropriate for clinical use.
The PCI is a multi-domain, simply-worded 33-item instrument which measures active and passive pain coping strategies using specific cognitive and behavioral strategies (pain transformation, distraction, reducing demands, retreating, worrying, and resting).57 It has moderate intra-rater reliability and internal consistency, strong inter-domain correlation, and varying degrees of convergent and discriminant validity when compared with VAS, age, and illness states.
The CPAQ is a 20-item measure of pain acceptance.59 It has two domains that measure activity engagement (pursuit of life activities regardless of pain) and pain willingness (recognition that avoidance and control are often unworkable methods of dealing with persistent pain). Acceptance of persistent pain is believed to allow patients to focus on engaging in meaningful and valued activities and goals. The CPAQ demonstrates moderate internal consistency, and divergent validity with the BDI.59 This instrument uses wordy items which may not be suitable for self-report from patients with low literacy.
Persistent pain management beliefs
Persistent pain management beliefs could be measured by the nine domain instrument CRPP, or the BPP.
The CRPP is a commercially available instrument which explores beliefs and attitudes that could interfere with pain management.60 The CRPP domains deal with philosophic beliefs about pain, denial that mood affects pain, denial that pain affects mood, perception of blame, inadequate support, disability entitlement, desire for medical breakthrough, scepticism of multidisciplinary approach and conviction of hopelessness. It has high internal consistency, and divergent validity with the PDI, CSQ, and MPQ.
The BPP is a composite measure of behavioral, physiological, and cognitive reactions to pain.61 It has six domains of health care avoidance, physiological responsivity, thoughts of disease progression, environmental influences, past and current treatment, and loss of control. The initial intent of the instrument was to use each patient’s answers to create a profile that predicts what nondrug methods might work best for managing pain. In the developmental literature the BPP showed poor intra-rater reliability, high internal consistency, and divergent validity with the BDI and body consciousness.61 This instrument appears worded for patients of usual literacy levels.
Table 4 provides the Ready Reckoner scores for short-listed psychological persistent pain assessment instruments, and highlights their variable quality in terms of psychometric properties and clinical utility.
Table 4.
Ready Reckoner for the short-listed psychological persistent pain assessment measures
| BDI | BPP | CES-D | CPAQ | CPCI suite | CPVI | CRPP | CSQ_24 | FABQ | HADS | K10 | MSPQ | PBC | PCI | PSEQ | TSK-11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Validity | Face | ✓ | |||||||||||||||
| Content | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Construct | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Comparison | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Sensitvity | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Factors | 5 | 4 | 4 | 8 | 6 | 9 | 2 | 2 | 2 | 2 | 1 | 2 | 6 | 1 | 2 | ||
| Reliability | Inter-tester | ✓ | |||||||||||||||
| Intra-tester | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Test–retest | ✓ | ✓ | |||||||||||||||
| Internal | ✓ | ||||||||||||||||
| Consistency | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Simple instructions, short, simply worded items | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| <20 items | (✓) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| • Number of items | 21 | 41 | 20 | 20 | 65 (abbrev) | 12 | 53 | 24 | 16 | 14 | 10 | 13 | 49 | 33 | 10 | 17 (11) | |
| Able to be scored manually | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| <15 minutes administration time | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| • Estimated average time to administer | 18 | 20 | 15 | 15 | 20 | 7 | 20 | 7 | 7 | 10 | 7 | 5 | 12 | 10 | 10 | 10 | |
| Norms | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Cut off scores | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Specific to NZ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| No cost | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| No registration/limitations | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Total ✓ | 14 | 8 | 7 | 6 | 8(9) | 9 | 4 | 10 | 14 | 9 | 14 | 13 | 10 | 11 | 13 | 13 |
Abbreviations: BDI, Beck Depression Inventory; BPP, Biobehavioral Pain Profile; CES-D, Center for Epidemiologic Studies-Depression Scale; CPAQ, Chronic Pain Acceptance Questionnaire; CPCI suite, Chronic Pain Coping Inventory suite; CPVI, Chronic Pain Values Inventory; CSQ_24, Coping Strategies Questionnaire; FABQ, Fear-Avoidance Beliefs Questionnaire; HADS, Hospital Anxiety and Depression Scale; K10, Kessler Psychological Distress Scale; MSPQ, Modified Somatic Perception Questionnaire; PBC, Pain Behavior Checklist; PCI, Pain Coping Inventory; PSEQ, Pain Self Efficacy Questionnaire; TSK-11, Tampa Scale for Kinesiophobia.
Category 3: Functional performance assessment instruments
A range of instruments which purported to measure function in persistent pain patients were considered for short-listing.
Instrument redundancy
The Work Limitations Questionnaire (WLQ) and the Work Limitations-26 (WL-26) were developed by the same group of researchers, and share over 90% items and wording.62,63 The WLQ is a 25-item measure of on-the-job impact of persistent health problems and/or treatment on work limitations (measuring capacity to work whilst workers are at work). The WLQ has four demand domains: physical, time, mental-interpersonal and output. It has been tested using a two and four-week recall period. It scores each item using a five-point Likert Scale: 0%, health makes the job demand difficult none of the time; 25%, a little of the time; 50%, some of the time; 75%, a great deal of the time; and 100%, health makes the job demand difficult all of the time. The WLQ correlates well with adverse events in the workplace such as employee injuries and sick leave. The instrument has high internal consistency and moderate criterion and construct validity with the Short Form Health-36 Survey Questionnaire (SF-36). It is useful for self-reporting. The WL-26 has 26-items of similar style, purpose and scoring to the WLQ. It defines the middle category on the Likert scale differently, as ‘approximately half the time’. Contact with the authors indicated that either instrument could be used to assess limitations in work functioning, however, they recommended the WLQ as the more robust measure. Both instruments require registration prior to use.
Occupation focus
Two instruments (WLQ and Occupational Role Questionnaire [OccRQ]) were identified, measuring different aspects of occupation. The OccRQ is an eight-item, two-domain instrument which tests attitudes to returning/remaining at work.64 It has two domains, assessing productivity and satisfaction. This instrument is suitable for self-report and administration in primary health care settings. The OccRQ has strong evidence of test-retest reliability and high internal consistency. It is moderately correlated with pain intensity (VAS).
General function
Three instruments were measures of general function, relevant in both primary and secondary health care settings: the 15-item Functional Abilities Confidence Scale (FACS), the 12-item Resumption of Activities of Daily Living (RADL) scale and the seven-item PDI.
The FACS measures confidence with general functional activities related to movements and postures affected by low back pain.65 The RADL measures self-reported resumption of usual daily activities estimating confidence regarding return to usual activities.66 These instruments were developed by the same research group, are strongly correlated, and have high internal consistency.
The PDI estimates impact on everyday activities and relationships.67 It is a measure of pain-related interference with role functioning, in domains of family/home responsibilities, recreation, social activity, occupation, sexual behavior, self-care and life-support activity. It is sensitive to responses from different patient groups, and correlates moderately with the BDI.
Patient-specific instruments
The importance of considering the patient’s perspective when measuring function was highlighted by Kliempt and colleagues.68 Two patient-specific instruments were identified, both with strong psychometric properties and tested across health conditions.
The Patient Goal Priority Questionnaire (PGPQ) is a patient-specific measure of patients’ behavioral goals.69 There are three measures: a questionnaire for assessing goals at baseline, a follow-up questionnaire, and a questionnaire for priority revision that can be used at any time after the commencement of treatment. The PGPQ can be used to identify and assess behavioral treatment goals, elaborate individual functional behavioral analyses relevant for everyday life functioning and determine the clinical signifiance of treatment. It is negatively and moderately correlated with the PDI, and is not intended for group analyses.
The Patient-Specific Functional Scale (PSFS) is a patient-specific clinical tool for eliciting, measuring and recording patients’ descriptions of their disabilities.70 It has strong evidence of construct validity, intra-rater reliability, sensitivity and specificity, and has an estimated amount of clinically significant change.71,72
Interference (disability) in functional activities
Two instruments measured the extent of interference in usual function (measures of disability); the Functional Assessment Screening Questionnaire (FASQ) and the Brief Pain Inventory–Interference (BPI_I).
The FASQ is a simply-worded 15-item instrument.73 It had reports of high internal consistency, good ability to differentiate between pain subgroups, and moderate divergence with pain intensity (VAS). No contact could be made with the developer, thus this instrument was excluded from consideration.
The BPI_I is a 7-item derivative of BPI_long form (BPI_LF), which is considered in the next section.74 The BPI_I measures interference in general activity, sleep, mood, and relationships. It can be quickly self- or interviewer-administered and is validated in other languages. Its wording may challenge patients of low-literacy. It has high internal consistency and moderate discriminant validity. The BPI_I has been modified into a 12-item instrument, adding items relevant to disability (self care, recreation, social activities, finding out information and communication).75 The BPI suite requires registration prior to use.
Impact on others
Two instruments assessed the influence of pain, and people close to the patient. One instrument assessed the impact of the patient’s pain on others (Family Impact of Pain Scale [FIPS]), and the other assessed the impact of the spouse (‘significant other’) on a patient’s pain responses (Spouse Response Inventory [SRI]).
The FIPS is a 10-item instrument measuring the extent to which family activities and interactions are affected by the patient’s persistent pain.76 It has two domains showing moderate test–retest and internal consistency. It is sensitive in identifying patients whose behavior significantly impacts on family members. It moderately correlates with the VAS, the BDI, and the CSQ.
The SRI assesses the extent to which spouse responses contribute to a patient’s pain and disability.77 It has four domains (solicitous and negative spouse response pain behaviors, and facilitating and negative spouse response well behaviors). It has moderate intra-rater reliability and poor to moderate construct validity with several other instruments (eg, PBC, VAS, BDI). The SRI requires registration with the author prior to use.
Functional performance
The Canadian Occupational Performance Measure (COPM) is a patient-centered measure of performance of activities and tasks of daily living.78–82 It is a semi-structured interview in which patients identify and rate areas of difficulty in occupational performance. It is a broad-ranging comprehensive instrument with strong evidence of construct and content validity, reliability in administration, test–retest, and correlation with other performance measures. It is a commercial product designed to be used by registered occupational therapists.
Table 5 reports the Ready Reckoner for the functional pain assessment instruments, highlighting their variable psychometric properties and clinical utility.
Table 5.
Ready Reckoner for the short-listed functional persistent pain assessment measures
| BPI_I | COPM | FACS | FIPS | OccRQ | PDI | PGPQ | PSFS | RADL | SRI | WLQ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Validity | Face | ✓ | ✓ | ✓ | ||||||||
| Content | ✓ | ✓ | ✓ | |||||||||
| Construct | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Comparison | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Sensitivity | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Factors | 1 | 2 | 2 | 2 | 2 | 5 | 4 | |||||
| Reliability | Inter-tester | ✓ | ✓ | |||||||||
| Intra-tester | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Internal | ||||||||||||
| Consistency | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Simple instructions, short, simply worded items | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| <20 items | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| • Number of items | 7 | 15 | 10 | 8 | 7 | 22 | 3 | 12 | 39 | 25 | ||
| Able to be scored manually | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| <15 minutes administration time | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| • Estimated average time to administer | 5 | 10 | 10 | 5 | 7 | 15 | 5 | 7 | 20 | 15 | ||
| Norms | ✓ | ✓ | ✓ | NA | NA | ✓ | ||||||
| Cut off scores | ✓ | ✓ | ✓ | ✓ | ✓ | NA | NA | ✓ | ✓ | |||
| Specific to NZ | ||||||||||||
| No cost | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| No registration/limitations | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Total ✓ | 8 | 10 | 12 | 13 | 13 | 11 | 9 | 10 | 11 | 5 | 10 |
Abbreviations: BPI_I, Brief Pain Inventory-Interference; COPM, Canadian Occupational Performance Measure; FACS, Functional Abilities Confidence Scale; FIPS, Family Impact of Pain Scale; OccRQ, Occupational Role Questionnaire; PDI, Pain Distress Inventory; PGPQ, Patient Goal Priority Questionnaire; PSFS, Patient-Specific Functional Scale; RADL, Resumption of Activities of Daily Living Scale; SRI, Spouse Response Inventory; WLQ, Work Limitations-Questionnaire.
Category 4: Multidimensional pain assessment instruments
Exclusions
Two instruments were excluded on grounds of redundancy. The Dartmouth Pain Questionnaire (DPQ) is a five-domain instrument adjunctive to the MPQ. It assesses the impact of pain on function.83 Other instruments measure this construct more comprehensively (eg, BPI). The BPI (32 items) and BPI_SF (15 items) are multidimensional psychometrically-sound measures of persistent pain features including pain severity, impact of pain on daily function, location of pain, pain medications and amount of pain relief in the past 24 hours or past week.74 The BPI has been validated in several languages. There is significant overlap between BPI and BPI_SF items, and therefore the BPI_SF was short-listed. The BPI suite is free to use after registration.
The Work Disability Diagnosis Interview (WDDI) was excluded on the basis of complexity and administration time. It includes several standard assessment instruments, a physical examination and an interview.84 Its estimated completion time exceeds three hours, constraining its utility in most clinical settings. This instrument however offers a comprehensive approach to persistent pain assessment, and may be useful in multidimensional multidisciplinary specialized assessment settings.
Retained instruments
The Obstacles to Return-to-work Questionnaire (ObsRWQ) measures prognosis for return to work, pain intensity and depression.85 It is moderately sensitive and specific around a cut-point of 150. Part I assesses depression and pain intensity; Part II assesses six domains of difficulties at work return, physical workload and harmfulness, social support at work, worry due to sick leave, work satisfaction, and family situation and support; and Part III is a single scale dealing with perceived prognosis of work return. The items in Part I and some of the items in Part III are taken from the Örebro Musculoskeletal Pain Screening Questionnaire (ÖMPSQ). This instrument correlates moderately with BDI.
The ÖMPSQ, if applied 4–12 weeks after injury, identifies the likelihood that workers with soft tissue injury will develop long-term disability.86 This provides an early opportunity to intervene to reduce the risk of long term disability in injured workers. Specific to low back pain, the instrument has been tested in several settings, providing thresholds for predicting risk of long-term disability with moderate evidence of validity.87–90
The West Haven–Yale Multidimensional Pain Inventory (WHYMPI) is a two-instrument suite (patient and ‘significant other’ versions). The patient version has 52 items in 12 domains divided into three parts, evaluating perceived interference of pain in occupational, social and family functioning; perceptions of responses of others to the patient’s pain, and participation in common daily activities.91 There are extensive norms, and strong psychometric properties. The ‘significant other’ version (WHYMPI_S) evaluates the influence of the patients’ pain on the partner/spouse, and partner influence on the patient.92 The WHYMPI requires registration prior to use.
Table 6 provides the Ready Reckoner for multidimensional pain assessment instruments. The generally high scores for these instruments indicate their value for health practitioners, in using one instrument to assess a number of constructs related to persistent pain.
Table 6.
Ready Reckoner for the short-listed multidimensional persistent pain assessment measures
| BPI_SF* | GPQ | OccRQ | ÖMPSQ | WHYMPI | WHYMPI_S | ||
|---|---|---|---|---|---|---|---|
| Validity | Face | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Content | ✓ | ✓ | |||||
| Construct | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Comparison | ✓ | ✓ | ✓ | ✓ | |||
| Sensitivity | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Factors | 2 | 5 | 9 | 3 | 3 | ||
| Reliability | Inter-tester | ✓ | ✓ | ✓ | |||
| Intra-tester | ✓ | ✓ | ✓ | ✓ | |||
| Test–retest | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Internal | ✓ | ||||||
| Consistency | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Simple instructions, short, simply worded items | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| <20 items | ✓ | ||||||
| • Number of items | 15 | 24 | 55 | 56 | 52 | 52 | |
| Able to be scored manually | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| <15 minutes administration time | ✓ | ✓ | |||||
| • Estimated average time to administer | 10 | 12 | 20 | 20 | 20 | 20 | |
| Norms | ✓ | ✓ | ✓ | ✓ | |||
| Cut off scores | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Specific to NZ | ✓ | ||||||
| No cost | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| No registration/limitations | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Total ✓ | 14 | 12 | 12 | 14 | 13 | 13 |
Abbreviations: BPI_SF, Brief Pain Inventory; GPQ, Glasgow Pain Questionnaire; OccRQ, Occupational Role Questionnaire; ÖMPSQ, Örebro Musculoskeletal Pain Screening Questionnaire; WHYMPI, WHYMPI_S, West Haven-Yale Multidimensional Pain Inventory.
Category 5: Quality of life/general health assessment instruments
A number of instruments were identified in this category, with different purposes in persistent pain assessment.
Exclusions
The EuroQol (EQ-5D), World Health Organization Quality of Life (WHOQOL; WHOQOL-Bref) and Quality of Well Being Scale (QWBS) instruments were developed specifically for population-health research and clinical trials.93–95 They were not intended for clinical application, and were thus excluded from further consideration.
The remaining instruments are all commercial, and could be used for clinical assessments. Prior to the development of the SF-36, the Sickness Impact Profile (SIP) was the best available quality-of-life measure.96 It assesses sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior and communication. These domains can be aggregated into broader physical and psychosocial domains. As it has now largely been superseded by the SF-36, the SIP was excluded from consideration. The Health Status Questionnaire (HSQ 2.0) contains the same items and domains as the SF-36, relevant to persistent pain.97 Given the lack of normative data, and overlap with the SF-36, the HSQ was also excluded from further consideration.
Retained instruments
The SF-36 contains eight domains (physical functioning, role limitations due to physical problems, bodily pain, social functioning, mental health, role limitations due to emotional problems, vitality, and overall/general health).98 International population and health condition norms are reported.99 Given its normative data, the large number of publications which report use of the SF-36, and its wide use in clinical and research environments, it was short-listed.
The Nottingham Health Profile (NHP) measures pain intensity, and impact of pain on general life areas.100 It has strong evidence of reliability, validity, sensitivity to change and domain construction (physical activities, pain, sleep, social isolation, emotional reactions, energy level, and whether their present level of health affects their life in occupation, personal relationships, hobbies etc). In a publication which compared the NHP and SF-36 for patients with lower limb ischaemia,101 the NHP was more sensitive in discriminating between ischemia levels. This highlights the importance of considering the sensitivity of any instrument for patients with different types of persistent pain.
Table 7 provides the Ready Reckoner scores for the general health/quality of life assessment instruments.
Table 7.
Ready Reckoner for the short-listed general health/quality of life persistent pain assessment measures
| NHP | SF-36 | ||
|---|---|---|---|
| Validity | Face | ✓ | ✓ |
| Content | ✓ | ✓ | |
| Construct | ✓ | ✓ | |
| Comparison | ✓ | ✓ | |
| Sensitivity | ✓ | ✓ | |
| Factors | 5 | 9 | |
| Reliability | Inter-tester | ✓ | ✓ |
| Intra-tester | ✓ | ✓ | |
| Test–retest | ✓ | ✓ | |
| Internal | |||
| Consistency | ✓ | ✓ | |
| Simple instructions, short, simply worded items | ✓ | ✓ | |
| <20 items | |||
| Number of items | 38 | 36 | |
| Able to be scored manually | |||
| <15 minutes administration time | ✓ | ||
| Estimated average time to administer | 10 | 20 | |
| Norms | ✓ | ✓ | |
| Cut off scores | |||
| Specific to NZ | ✓ | ||
| No cost | |||
| No registration/limitations | |||
| Total score | 12 | 12 |
Abbreviations: NHP, Nothngham Health Profile; SF-36, Short Form Health Survey-36.
Discussion
This review identified many assessment instruments published over the past 20 years, dealing with many constructs related to persistent pain presentations and behaviors. Only a few instruments were specific to single health disciplines, or single persistent pain constructs. The publication of assessment instruments appears to mirror developments in under-standing persistent pain behaviors and presentations, in terms of neuroanatomy, biology, physiology, psychology, and social science.5–14 However, no one instrument currently appears to assess all features of persistent pain, and thus multiple instruments are required to provide a comprehensive description of an individual with persistent pain. This is perhaps not surprising, as given the variability in etiology, location and nature of persistent pain presentations, and the variable individual time taken for such pain to develop, it would be a challenge to develop one instrument which addressed all aspects of persistent pain for all patients.6–10 Given the time required to administer even the shortest instrument in every assessment category identified in this review, this could be daunting and burdensome for patients and health providers. Thus, health practitioners using persistent pain assessment instruments for the first time would be advised to start with a multidimensional instrument which provides an overview of the patient. Once core areas of concern have been identified, more specific assessment instruments could be used.
There was considerable overlap in item intent and wording within- and between-persistent pain assessment categories in the short-listed instruments. It appears that instruments measuring similar constructs have been developed by different research groups with apparently little reflection on already published instruments. Choosing between similarly worded and constructed instruments with similar purposes, and similar psychometric properties, thus provides a challenge for health practitioners. The review ighlighted how some instruments changed over time, with little explanation of wording change, change in item order, or addition or removal of items. This suggests an opportunity for international research groups to collaborate on collating, critically appraising and then distilling essential items from key instruments, in order to develop a comprehensive battery of assessment questions which would address all aspects of persistent pain presentation.
The generalizability of many instruments was constrained by the size of study samples, and/or the specific nature of populations on which instruments were tested. This reduced the usefulness of many instrument’s population norms, or cut-points, and raised concerns that if these were inappropriately applied, patients may be inaccurately classified regarding persistent pain behaviors. This highlights the need for instrument developers to research their instrument’s performance in different patient subgroups, with different demographic characteristics and health conditions. This review also flagged the importance of improved understanding of the social and cultural implications of describing pain experiences, to test the stability and generalizability of persistent pain descriptors across population groups and settings.
There was little consistency in choices of statistics tests for psychometric properties, or ways in which psychometric properties were reported. This meant that, despite the volume of published research in this area, little information was presented in useful terms to encourage clinical uptake of any persistent pain assessment instrument. Thus for many health practitioners, the wealth and variability of published material on persistent pain assessment may be overwhelming with respect to instrument choice. For instance, there was little convincing evidence to support the use of many long versus short/revised instruments, in terms of psychometric properties. Choice would appear to be based on personal preference, and/or clinical imperatives. This finding highlights the need for developers of new instruments to justify item intent, number, and wording, and to provide evidence of psychometric properties to convince users that their instrument provides the most useful assessment information in specific circumstances.
Fewer than 25% of instruments initially identified in the literature search were short-listed. The short-listing process relied on the quality of background literature reporting. Had more detail been available on psychometric properties or clinical utility, other instruments may have been short-listed. Some instruments, whilst appearing promising, reported psychometric properties in unpublished conference proceedings or research theses. As these were often not available via library databases, the instrument was excluded in line with the review criteria. Some instruments showed promise on initial test results published in one or two research papers, however there was little subsequent research to support its use. On the other hand, some instruments with less robust psychometric properties were regularly reported as research tools, and would be thus more familiar to health practitioners. Most instrument developers could be contacted, and many willingly provided additional (although often unpublished) details on their instrument’s performance.
The Ready Reckoner, although unvalidated, provided a comprehensive way of collating key information on psychometric properties and clinical utility for efficient clinical decision-making. It highlighted that many instruments did not have good clinical utility despite having sound psychometric properties. Clinical utility could be constrained by lengthy or unwieldy questions, complex wording, or multiple intentions in one question. Administration time for such instruments often precluded their ready clinical use. This review sought to identify instruments relevant to primary health care settings. These were generally short, efficient to deliver and score and sensitive to persistent pain problems. A resource of high quality instruments for primary care providers should increase the frequency with which persistent pain patients are identified early.
The importance of considering the patient in an holistic manner was supported not only by the different categories of persistent pain assessment, but also by the availability of assessment instruments for ‘significant others’. These instruments recognize that patients with persistent pain rarely exist in isolation, and that their pain behaviors have an effect on, and are influenced by, the behaviors of people around them.
Conclusion
Early identification of patients at-risk of developing persistent pain is essential to ensure appropriate and timely intervention, and reduce avoidable individual, social, community and work-related costs. No one assessment instrument captured all the constructs of persistent pain. While the compendium focuses clinicians’ choices on high quality, clinically useful instruments, clinicians should use multiple instruments to ensure comprehensive assessment of adults with persistent pain.
The compendium is available from New Zealand Accident Compensation Corporation. Health practitioners who have not, to date, used standard assessment instruments for persistent pain patients are encouraged to choose instruments from those provided in the compendium.
Appendix 1
Search strategy
The search strategy incorporated all possible variations of nomenclature relating to persistent pain assessment. The search sought to identify:
all questionnaires, surveys, instruments and rating scales developed to assess persistent pain in adults who had noncancer pain, and
developmental literature including psychometric testing of these instruments.
Concurrently, background literature was sought on:
biomedical, functional, behavioral, and psychosocial aspects relevant to persistent pain assessment
risk factors for developing persistent (noncancer) pain
special populations (indigenous, rural, and remote) and instruments specifically developed to address their needs, and
different methods of administration of assessment instruments (in person, via telephone or mail).
Search terms
MeSH subject headings: pain measurement, persistent pain, questionnaires, indigenous population
Non-MeSH search terms: tool*, scale*, inventor*, questionnaire*, protocol*, survey*, profile*, model*, drawing*, checklist*, index, pain, pain assessment, persist*, functional, biomedical, psychosocial, psycholog*, behaviour*, behavior*, risk, assess*, screen*, rural, indigenous, maori, postal, telephone, psychometric, valid, reliab*, respons*. The search terms and strategies were amended as required for other databases.
Library databases and internet sites
All available databases were accessed through university library sources. The ‘parent’ data bases sourced mainstream academic databases which list the peer reviewed literature, including OVID (AMED, CINAHL, MEDLINE, EMBASE) and INFORMIT (AUSThealth, INFORMIT Plus Text [Meditext], INFORMIT e-library). These principal databases were interrogated first and publications sourced from these databases contributed more than 95% of the literature reviewed. To ensure rigor in searching of literature, other secondary databases to validate the initial findings were then searched, including, but not limited to, PsychINFO, PsycARTICLES, Science Direct, Web of Science, PubMed, etc. Interrogation of these databases revealed mostly redundant duplicate publications, already identified from the larger databases.
Appendix 2
Persistent pain severity
| Unidimensional measure of pain severity | Visual Analogue Scale (VAS) [10 cm line] (reported,22 discriminant values102 Verbal Rating Scale (VRS) [word descriptors]15 Numeric Rating Scale (NRS) [0–10 categories]15 |
| Multidimensional pain assessment instruments | Chronic Pain Grade (CPG)17 McGill Pain Questionnaire Long form (MPQ_LF)22 McGill Pain Questionnaire Short form (MPQ_SF)23 Neuropathic Pain Scale (NPS)103 Neuropathic Pain Symptom Inventory (NPSI)104 |
| Discriminating neuropathic and nonneuropathic pain | Douleur Neuropathique 4 (DN4)25 IDPain105 Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)27 (PHC) Neuropathic Pain Questionnaire Long Form (NPQ_LF)106 Neuropathic Pain Questionnaire Short Form (NPQ_SF)106 |
Psychological assessment of chronic pain
| Psychological states not directly related to persistent pain | Depression, Anxiety, and Positive Outlook (DAPOS)107 |
| Measurement of anxiety, depression, and mood | Beck Depression Inventory (BDI)108 (peer reviewer) |
| Center for Epidemiologic Studies-Depression Scale (CES-D)36 | |
| Hospital Anxiety and Depression Scale (HADS)109 (ACC Pain Focus Group) | |
| Kessler Psychological Distress Scale (K10)34 | |
| Zung Self-Rating Depression Scale (ZSRD)110 | |
| Physiological manifestations of anxiety and depression relative to persistent pain | Modified Somatic Perception Questionnaire (MSPQ)41 |
| Pain cognition | Cognitive Coping Strategies Inventory-Revised (CCSI-R)111 |
| Pain Anxiety Symptoms Scale (PASS-SF(20), PASS-40, PASS-72)112 | |
| Pain Cognition List (PCL)113 | |
| Pain Cognitions Questionnaire (PCQ)114 | |
| Catastrophizing, negative thoughts, fear | Negative thoughts about pain |
| Inventory of Negative Thoughts in Response to Pain (INTRP)115 | |
| Catastrophizing | |
| Pain Catastrophizing Scale (PCS) (includes a scale for the significant other/partner PCS_S)116 | |
| Persistent pain fear | |
| 1. Fear-Avoidance Beliefs Questionnaire (FABQ)43 | |
| 2. Fear Avoidance of Pain Scale (FAPS)117 | |
| 3. Fear of Pain Comparisons (FPC_11)118 | |
| 4. Tampa Scale for Kinesiophobia (TSK)119 | |
| 5. Tampa Scale for Kinesiophobia Short version (TSK-11)45 | |
| Pain distress | |
| Pain Distress Inventory (PDI)46 | |
| Vigilance, preoccupation, and awareness of pain | |
| Pain Vigilance and Awareness Questionnaire (PVAQ)120 | |
| Psychopathology in persistent pain | |
| Symptoms of Chronic Pain List (SCL_27)121 | |
| Risk factor identification | Distress and Risk Assessment Method (DRAM)122 |
| Pain function, coping & management | Ability to function with persistent pain |
| Pain and Impairment Relationship Scale (PAIRS)123 | |
| Persistent pain coping | |
| 1. Chronic Pain Coping Inventory suite (CPCI_65 (CPCI_significant other, CPCI_42, and CPCI_abbreviated)52 | |
| 2. Coping Strategies Questionnaire (CSQ_50)124 which also includes a revised (CSQ_R(27)), a shorter version (CSQ24)56 (and an abbreviated version (CSQ_abbreviated)54 | |
| 3. Stoicism (Pain Attitudes Questionnaire-Revised (PAQ-R)) 125 Pain-Coping Inventory (PCI)57 | |
| 4. Pain Coping Style Inventory (PCSI)126 | |
| 5. Vanderbilt Multidimensional Pain Management Inventory (VMDPMI)127 | |
| Acceptance of persistent pain | |
| Chronic Pain Acceptance Questionnaire (CPAQ)128 | |
| Persistent pain management beliefs | |
| Cognitive Risk Profile for Pain (CRPP)60 | |
| Reactions to persistent pain | |
| 1. Brief Pain Coping Inventory (BPCI)129 | |
| 2. Biobehavioral Pain Profile (BPP)61 | |
| Pain attitudes | |
| Survey of Pain Attitudes (SOPA_57),130 which includes a 35 item version (SOPA_35) and a short version (SOPA_S) | |
| Pain beliefs and consequences | |
| Pain Beliefs Questionnaire (PBQ)131 | |
| Pain beliefs and knowledge | |
| Pain Beliefs and Perceptions Inventory (PBPI)132 | |
| Psychological pain functioning | |
| 1. Multidimensional Locus of Pain Control Questionnaire (MLPC)133 | |
| 2. Pain Solutions Questionnaire (PaSolQ)134 | |
| 3. Pain Patient Profile (PPP)135 | |
| Behavioral change readiness | Chronic pain self-efficacy scale (CPS-ES)136 |
| Chronic Pain Values Inventory (CPVI)49 | |
| Multidimensional Pain Readiness to Change Questionnaire (MPRCQ)50 | |
| Pain Behavior Checklist (PBC)137 | |
| Pain Stages of Change Questionnaire (PSCQ)138 | |
| Pain Self Efficacy Questionnaire (PSEQ)139 |
Eighteen instrument suites (25 instruments) were identified, these being BDI, BPP, CES-D, CPAQ, CPCI suite of four instruments, CPVI, CRPP, CSQ suite of four instruments, FABQ, HADS, K10, MPRCQ, MSPQ, PBC, PCI, PSEQ, TSK suite of 2 instruments, ZSRD.
Functional capacity
| Occupation focus | Work Limitations Questionnaire suite (WLQ)62 |
| Work Limitations-26 (WL-26)140 | |
| Occupational Role Questionnaire (OccRQ)64 | |
| Risk Factor Questionnaire (RFQ)141 | |
| General function (women only) | Inventory of Functional Status – chronic pain (IFS-CP)142 |
| General function | Functional Abilities Confidence Scale (FACS)65 |
| Resumption of Activities of Daily Living Scale (RADL)66 | |
| General function (Indigenous health) | Kempsey Survey of Muscle, Joint and Bone Conditions (KSMBJC)143 |
| Interference (disability) in functional activities | Brief Pain Inventory-Interference (BPI_I)144 (7-item derivative of BPI_LF,74 developed into a 12-item instrument75 for disabled persons) |
| Functional Assessment Screening Questionnaire (FASQ)73 | |
| Functional Interference Estimate (FIE)145 | |
| Pain Disability Index (PDI)146 | |
| Pain Disability Questionnaire (PDQ)147 | |
| Impact on others | Family Impact of Pain Scale (FIPS)76 |
| Spouse Response Inventory (SRI)148 | |
| Patient-specific instruments | Patient Goal Priority Questionnaire (PGPQ)69 |
| Patient-Specific Functional Scale (PSFS)70 | |
| Performance | Canadian Occupational Performance Measure (COPM)78 |
Multidimensional assessment of persistent pain
| Occupational issues | Obstacles to Return-to-Work Questionnaire (ObsRWQ)85 |
| Expectations | Patient Centered Outcomes Questionnaire (PCOQ)149 |
| Medical Rehabilitation Follow Along measure (MRFA) – Musculoskeletal form150 | |
| Yellow flags | Örebro Musculoskeletal Pain Screening Questionnaire (ÖMPSQ). This uses exactly the same wording as the Acute Low Back Screening Questionnaire (Yellow Flgs) already in use by ACC in the Acute Low Back Pain guidelines.151 |
| The ÖMPSQ includes a wide range of body parts likely to be affected by musculoskeletal pain. | |
| Obstacles to Return-to-Work Questionnaire (ObsRWQ) (listed above)85 contains items relating to depression | |
| Pain dimensions | Brief Pain Inventory (BPI)144 (original (also known as the long form)) |
| Brief Pain Inventory (BPI-SF)144 (short form) | |
| Glasgow Pain Questionnaire (GPQ)152 | |
| Health Background Questionnaire for Pain (HBQP)153 | |
| Hunter Integrated Pain Service (HIPS) Patient Screening Questionnaire (HIPSPSQ) (no published reference)154 | |
| Obstacles to Return-to-Work Questionnaire (ObsRWQ)85 (listed above and reporting pain experiences). | |
| Pain Outcomes Questionnaire suite of six instruments (POQ) Intake and Pain Outcomes Questionnaire-Short Form (POQ-42, POQ-SF, POQ-DC-28 [discharged from acute care], POQ-FU-34 [at follow-up], POQ-DC-5 [satisfaction scale extracted from POQ-DC-28])155 | |
| West Haven-Yale Multidimensional Pain Inventory (WHYMPI)91 including the WHYMPI_S (Significant Other Instrument) | |
| Prediction of future disability (LBP only) | Vermont Disability Prediction Questionnaire (VDPQ)156 |
| Pain profiling and outcome prediction instruments | Profile of Chronic Pain: Screen (PCP:S)157 |
| Profile of Chronic Pain: Extended Assessment Battery (PCP:ES)158 | |
| Pain Outcomes Profile (POP)155 | |
| Compilation or adaptation of existing instruments | Dartmouth Pain Questionnaire (DPQ) (enhancing items from McGill Pain Questionnaire [MPQ])83 |
| Work Disability Diagnosis Interview (WDDI)84 involves interview which consists partly of questions compiled by instrument authors and partly of a range of standardised questionnaires such as the Oswestry Disability Index, Fear-Avoidance Beliefs Questionnaire and Work APGAR. |
General health/Quality of life assessment
| EuroQol (EQ-5D)93 |
| Health Status Questionnaire 2.0 (HSQ)159 |
| Nottingham Health Profile (NHP)160 |
| Quality of Well-Being Scale – Self-Administered (QWBS)95 |
| World Health Organization Quality of Life (WHOQOL)94 (including a short form WHOQOL-Bref) |
| Short Form-36 Health Survey (SF36)98 |
| Sickness Impact Profile (SIP)96 |
Appendix 3
Excluded instruments
Persistent pain severity
Lack of published literature
At the time of the review, there was no published literature on psychometric properties of the instrument IDPain, and this instrument was excluded from further consideration.
Contact with author
Despite repeated attempts, no contact was able to be made with the author of the NPS (Galer) and thus this instrument was excluded after psychometric property evaluation.
Primary function
The NPSI is mainly reported in the literature as an outcome measure, not an assessment instrument, and it was thus excluded from further consideration.
Psychological assessment of persistent pain
Twenty-six instrument suites (31 instruments) were excluded during Step 2. The instruments, and primary reasons for exclusion, are listed below.
Brief Pain Coping Inventory (BPCI) no information on administration or scoring
Cognitive Coping Strategies Inventory-Revised (CCSI-R) no psychometric properties
Chronic Pain Self-Efficacy Scale (CPS-ES) limited evidence of strong psychometric properties
Depression, Anxiety and Positive Outlook (DAPOS) limited evidence of strong psychometric properties
Distress and Risk Assessment Method (DRAM) replicates questions in MSPQ and Zung instruments, minimum psychometric properties
Fear Avoidance of Pain Scale (FAPS) No information on psychometric properties
Fear of Pain Comparisons (FPC) uncertain clinical utility161. Found it was less useful than the PASS and FABQ for understanding chronic pain
Inventory of Negative Thoughts in Response to Pain (INTRP) limited evidence of strong psychometric properties
Multidimensional Locus of Pain Control Questionnaire (MLPC) authors stated that further work is required on the instrument’s psychometric properties, particularly aspects of validity. The PSCQ covers this area better.
Pain and Impairment Relationship Scale (PAIRS) limited evidence of strong psychometric properties
Pain Attitudes Questionnaire-Revised (PAQ-R) limited evidence of strong psychometric properties
Pain Solutions Questionnaire (PaSolQ) validity untested in English language
Pain Anxiety Symptoms Scale (PASS-20, PASS-40, PASS-72) limited evidence of strong psychometric properties, wordy instrument with multiple items in the long forms, uncertain psychometric properties in the short form
Pain Beliefs and Perceptions Inventory (PBPI) limited evidence of strong psychometric properties
Pain Beliefs Questionnaire (PBQ) unable to trace authors, minimum evidence of psychometric properties
Pain Cognition List (PCL) limited evidence of strong psychometric properties, scoring instructions only available in Dutch
Pain Cognitions Questionnaire (PCQ) unable to trace the authors
Pain Catastrophizing Scale (PCS) limited evidence of strong psychometric properties
Pain Coping Style Inventory (PCSI) uses AVAS for scoring all items, minimum psychometric properties, replicates other more readily scored instruments
Pain Distress Inventory (PDI) could not contact developers
Pain Patient Profile (PPP) limited information on psychometric testing
Pain Stages of Change Questionnaire (PSCQ) limited evidence of strong psychometric properties
Pain Vigilance and Awareness Questionnaire (PVAQ) limited evidence of strong psychometric properties
Symptoms of Chronic Pain List (SCL_27) limited evidence of strong psychometric properties
Survey of Pain Attitudes (SOPA) (including SOPA_35, SOPA_57, SOPA_short) limited evidence of strong psychometric properties
Vanderbilt Multidimensional Pain Management Inventory (VMDPMI) limited access to background documentation, limited evidence of psychometric properties, overlaps with other better credentialed instruments
Functional assessment of persistent pain
The excluded instruments during Step 2 comprised FIE, IFS_CP, KSMBJC, PDQ, RFQ (all for poor evidence of psychometric testing).
Multidimensional assessment of persistent pain
Six instruments (one instrument suite) were excluded because the primary function was to measure outcome (POQ suite). Eight instruments were excluded during Step 2 for poor evidence of psychometric testing, comprising HBQP, HIPSPSQ, MRFA, PCOQ, PCP_Screen, PCO_Ext, POP and VDPQ.
General health/Quality of life assessment of persistent pain
No instruments were excluded during Step 2.
Footnotes
Disclosures
None of the authors have conflicts of interest to disclose.
References
- 1.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based Medicine: How to practice and teach. London: Churchill Livingston; 2000. [Google Scholar]
- 2.Bowling A. Research Methods in Health: Investigating health and health services. 2nd ed. Philadelphia: Open University Press, Buckingham; 2002. [Google Scholar]
- 3.Streiner D. Clinimetrics vs psychometrics: an unnecessary distinction. J Clin Epidemiol. 2003;56(12):1142–1145. doi: 10.1016/j.jclinepi.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Wind H, Gouttebarge V, Kuijer PP, Frings-Dresen MH. Assessment of functional capacity in the musculoskeletal system in the context of work, daily living and sport: a systematic review. J Occup Rehabil. 2005;15(2):253–272. doi: 10.1007/s10926-005-1223-y. [DOI] [PubMed] [Google Scholar]
- 5.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: Implications for clinical management. Anesth Analg. 2004;99(2):510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 6.Cousins MJ. Persistent Pain: A Disease Entity. J Pain Symptom Manage. 2007;33(2):S4–S10. [Google Scholar]
- 7.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia. A prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 8.Blyth FM, March LM, Cousins MJ. Chronic pain-related disability and use of analgesic and health services in a Sydney community. Med J Aust. 2003;179:84–87. doi: 10.5694/j.1326-5377.2003.tb05441.x. [DOI] [PubMed] [Google Scholar]
- 9.Blyth FM, March LM, Nicholas M, Cousins MJ. Chronic pain, work performance and litigation. Pain. 2003;103:41–47. doi: 10.1016/s0304-3959(02)00380-9. [DOI] [PubMed] [Google Scholar]
- 10.Blyth FM, March LM, Brnabic AJM, Cousins MJ. Chronic pain and frequent use of health care. Pain. 2004;111:51–58. doi: 10.1016/j.pain.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Indahl A, Haldorsen EMH, Holm S, Reikeras O, Olav MD. Five-year follow-up study of a controlled clinical trial using light mobilization and an informative approach to low back pain. Spine. 1998;23(23):2625–2630. doi: 10.1097/00007632-199812010-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hagen EM, Eriksen HR, Ursin H. Does early intervention with a light mobilization program reduce long-term sick leave for low back pain? Spine. 2000;25(15):1973–1976. doi: 10.1097/00007632-200008010-00017. [DOI] [PubMed] [Google Scholar]
- 13.Gatchell RJ, Polatin PB, Noe C, Gardea M, Pulliam C, Thompson J. Treatment- and cost-effectiveness of early intervention for acute low-back pain patients: a one-year prospective study, J Occup Rehabil. 2003;13(1):1–9. doi: 10.1023/a:1021823505774. [DOI] [PubMed] [Google Scholar]
- 14.Wand BM, Bird C, McAuley JH, Dore CJ, MacDowell M, De Souza LH. Early intervention for the management of acute low back pain: a single-blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine. 2004;29(21):2350–2356. doi: 10.1097/01.brs.0000143619.34308.b4. [DOI] [PubMed] [Google Scholar]
- 15.AHCPR. Acute Pain Management: Operative or Medical Procedures and Trauma, Clinical Practice Guideline No. 1, Publication No 92–0032. 1992:116–117. [Google Scholar]
- 16.Texas Cancer Council. Guidelines for treatment of cancer pain: The revised pocket edition of the final report of the Texas cancer council’s workgroup on pain control in cancer patients. [publication on the Internet]. 1997; [cited October 11, 2006] Available from http://www.texascancercouncil.org.
- 17.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 18.Elliott AM, Smith BH, Smith WC, Chambers WA. Changes in chronic pain severity over time: the chronic pain grade as a valid measure. Pain. 2000;88(3):303–308. doi: 10.1016/S0304-3959(00)00337-7. [DOI] [PubMed] [Google Scholar]
- 19.Elliott AM, Smith BH, Hannaford PC, Smith WC, Chambers WA. Assessing change in chronic pain severity: the chronic pain grade compared with retrospective perceptions. Br J Gen Pract. 2002;52(477):269–274. [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25(24):3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Smith CA, Wallston KA, Dwyer KA, Dowdy SW. Beyond good and bad coping: a multidimensional examination of coping with pain in persons with rheumatoid arthritis. Ann Behav Med. 1999;19(1):11–21. doi: 10.1007/BF02883422. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 24.Bouhassira D. The DN4 questionnaire: a new tool for the diagnosis of neuropathic pain. Douleurs. 2005;6(5):297–300. [Google Scholar]
- 25.Bouhassira D, Attal N, Alchaar H. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Benzon HT. The neuropathic pain scales. Reg Anesth Pain Med. 2005;30(5):417–421. doi: 10.1016/j.rapm.2005.07.185. [DOI] [PubMed] [Google Scholar]
- 27.Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1–2):147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 28.Backonja M. Need for differential assessment tools of neuropathic pain and the deficits of LANSS pain scale. Pain. 2002;98(1–2):229–230. doi: 10.1016/s0304-3959(02)00115-x. [DOI] [PubMed] [Google Scholar]
- 29.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Benzon HT. The neuropathic pain scales. Reg Anesth Pain Med. 2005;30(5):417–421. doi: 10.1016/j.rapm.2005.07.185. [DOI] [PubMed] [Google Scholar]
- 31.Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds assessment of neuropathic symptoms and signs pain scale. Reg Anesth Pain Med. 2005;30(5):422–428. doi: 10.1016/j.rapm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Orhan EK, Yucel A, Senocak M, Ertas M. The validation study results of LANSS pain scale. Agri-Istanbul. 2002;14(3):49–54. [Google Scholar]
- 33.Beck AT, Steer RA, Garbin MG. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 34.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychological Medicine. 2002;32(6):959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 35.Andrews G, Slade T. Interpreting scores on the kessler psychological distress scale (K10) Aust N Z J Public Health. 2001;25(6):494–497. doi: 10.1111/j.1467-842x.2001.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 36.Radloff LS. The CED-D scale: a self depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 37.Turk DC, Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behav Res Ther. 1994;32(1):9–16. doi: 10.1016/0005-7967(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 38.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13(2):163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcome. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 41.Main CJ. The modified somatic perception questionnaire (MSPQ) J Psychosom Res. 1983;27(6):503–514. doi: 10.1016/0022-3999(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 42.Deyo RA, Walsh NE, Schoenfeld LS, Ramamurthy S. Studies of the modified somatic perceptions questionnaire (MSPQ) in patients with back pain. Psychometric and predictive properties. Spine. 1989;14(5):507–510. doi: 10.1097/00007632-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 44.Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than fear itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80(1–2):329–339. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- 45.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa scale for kinesiophobia. Pain. 2005;117(1–2):137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Butler A, Bagge CL. The Pain Distress Inventory: development and initial psychometric properties. J Clin Psychol. 2003;59(7):767–785. doi: 10.1002/jclp.10173. [DOI] [PubMed] [Google Scholar]
- 47.Philips HC, Jahanshahi M. The components of pain behaviour report. Behav Res Ther. 1986;24(2):117–125. doi: 10.1016/0005-7967(86)90082-3. [DOI] [PubMed] [Google Scholar]
- 48.Anciano D. The pain behaviour checklist: factor analysis and validation. Br J Clin Psychol. 1986;25(4):301–302. doi: 10.1111/j.2044-8260.1986.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 49.McCracken LM, Yang SY. The role of values in a contextual cognitive-behavioural approach to chronic pain. Pain. 2006;123(1–2):137–145. doi: 10.1016/j.pain.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Nielson W, Jensen MP, Kerns RD. Initial development and validation of a multidimensional pain readiness to change questionnaire. J Pain. 2003;4(3):148–158. doi: 10.1054/jpai.2003.436. [DOI] [PubMed] [Google Scholar]
- 51.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Jensen MP, Turner JA, Romano JM, Strom SE. The chronic pain coping inventory: development and preliminary validation. Pain. 1995;60(2):203–216. doi: 10.1016/0304-3959(94)00118-X. [DOI] [PubMed] [Google Scholar]
- 53.Romano JM, Jensen MP, Turner JA. The chronic pain coping inventory-42: reliability and validity. Pain. 2003;104(1–2):65–73. doi: 10.1016/s0304-3959(02)00466-9. [DOI] [PubMed] [Google Scholar]
- 54.Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One-and two-item measures of pain beliefs and coping strategies. Pain. 2003;104(3):453–469. doi: 10.1016/S0304-3959(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 55.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983b;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 56.Harland NJ, Georgieff K. Development of the coping strategies questionnaire 24, a clinically utilitarian version of the coping strategies questionnaire. Rehabil Psychol. 2003;48(4):296–300. [Google Scholar]
- 57.Kraaimaat FW, Bakker A, Evers AW. Pain coping strategies in chronic pain patients: the development of the pain coping inventory (PCI) Gedragstherapie. 1997;30(3):185–201. [Google Scholar]
- 58.McCracken LM. Behavioural constituents of chronic pain acceptance: results from factor analysis of the chronic pain acceptance questionnaire. J Back Musculoskeletal Rehabil. 1999;13(2–3):93–100. [Google Scholar]
- 59.McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107(1–2):159–166. doi: 10.1016/j.pain.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Cook AJ, Degood DE. The cognitive risk profile for pain: development of a self-report inventory for identifying beliefs and attitudes that interfere with pain management. Clin J Pain. 2006;22(4):332–345. doi: 10.1097/01.ajp.0000209801.78043.91. [DOI] [PubMed] [Google Scholar]
- 61.Dalton JA, Feuerstein M, Carlson J, Roghman K. Biobehavioral pain profile: development and psychometric properties. Pain. 1994;57(1):95–107. doi: 10.1016/0304-3959(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 62.Lerner D, Amick BC, Rogers WH, Malspeis S, Bungay K, Cynn D. The work limitations questionnaire. Med Care. 2001;39(1):72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Lerner D, Reed JI, Massarotti E, Wester LM, Burke TA. The work limitations questionnaire’s validity and reliability among patients with osteoarthritis. J Clin Epidemiol. 2002;55(2):197–208. doi: 10.1016/s0895-4356(01)00424-3. [DOI] [PubMed] [Google Scholar]
- 64.Kopec JA, Esdaile JM. Occupational role performance in persons with back pain’, Disability and Rehabilitation. 1998;20(10):373–379. doi: 10.3109/09638289809166096. [DOI] [PubMed] [Google Scholar]
- 65.Williams RM, Myers AM. Functional abilities confidence scale: a clinical measure for injured workers with acute low back pain. Phys Ther. 1998b;78(6):624–634. doi: 10.1093/ptj/78.6.624. [DOI] [PubMed] [Google Scholar]
- 66.Williams RM, Myers AM. A new approach to measuring recovery in injured workers with acute low back pain: resumption of activities of daily living scale. Phys Ther. 1998a;78(6):613–623. doi: 10.1093/ptj/78.6.613. [DOI] [PubMed] [Google Scholar]
- 67.Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59(3):974. doi: 10.2466/pms.1984.59.3.974. [DOI] [PubMed] [Google Scholar]
- 68.Kliempt P, Ruta D, McMurdo M. Measuring the outcomes of care in older people: a non-critical review of patient-based measures. III. Pain, physical disability and handicap, and social health instruments. Rev Clin Gerontol. 2000;10:235–244. [Google Scholar]
- 69.Åsenlöf P, Denison E, Lindberg P. Behavioural goal assessment in patients with persistent musculoskeletal pain. Physiother Theory Pract. 2004;20(4):243–254. [Google Scholar]
- 70.Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: a report of a patient specific measure. Physiother Can. 1995;47(4):258–263. [Google Scholar]
- 71.Chatman AB, Hyams SP, Neel JM, Binkley JM, Stratford PW, Schomberg A, Stabler M. The patient-specific functional scale: measurement properties in patients with knee dysfunction. Phys Ther. 1997;77(8):820–829. doi: 10.1093/ptj/77.8.820. [DOI] [PubMed] [Google Scholar]
- 72.Westaway MD, Stratford PW, Binkley JM. The patient-specific functional scale: validation of its use in persons with neck dysfunction. J Orthop Sports Phys Ther. 1998;27(5):331–338. doi: 10.2519/jospt.1998.27.5.331. [DOI] [PubMed] [Google Scholar]
- 73.Millard RW. The functional assessment screening questionnaire: application for evaluating pain-related disability. Arch Phys Med Rehabil. 1998;70(4):303–307. [PubMed] [Google Scholar]
- 74.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 75.Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43(12):704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- 76.Newton-John TR. The family impact of pain scale: preliminary validation. J Clin Psychol Med Settings. 2005;12(4):349–358. [Google Scholar]
- 77.Schwartz L, Kraft GH. The role of spouse responses to disability and family environment in multiple sclerosis. Am J Phys Med Rehabil. 1999;78(6):525–532. doi: 10.1097/00002060-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Law M, Baptiste S, McColl MA, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57(2):82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- 79.Carpenter L, Baker GA, Tyldesley B. The use of the canadian occupational performance measure as an outcome of a pain management program. Can J Occup Ther. 2001;68(1):16–22. doi: 10.1177/000841740106800102. [DOI] [PubMed] [Google Scholar]
- 80.Jolles BM, Buchbinder R, Beaton DE. A study compared nine patient-specific indices for musculoskeletal disorders. J Clin Epidemiol. 2005;58(8):791–801. doi: 10.1016/j.jclinepi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Persson E, Rivano-Fischer M, Eklund M. Evaluation of changes in occupational performance among patients in a pain management program. J Rehabil Med. 2004;36(2):85–91. doi: 10.1080/16501970310019142. [DOI] [PubMed] [Google Scholar]
- 82.Walsh DA, Kelly SJ, Johnson SP, Rajkumar S, Bennetts K. Performance problems of patients with chronic low-back pain and the measurement of patient-centered outcome. Spine. 2004;29(1):87–93. doi: 10.1097/01.BRS.0000105533.09601.4F. [DOI] [PubMed] [Google Scholar]
- 83.Corson JA, Schneider MJ. The Dartmouth pain questionnaire: an adjunct to the McGill pain questionnaire. Pain. 1984;19(1):59–69. doi: 10.1016/0304-3959(84)90065-4. [DOI] [PubMed] [Google Scholar]
- 84.Durand MJ, Loisel P, Hong QN, Charpentier N. Helping clinicians in work disability prevention: the work disability diagnosis interview. J Occup Rehabil. 2002;12(3):191–204. doi: 10.1023/a:1016846712499. [DOI] [PubMed] [Google Scholar]
- 85.Marhold C, Linton SJ, Melin L. Identification of obstacles for chronic pain patients to return to work: evaluation of a questionnaire. J Occup Rehabil. 2002;12(2):65–75. doi: 10.1023/a:1015056429505. [DOI] [PubMed] [Google Scholar]
- 86.Linton SJ, Hallden K. Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain. 1998;14(3):209–215. doi: 10.1097/00002508-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Boersma K, Linton SJ. Screening to identify patients at risk: profiles of psychological risk factors for early intervention. Clin J Pain. 2005;21(1):38–43. doi: 10.1097/00002508-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Dunstan DA, Covic T, Tyson GA, Lennie IG. Does the Orebro musculoskeletal pain questionnaire predict outcomes following a work-related compensable injury? Int J Rehabil Res. 2005;28(4):369–370. doi: 10.1097/00004356-200512000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Grotle M, Vollestad NK, Brox JI. Screening for yellow flags in first-time acute low back pain: reliability and validity of a Norwegian version of the acute low back pain screening questionnaire. Clin J Pain. 2006b;22(5):458–467. doi: 10.1097/01.ajp.0000208243.33498.cb. [DOI] [PubMed] [Google Scholar]
- 90.Hurley DA, Dusoir TE, McDonough SM, Moore AP, Baxter GD. How effective is the acute low. Clin J Pain. 2001;17(3):256–263. doi: 10.1097/00002508-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale multidimensional pain inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 92.Kerns RD, Rosenberg R. Pain-relevant responses from significant others: development of a significant-other version of the WHYMPI scales. Pain. 1995;61(2):245–249. doi: 10.1016/0304-3959(94)00173-C. [DOI] [PubMed] [Google Scholar]
- 93.Euroqol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 94.WHOQOL Group. The world health organization quality of life assessment (WHOQOL): position paper from the world health organization. Soc Sci Med. 1995;41(10):1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan RM, Bush JW, Berry CC. Health status: types of validity and the index of well-being,’. Health Serv Res. 1976;11(4):78–507. [PMC free article] [PubMed] [Google Scholar]
- 96.Bergner M, Bobbitt RA, Kressel S, Pollard WE, Gilson BS, Morris JR. The sickness impact profile: conceptual formulation and methodology for the development of a health status measure. Int J Health Serv. 1976;6(3):393–415. doi: 10.2190/RHE0-GGH4-410W-LA17. [DOI] [PubMed] [Google Scholar]
- 97.Claiborne N, Krause TM, Heilman AE, Leung P. Measuring quality of life in back patients: comparison of health status questionnaire 2.0 and quality of life inventory. Soc Work Health Care. 1999;28(3):7–94. doi: 10.1300/j010v28n03_05. [DOI] [PubMed] [Google Scholar]
- 98.Ware JR, Sherbourne C. The MOS-36 item short form health survey 1: conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 99.Scott KM, Tobias MI, Sarfati D, Haslett SJ. SF-36 health survey reliability, validity and norms for New Zealand. Aust N Z J Public Health. 1999;23(4):401–406. doi: 10.1111/j.1467-842x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 100.Ebrahim S, Barer D, Nouri F. Use of the Nottingham health profile with patients after a stroke. J Epidemiol Community Health. 1986;40(2):166–169. doi: 10.1136/jech.40.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wann-Hansson C, Hallberg IR, Risberg B, Klevsgård R. A comparison of the Nottingham health profile and short form 36 health survey in patients with chronic lower limb ischaemia in a longitudinal perspective. Health Qual Life Outcomes. 2004;2:9. doi: 10.1186/1477-7525-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1–2):95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 103.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the neuropathic pain scale. Neurology. 1997;48(2):332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 104.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108(3):248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 105.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID pain. Curr Med Res Opin. 2006;22(8):1555–1565. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 106.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19(5):306–314. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Pincus T, Williams AC, Vogel S, Field A. The development and testing of the depression, anxiety, and positive outlook scale (DAPOS) Pain. 2004;109(1–2):181–188. doi: 10.1016/j.pain.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 108.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 109.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 110.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 111.Butler RW, Damarin FL, Beaulieu C, Schwebel AI, Thorn BE. Assessing cognitive coping strategies for acute post-surgical pain. Psychol Assess. 1989;1(1):41–45. [Google Scholar]
- 112.McCracken LM, Zayfert C, Gross RT. The pain anxiety symptoms scale: development and validation of a scale to measure fear of pain. Pain. 1992;50(1):67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- 113.Vlaeyen JW, Geurts SM, Kole-Snijders AM, Schuerman JA, Groenman NH, van Eek H. What do chronic pain patients think of their pain? Towards a pain cognition questionnaire. Br J Clin Psychol. 1990;29(Pt 4):383–394. doi: 10.1111/j.2044-8260.1990.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 114.Boston K, Pearce SA, Richardson PH. The pain cognitions questionnaire. J Psychosom Res. 1990;34(1):103–109. doi: 10.1016/0022-3999(90)90013-t. [DOI] [PubMed] [Google Scholar]
- 115.Gil K, Williams DA, Keefe FJ, Beckham JC. The relationship of negative thoughts to pain and psychological distress. Behav Ther. 1990;21(3):349–362. [Google Scholar]
- 116.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
- 117.Crowley D, Kendall NA. Development and initial validation of a questionnaire for measuring fear-avoidance associated with pain: the fear-avoidance of pain scale. J Muscoskel Pain. 1999;7(3):3–19. [Google Scholar]
- 118.Rainwater AJ. PhD Thesis. USA: Oklahoma State University; 1999. The role of experienced pain in the assessment of fear of pain: a predictive validity study of the fear of pain questionnaire- III. [Google Scholar]
- 119.Miller R, Kori S, Todd D. The Tampa Scale. Ministry of Health NZ; 1999. [cited November 11, 2006]. Available from: http://www.moh.govt.nz/moh.nsf/f872666357c511eb4c25666d000c8888/fac6e672dcee5aa8cc256edf00742bb7?OpenDocument. [Google Scholar]
- 120.McCracken LM. Attention to pain in persons with chronic pain: a behavioural approach,’. Behav Ther. 1997;28(2):271–284. [Google Scholar]
- 121.Hardt J, Gerbershagen HU. Cross-validation of the SCL-27: a short psychometric screening instrument for chronic pain patients. Eur J Pain. 2001;5(2):187–197. doi: 10.1053/eujp.2001.0231. [DOI] [PubMed] [Google Scholar]
- 122.Main CJ, Wood PL, Hollis S, Spanswick CC, Waddell G. The distress and risk assessment method. A simple patient classification to identify distress and evaluate the risk of poor outcome. Spine. 1992;17(1):42–52. doi: 10.1097/00007632-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 123.Riley JF, Ahern DK, Follick MJ. Chronic pain and functional impairment: assessing beliefs about their relationship. Arch Phys Med Rehabil. 1988;69(8):579–582. [PubMed] [Google Scholar]
- 124.Rosenstiel AK, Keefe FJ. Coping strategies questionnaire (CSQ) Athens, OH: Ohio University; 1983a. [Google Scholar]
- 125.Yong HH, Bell R, Workman B, Gibson SJ. Psychometric properties of the pain attitudes questionnaire (revised) in adult patients with chronic pain. Pain. 2003;104(3):673–681. doi: 10.1016/S0304-3959(03)00140-4. [DOI] [PubMed] [Google Scholar]
- 126.Crow CS, Olivet LW, Burry-Stock J, VanderMeer JL. Assessment of pain coping styles: development of an inventory. J Adv Nurs. 1996;24(5):890–898. doi: 10.1111/j.1365-2648.1996.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 127.Smith BH, Penny KI, Purves AM. The chronic pain grade questionnaire: validation and reliability in postal research. Pain. 1997;71(2):141–147. doi: 10.1016/s0304-3959(97)03347-2. [DOI] [PubMed] [Google Scholar]
- 128.Geiser DS. Unpublished doctoral dissertation. Reno: University of Nevada; 1992. A comparison of acceptance-focused and control-focused psychological treatments in a chronic pain treatment center. [Google Scholar]
- 129.McCracken LM, Eccleston C, Bell L. Clinical assessment of behavioural coping responses: preliminary results from a brief inventory. Eur J Pain. 2005;9(1):69–78. doi: 10.1016/j.ejpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 130.Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients’ attitudes toward pain. J Psychosom Res. 1987;31(3):393–400. doi: 10.1016/0022-3999(87)90060-2. [DOI] [PubMed] [Google Scholar]
- 131.Edwards LC, Pearce SA, Turner-Stokes L, Jones A. The pain beliefs questionnaire: an investigation of beliefs in the causes and consequences of pain. Pain. 1992;51(3):267–272. doi: 10.1016/0304-3959(92)90209-T. [DOI] [PubMed] [Google Scholar]
- 132.Williams DA, Thorn BE. An empirical assessment of pain beliefs. Pain. 1989;36(3):351–8. doi: 10.1016/0304-3959(89)90095-X. [DOI] [PubMed] [Google Scholar]
- 133.ter Kuile MM, Linssen ACG, Spinhoven P. The development of the multidimensional locus of pain control questionnaire (MLPC): factor structure, reliability, and validity. J Psychopathol Behav Assess. 1993;15(4):387–404. [Google Scholar]
- 134.De Vlieger P, Bussche EV, Eccleston C, Crombez G. Finding a solution to the problem of pain: conceptual formulation and the development of the pain solutions questionnaire (PaSol) Pain. 2006;123(3):285–293. doi: 10.1016/j.pain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Tollison DC, Langley JC. Pain patient profile manual. Minneapolis: National Computer Services; 1995. [Google Scholar]
- 136.Anderson K, Dowds B, Pelletz R, Edwards W, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 137.Zarkowska AW. M.Phil thesis. London: University of London; 1981. The relationship between subjective and behavioural aspects of pain in people suffering from lower back pain. [Google Scholar]
- 138.Kerns RD, Rosenberg R, Jamison RN, Caudill MA, Haythornthwaite J. Readiness to adopt a self-management approach to chronic pain: the pain stages of change questionnaire (PSOCQ) Pain. 1997;72(1–2):227–234. doi: 10.1016/s0304-3959(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 139.Nicholas M. PhD thesis. Sydney: University of New South Wales; 1988. An evaluation of cognitive, behavioural and relaxation treatments for chronic low back pain. [Google Scholar]
- 140.Amick B, Lerner D, Rogers W, Rooney T, Katz J. A review of health-related work outcome measures and their uses, and recommended measures. Spine. 2000;25(24):3152–3160. doi: 10.1097/00007632-200012150-00010. [DOI] [PubMed] [Google Scholar]
- 141.Halpern M, Hiebert R, Nordin M, Goldsheyder D, Crane M. The test-retest reliability of a new occupational risk factor questionnaire for outcome studies of low back pain. Appl Ergon. 2001;32(1):39–46. doi: 10.1016/s0003-6870(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 142.Vallerand AH. Development and testing of the inventory of functional status – chronic pain. J Pain Symptom Manage. 1998;15(2):125–133. doi: 10.1016/s0885-3924(98)80010-9. [DOI] [PubMed] [Google Scholar]
- 143.Vindigni D, Parkinson L, Rivett D, et al. Developing a musculoskeletal screening survey for Indigenous Australians living in rural communities. Rural Remote Health. 2006;6(1):321. [PubMed] [Google Scholar]
- 144.Cleeland CS. Brief pain inventory (BPI) Fabry Registry. 1982. [cited 2 August 2007]. Available from: http://www.mdanderson.org/pdf/bpisf.pdf.
- 145.Ferguson RJ, Seville J, Cole B, et al. Psychometric update of the functional interference estimate: a brief measure of pain functional interference. J Pain Symptom Manage. 2004;28(4):389–395. doi: 10.1016/j.jpainsymman.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 146.Pollard CA. PhD thesis. San Diego: California School of Professional Psychology; 1981. The relationship of family environment to chronic pain disability. [Google Scholar]
- 147.Anagnostis C, Gatchel R, Mayer T. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine. 2004;29(20):2290–2302. doi: 10.1097/01.brs.0000142221.88111.0f. [DOI] [PubMed] [Google Scholar]
- 148.Schwartz L, Jensen MP, Romano JM. The development and psychometric evaluation of an instrument to assess spouse responses to pain and well behaviour in patients with chronic pain: the spouse response inventory. J Pain. 2005;6(4):243–252. doi: 10.1016/j.jpain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 149.Robinson ME, Brown JL, George SZ, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med. 2005;6(5):336–345. doi: 10.1111/j.1526-4637.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 150.Granger CV, Ottenbacher KJ, Baker JG, Sehgal A. ‘Reliability of a brief outpatient functional outcome assessment measure. Am J Phys Med Rehabil. 1995;74(6):469–475. doi: 10.1097/00002060-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 151.Linton SJ, Hallden K. Proceedings of the 8th World Congress on Pain. Seattle: IASP Press; 1996. Risk factors and the natural course of acute and recurrent musculoskeletal pain: developing a screening instrument. [Google Scholar]
- 152.Thomas RJ, McEwen J, Asbury AJ. The Glasgow pain questionnaire: a new generic measure of pain; development and testing. Int J Epidemiol. 1996;25(5):1060–1067. doi: 10.1093/ije/25.5.1060. [DOI] [PubMed] [Google Scholar]
- 153.Mossey JM, Kerr ND, Welz-Bosna M, Gallagher RM. Preliminary evaluation of the health background questionnaire for pain and clinical encounter form for pain. Pain Med. 2005;6(6):443–451. doi: 10.1111/j.1526-4637.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 154.Hunter New England NSW Health. Hunter Integrated Pain Service (HIPS) Patient Screening Questionnaire (HIPSPSQ) [cited August 2, 2007]. Available from: www.hunter.health.nsw.gov.au/docs/HIPS-PatientScreeningQuestionnaire-V4.pdf> [website updated May 2008: [cited 16 December 2008] available from: http://www.hnehealth.nsw.gov.au/__data/assets/pdf_file/0020/45812/Patient_Screening_QuestionnaireMay_08.pdf.
- 155.Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381–395. doi: 10.1682/jrrd.2003.09.0381. [DOI] [PubMed] [Google Scholar]
- 156.Waddell G, Burton AK, Main CJ. Screening to identify people at risk of long-term incapacity for work: a conceptual and scientific review. London: Royal Society of Medicine Press; 2003. [Google Scholar]
- 157.Ruehlman LS, Karoly P, Newton C, Aiken LS. The development and preliminary validation of brief measure of chronic pain impact for use in the general population. Pain. 2005;113(1–2):82–90. doi: 10.1016/j.pain.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 158.Ruehlman LS, Karoly P, Newton C, Aiken LS. The development and preliminary validation of the profile of chronic pain: extended assessment battery. Pain. 2005;118(3):380–389. doi: 10.1016/j.pain.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 159.Health Outcomes Institute. Health Status Questionnaire 2.0: user guide. Medical Center; Boston, MA: 1993. [Google Scholar]
- 160.Hunt SM, McKenna SP, Williams Reliability of a population survey tool for measuring perceived health problems: a study of patients with osteoarthrosis. J Epidemiol Community Health. 1981;35(4):297–300. doi: 10.1136/jech.35.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCracken LM, Gross RT, Aikens J, Carnrike CL. The assessment of anxiety and fear in persons with chronic pain: a comparison of instruments. Behav Res Ther. 1996;34(11–12):927–933. doi: 10.1016/s0005-7967(96)00057-5. [DOI] [PubMed] [Google Scholar]