Abstract
The T helper type 17 (Th17) lineage of CD4+ T-cells produce several effector molecules including IL-17A, IL-17F, IL-21, and IL-22. In addition to CD4+, αβ T-cells, these cytokines can be produced by natural killer and γδ T-cells. These effector cytokines can be produced rapidly upon infection at mucosal sites and evidence to date strongly implicates that this arm of the immune system plays a critical role in mucosal immunity to many extracellular pathogens. Moreover these cytokines can also coordinate adaptive immunity to some intracellular pathogens. In this review, we will highlight recent progress in our understanding of these cytokines, and mechanisms of their effector function in the mucosa.
Keywords: Th17, Infection, mucosa
Introduction
CD4+ T helper cells are critical mediators of adaptive immune responses. Following interaction with class II MHC+ dendritic and other antigen presenting cells, naïve T cells receive signals by engagement of the T cell receptor (signal 1), costimulatory molecules (signal 2) and cytokine signals (signal 3) and undergo activation and differentiation into effector CD4+ T cells. Some of the strongest evidence that these cells are critical effector cells at mucosal surfaces is clearly evidenced by the HIV epidemic that leads to the depletion of these cells from the mucosa and periphery [1–3] leading to the acquired immunodeficiency syndrome (AIDS).
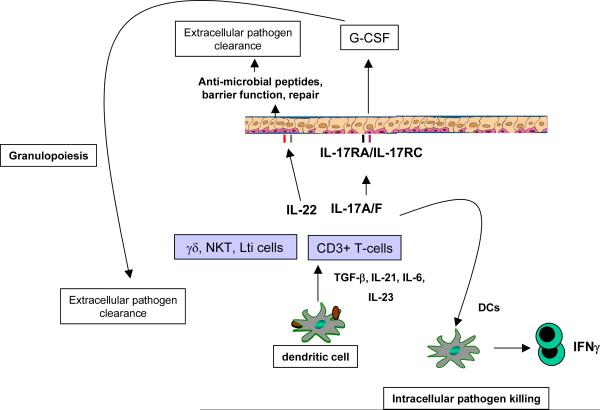
Pioneering work by Mossmann and Coffman described the first two CD4+ T-cells subsets based on discrete cytokine profiles [4]. Th1 effectors produce Interferon-gamma (IFN-γ) and regulate cellular immunity against intracellular infections, whereas Th2 cells produce Interleukin (IL)-4, IL-5 and IL-13 and mediate humoral immunity against parasite infections. However these two T-cell subsets do not completely account for some of the opportunistic infections seen in the context of congenital or acquired absence of CD4+ T-cells such as mucosal candidiasis, Pneumocystis carinii pneumonia, or some bacterial pneumonias. Mice deficient in Th1, Th2 responses (or both) are not permissive for P. carinii pneumonia [5;6], a hallmark infection in AIDS patients with low CD4+ T-cell counts. These data suggested other CD4+ T-cell lineages are critical for host defenses against opportunistic infections. IL-17 was cloned from CD4+ memory cells in 1993 [7] and Infante-Duarte demonstrated that IL-17 cells could be produced in response to bacterial lipopeptides [8]. Importantly this early study showed that IL-17 producing cells were distinct from Th1 cells and thus this provided the first evidence that T-cell derived IL-17 may have unique effector functions in host resistance against pathogens [8]. Recent evidence has shown that a major subset of cells that produce IL-17, are indeed distinct from Th1 and Th2 cells, and consist of a third subset of T cells referred to as Th17 cells [9–11]. Th17 cells produce the cytokines IL-17A (IL-17) [10;11] and IL-17F [9], as well as the cytokines IL-21 [12;13] and IL-22 [14;15] (Figure 1). This new Th17 cell lineage fills in some of the holes in host immunity not fully explained by the Th1/Th2 paradigm.
Figure 1. Schema of Th17 effector in mucosal immunity.
IL-17 and IL-22 can be produced by several immune cells including αβ, and γβT-cells, as well as NK cells. Receptors for IL-17a, IL-17F, and IL-22 are expressed on mucosal epithelial cells. Stimulation of epithelial cells with IL-17 and IL-22 induces G-CSF and CXC chemokine production. The combination of G-CSF and CXC chemokines such as CXCL1, CCXL2, CXCL5 and CXCL8 result in neutrophil recruitment required for bacterial and fungal clearance at mucosal sites. IL-22 and IL-17 can also augment the expression of antimicrobial peptides. IL-22, in part via the activation of STAT3 can also mediate epithelial repair which is critical to control of extracellular bacterial pathogens. In the setting of intracellular pathogens IL-17 can induce the production of IL-12 in DCs and drive Th1 immunity and intracellular bacterial control.
Th17 Effector Cytokines: IL-17A, IL-17F, and IL-22
IL-17 is the prototype of Th17 cytokines and is the best studied of the Th17 cytokines. The first identified receptor for IL-17, IL-17RA is a Type I transmembrane protein and is ubiquitously expressed on various organs including lung, kidney and spleen [16]. This receptor can bind at least three members of the IL-17 family including IL-17A, the closely related molecule IL-17F, as well as the most distally related IL-17 family member IL-17E [17]. The best studied examples of cells that express the receptor for IL-17 are leucocytes, epithelial cells, mesothial cells, vascular endothelial cells, keratinocytes and fibroblasts and respond to IL-17R-mediated signaling by production of granulocyte colony-stimulating factor (G-CSF), IL-6, IL-8 and mediate granulopoiesis, neutrophil recruitment and inflammatory responses (reviewed in [18], Figure 1). However, more recently it has been shown that macrophages [19] and dendritic cells [20;21] also express IL-17RA receptors and respond to IL-17 by producing cytokines and chemokines. Indeed over-expression of IL-17 in mice results in massive extramedullary hematopoiesis [22] via the induction of G-CSF and stem cell factor [23]. IL-17RA KO mice can develop spontaneous infection with S. aureus infection around the eyes and nares [24] and this phenotype is also observed in mice deficient in two of the three ligands for this receptor, IL-17A/F double KO mice [19]. Interestingly this phenotype is not observed in IL-17A or IL-17F single KO mice [19] suggesting that these two cytokines can compensate for each other in this model. Both IL-17A and IL-17RA KO mice show susceptibility to pulmonary infection with the extracellular gram negative bacteria K. pneumoniae again with IL-17RA KO mice showing greater susceptibility than IL-17A KO mice [25]. In this model IL-17RA KO mice fail to recruit neutrophils into the lung in part due to reduced CXC chemokine production [26] but also reduced granulopoiesis likely through reduced G-CSF production [26]. Also through its effects on dendritic cells, IL-17 has been shown to regulate IL-12p70 production by dendritic cells and thereby regulating Th1 response to the intracellular pathogen Francisella tularensis [21] and Chlamydia muridarum [20]. In contrast, IL-17 appears to be dispensable for Th1 immunity to Mycobacterium tuberculosis and Listeria [25;27], but may be important in generation of mycobacterial granulomas [28]. Thus data to date strongly implicate Th17 cytokines in host immunity to both extracellular pathogens and certain intracellular pathogens.
IL-22 was initially known as IL-10-related T cell derived inducible factor (IL-TIF) when it was first characterized by Dumoutier et al. It was induced in mouse T lymphoma cells by IL-9 and had 22% amino acid identity with IL-10 [29]. IL-TIF did not appear to have effect on proliferation of T cells or mast cells; however IL-22 activates STAT3 in kidney mesangial cells [30] and keratinocytes [31]. IL-TIF was renamed IL-22 after the human protein was discovered, and other structurally related cytokines (IL-19, IL-20, IL-24, IL-26, IL-28αβ and IL-29) joined IL-22 to become part of the IL-10 cytokine family [30]. The IL-22 receptor is part of the cytokine receptor family class 2, and consists of two subunits, IL-22R1 and IL-10R2 [30;31]. IL-10R2 is widely expressed on immune cells (T, B and NK cells), unlike IL-22R1 [31]. Wolk et al had demonstrated that IL-22R1 expression is present in a variety of non-immune tissues: skin, lung, small intestine, liver, colon, kidney and pancreas [31]. They also showed activation of STAT3 in human keratinocytes by IL-22 [31]. IL-22R1 is also expressed on mucosal epithelium GI tract and lung [32] as well as on macrophages [33]. IL-22 can be co-expressed by Th17 cells [15], γδ T-cells [34;35] as well as NK cells [36–38].
Regulation of IL-17 and IL-22 in the lung
The differentiation of Th17 cells is determined by the exposure to polarizing cytokines such as TGF-β, IL-6, IL-21, while IL-23 further stabilizes the commitment of Th17 cells to this lineage (reviewed in [39]. These polarizing cytokines act on newly primed cells to induce the expression of the transcription factor RORγT and RORα which are considered master regulators of Th17 differentiation [40;41]. RORγT also control the expression of IL-23 inducible receptors on newly primed T cells, to expand their responsiveness to IL-23 and sustain the T lineage specific responses. The gp-130-Stat3 pathway is essential for expression of RORγT and Th17 development. Recently, IL-21 has been identified as a downstream mediator of Th17 differentiation and functions in an autocrine manner. Th17 cells also express high levels or IL-R1 [42] and TLR2 [43] and both IL-1β and TLR2 ligands can activate these cells to produce IL-17 and IL-22. Cellular sources of IL-17 in acute primary pulmonary infection with K. pneumoniae [44], M. tuberculosis [45], F.tularensis [21], or Influenza [46] include γδ T-cells, iNKT cells and possibly LTi cells [47]. In many infections, the γδ T-cell response can be the predominant source and the cellular production of IL-17 by these cells is critically regulated by both IL-23 and IL-1β [34;35]. What remains unclear is the cellular sources of IL-23 and IL-1β in vivo and the γδ T-cell subsets that respond to these signals. In a pulmonary model of Aspergillus infection, Vγ1 T-cells are the dominant source of IL-17 [48]. However in a model of chronic Bacillus subtilis infection the Vγ6 subset dominates [49], while in a pulmonary model of M. bovis BCG both Vγ6 and Vγ4 predominate [50].
Since over-induction of Th17 cells can impact tissue damage due to induction of inflammatory pathways, the generation of Th17 cells is strictly regulated. For example, cytokines such as IL-27 [51;52], those belonging to Th1 (IFNγ) and Th2 pathway (IL-4) [10;11] and IL-2 [53] tightly regulate the induction of Th17 cells. Endogenous lipid mediators like prostaglandin E2 (PGE2) which are released under inflammatory conditions promote induction of IL-23 through the EP2 and EP4 receptors expressed on DCs [54] to direct subsequent Th17 cell differentiation. Further PGE2 also synergizes with IL-1B and IL-23 to upregulate RORγt [54]. These studies, suggest that external infection-induced lipid mediators can also tip the decision towards Th17 responses. Accordingly, PGE2 is induced in response to respiratory Francisella [55] (Woolard et al, Iand I 2008) and Bordetella [56] infection drives Th17 responses.
Th17 effector responses in the mucosa
Human bronchial lung epithelial (HBE) cells express both IL-17RA, IL-17RC, IL-22R and IL-10R2 and thus can responses to IL-17A, IL-17F, and IL-22 [25;57–59]. The expression of IL-17RA and IL-17RC also appear to be expressed in a basolateral dominant fashion [57;59] such that signaling only occurs in polarized epithelial cells are exposed to ligand provided to basolateral surface [59;60]. Treatment of HBE cells with IL-17 induce CXC chemokines such as IL-8 [57;61], G-CSF [57], and antimicrobial proteins such as human β-defensin 2 [62]. IL-17 treatment also induced IL-19 [63] which may be important in regulating Th2 responses in the mucosa. IL-17 also induced the expression of the polymeric immunoglobulin receptor [25] and indeed Th17 cytokines have been shown to be critical in generating mucosal IgA responses [64]. IL-22 can activate STAT3 [30] in both murine and human lung epithelial cells and synergizes with IL-17 to increases the expression of human antimicrobial genes such as HBD2, lipocalin [25] and the calgranulins [25]. An activity that is unique to IL-22 and not shared by IL-17A or IL-17F in human lung epithelial cells is the fact that IL-22 can augment the clonogenic potential of these cells and accelerate wound repair [25]. In skin keratinocytes, IL-22 can also cause acanthosis and hyper-proliferation [65]. Thus IL-22 may be important in epithelial repair in infection as well as augmenting barrier function (Figure 1). To this end neutralizing IL-22 in vivo leads to rapid dissemination of bacteria from the lung during K. pneumoniae infection [25]. Mucosal IL-22 administration can reduce local bacterial growth as well as prevent dissemination in this model [25]. The cellular source of IL-22 in the model is unclear but IL-22 producing cells are present in Rag 2−/− mice but not Rag 2−/−, γC double KO mice demonstrating that IL-22 producers are γC dependent (ref). Interestingly, IL-22 treatment of human macrophages has also been shown to induce phagolysosomal fusion and enhance mycobacterial killing [33].
Th17 cytokines and bacterial infections at the mucosa
As mentioned above, early work in IL-17RA KO mice demonstrating increased sensitivity to cutaneous S. aureus [24;66;67] and pulmonary K. pneumoniae [26] infection established critical role for IL-17 in protective immunity against extracellular bacteria. Further studies also show that Th17 cells play protective roles against extracellular bacterial infections in the gut mucosa. Citrobacter rodentum, a naturally occurring mouse pathogen requires the generation of IL-23-dependent protective Th17 cells in the lamina propria for protection [66;68]. Also, IL-22 contributes to the early host defense against C.rodentium through the direct induction of the Reg family of antimicrobial proteins in colonic epithelial cells [69]. IL-17 signaling also appears to be host-protective in the oral mucosa, as IL-17R-deficient mice are highly susceptible to infection by the gram-negative anaerobic periodontal pathogen, Porphyromonas gingivalis, due to reduced neutrophil mobilization and recruitment [70].
Although these studies strongly suggest that Th17 cytokine responses are protective against most extracellular pathogens, in some cases Th17 responses contribute to tissue pathology. This was first shown in response to chronic biofilm infection with Pseudomonas aeruginosa where IL-23 deficient mice have markedly reduced IL-17 responses and less tissue pathology in response to chronic mucoid P. aeruginosa infection [71]. Furthermore, in whooping cough, an infection caused by B. pertussis, a gram negative extracellular bacterial infection in the respiratory tract results in persistent cough as one of the hallmarks of the clinical disease. It is becoming clear that the bias towards Th17 responses may be the basis for the severe pathology including bronchiectasis and persistent cough seen following B. pertussis infection. Furthermore, the Th17 responses following respiratory Bordetella infection are dependent on innate IL-1β induction and activation of NALP3 inflammasome [72]). . This hypothesis is further supported by another cause of bronchiectasis, cystic fibrosis which is highly associated with bronchiectasis due to chronic biofilm infection with P. aeruginosa and increased expression of Th17 cytokine production [25]. Following M. tuberculosis pulmonary infection, the inflammation caused in the lung due to repeated vaccinations with BCG results in pathological consequences caused by neutrophil-mediated influx that is IL-17 dependent [73]. These disease models may serve as an example for the role of IL-17 in mediating pathology while conferring protection against bacterial infections in the respiratory mucosa. These findings are not confined to the lung. In the gastrointestinal tract, in H.pylori infection the Th17 response may culminate in a pathogenic inflammatory response in the gut mucosa [74;75]. Accordingly, IL-17 deficiency resulted in decreased bacterial growth and inflammation and was associated with decreased Th1 responses [76]. In contrast, certain intracellular pathogens that may require both CD4 Th1 cells and neutrophils for protection at mucosal sites may be dependent on IL-23/IL-17 axis for pathogen control. For example, the induction of IL-17 and IL-17F production following acute Mycoplasma pneumonia pulmonary infection is IL-23-dependent and contributes to neutrophil recruitment and mediates protection against the infection [77]. Also, infection with Salmonella typhimurium, which can exist as an intracellular pathogen, results in induction of IL-17 and IL-22 in the ileal mucosa and the absence of IL-17R signaling results in increased translocation of the bacteria to the mesenteric lymph nodes, reduced induction of chemokines, anti-microbials and reduced neutrophilic recruitment to the ileal mucosa [78]. Using a macaque model of Simian Immunodeficiency Virus (SIV) to study HIV human disease and related complications arising due to bacterial coinfections such as S.typhimurium, it was found that SIV coinfection selectively inhibits Th17 responses elicited by S. typhimurium, probably due to depletion of CD4+ T cells in the ileal mucosa [78]. This results in an inability of SIV-infected macaques to mount normal mucosal inflammatory response to S. typhimurium infection and allows dissemination of bacteria to the mesenteric lymph node. This data may provides a basis for the observation that people with HIV are at a increased risk of developing bacteremia due to dissemination of bacteria resulting from reduced CD4 Th17 responses. Accordingly, HIV-infected individuals that receive antiretroviral therapy undergo effective CD4 T-cell restoration and this is associated with enhanced CD4 Th17-cell accumulation [79].
Th17 cytokines and fungal infections at the mucosa
The role of Th17 cytokines in fungal infection have been controversial but evidence in both experimental animal models and humans is clearly emerging that these cytokines play a critical role in mucosal infections. Initial observations suggested that Th17 cytokines, particularly IL-17 contributes to tissue pathology in invasive aspergillus infection in the lung particularly in the setting of NADPH oxidase deficiency [48]. However IL-17 and IL-17RA signaling are critical for host resistance to oro-pharyngeal candidiasis and for the expression of mouse β-defensin 3 that has anti-Candicidal activity [80]. Patients with Hyper IgE syndrome (HIES) that frequently suffer from oropharyngeal candidiasis have mutations in STAT3 [81;82] thus fail to generate Candida specific Th17 cells adding further evidence that this phenotype is tightly linked to Th17 immunity [83;84]. Indeed, treatment of skin keratinocytes with Th17 cytokines markedly increases anti-Candicidal activity in vitro and activated T-cells from HIES patients are unable to induce this anti-Candicidal activity in vitro. It has recently been shown that patients with chronic mucocutaneous candidiasis also have antibodies that can block IL-17 and or IL-22 which may also explain the susceptibility of these patients to mucosal Candida infection [85]. In respiratory tract models of fungal infections using Pneumocytis carnii [86] and Aspergillus fumigatus [87], induction of IL-23 and IL-17 following pathogen challenge is protective, since IL-23KO mice or neutralization of the IL-23/IL-17 axis resulted in impaired clearance of the pathogen.
Th17 cytokines and viral infection
The role of Th17 cytokines in viral infection is a rapidly emerging area of research. Herpes simplex virus (HSV-1) infection of the cornea results in early induction of both IL-23 [88] and IL-17 [89] and IL-17RA KO mice have reduced early infiltration of neutrophils and corneal opacity following HSV infection [89]. In contrast, IL-23KO mice have exacerbated disease and pathology possibly due to HSV increased IL-12 responses and increased IFNγ producing cells [88]. In pulmonary influenza infection IL-17R KO mice had recued tissue pathology and weight loss suggesting that in this model, IL-17 contributes to tissue pathology as well [46]. However in a heterotypic influenza model, CD8+ cells that produce IL-17 can mediate protection against influenza challenge [90]. Thus the role of the IL-17 response to primary influenza infection versus heterotypic infection appears to differ. Human rhinovirus (HRV) infections are associated with exacerbations of asthma and chronic obstructive pulmonary infiltration and IL-17 was shown to function synergistically with HRV to induce IL-8 from epithelial cells and may contribute to the recruitment of neutrophils, immature DCs and memory T cells to the lung contributing to severe inflammatory profiles seen during viral exacerbations of airway disease [91].
Conclusions
We specifically did not address the role of Th17 cytokines in vaccine induced immunity as this has been recently reviewed [92]. Data to date suggest that Th17 cells have evolved to mediate protective immunity against a variety of pathogens at different mucosal sites. Moreover, deficient Th17 responses explain in part the increased susceptibility to certain infections such as mucocutaneous in HIES patients and depletion of Th17 cells by HIV explains some of the opportunistic infections in AIDS. Taken together strategies to augment these cells or recover these cell populations will be important for improved vaccine or therapeutic efficacy in these patients. It is important to remember that IL-17 is evolutionary conserved and the gene exists in mollusks and Ciona intestinalis which predates the development of adaptive T-cell immunity and thus this cytokine likely bridged innate and adaptive immunity. What remains unclear how did evolutionary pressure force this gene to be expressed in memory CD4+ T-cells in mammals? This will be an important area of future investigation.
Biographies

Dr. Jay K. Kolls is Professor of Genetics and Pediatrics and Chair, Department of Genetics at the Louisiana State University Health Sciences Center in New Orleans. Dr Kolls was recruited on January 2009 from the Children's Hospital of Pittsburgh at the University of Pittsburgh in Pittsburgh, Pennsylvania where he was the Niels K Jerne Professor Pediatrics and Immunology. He earned his medical degree at the University of Maryland and completed his residency training in Internal Medicine/Pediatrics at Charity Hospital in New Orleans, LA. After that he completed Fellowships in Adult and Pediatric Pulmonology at LSU and Tulane Health Sciences Center respectively. He performed his research fellowship in the laboratory of Dr. Bruce Beutler at Howard Hughes Medical Institute, UT Southwestern Medical Center, Dallas, TX. Dr. Kolls is a member of the American Association of Immunology, American Society of Microbiology, and the American Society of Clinical Investigation. Dr. Kolls has authored or coauthored more than 160 peer-reviewed articles. The major goal of Dr. Kolls' research is to investigate mechanisms of mucosal host defenses in normal and immunocompromised hosts using genetic models. Presently, his lab is investigating how IL-23 and IL-17 and IL-22 regulate host defense against extracellular pathogens and epigenetic regulation of macrophage function. Additionally, he researches host susceptibility to opportunistic infection such as Pneumocystis and is developing pre-clinical gene-based vaccines against this pathogen.

Dr. Khader received her PhD in Biotechnology from Madurai Kamaraj University, India where she studied host-pathogen interactions during the mycobacterial disease, leprosy. Dr. Khader then carried out her Post-doctoral training at the Trudeau Institute, NY where she continued studying host immune responses to another mycobacterial disease, Tuberculosis. During her stay at the Trudeau Institute, Dr. Khader demonstrated a critical role for the cytokine Interleukin-17 in vaccine-induced immunity to tuberculosis. Dr. Khader joined the University of Pittsburgh in 2007 as Assistant Professor in the Department of Pediatrics where her lab continues to study the role of T helper subsets in immunity to intracellular pathogens. Recently, Dr. Khader received a Pathway to Independence Award from NIH and the Young Investigator Award from the International Cytokine Society. Dr. Khader is also an Associate Editor of the Journal of Immunology and on the Editorial Board of the Journal of Infectious Diseases and Immunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal. Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- [3].Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- [4].Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- [5].Garvy BA, Ezekowitz RA, Harmsen AG. Role of gamma interferon in the host immune and inflammatory responses to Pneumocystis carinii infection. Infect. Immun. 1997;65:373–379. doi: 10.1128/iai.65.2.373-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infection & Immunity. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- [8].Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- [9].Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- [11].Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- [13].Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- [15].Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yao Z, Fanslow WC, Seldin MF, Rousseau A-M, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- [17].Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- [20].Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol. 2009;183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- [21].Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schwarzenberger P, La RV, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, et al. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- [23].Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, Bagby G, Nelson S, Kolls JK. Requirement of Endogenous Stem Cell Factor and Granulocyte-Colony- Stimulating Factor for IL-17-Mediated Granulopoiesis. J. Immunol. 2000;164:4783–4789. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- [24].Schwarzenberger P, Kolls JK. Interleukin 17: an example for gene therapy as a tool to study cytokine mediated regulation of hematopoiesis. J. Cell Biochem. Suppl. 2002;38(Suppl):88–95. doi: 10.1002/jcb.10054. [DOI] [PubMed] [Google Scholar]
- [25].Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- [28].Okamoto YY, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- [29].Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 2000;164:1814–1819. doi: 10.4049/jimmunol.164.4.1814. [DOI] [PubMed] [Google Scholar]
- [30].Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin. Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- [31].Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- [32].Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol. Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- [33].Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009;183:6639–6645. doi: 10.4049/jimmunol.0902587. [DOI] [PubMed] [Google Scholar]
- [34].Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- [35].Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [36].Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- [38].Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- [40].Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- [41].Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, et al. Cutting Edge: Roles of Toll-Like Receptor 4 and IL-23 in IL-17 Expression in Response to Klebsiella pneumoniae Infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- [46].Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- [49].Simonian PL, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl. Res. 2009;154:222–227. doi: 10.1016/j.trsl.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- [51].Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, De Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- [52].Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- [53].Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- [54].Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal MR. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp. Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 2008;76:2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Siciliano NA, Skinner JA, Yuk MH. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J. Immunol. 2006;177:7131–7138. doi: 10.4049/jimmunol.177.10.7131. [DOI] [PubMed] [Google Scholar]
- [57].McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, et al. Role of IL-17A, IL-17F, and the IL-17 Receptor in Regulating Growth-Related Oncogene-{alpha} and Granulocyte Colony-Stimulating Factor in Bronchial Epithelium: Implications for Airway Inflammation in Cystic Fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- [60].Uesugi T, Froh M, Arteel GE, Bradford BU, Gabele E, Wheeler MD, Thurman RG. Delivery of IkappaB superrepressor gene with adenovirus reduces early alcohol-induced liver injury in rats. Hepatology. 2001;34:1149–1157. doi: 10.1053/jhep.2001.29400. [DOI] [PubMed] [Google Scholar]
- [61].Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- [62].Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- [63].Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, Wu R. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J. Allergy Clin. Immunol. 2008;121:1415–21. 1421. doi: 10.1016/j.jaci.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: Lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- [66].Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential roles of IL-17A and IL-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009 doi: 10.1016/j.immuni.2008.11.009. In press. [DOI] [PubMed] [Google Scholar]
- [67].Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- [69].Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, De Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- [70].Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect. Immun. 2008;76:4206–4213. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J Physiol Lung Cell Mol. Physiol. 2006 doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- [73].Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- [75].Caruso R, Fina D, Paoluzi OA, Del Vecchio BG, Stolfi C, Rizzo A, Caprioli F, Sarra M, Andrei F, Fantini MC, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur. J. Immunol. 2008;38:470–478. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- [76].Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, et al. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol. 2010;184:5121–5129. doi: 10.4049/jimmunol.0901115. [DOI] [PubMed] [Google Scholar]
- [77].Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes. Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal. Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- [80].Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- [82].Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- [83].Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].de BL, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von BH, Samarina A, Janniere L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim B, Sarangi PP, Azkur AK, Kaistha SD, Rouse BT. Enhanced viral immunoinflammatory lesions in mice lacking IL-23 responses. Microbes. Infect. 2008;10:302–312. doi: 10.1016/j.micinf.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J. Leukoc. Biol. 2008;83:401–408. doi: 10.1189/jlb.0807571. [DOI] [PubMed] [Google Scholar]
- [90].Hamada H, Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wiehler S, Proud D. Interleukin-17A modulates human airway epithelial responses to human rhinovirus infection. Am. J. Physiol Lung Cell Mol. Physiol. 2007;293:L505–L515. doi: 10.1152/ajplung.00066.2007. [DOI] [PubMed] [Google Scholar]
- [92].Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal. Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]