Abstract
Most of the literature on bipolar disorder has focused on abnormalities of neurotransmitters rather than brain circuits. The few discussions of circuits have primarily focused on the limbic system. Evidence is accumulating, however, that basal ganglia-thalamic-cortical reentrant circuits play a major role in the affective, motor and cognitive symptoms of a number of neuropsychiatric conditions including bipolar disorder. In this paper, the authors argue that there is compelling direct and indirect evidence of frontal-subcortical circuit abnormalities in patients with bipolar affective disorder.
Introduction
Bipolar affective disorder is a complex neurobiological illness1 and the pathophysiology of this condition remains poorly understood.2,3 Most of the literature on this disorder has focused on abnormalities of neurotransmitters rather than brain circuits. The few discussions of circuits have primarily focused on the limbic system. The basal ganglia-thalamic-cortical reentrant circuits, generally known as frontal-subcortical (FSC) circuits, are one of the primary brain networks and are involved in motor, cognitive and emotional processing.4–6 There is accumulating evidence, however, that these circuits play a major role in the affective, motor and cognitive symptoms of a number of neuropsychiatric conditions including bipolar disorder.4–14
Overview of Frontal-Subcortical (FSC) Circuits
The five FSC circuits are one of the primary organizational networks of the brain and are highly involved in brain-behavior relationships.4,5,9 The five circuits are the motor, oculomotor, dorsolateral prefrontal (DLPF), anterior cingulate (AC), and orbitofrontal (OF). These circuits share a general structure consisting of the cortex, basal ganglia and thalamus (Figure 1). Information originates from the cerebral cortex, travels first to the basal ganglia then on to the thalamus, and finally returns to numerous areas of the cortex.6,15 This feedback to multiple cortical areas results in FSC processing in cognitive, emotional, and sensory as well as motor domains.5,9,16 All FSC circuits have both a direct and an indirect pathway, which have “opposite” or reciprocal functions (Figure 2).
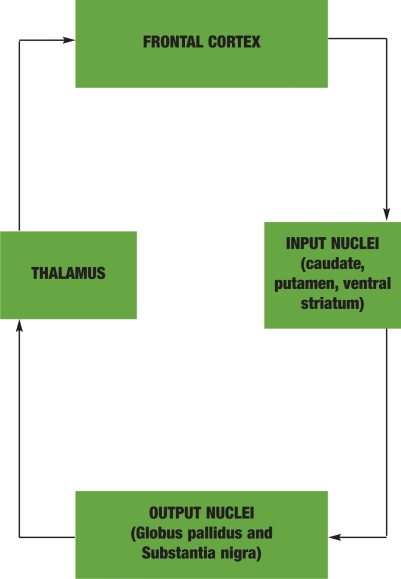
Figure 1.
SIMPLIFIED FRONTAL-SUBCORTICAL CIRCUIT STRUCTURE. This figure depicts the general organizational structure of the frontal-subcortical circuits. This is a simplified illustration showing only the direct pathway. All FSC circuits share a general structure consisting of an information loop connecting the cortex, basal ganglia, and thalamus. Information originates from many areas in the cerebral cortex, travels first to the basal ganglia, then on to the thalamus, and finally returns to the cortex. The caudate, putamen, and ventral striatum make up the input nuclei and receive excitatory glutamate projections from multiple areas of the cortex. The input nuclei then connect by way of inhibitory GABA fibers to the major output nuclei, which consist of the internal segment of the globus pallidus and the pars reticulata of the substantia nigra. The output nuclei send inhibitory GABA efferents to the thalamus. Finally, the circuit is closed by way of excitatory glutamate fibers that project back to the cortex.
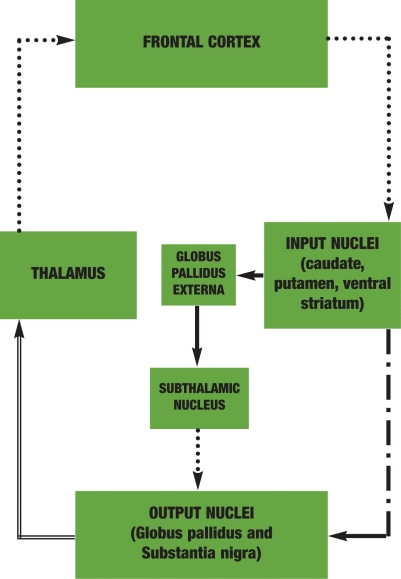
Figure 2.
DIRECT AND INDIRECT FRONTAL-SUBCORTICAL CIRCUITS. This figure illustrates the direct and indirect frontal-subcortical circuits. The components of the indirect pathway are shown by the two solid lines (GABA) and the dotted line (glutamate) between the subthalamic nucleus and the output nuclei. The subthalamic nucleus also receives direct input from the frontal cortex by way of excitatory glutamate fibers, which are not shown in this illustration. The direct and indirect pathways have reciprocal processing functions. Information that flows by way of the direct pathway increases excitatory outflow to the cortex while the indirect pathway decreases excitatory drive.
Glutamate fibers • • • • • • • • • • • •
GABA fibers (direct pathway) ■■■■■ ■ ■■■■■ ■ ■■■■■
GABA fibers (indirect pathway) ■■■■■■■■■■■■
GABA fibers (common to both pathways) ════════════
Direct FSC pathway. Excitatory. Information that flows by way of the direct FSC pathway increases excitatory flow to the cortex. The basal ganglia can be characterized as either “input” or “output” nuclei.16 The caudate, putamen, and ventral striatum make up the input nuclei and receive excitatory glutamate projections from multiple areas of the cortex.15–17 The input nuclei then connect by way of inhibitory gamma aminobutyric acid (GABA) fibers to the major output nuclei, which consist of the internal segment of the globus pallidus and the pars reticulata of the substantia nigra. The output nuclei send inhibitory GABA efferents to thalamic nuclei that project back to the cortex by way of excitatory glutamate fibers. This circuit is known as the direct FSC circuit (Figure 2).
Indirect FSC pathway. Inhibitory. The indirect pathway tends to inhibit the thalamus and decreases excitatory drive to the cortex.18,19 This circuit involves GABA projections, first from the striatum to the globus pallidus externa, then on to the subthalamic nucleus (Figure 2). Projections then connect with the globus pallidus interna and substantia nigra by way of glutamate neurons. The indirect pathway also receives excitatory glutamatergic input from many areas of the cortex directly to the subthalamic nucleus.
This reciprocity of the direct versus indirect pathways is believed be involved in the initiation and cessation of behaviors required for adaptive functioning.19 In the normally functioning brain, a dynamic balance of neural tone in both pathways is required to allow the appropriate expression and cessation of behaviors.19
FSC Neurochemistry
Several neurotransmitters thought to play a role in bipolar affective disorder are utilized in FSC circuits including glutamate, GABA, dopamine, acetylcholine and serotonin.5,9,18 Interactions between glutamate and GABA fibers determine whether FSC circuit output to the cortex will be excitatory or inhibitory.18 The role of the other neurotransmitters is primarily one of modulation.18
Dopamine has complex facilitation and inhibition effects on FSC circuits.6 Dopaminergic fibers project from the substantia nigra pars compacta (SNpc) and ventral tegmental area to the striatum. The SNpc receives input from the limbic circuits including the ventral striatum. Thus, the ventral striatum, by way of projections to the SNpc, is able to exert global regulatory influence on dopaminergic input to the entire striatum. It has been hypothesized that this provides the anatomical basis for the diverse effects of dopaminergic agents on motivation, thought, behavior and motor activity.20 Further, this mechanism is thought to provide “motivational” input to modulate motor behavior and reinforce successful experience.5
Hypothetical Role of FSC Dysfunction in Bipolar Disorder
Several authors have hypothesized that orbitofrontal FSC circuit abnormalities may play a role in the etiology of mood disorders.7–10,12–14,21 Strakowski and Sax have hypothesized that secondary bipolar disorder is the result of decreased excitatory drive to the orbitofrontal cortex.12 They theorize that injury to the caudate may lead to a subsequent reduction in inhibitory GABAergic transmission to the globus pallidus with a corresponding increase in inhibitory signal to the thalamus and finally decreased glutamate mediated excitatory transmission from the thalamus to the orbitofrontal cortex. They also state that injury to the thalamus would lead to decreased excitation to the orbitofrontal cortex by a similar mechanism.
Caligiuri and colleagues have also suggested that a disturbance of FSC circuit inhibition of the cortex may be related to affective state in BD.8 Their hypothesis is that cortical activity would increase during manic states with corresponding changes in FSC activation patterns. They also suggest that the lateralized findings in their study may represent a compensation mechanism in the opposite hemisphere.
Though similar in many respects, the key difference between these hypotheses is whether thalamic drive and subsequent frontal activation is consistently decreased in bipolar affective disorder or if this fluctuates in synchrony with mood state.
FSC Syndromes with Symptoms Similar to Bipolar Disorder
Lesions of three of the five FSC circuits produce circuit-specific behavior syndromes.5,9,18 Three syndromes produce symptoms similar to those seen in bipolar affective disorder, which suggests that one or all of these circuits may be involved in the etiology of the illness.
Akinetic mutism (anterior cingulated syndrome). The anterior cingulate syndrome, sometimes termed “akinetic mutism” in its most severe form produces profound apathy, motor and verbal inactivity and indifference to thirst or hunger.5,9 Less severe forms result in loss of motivation, psychomotor slowing and blunted affect.18 This syndrome has similarities with the depressive symptom cluster often seen in bipolar depression.1
Orbitofrontal syndrome. The orbitofrontal syndrome involves behavioral disinhibition and emotional lability.5,9,18 The syndrome typically involves disinhibited, tactless, and impulsive behaviors, impaired restraint in social interactions, and has many similarities with mania.18
Dorsolateral prefrontal syndrome. Finally, the dorsolateral prefrontal syndrome includes symptoms of executive dysfunction, such as difficulty focusing and sustaining attention as well as reduced verbal fluency and motor programming.5 These symptoms are similar to those seen in both mania and depression.
FSC Circuits in Neurological Disorders having Psychiatric Symptoms
Accumulating evidence suggests abnormalities in FSC circuits play a role in the etiology of several neuropsychiatric disorders. Many of these disorders are frequently comorbid and/or have significant symptom overlap with BD. The neurobiology of movement disorders is thought to involve a disruption of the normal balance between activities of the direct and indirect FSC pathways.22
Parkinson's disease. The currently accepted model for Parkinson's disease is that a loss of striatal dopaminergic projections from the substantia nigra to putamen results in a decrease in motor cortical activation by way of increased indirect and decreased direct pathway influence.22 Further, impaired dopaminergic neurotransmission to the caudate and subsequent impairment of the DLPF circuit is thought to underlie the executive dysfunction and dementia associated with Parkinson's disease.22 Depression is common in Parkinson's disease but mania does not occur except secondary to treatment with dopamine agonists.22
Wilson's disease. Wilson's disease is an inherited disorder of copper metabolism, which results in degeneration of the putamen, globus pallidus, and thalamus.22 Psychiatric symptoms are common with this disorder and include cognitive deficits, major depression, mania, and behavioral disinhibition.22 It is thought that the behavioral disinhibition is mediated by way of OF circuit dysfunction at pallidal and thalamic levels.22
Huntington's disease. Huntington's disease is a neurodegenerative disorder that involves the caudate.9 Psychiatric symptoms include dementia, unipolar depression, anxiety symptoms, and bipolar disorder.9,22 Dementia is likely a result of interruption of the DLPF circuit.22 It is postulated that the hyperkinetic symptoms seen with this disorder are a result of impairment of the indirect pathways of the AC and OF circuits.22
Sydenham's chorea. Sydenham's chorea follows antecedent infection with group A streptococcus and is thought to result in an inflammatory process in the caudate, putamen, and globus pallidus.22 Obsessive-compulsive symptoms, hyperactivity impulsivity, and distractibility are thought to be the result of a relative preponderance of direct versus indirect tone in the OF circuit.22
Tourette's syndrome. Tourette's syndrome is characterized by the presence of chronic motor and vocal tics. In addition to tics, patients often experience hyperactivity, inattention, and obsessive-compulsive symptoms.22 It is hypothesized that the neuropathology of this disorder involves increased relative direct pathway tone in the OF, AC, and DLPF circuits at the level of the caudate and globus pallidus.22 Hyperinnervation of the striatum by dopaminergic neurons may play a role also by shifting the balance of neurotransmission in favor of the direct pathways.22
Focal brain injury. Numerous studies have reported associations between specific brain lesions and bipolar symptoms.23–33 These studies (for a complete review, refer to Strakowski and Sax3) suggest that different localizations of lesions result in unipolar secondary mania (episodes of mania only) versus secondary bipolar disorder (episodes of mania and depression). Specifically, cortical injury to the nondominant orbitofrontal or basal temporal cortex appears to produce unipolar mania in some patients. However, subcortical injury of either the head of the caudate or thalamus appears to be necessary for a secondary cycling disorder to develop.12 In regard to other FSC structures, injuries to the globus pallidus and ventral striatum do not result in secondary mania.3,9
Other Psychiatric Disorders with Likely FSC Circuit Abnormalities
A number of psychiatric disorders in addition to bipolar affective disorder are now thought to involve FSC circuit abnormalities:
Obsessive-compulsive disorder. Baxter and colleagues have proposed a model of obsessive-compulsive disorder in which there is increased direct pathway OF circuit tone mediated at the level of the caudate and thalamus.34
Schizophrenia. FSC circuit abnormalities have been implicated in schizophrenia.35 It is thought that in this illness, hypofrontal activity, predominately in the dorsolateral prefrontal cortex, is associated with chronic deficits in striatal glutamate tone resulting in hyperactive FSC dopaminergic neurotransmission.35 Further, the primary neuropathological process is now thought to be the cortical deficiency in cortical glutamate neurotransmission rather than hyperdopaminergia as was previously believed.35 Though the exact pathophysiological mechanisms underlying FSC abnormalities remain incompletely understood, the thalamus has been implicated in schizophrenia, because of its role in sensory gating, which is disturbed in psychosis.36 Further, there is evidence of abnormalities of dopamine and glutamatergic systems at the level of the nucleus accumbens.35 These abnormalities likely are mediated by way of the DLPF circuit.5 The hypofrontal activity would suggest a preponderance of indirect over direct activity.
Attention-deficit/hyperactivity disorder. Attention-deficit/hyperactivity disorder is a common childhood psychiatric disorder. There is considerable overlap between the symptoms of hyperactivity seen with this illness and mania. Imaging studies of those with the disorder have revealed abnormalities in the caudate and globus pallidus.37 Voeller has suggested that attention-deficit/hyperactivity disorder involves dysfunction of the AC, OF and DLPF circuits.37 This would likely be mediated by a relative increase in direct pathway activity for the OF, and DLPF circuits resulting in hyperactive symptoms and increased indirect activity in the AC circuit resulting in inattentive symptoms.
Unipolar depression. Several authors have hypothesized that alterations in FSC circuits may play a role in the etiology of unipolar depression.9,10,21 Neurological models of depression have consistently reported involvement of the striatum and stroke induced mood changes are associated with infarctions of the caudate.9,21,38 Functional imaging studies in primary depression have consistently revealed decreased frontal lobe activation and some authors have reported changes in the amygdala, anterior temporal lobe, cingulate, basal ganglia, and thalamus.21 In regard to FSC circuit structures, cortical blood flow and metabolism are decreased in the ventral striatum but increased in the globus pallidus, thalamus, and amygdala.10 The anatomical localization of hypofrontal activity has been found most consistently in the dorsolateral prefrontal and orbitofrontal areas.21 Therefore, unipolar depression may involve increased indirect activity in those pathways.
Structural Imaging, Neuropathologic, and Pharmacologic Findings Supporting FSC Involvement in Bipolar Affective Disorder and Other Psychiatric Disorders
A comparison of structural imaging to post mortem neurohistological studies of schizophrenia and mood disorders concluded there is overlap between the abnormalities found in both categories of disorders but that subtle structural change in the basal ganglia may be the primary alteration in mood disorders.39
There is evidence that pharmacologic agents effective for mood and psychotic disorders act on structures involved with FSC circuits. Antidepressant treatment has been reported to increase metabolism in the basal ganglia as well as the cortex.40 Caudate enlargement has been associated with antipsychotic use in schizophrenia.41 The actions of psychostimulants on the brain may provide some information about potential abnormalities in bipolar affective disorder. Psychostimulants have the potential to induce mania and many stimulant abusers have mood disturbances.42 There is evidence that FSC structures may be affected by these agents. For example, both the cortex and basal ganglia may be altered by methamphetamine abuse. Specifically, postmortem studies of methamphetamine abusers have revealed deficits in striatal dopaminergic markers.43 In addition, a recent PET study of methamphetamine abusers found abnormal glucose metabolism in multiple brain areas including the ventral striatum.42 Recent pharmacologic studies suggest that mood stabilizers have significant neuroprotective and neurotropic effects in areas involving FSC circuits.2,10,44,45
Structural imaging studies of patients with bipolar affective disorder support the concept of abnormalities in brain regions associated with FSC circuits in bipolar patients. Structural imaging studies in BD have not reported general cortical atrophy with exception of one study, which reported cerebral atrophy in females.46 In regard to specific cortical regions, decreased prefrontal cortical volume has been reported in most studies. Magnetic resonance imaging (MRI) studies have found gray matter volume reductions of approximately 40 percent in the prefrontal cortex and decreased gray matter in the left temporal pole.10,47,48 A number of studies have evaluated the basal ganglia and thalamus in bipolar disorder. Several studies reported no difference in the volumes of the basal ganglia as compared to normal controls.49–54 Other research has reported increased caudate volumes,11,55 no change in the caudate,56 general striatal enlargement,57 increased putamen volumes,56 and increased pallidal volumes57 in bipolar disorder patients. In regard to the thalamus, increased volume,52,58,60,69 reduced volume in adolescent bipolar patients,60 and no change in volume compared to controls49–54 have all been reported.
Functional imaging studies in bipolar disorder are limited by small sample size and are confounded by the variable of treatment with psychotropic medication. However, these studies generally support hypoactivity of the frontal cortex during bipolar depression.19,61–68 In mania, there is evidence for increased activity in the anterior cingulate cortex69,70 and decreased activity in other areas,71–74 including the orbitofrontal cortex.72 In the basal ganglia, there is evidence of a general increase in activity during mania75 as well a specific increase in the globus pallidus,8 ventral striatum,76 and caudate.69 During depression, increased activity of the thalamus and caudate have been reported.61
Discussion and Conclusions
A substantial body of evidence supports the existence of FSC circuit abnormalities in bipolar affective disorder as well as other neuropsychiatric conditions. Neurological syndromes affecting the FSC circuits produce symptoms seen in bipolar disorder. Neuropsychiatric disorders with FSC pathology have symptom overlap with BD and damage to FSC structures can result in secondary BD. Postmortem, spectroscopic, and structural imaging studies have revealed abnormalities in FSC structures of BD patients. Functional neuroimaging studies have reported abnormalities in the ventral striatum, putamen, caudate, globus pallidus, thalamus, and cortex. Recent pharmacologic studies suggest that mood stabilizers have significant neuroprotective and neurotropic effects in areas involving FSC circuits. Despite compelling evidence for FSC circuit dysfunction in bipolar affective disorder, the exact nature of FSC pathology is unknown. Further, it is unclear if there are specific differences between BD and other neuropsychiatric conditions with FSC circuit pathology.
The FSC circuits mediate motivation, social behavior, motor activity, and executive functioning. It is likely that many, if not all, neurological and psychiatric disorders with dysfunction in these areas share FSC circuit pathology, and specific symptoms of each disorder reflect which circuits are involved and whether there is a preponderance of direct versus indirect activity. In general, hyperkinetic syndromes involve a preponderance of direct activity while hypokinetic disorders involve a relative increase in indirect activity. Therefore, FSC circuit dysfunction may represent a final common pathway of symptom generation for a variety of conditions. The underlying cause of the circuit abnormality may be highly variable and range from neurodegeneration of FSC circuit structures to abnormalities of neurotransmitter circuit modulation.
References
- 1.Marchand WR. Recognizing and treating bipolar disorder. Hospital Physician. 2003;39:21–30. [Google Scholar]
- 2.Manji HK, Lenox RH. Signaling: Cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–30. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 3.Strakowski SM, Sax KW. Secondary Mania: A model of the pathophysiology of bipolar disorder? In: Soares JC, Gershon S, editors. Bipolar Disorders: Basic Mechanisms and Therapeutic Implications. New York: Marcel Dekker, Inc.; 2000. pp. 13–29. [Google Scholar]
- 4.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–39. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- 5.Lichter DG, Cummings JL. Introduction and overview. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 1–43. [Google Scholar]
- 6.Mink JW. Basal ganglia dysfunction in Tourette's syndrome: A new hypothesis. Pediatr Neurol. 2001;25:190–8. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg HP, Stern E, Martinez D, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–52. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 8.Caligiuri MP, Brown GB, Meloy MJ, et al. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 2003;123:171–82. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 10.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 11.Soares JC, Mann JJ. The anatomy of mood disorders-review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Strakowski SM, Sax KW. Secondary Mania: A model of the pathophysiology of bipolar disorder? In: Soares JC, Gershon S, editors. Bipolar Disorders: Basic Mechanisms and Therapeutic Implications. New York: Marcel Dekker, Inc.; 2000. pp. 13–29. [Google Scholar]
- 13.Strakowski SM, DelBello MP, Adler C, et al. Neuroimaging in bipolar disorder. Bipolar Disorders. 2000;2:148–64. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striato-pallido-thalamus function. Behavioral and Brain Sciences. 1987;10:197–254. [Google Scholar]
- 15.Kingsley RE. Concise Text of Neuroscience. Second Edition. Baltimore: Lippincott Williams & Watkins; 2000. Motor systems III: The basal ganglia; pp. 285–310. [Google Scholar]
- 16.Middleton FA, Strick PL. A revised neuroanatomy of frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 44–58. [Google Scholar]
- 17.Jankovic J, De Leon ML. Basal ganglia and behavioral disorders. In: Schiffer RB, Rao SM, Fogel BS, editors. Neuropsychiatry. Second Edition. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 935–46. [Google Scholar]
- 18.Bronstein YL, Cummings JL. Neurochemistry of frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 59–91. [Google Scholar]
- 19.Baxter LR, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–50. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 20.Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358–70. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- 21.Mayberg H. Depression and frontal-subcortical circuits: Focus on prefrontal-limbic interactions. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 177–206. [Google Scholar]
- 22.Lichter DG. Movement disorders and frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 260–313. [Google Scholar]
- 23.Berthier ML, Kulisevsky J, Gironell A, Benitez JAF. Poststroke bipolar affective disorder: clinical subtypes, concurrent movement disorders and anatomical correlates. J Neuropsychiatry Clin Neurosci. 1996;8:160–7. doi: 10.1176/jnp.8.2.160. [DOI] [PubMed] [Google Scholar]
- 24.Bogousslavsky J, Ferrazzini M, Regli F, et al. Manic delirium and frontal-like syndrome with paramedian infarction of the right thalamus. J Neurol Neurosurg Psychiatry. 1988;51:116–19. doi: 10.1136/jnnp.51.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings JL, Mendez MF. Secondary mania with focal cerebrovascular lesions. Am J Psychiatry. 1984;141:1084–7. doi: 10.1176/ajp.141.9.1084. [DOI] [PubMed] [Google Scholar]
- 26.Jorge RE, Robinson RG, Starkstein SE, et al. Secondary mania following traumatic brain injury. Am J Psychiatry. 1993;150:916–21. doi: 10.1176/ajp.150.6.916. [DOI] [PubMed] [Google Scholar]
- 27.Kulisevsky J, Berthier ML, Pujol J. Hemiballismus and secondary mania following right thalamic infarction. Neurology. 1993;43:1422–4. doi: 10.1212/wnl.43.7.1422. [DOI] [PubMed] [Google Scholar]
- 28.Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: causal factors. Am J Psychiatry. 1988;145:172–8. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- 29.Starkstein SE, Pearlson GD, Boston J, Robinson RG. Mania after brain injury: A controlled study of causative factors. Arch Neurol. 1987;44:1069–73. doi: 10.1001/archneur.1987.00520220065019. [DOI] [PubMed] [Google Scholar]
- 30.Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: Twelve case reports and review of the literature. J Nerv Ment Dis. 1988;176:87–100. doi: 10.1097/00005053-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Starkstein SE, Mayberg HS, Berthier ML, et al. Mania after brain injury: neuroradiological and metabolic findings. Arch Neurol. 1990;27:652–59. doi: 10.1002/ana.410270612. [DOI] [PubMed] [Google Scholar]
- 32.Starkstein SE, Fedoroff P, Berthier ML, Robinson RG. Manic-depressive and pure manic states after brain lesions. Biol Psychiatry. 1991;29:149–58. doi: 10.1016/0006-3223(91)90043-l. [DOI] [PubMed] [Google Scholar]
- 33.Vuilleumier P, Ghika-Schmid F, Bogoussalaavsky J, et al. Persistent recurrence of hypomania and prosopoaffective agnosia in a patient with right thalamic infarct. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:40–4. [PubMed] [Google Scholar]
- 34.Baxter LR, Clark EC, Iqbal M, et al. Cortical-subcortical systems in the mediation of obsessive-compulsive disorder. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 207–30. [Google Scholar]
- 35.West AR, Grace AA. The role of frontal-subcortical circuits in the pathophysiology of schizophrenia. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 372–400. [Google Scholar]
- 36.Harrison PJ. The neuropathology of schizophrenia: A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 37.Voeller KS. Attention-deficit/hyperactivity disorder as a frontal-subcortical disorder. In: Lichter DG, Cummings JL, editors. Frontal-Subcortical Circuits in Psychiatric and Neurological Disorders. New York: Guilford Press; 2001. pp. 334–71. [Google Scholar]
- 38.Stoll AL, Renshaw PF, Yurgelun-Todd DA, Cohen BM. Neuroimaging in bipolar disorder: What have we learned. Biol Psychiatry. 2000;48:505–17. doi: 10.1016/s0006-3223(00)00982-3. [DOI] [PubMed] [Google Scholar]
- 39.Baumann B, Bogerts B. The pathomorphology of schizophrenia and mood disorders: Similarities and differences. Schizophr Res. 1999;39:141–8. doi: 10.1016/s0920-9964(99)00113-9. [DOI] [PubMed] [Google Scholar]
- 40.Rubin E, Sackeim H, Nobler MS. Brain imaging studies of antidepressant treatments. Psychiatr Ann. 1994;24:653–61. [Google Scholar]
- 41.Chakos MH, Lieberman JA, Bilder RM, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–6. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 42.London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 44.Gray NA, Zhou R, Du J, et al. The use of mood stabilizers as plasticity enhancers in the treatment of neuropsychiatric disorders. J Clin Psychiatry. 2003;64(suppl 5):3–17. [PubMed] [Google Scholar]
- 45.Ketter TA, Wang PW. The emerging differential roles of GABAergic and antiglutamatergic agents in bipolar disorders. J Clin Psychiatry. 2003;64(suppl 3):15–20. [PubMed] [Google Scholar]
- 46.Lewine RRJ, Hudgins P, Brown F, et al. Differences in qualitative brain morphology findings in schizophrenia, major depression, bipolar disorder and normal volunteers. Schizophr Res. 1995;15:253–9. doi: 10.1016/0920-9964(94)00055-d. [DOI] [PubMed] [Google Scholar]
- 47.Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry. 2003;60:1069–77. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- 48.Sharma V, Menon R, Carr TJ, et al. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord. 2003;77:167–71. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 49.Brambilla P, Harenski K, Nicoletti MA, et al. Anatomical MRI study of basal ganglia in bipolar disorder patients. Psychiatry Research: Neuroimaging. 2001;106:65–80. doi: 10.1016/s0925-4927(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 50.Caetano SC, Sassi R, Brambilla P, et al. MRI study of thalamic volumes in bipolar and unipolar patients and healthy individuals. Psychiatry Research: Neuroimaging. 2001;108:161–8. doi: 10.1016/s0925-4927(01)00123-8. [DOI] [PubMed] [Google Scholar]
- 51.Dupont RM, Butters N, Schafer K, et al. Diagnostic specificity of focal white matter abnormalities in bipolar disorder and unipolar mood disorder. Biol Psychiatry. 1995;38:482–6. doi: 10.1016/0006-3223(95)00100-u. [DOI] [PubMed] [Google Scholar]
- 52.Sax KW, Strakowski SM, Zimmerman ME, et al. Frontosubcortical neuroanatomy and the Continuous Performance Test in mania. Am J Psychiatry. 1999;56:139–41. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 53.Strakowski SM, Wilson DR, Tohen M, et al. Structural brain abnormalities in first-episode mania. Biol Psychiatry. 1993;33:602–9. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- 54.Swayze VW II, Andreasen NC, Alliger RJ, et al. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–40. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 55.Aylward EH, Roberts-Twillie JV, Barta PE, et al. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–93. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- 56.Strakowski SM, DelBello MP, Zimmerman ME, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–7. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 57.Strakowski SM, DelBello MP, Adler C, et al. Neuroimaging in bipolar disorder. Bipolar Disorders. 2000;2:148–64. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 58.Altshuler LL, Conrad AJ, Hauser P, et al. Reduction of temporal lobe volume in bipolar disorder: A preliminary report of magnetic resonance imaging. Arch Gen Psychiatry. 1991;48:221–40. doi: 10.1001/archpsyc.1991.01810290094018. [DOI] [PubMed] [Google Scholar]
- 59.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 60.Dasari M, Friedman L, Jesberger J, et al. A magnetic resonance imaging study of thalamic area in adolescent patients with either schizophrenia or bipolar disorder as compared to healthy controls. Psychiatry Res. 1999;91:155–62. doi: 10.1016/s0925-4927(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 61.Baxter LR, Phelps MR, Mazziotta JC, et al. Cerebral metabolic rates for glucose in mood disorders: studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry. 1985;42:441–7. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- 62.Buchsbaum MS, DeLisi LE, Holcomb HH, et al. Anterioposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch Gen Psychiatry. 1984;41:1159–66. doi: 10.1001/archpsyc.1984.01790230045007. [DOI] [PubMed] [Google Scholar]
- 63.Buchsbaum MS, Wu J, DeLisi LE, et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord. 1986;10:137–52. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 64.Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 65.Kegeles LS, Malone KM, Slifstein M, et al. Response of cortical metabolic deficits to serotonergic challenge in familial mood disorders. Am J Psychiatry. 2003;160:76–82. doi: 10.1176/appi.ajp.160.1.76. [DOI] [PubMed] [Google Scholar]
- 66.Bonne O, Krausz Y, Gorfine M, et al. Cerebral hypoperfusion in medication resistant, depressed patients assessed by Tc99m HMPAO SPECT. J Affective Disord. 1996;41:163–71. doi: 10.1016/s0165-0327(96)00058-4. [DOI] [PubMed] [Google Scholar]
- 67.Delvenne V, Delecluse G, Hubain PP, et al. Regional blood flow in patients with affective disorders. Br J Psychiatry. 1990;157:359–65. doi: 10.1192/bjp.157.3.359. [DOI] [PubMed] [Google Scholar]
- 68.Ito H, Kawashima R, Awata S, et al. Huypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med. 1996;37:410–4. [PubMed] [Google Scholar]
- 69.Blumberg HP, Stern E, Martinez D, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–52. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 70.Goodwin GM, Cavanagh JTO, Glabus MR, et al. Uptake of 99mTc-exametazime by single photon emission computed tomography before and after lithium withdrawal in bipolar patients: associations with mania. Br J Psychiatry. 1997;170:426–30. doi: 10.1192/bjp.170.5.426. [DOI] [PubMed] [Google Scholar]
- 71.Al-Mousawi AH, Evans N, Ebmeier KP, et al. Limbic dysfunction in schizophrenia and mania: A study using 18F-labled fluorodeoxyglucose and positron emission tomography. Br J Psychiatry. 1996;169:509–16. doi: 10.1192/bjp.169.4.509. [DOI] [PubMed] [Google Scholar]
- 72.Blumberg H, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–8. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 73.Rubin E, Sackeim HA, Prohovnik I, et al. Regional cerebral blood flow in mood disorders: IV. Comparison of mania and depression. Psychiatry Res. 1995;61:1–10. doi: 10.1016/0925-4927(95)02594-n. [DOI] [PubMed] [Google Scholar]
- 74.Migliorelli R, Starkstein SE, Teson A, et al. SPECT findings in patients with primary mania. J Neuropsychiatry Clin Neurosci. 1993;5:379–83. doi: 10.1176/jnp.5.4.379. [DOI] [PubMed] [Google Scholar]
- 75.O'Connell RA, van Heertum RL, Luck D, et al. Single-photon emission computed tomography of the brain in acute mania and schizophrenia. J Neuroimaging. 1995;5:101–4. doi: 10.1111/jon199552101. [DOI] [PubMed] [Google Scholar]
- 76.Guze BH, Baxter LR, Jr, Schwartz JM, et al. Changes in glucose metabolism in dementia of the Alzheimer type compared with depression: A preliminary report. Psychiatry Res. 1991;40:195–202. doi: 10.1016/0925-4927(91)90010-n. [DOI] [PubMed] [Google Scholar]