Abstract
Background
There are limited data on the immune profiles of HIV-positive children, compared with healthy controls, and no such data for Asian children.
Objectives
To immunophenotype HIV-positive Asian children, including long-term non-progressors (LTNPs), compared with age-matched healthy controls.
Methods
We used flow cytometry to analyze 13 lymphocyte and monocyte subsets from 222 untreated, HIV-positive children with 15%–24% CD4+ T cells and no AIDS-related illnesses and 142 healthy children (controls). Data were compared among age categories. Profiles from LTNPs (n=50), defined as children≥ 8 years old with CD4+ T-cell counts ≥ 350 cells/mm3, were compared with data from age-matched non-LTNPs (n=17) and controls (n=53).
Results
Compared with controls, HIV-positive children had lower values (cell count per mm3 and percent distribution) for helper T cells and higher values for cytotoxic T cells, with reductions in populations of naïve helper and cytotoxic T cells, B cells, and natural killer (NK) cells. HIV-positive children had high values for activated helper and cytotoxic T cells. Compared with non-LTNPs, LTNPs had higher values of helper and cytotoxic T cells, naïve and memory T-cell subsets, and B and NK cells. Surprisingly, counts of activated helper and cytotoxic T cells were also higher among LTNPs. LNTPs were more frequently male.
Conclusions
Untreated, HIV-infected Asian children have immune profiles that differ from those of controls, characterized by low values for helper T cells, naive T cells, B cells, and NK cells but high values for cytotoxic, activated helper, and cytotoxic T cells. The higher values for activated T cells observed in LTNPs require confirmation in longitudinal studies.
Clinical Implications
The distinct immunologic profile of LTNPs might identify lymphocyte subsets associated with HIV disease progression.
Keywords: HIV, children, lymphocyte, monocyte, phenotyping, long-term non-progressors, antiretroviral therapy, Asia, disease progression, pediatric AIDS
Introduction
HIV-positive children were generally infected with HIV at birth, from their mothers; about one-third progress to AIDS within the first year of life without antiretroviral therapy (ART)1 and most become ill from HIV by age 52. Less than 10% remain healthy by age 8 without ART—they are called HIV long-term non-progressors (LTNPs)3.
Hallmarks of HIV infection are high turnover of CD4+ T cells and activation of polyclonal B cells and persistent immune activation4, 5. Immune activation is characterized by expression of activation markers on T cells, which is associated with decreases in numbers of CD4+ T cells, increases in HIV RNA, and progression of HIV disease. ART partially reverses these defects. CD4+ T-cell regeneration following ART is better in children than adults because of children's actively functioning thymic glands6.
It is not clear why LTNPs can control HIV. Lessons can be learned from so-called elite controllers, who account for < 1% of the HIV population and keep HIV at less than 50 copies/ml without ART7. Elite controllers have a robust, HIV-specific CD8+ T-cell response (particularly against the HIV protein gag), preserve the naïve T-cell population that mediates responses to new antigens, generate polyfunctional T cells, and have low-level immune activation. Some also have mutations in the chemokine co-receptor CCR5, HLA haplotypes that can select immunogenic HIV epitopes, or infections with defective forms of HIV that replicate poorly8-11. These natural states of immune control of HIV are not seen in the general, HIV-infected population, despite the success of ART12.
Several studies have reported low counts of CD4+ T cells, high counts of CD8+ T cells, and high counts of activated CD8+ T cells in children with HIV4, 13; characterizations of other cell subsets and comparisons with age-matched healthy controls have not been systematically performed. Studies in healthy controls in the United States and Africa have shown that lymphocyte counts, particularly of CD4+ T cells, can be affected by age, sex, and ethnicity14, 15
This study is the first in Asia to evaluate lymphocyte and monocyte subsets in HIV-positive children and age-matched healthy controls. We also examined the immunologic profile of pediatric LTNPs, compared with non-LTNPs and healthy controls. The results might be used to better characterize immune profile of pediatric HIV and to understand the immunologic profile of LTNPs.
Methods
We used data from first visits with 222 HIV-positive children from 6 sites in Thailand and 2 sites in Cambodia who enrolled in the Pediatric Randomized to Early vs. Deferred Initiation in Cambodia and Thailand Study (PREDICT, http://www.clinicaltrials.gov/ct/show/NCT00234091). These children were 1–12 years old and never treated with ART, except as part of prevention of mother to child HIV transmission, and had percentages of CD4+ T cells in the moderate immune suppression range (CD4+ T cell 15%–24%) without severe symptoms of HIV infection (Center for Disease Control and Prevention [CDC] class C). The age-matched healthy controls were enrolled at the Well-Child Care clinic at Chulalongkorn University hospital in Bangkok, one of the PREDICT study sites. Healthy controls were excluded for abnormal growth (below 3rd or above 97th percentile, according to the Thai growth chart), febrile illness, respiratory and other infections at screening, or medical illnesses that might result in abnormal immunity such as HIV infection or exposure and allergic conditions. Medical histories were provided for groups, which each underwent physical examinations and blood sample collection. HIV RNA levels were measured in blood samples from HIV-positive children. Our case definition for LTNP was ≥8 years in age with no indication for ART (CD4+ T cells ≥ 350 cells/mm3). Because age affects lymphocyte subsets, we chose children of similar ages as controls for LTNPs: the first group was HIV-positive children ≥ 8 years old with CD4 < 350 cells/mm3 (non-LTNPs); the second group was healthy children ≥ 8 years old. Each study was approved by national and local institutional review boards. All caregivers consented to the study and healthy children ≥ 7 years old also gave assent.
Flow cytometer set-up, gating, and marker placement in each laboratory were dictated by guidelines established by the National Institute of Allergy and Infectious Diseases-Division of AIDS (NIAID-DAIDS) and the advanced flow cytometry working group of the Pediatric AIDS Clinical Trials Group, in collaboration with the technical divisions of the manufacturers of the flow reagents16. All laboratories were approved by NIAID-DAIDS and passed the annual quality assurance programs of the United Kingdom National External Quality Assessment Service and Mahidol University, Bangkok, Thailand. By convention, we used clusters of designation (CD) numbers only when necessary in the text and elsewhere when presence and absence of markers is necessary. More details are in the Online Repository.
Statistical analyses
Analyses were performed with Stata 11 (College Station, Tx, USA). Demographic and baseline data were described according to HIV status or as healthy controls. For descriptive analyses, median interquartile range (IQR) and percentage distribution were used; T-test and Mann-Whitney-U test were used to compare differences between groups including distributions of T-cell subsets; the Chi-square χ2 test was performed for categorical variables. We classified children according to whether they were LTNPs and used Kruskal Wallis tests or Mann Whitney-U tests to make comparisons among LTNPs, non-LTNPs, and healthy controls. More details are in the Online Repository.
We compared counts and percentages of T-cell subsets between healthy controls and HIV–positive children using regression models stratified by 4 age categories and adjusted for sex. Separate regressions were performed for each age group. Additional regression models were run, with age expressed first as a categorical covariate, and then as a continuous covariate adjusted for HIV-status and sex. Counts and percentages of T-cell subsets were transformed by log10 or Box-Cox power transformations to more normally distribute residual errors. Logistic regression analysis assessed whether T-cell subset distributions predicted which children would be LTNPs. Odds ratios (OR) and 95% confidence intervals (95% CI) were reported relative to the LTNP group.
Results
We enrolled 222 untreated HIV-positive children and 147 age-matched healthy controls (Table I). The HIV-positive group included Thai and Cambodian children where as the HIV-negative group included only Thai children. The median age was approximately 6.5 years; healthy controls were slightly older. Children with HIV were mainly mildly symptomatic (CDC class A). The median number of CD4+ T cells was lower among HIV-positive children; the median amount of HIV RNA was 4.8 log10 copies/ml.
Table I. Demographics of HIV-positive and healthy children.
| Characteristic | Overall (n=364) |
HIV positive (n=222) |
Healthy controls (n=142) |
P-value |
|---|---|---|---|---|
| Mean age, years (SD) | 6.6 (2.9) | 6.3 (2.8) | 7.0 (2.9) | 0.03 |
| Median age, years (IQR) | 6.5 (4.3-8.7) | 6.4 (4.1-8.4) | 6.8 (4.6-9.2) | 0.04 |
| Age categories, n (%) | 0.12 | |||
| <2-3yrs | 79 (21.7) | 54 (24.3) | 25 (17.6) | |
| 4-5 yrs | 74 (20.3) | 47 (21.2) | 27 (19.0) | |
| 6-8 yrs | 135 (37.1) | 83 (37.4) | 52 (36.6) | |
| 9-12 yrs | 76 (20.9) | 38 (17.1) | 38 (26.8) | |
| % male in age groups, n (%) | ||||
| <2-3yrs | 37 (46.84) | 30 (55.56) | 7 (28.00) | 0.01 |
| 4-5 yrs | 32 (43.24) | 22 (46.81) | 10 (37.04) | 0.35 |
| 6-8 yrs | 57 (42.22) | 35 (42.17) | 22 (42.31) | 0.94 |
| 9-12 yrs | 22 (28.95) | 8 (21.05) | 14 (36.84) | 0.01 |
| Sex M:F | 148: 216 (41: 59) |
95: 127 (43: 57) |
53:89 (37:63) |
0.30 |
| Nationality, n (%) | <0.001 | |||
| Thai | 264 (72.5) | 122 (55.0) | 142 (100.0) | |
| Cambodia | 100 (27.5) | 100 (45.0) | 0 (0.0) | |
| %CDC N:A:B | 1:62:37 | |||
| Median CD4% (IQR) | 24.5 (18.6-32.3) | 20.2 (16.5-23.5) | 34.8 (30.2-38.6) | <0.001 |
| Median CD4 count, cells/mm3 (IQR) | 835 (578-1130) | 631 (434-887) | 1099 (895-1452) | <0.001 |
| Median HIV RNA log10 copies/ml (IQR) | - | 4.8 (4.3-5.0) | - | - |
Figures 1A to 1E and Figures E1 to E6- (Online Repository)
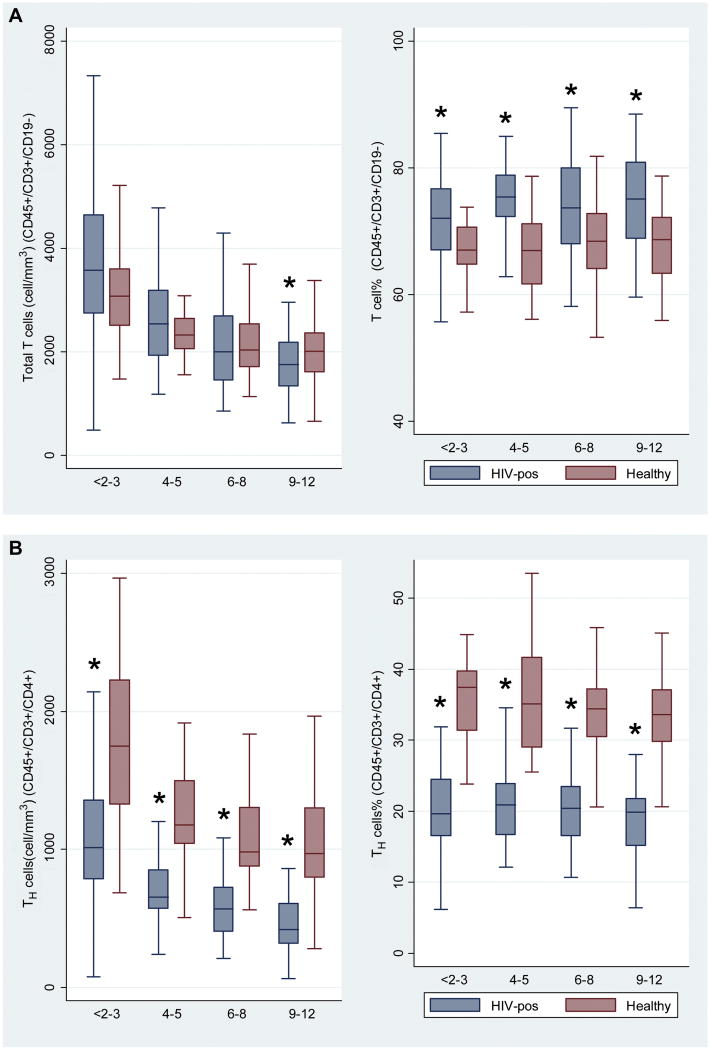
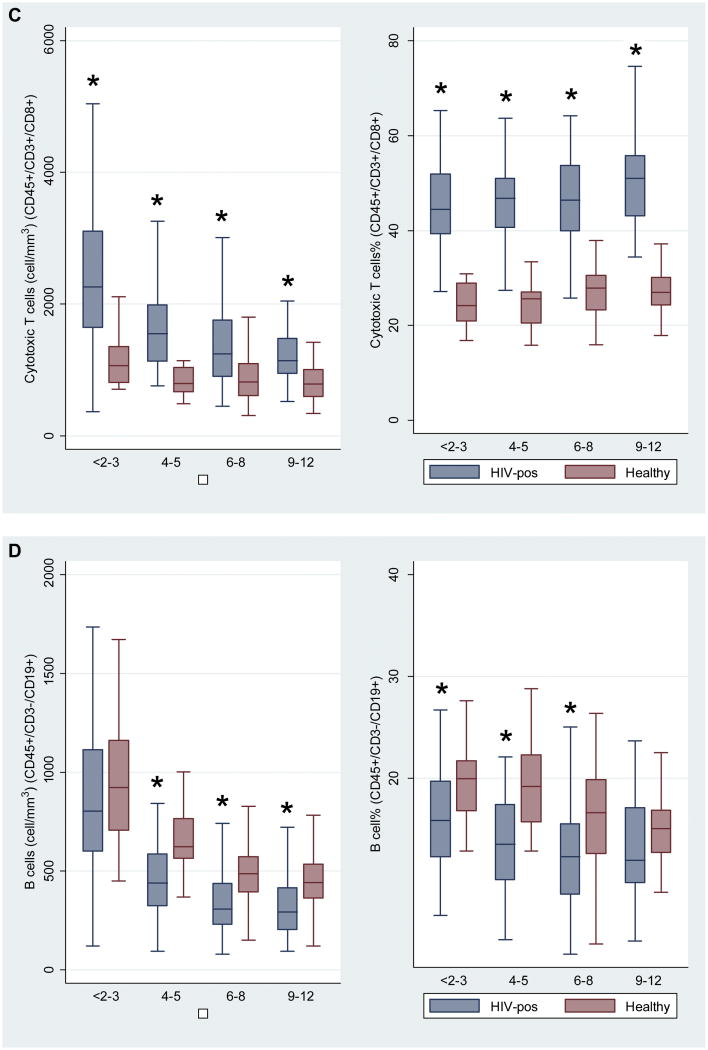
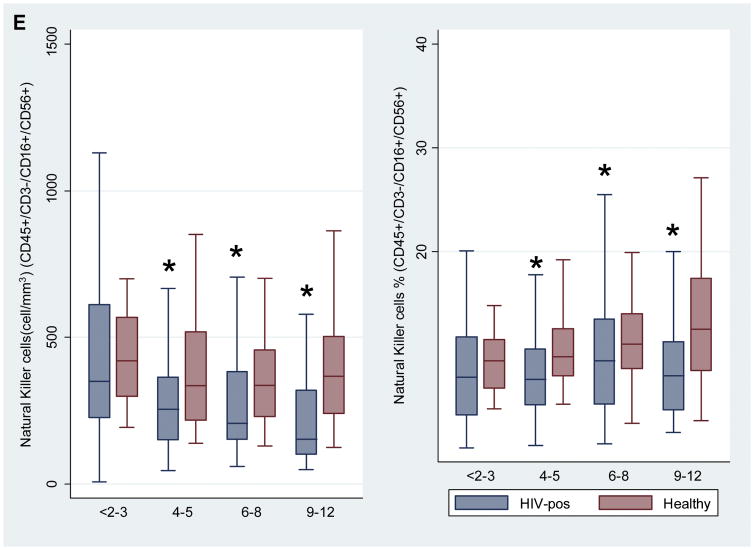
Figures 1A to 1E. Comparison of cell subsets among 4 age groups of HIV-negative and HIV-positive children.
Figure 1A: CD45+/3+/19- (Total T cells), Figure 1B: CD45+/3+/4+ (Helper T cells), Figure 1C: CD45+/3+/8+ (Cytotoxic T cells), Figure 1D: CD45+/3-/19+ (B cells), Figure 1E: CD45+/3-/16+/56+ (Natural killer cells).
Asterisk * denotes P value < 0.05.
Number of children: <2-3 years (n=79), 4-5 years (n=74), 6-8 years (n=135), 9-12 years (n=76).
We compared counts and percentages of cell subsets between HIV positive children and healthy controls in 4 age categories (<2–3 years, 4–5 years, 6–8 years, 9–12 years). Within these groups, sex was generally balanced between HIV-positive children and healthy controls (Table I). HIV-positive children had significantly lower total counts of T cells in the 9–12-year age group, and lower total percentages of T cells across all age groups than healthy controls (Figure 1A). Counts and percentages of helper T cells and naive and memory cell subsets differed significantly between HIV-infected children and healthy controls, across all age groups. HIV-positive children had lower counts and percentages of helper T cells (Figure 1B) and higher counts and percentages of cytotoxic T cells (Figure 1C). Counts and percentages of B cells (Figure 1D) and natural killer (NK) cells (Figure 1E) were significantly lower in some age groups of HIV-positive children. The HIV-positive children had lower counts of naive helper T cells (Online Repository Figure E1) and memory helper T cells (Online Repository Figure E2), but higher counts and percentages of activated helper T cells (Online Repository Figure E3). There were lower counts and percentages of naive cytotoxic T cells (Online Repository Figure E4), and higher counts and percentages of memory cytotoxic T cells (Online Repository Figure E5) and activated cytotoxic T cells (Online Repository Figure E6) in HIV-positive children, compared with healthy controls. Numbers of activated and perivascular monocytes did not differ between HIV-positive children and healthy controls (data not shown).
In the regression analyses (with age as either categorical or continuous values), age had a significant impact on percentages and counts of helper T cells, memory helper T cells, B cells, NK cells, activated monocytes, and perivascular monocytes, but only affected cell counts for the other subsets. Analysis by sex did not affect cell subsets, except in a univariate analysis, girls had higher percentages of CD4+ T cells (OR 2.17, 95% confidence intervals [CI] 0.27–4.08, P=0.025) and lower percentages of memory cytotoxic T cells (−0.07, 95% CI 0-15, P=0.043) than boys.
The characteristics of children ≥8 years old categorized as LTNPs (n=50), non-LTNPs (n=17), and healthy controls (n=53) generally matched (Online Repository Table E1). Counts of cell subsets were significantly different in LTNPs compared with non-LTNPs and to healthy controls (Online Repository Table E2). In general, values for cell subset counts of LTNPs were between those of non-LTNPs and healthy controls. Importantly, the LTNPs had higher counts of helper and cytotoxic T cells, as well as naive and memory cell subsets, and higher counts of B cells and NK cells, than non-LTNPs, but values for LTNPs were lower than those of healthy controls, except for counts of total cytotoxic and memory cytotoxic T cells. The LTNPs had higher counts of activated helper and cytotoxic T cells than the healthy controls, and surprisingly, also higher counts than the non-LTNPs. Counts of the 2 monocyte subsets did not differ between groups. There were fewer significant differences between LTNPs and non-LTNPs in percentages of cell subsets (Online Repository Table E3). Only the total percentage of helper T cells was significantly higher in LTNPs, whereas the percentage of activated monocytes was lower among LTNPs, compared with non-LTNPs. There were, however, significant differences between LTNPs and healthy controls in percentages of all subsets except the 2 monocyte subsets.
Data were further analyzed using more stringent criteria for LTNPs: using CD4+ T cell counts ≥ 500 T cells/mm3 (instead of CD4 ≥ 350 T cells/mm3) and removing from the analysis data from children with CDC B classification (mainly history of pneumonia). These datasets included 29 LTNPs, 38 non-LTNPs, and 53 healthy controls in the first analysis and 35 LTNPs, 12 non-LTNPs, and 53 healthy controls in the second analysis. Findings from each analysis were similar to data derived using the original definition of LTNP (data not shown).
We examined whether sex, CDC category, HIV RNA level, and lymphocyte cell-subset values could identify which children were LTNPs (Table II). Factors that identified LTNPs, by univariate and multivariate analyses, were male sex; higher counts of total T cells, helper T cells, cytotoxic T cells and their naive and memory subsets; and higher counts of B cells. Surprisingly, higher counts of activated helper T cells also identified LTNPs, where as there were no statistically significant differences in counts of activated cytotoxic T cells between LTNPs and non-LTNPs. Levels of HIV RNA and CDC category (N, A, or B) did not identify LTNPs.
Table II. Univariate and multivariate regression analyses of predicting factors for long-term non-progressors (LTNPs).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95%CI | P-value | OR | 95%CI | P-value |
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | 4.6 | 0.9, 22.4 | 0.032 | 10.2 | 1.1, 96 | 0.021 |
| CDC stage | ||||||
| N/A | 1 | |||||
| B | 1.0 | 0.31-3.4 | 0.963 | |||
| Log HIV-RNA at baseline | 0.4 | 0.1- 1.2 | 0.081 | 2.1 | 5.8, 472.8 | 0.361 |
| Total T cells | ||||||
| < 1412 | 1 | 1 | ||||
| ≥1412 | 37.4 | 8.2-170.3 | <0.001 | 67.1 | 9.3,485 | <0.001 |
| Naïve helper T cells | ||||||
| < 128 | 1 | 1 | ||||
| ≥128 | 21.6 | 5.4-87.0 | <0.001 | 32.2 | 5.9,175.3 | <0.001 |
| Memory helper T cells | ||||||
| < 138 | 1 | 1 | ||||
| ≥138 | 21.6 | 5.4-87.0 | <0.001 | 26.5 | 5,139.7 | <0.001 |
| Activated helper T cells | ||||||
| <35.5 | 1 | 1 | ||||
| ≥35.5 | 4.0 | 1.2-13.4 | 0.0219 | 3.6 | 1,12.9 | 0.047 |
| Cytotoxic T cells | ||||||
| <954 | 1 | 1 | ||||
| ≥954 | 8.8 | 2.5-30.7 | 0.0004 | 9.7 | 2.4,38.9 | 0.001 |
| Naive cytotoxic T cells | ||||||
| <176 | 1 | 1 | ||||
| 176-292 | 4.6 | 1.1-20.4 | 5.3 | 1.1,26.5 | ||
| ≥292 | 14.3 | 3.1-66.0 | <0.001 | 16 | 3,84.9 | 0.002 |
| Memory cytotoxic T cells | ||||||
| <411 | 1 | 1 | ||||
| ≥411 | 5.9 | 1.8-19.9 | 0.0037 | 6.7 | 1.8,25.5 | 0.004 |
| Activated cytotoxic T cells | ||||||
| <379 | 1 | 1 | 1 | |||
| ≥379 | 2.8 | 0.9-9.2 | 0.0930 | 2.7 | 0.7,9.8 | 0.135 |
| B cells | ||||||
| <216 | 1 | 1 | ||||
| 216-285 | 3.4 | 0.83-14.2 | 5.5 | 1.1,29 | ||
| ≥ 286 | 22.1 | 3.9-124.3 | <0.001 | 30.7 | 4.4,214.5 | <0.001 |
| Natural killer cells | ||||||
| <108 | 1 | 1 | ||||
| ≥108 | 1.9 | (0.6-6.4) | 0.2873 | 1.5 | 0.4,5.5 | 0.569 |
LTNP is defined as antiretroviral-naive, HIV-positive children aged ≥ 8 years with CD4 ≥ 350 cells/mm3; non-LTNP is defined as antiretroviral-naive, HIV-positive children aged ≥ 8 years with CD4 < 350 cells/mm3; Healthy controls is defined as healthy children aged ≥ 8 years. For lymphocyte subsets, values are expressed in cell count (cells/mm3)
Discussion
This is the first study to show that the immunologic profile of untreated, HIV-positive Asian children differs significantly from that of age-matched healthy controls. Children with HIV had lower counts and percentages of helper T cells and naive and memory T-cell subsets. Values for cytotoxic T cells and the memory subset were higher in HIV-infected children than in healthy controls Notably, HIV-positive children had lower counts and percentages of naive cytotoxic T cells and higher counts and percentages of activated helper and cytotoxic T cells than controls.
Several studies have described the immunologic profile of HIV-positive children but did not include extensive flow cytometry analyses of lymphocyte subsets, using more than 2 fluorochromes, or age-matched healthy controls, as this study has13, 17, 18. We found that the profile of HIV-infected children is characterized by low values for helper T cells and high values for cytotoxic T cells, as well as a reduced naive T-cell population. After birth, there is an expansion of the naive T-cell pool from T-cell proliferation. HIV infection leads to continuous activation of naive T cells and loss of this population19. Even neonates that have been exposed to HIV but not infected have lower values of naive T cells, compared with HIV-unexposed neonates.20 The HIV-infected children also had increased values for memory CD8+ T cells. Other studies have shown this to be associated with HIV viremia and decreases in CD4+ T cells.17, 21
The HIV-positive children in this study had a remarkable increase in activation of helper and cytotoxic T cells, compared with controls. Persistent immune activation is associated with unfavorable clinical outcome from HIV. It is largely driven by HIV viremia, which explains the high numbers of activated T cells observed in untreated HIV-positive children in this study18, 22 The T-cell activation marker CD38 DR (on cytotoxic T cells) correlated with high HIV RNA and the rate of HIV disease progression in children and adults13, 17, 18, 23. The lower values for B cells observed in the HIV-positive children might arise during HIV replication, which causes lysis of B cells in germinal centers of lymph nodes5, 24. NK cells are important for the lysis of HIV-infected cells; numbers of these cells were also reduced in the HIV-positive children, compared with controls23. Low concentrations of activated monocytes and perivascular monocytes exist in the peripheral blood and have been identified in children and adults with HIV-associated neurologic diseases25, 26. The HIV-positive children in this study did not have detectable neurologic disease, which could explain the lack of differences observed in monocyte subsets, compared with healthy controls. The effects of age, sex, and ethnicity on lymphocyte counts have been evaluated in several studies of healthy individuals14, 27, 28. Counts of lymphocytes—particularly of helper T cells, B cells, and NK cells—tend to peak in early childhood then decrease with age, where as percentages of lymphocyte vary less with age15, 27-30; we also observed this effect in our study population. Similar to this study, girls in Malawi had higher counts of CD4+ T cells than boys15. Flow cytometry patterns of the HIV-positive, Asian children in this study were similar to those reported in non-Asians3, 13, 17, 21, 22. A comparison of flow cytometry patterns between healthy Asian children and those of other ethnic groups is an important topic for future study.
There are few studies of pediatric LTNPs31, 32. The Women and Infant Transmission Study followed 137 HIV-positive children from birth and found 10 to be LTNPs, defined as children with >25% and >500 cells/mm3 CD4+ T cells at 8 years of age. The investigators found that activated cytotoxic T cells, levels of CD8 DR below 5%, and levels of CD8 and CD38 below 25% at 2 months of age predicted which children would become LTNPs3. Children who are slow progressors have been found to have lower percentages of memory CD4+ T cells and higher percentage of naive CD4+ T cells 32. Overall, the LTNPs in this study had counts and percentages of cell subsets that were closer to those of healthy controls than of the non-LTNPs. Compared with the non-LTNPs, the LTNPs had higher counts of helper and cytotoxic T cells, of naive and memory cell subsets, and B and NK cells. The higher counts of memory cell and NK cells observed in the LTNPs in this study contradict previous reports21, 32. More surprisingly, the LTNPs had higher counts, but not percentages, of activated T cells, particularly of helper T cells, than the non-LTNPs. This is in contrast to studies in children and adults from other ethnic groups3, 7, 13, 32, 33. The reasons for the differences are unclear but could include greater variation in cell counts, compared with cell percentages, and the cross-sectional measurement, which can vary with time. Being male was significantly associated with LTNP in this study; this finding differs from other pediatric LTNP studies3, 31 and requires confirmation in larger studies with longer periods of observation. We did not associate levels of HIV RNA with LTNP, although HIV RNA levels generally associated with decreases in CD4+ T cells17. Another difference in our study was that CDC class did not predict long-term non-progression, likely because none of the children in this study had severe HIV disease. Longitudinal analyses of cell subsets are underway in the PREDICT study; these might identify lymphocyte subsets associated with long-term non-progression in children in the deferred arm of the study.
This study differs from other studies of pediatric HIV in the large number of HIV-positive children without severe immune suppression, the inclusion of healthy controls, the detailed examination of lymphocyte and monocyte subsets, and the Asian ethnicity of all children. Importantly, we were able to characterize the immunologic profile of a relatively large number of LTNPs in comparison with non-LTNPs and healthy controls. The study is limited by possible variations in percentages and counts of cell subsets between time points. A longitudinal examination of cell subsets would allow groups to be more accurately compared and for LTNPs to be compared with non-LTNPs.
This study used immunophenotype analysis to characterize the immunologic profile of HIV-positive Asian children, compared to their age-matched healthy controls. The longitudinal phase of the PREDICT study could identify lymphocyte subsets that predict HIV disease progression. Such knowledge could have a significant public health impact in identifying children who require early ART and those for whom ART could be deferred.
Supplementary Material
Acknowledgments
We thank The Immunology Research Fund, Texas Children's Hospital, Houston, TX, USA for the gift of monoclonal antibody reagents for the pilot study. The PREDICT study is sponsored by National Institute of Allergy and Infectious Disease (NIAID), Grant number U19 AI053741, Clinical trial.gov identification number NCT00234091. The lymphocyte subset study in HIV positive children is sponsored by NIAID, Grant 1R01AI075408-0 and The Thai Research Council sponsored the study in healthy children. KP is Thailand Research Fund-Senior Research Scholar. Antiretroviral therapy for PREDICT is provided by GlaxoSmithKline, Boehringer Ingelheim, Merck, Abbott and Roche. We thank Ms. Piraporn June Ohata for her assistance in preparing the manuscript.
Abbreviations
- ART
Antiretroviral therapy
- CDC
Center for Disease Control and Prevention
- HIV
Human Immunodeficiency Virus
- LTNP
Long term non progressor
- WHO
World Health Organization
Footnotes
Conflict of interest: Authors declare no conflict of interest
A complete list of the PREDICT Study Team can be found in the Online Repository.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. AIDS Epidemic Update 2009. [22 July 2010]; http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2009/default.asp.
- 2.Chearskul S, Chotpitayasunondh T, Simonds RJ, et al. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics. 2002;110(2 Pt 1):e25. doi: 10.1542/peds.110.2.e25. [DOI] [PubMed] [Google Scholar]
- 3.Paul ME, Mao C, Charurat M, et al. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol. 2005;115(4):848–55. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 4.Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S195–203. doi: 10.1016/j.jaci.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Puronen CE, Sereti I. Immunopathogenesis of asymptomatic chronic HIV Infection: the calm before the storm. Curr Opin HIV AIDS. 2009;4(3):206–14. doi: 10.1097/COH.0b013e328329c68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Resino S, Resino R, Micheloud D, et al. Long-term effects of highly active antiretroviral therapy in pretreated, vertically HIV type 1-infected children: 6 years of follow-up. Clin Infect Dis. 2006;42(6):862–9. doi: 10.1086/500412. [DOI] [PubMed] [Google Scholar]
- 7.Saag M, Deeks SG. How do HIV elite controllers do what they do? Clin Infect Dis. 2010;51(2):239–41. doi: 10.1086/653678. [DOI] [PubMed] [Google Scholar]
- 8.Streeck H, Jolin JS, Qi Y, et al. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol. 2009;83(15):7641–8. doi: 10.1128/JVI.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananworanich J, Pancharoen C, Sirivichayakul S, et al. Undetectable plasma HIV-1 RNA with strong gag-pol specific interferon-gamma ELISPOT response in an HIV-1 clade A/E-infected child untreated with antiretroviral therapy. Asian Pac J Allergy Immunol. 2004;22(2-3):165–9. [PubMed] [Google Scholar]
- 10.Miura T, Brumme ZL, Brockman MA, et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol. 2010 doi: 10.1128/JVI.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197(4):563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 12.Migueles SA, Weeks KA, Nou E, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83(22):11876–89. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul ME, Shearer WT, Kozinetz CA, Lewis DE. Comparison of CD8(+) T-cell subsets in HIV-infected rapid progressor children versus non--rapid progressor children. J Allergy Clin Immunol. 2001;108(2):258–64. doi: 10.1067/mai.2001.117179. [DOI] [PubMed] [Google Scholar]
- 14.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Mandala WL, MacLennan JM, Gondwe EN, Ward SA, Molyneux ME, MacLennan CA. Lymphocyte subsets in healthy Malawians: implications for immunologic assessment of HIV infection in Africa. J Allergy Clin Immunol. 2010;125(1):203–8. doi: 10.1016/j.jaci.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelman R, Cheng SC, Kidd P, Waxdal M, Kagan J. Assessment of the effects of instrumentation, monoclonal antibody, and fluorochrome on flow cytometric immunophenotyping: a report based on 2 years of the NIAID DAIDS flow cytometry quality assessment program. Clin Immunol Immunopathol. 1993;66(2):150–62. doi: 10.1006/clin.1993.1019. [DOI] [PubMed] [Google Scholar]
- 17.Navarro J, Resino S, Bellon JM, et al. Association of CD8+ T lymphocyte subsets with the most commonly used markers to monitor HIV type 1 infection in children treated with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17(6):525–32. doi: 10.1089/08892220151126607. [DOI] [PubMed] [Google Scholar]
- 18.Benito JM, Lopez M, Lozano S, et al. Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;38(4):373–81. doi: 10.1097/01.qai.0000153105.42455.c2. [DOI] [PubMed] [Google Scholar]
- 19.Hazenberg MD, Otto SA, van Rossum AM, et al. Establishment of the CD4+ T-cell pool in healthy children and untreated children infected with HIV-1. Blood. 2004;104(12):3513–9. doi: 10.1182/blood-2004-03-0805. [DOI] [PubMed] [Google Scholar]
- 20.Ono E, Nunes dos Santos AM, de Menezes Succi RC, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res. 2008;41(8):700–8. doi: 10.1590/s0100-879x2008000800011. [DOI] [PubMed] [Google Scholar]
- 21.Resino S, Navarro J, Bellon JM, Gurbindo D, Leon JA, Munoz-Fernandez MA. Naive and memory CD4+ T cells and T cell activation markers in HIV-1 infected children on HAART. Clin Exp Immunol. 2001;125(2):266–73. doi: 10.1046/j.1365-2249.2001.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott-Algara D, Rouzioux C, Blanche S, et al. In untreated HIV-1-infected children, PBMC-associated HIV DNA levels and cell-free HIV RNA levels are correlated to distinct T-lymphocyte populations. J Acquir Immune Defic Syndr. 2010;53(5):553–63. doi: 10.1097/QAI.0b013e3181cf060f. [DOI] [PubMed] [Google Scholar]
- 23.Douek DC, Roederer M, K RA. Emerging Concepts in the Immunopathogenesis of AIDS. Annual Review of Medicine. 2009;60:471–84. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cagigi A, Palma P, Nilsson A, et al. The impact of active HIV-1 replication on the physiological age-related decline of immature-transitional B-cells in HIV-1 infected children. Aids. 2010 doi: 10.1097/QAD.0b013e32833c3298. [DOI] [PubMed] [Google Scholar]
- 25.Ratto-Kim S, Chuenchitra T, Pulliam L, et al. Expression of monocyte markers in HIV-1 infected individuals with or without HIV associated dementia and normal controls in Bangkok Thailand. J Neuroimmunol. 2008;195(1-2):100–7. doi: 10.1016/j.jneuroim.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Ramon S, Bellon JM, Resino S, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003;111(2):E168–75. doi: 10.1542/peds.111.2.e168. [DOI] [PubMed] [Google Scholar]
- 27.Bunders M, Cortina-Borja M, Newell ML. Age-related standards for total lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe. Pediatr Infect Dis J. 2005;24(7):595–600. doi: 10.1097/01.inf.0000168835.01233.64. [DOI] [PubMed] [Google Scholar]
- 28.Bunders M, Thorne C, Newell ML. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. Aids. 2005;19(10):1071–9. doi: 10.1097/01.aids.0000174454.63250.22. [DOI] [PubMed] [Google Scholar]
- 29.van Gent R, van Tilburg CM, Nibbelke EE, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133(1):95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Kupka R, Msamanga GI, Aboud S, Manji KP, Duggan C, Fawzi WW. Patterns and predictors of CD4 T-cell counts among children born to HIV-infected women in Tanzania. J Trop Pediatr. 2009;55(5):290–6. doi: 10.1093/tropej/fmn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofer CB, Oliveira RH, Machado ES, et al. Neonatal factors associated with HIV long term non-progressors in a cohort of vertically infected children in Rio de Janeiro, Brazil (‘Peixe’ Project) Braz J Infect Dis. 2009;13(4):276–9. doi: 10.1590/s1413-86702009000400007. [DOI] [PubMed] [Google Scholar]
- 32.Resino S, Abad ML, Navarro J, Bellon JM, Sanchez-Ramon S, Angeles Munoz-Fernandez M. Stimulated proliferative responses in vertically HIV-infected children on HAART correlate with clinical and immunological markers. Clin Exp Immunol. 2003;131(1):130–7. doi: 10.1046/j.1365-2249.2003.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resino S, Seoane E, Gutierrez MD, Leon JA, Munoz-Fernandez MA. CD4(+) T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42(3):269–76. doi: 10.1097/01.qai.0000222287.90201.d7. [DOI] [PubMed] [Google Scholar]
- 34.Kim WK, Alvarez X, Fisher J, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168(3):822–34. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.