Abstract
BACKGROUND
Targeting the tumor microenvironment and angiogenesis is a novel lymphoma therapeutic strategy. We report safety, activity and angiogenic profiling with the RT-PEPC regimen (rituximab with thalidomide, and prednisone, etoposide, procarbazine and cyclophosphamide) in recurrent mantle cell lymphoma (MCL).
METHODS
RT-PEPC includes induction (months 1–3) of weekly rituximab × 4, daily thalidomide (50 mg) and PEPC, then maintenance thalidomide (100 mg), oral PEPC titrated to neutrophil count, and rituximab every 4 months. Endpoints included safety, efficacy, quality of life (QoL), and translational studies including tumor angiogenic phenotyping, plasma VEGF and circulating endothelial cells.
RESULTS
Twenty-five pts were enrolled (22 evaluable) with median age 68 yrs (range 52–81), 24 (96%) stage III/IV, 18 (72%) IPI 3–5, 20 (80%) high risk MIPI, median 2 prior therapies (range 1–7), and 15 (60%) bortezomib progressors. At a median follow-up of 38 months, ORR was 73% (32% CR/CRu, 41% PR, n=22) and median PFS 10 months. Four CRs are ongoing (6+, 31+, 48+ and 50+ months). Toxicities included grade 1–2 fatigue, rash, neuropathy and cytopenias including grade 1–2 thrombocytopenia (64%) and grade 3–4 neutropenia (64%). Two thromboses and 5 grade 3–4 infections occurred. QoL was maintained or improved. Correlative studies demonstrated tumor autocrine angiogenic loop (expression of VEGFA and VEGFR1) and heightened angiogenesis and lymphangiogenesis in stroma. Plasma VEGF and circulating endothelial cells trended down with treatment.
CONCLUSIONS
RT-PEPC has significant and durable activity in MCL, with manageable toxicity and maintained QoL. Novel low-intensity approaches warrant further evaluation, potentially as initial therapy in elderly patients.
INTRODUCTION
Mantle cell lymphoma (MCL) represents 5–8% of non-Hodgkin’s lymphoma and occurs primarily in the elderly. Median failure-free survival is approximately 8 to 20 months with standard regimens 1–3, although high-intensity chemotherapy with or without stem-cell transplantation provide longer progression-free survival 4, 5. Disease course is characterized by relapses with a reported median survival of 4–5 years 6.
Management of recurrence is limited by age and comorbidities. Single-agents such as the proteasome inhibitor bortezomib, mTOR inhibitor temsirolimus, and immunomodulatory drug (iMiD) lenalidomide provide modest response rates (22–53%) with durations of response of approximately 4.8–9.2 months 7–12. Without a curative option in general, regimens offering disease control with convenience and tolerability (to preserve quality of life) are valuable to patients with MCL – particularly the elderly. One strategy is represented by novel regimens, including metronomic therapy, which target the tumor microenvironment and angiogenesis.
The PEPC (C3) metronomic regimen 13 consists of oral prednisone (20 mg), etoposide (50 mg), procarbazine (50 mg) and cyclophosphamide (50 mg) administered orally on a continuously schedule. In a retrospective analysis of 22 recurrent MCL patients, we reported a response rate of 82% (including 46% CR) and a median time on therapy of 17 months. Treatment was well tolerated, though precise characterization was limited by the retrospective assessment 13. We hypothesized that addition of another putative anti-angiogenic agent (thalidomide) could potentially augment therapy. The combination of rituximab and thalidomide (RT) demonstrated activity in a pilot MCL study 14, with 13 of 16 evaluable subjects having responses (5 CR, 8 PR) and median progression-free survival measured at 20 months. However, toxicity mandated significant thalidomide dose reductions.
We report a prospective phase II study of the RT-PEPC combination incorporating low-dose thalidomide (50–100 mg), with assessment of safety, efficacy and quality-of-life in relapsed and refractory MCL. Maintenance rituximab was incorporated with an attempt to maximize duration of responses 15. Translational studies explored the angiogenic profiles of primary tumor cells and tumor vasculature, and correlated anti-angiogenesis with clinical response by measurement of both soluble and cellular markers of angiogenesis including plasma VEGF (vascular endothelial growth factor) and circulating endothelial cells.
PATIENTS AND METHODS
Eligibility
Subjects had confirmed MCL with t(11; 14)(q13;q32) translocation or cyclin D1 overexpression; recurrent or persistent disease after at least one therapy; measurable disease and Karnofsky performance status (KPS) 50% or higher. Absolute neutrophil count (ANC) ≥ 1000 cell/μL, platelets ≥ 50,000 cells/μL, total bilirubin/AST/ALT ≤ 2X upper limit of normal (ULN), and creatinine ≤ 2X ULN were required. Key exclusion criteria included central nervous system lymphoma or HIV; prior therapy or major surgery within 3 weeks; and pre-existing > grade 2 peripheral neuropathy.
Study Design
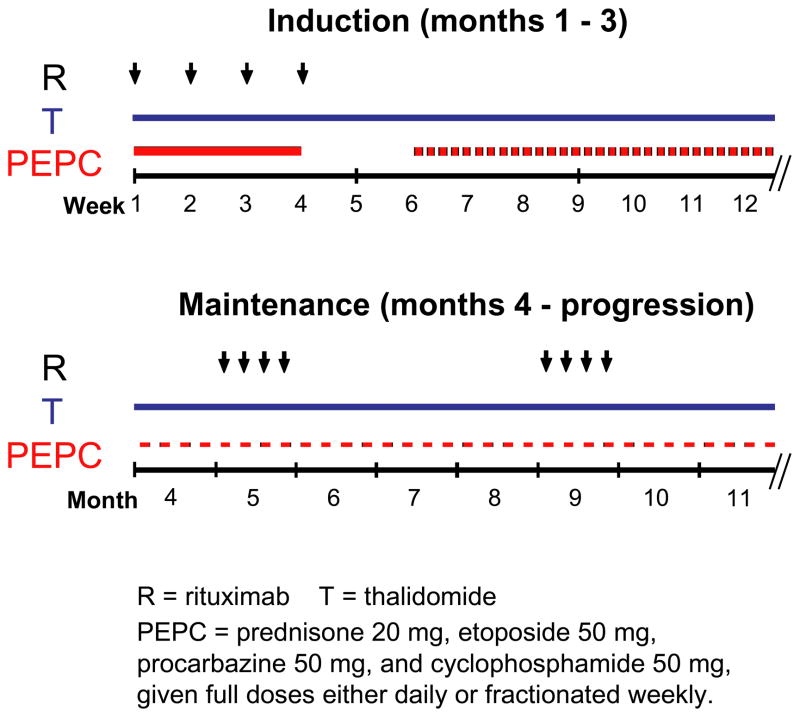
This study was approved by the Weill Cornell Medical College Institutional Review Board. Written informed consent was obtained in all study participants. RT-PEPC therapy includes an induction phase (months 1–3) of weekly rituximab × 4 at month 1, daily thalidomide (50 mg months 1–2, then 100 mg after month 2) and daily PEPC with prednisone 20 mg, etoposide 50 mg, procarbazine 50 mg, and cyclophosphamide 50 mg. PEPC was held when ANC dropped to < 2K/μL, and restarted at alternate day or fractionated weekly basis when ANC returned to ≥ 2K/μL. A maintenance phase (month 4 until progression) continued with daily thalidomide at 100 mg, PEPC dosing titrated to ANC > 2K/μL, and weekly rituximab × 4 every 4 months (Figure 1). Daily aspirin 81 mg was initiated for thromboprophylaxis after two episodes of deep vein thrombosis occurred.
Figure 1. RT-PEPC treatment schedule.
Treatment consisted of induction and maintenance phases. Patients received weekly rituximab × 4 at month 1, daily thalidomide at 50 mg (months 1–2, then dose-escalated to 100 mg if tolerated after month 2) and daily PEPC with prednisone 20 mg, etoposide 50 mg, procarbazine 50 mg, and cyclophosphamide 50 mg during induction. PEPC was put on hold when ANC dropped to < 2K/μL, and restarted at alternate day or fractionated weekly basis to maintain ANC ≥ 2K/μL. A maintenance phase (month 4 until progression) continued with daily thalidomide at 50–100 mg, PEPC dosing titrated to ANC ≥ 2K/μL, and maintenance rituximab every 4 months
Efficacy and Safety Assessments
Response criteria followed the guidelines reported by Cheson et al 16. Toxicities were graded according to NCI Common Terminology Criteria for Adverse Events version 3.0. Thalidomide was dose-reduced or discontinued for persistent neuropathy ≥ grade 3. During induction, patients were evaluated weekly at month 1 and monthly for months 2–3 for toxicity assessment. Response measurement began at month 3. During maintenance, patients were evaluated every 3 months for the first year, and then every 4–6 months until disease progression and indefinitely for survival.
Statistical Analysis
Study endpoints included overall (OS) and progression-free survival (PFS) summarized using the Kaplan-Meier curves, and objective response (CR/CRu/PR) summarized by an exact 95% confidence interval. The 2-year OS and PFS rates were estimated based on the Kaplan-Meier estimates. Cox proportional hazards regression analysis was used to evaluate response status and risk factors associated with PFS. All analyses were performed in S-PLUS version 8.0 for Windows. For correlative studies, statistical significance between samples was analyzed using a 2-tailed t test. P-values of the pair-wise comparisons were adjusted using Bonferroni method. ANOVA was used to compare the difference in means among the different time points.
Quality-of-Life Assessment
Quality-of-life (QoL) assessments were obtained with the Functional Assessment of Cancer Therapy – General (FACT-G version 3) instrument at baseline, every 2 months until month 6, and every 6 months until disease progression 17. FACT-G questionnaires include five subscales (physical well-being, social/family well-being, relationship with doctor, emotional well-being, and functional well-being). The sum of physical well-being and functional well-being scores was defined as the modified Trial Outcome Index (TOI). ANOVA was used to compare the difference in the means of QoL total score and modified TOI among the different time points.
Angiogenesis Biomarker Studies
Quantitative PCR analysis of VEGF receptor expression on MCL B-cells
Baseline MCL B-cells were isolated from either lymph nodes or peripheral blood if involved by disease using CD19+ magnetic beads (Miltenyi Biotec, Auburn, CA), and subjected to RNA extraction with Trizol (Invitrogen, Carlsbad, CA). MCL cell line Jeko-1 and CD19-selected normal donor peripheral B-cells were used for comparisons. Quantitative VEGF receptor expression analysis was performed with Taqman expression assays for VEGFR-1, VEGFR-2 and VEGFR-3 (Applied Biosystems, Foster City, CA).
Immunohistochemistry
Frozen lymph node tissue was obtained at baseline in 7 patients, and paraffin tissues in 10 patients for correlative studies. Frozen tissue sections were stained with anti-VEGFR-1 (FB5, ImClone), VEGFR-2 (1121, ImClone), VEGFR-3 (3C5, ImClone), VEGF-A (VG1, Zymed), CD34 (QBEnd10, Dako) and Lyve-1 (Abcam). Paraffin tissue sections were stained with anti-CD34, α-smooth muscle actin (1A4, Sigma), and podoplanin (Angio-Bio Co.). Double-staining was performed with the following antibodies: VEGFR-2/CD34, VEGFR-2/VEGF-A, CD34/Lyve-1, α-SMA/CD34, and CD34/podoplanin. All staining experiments included positive and negative controls.
VEGF ELISA
Blood samples were collected at baseline, monthly for the first 3 months, and then every 3 months (until at least month 6). Plasma VEGF was quantified using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (R&D Systems, Minneapolis, MN).
FACS analysis of circulating endothelial cells (CECs)
Mononuclear cells were isolated using Ficoll-Paque™ PLUS (GE Healthcare, Piscataway, NJ) gradient centrifugation from 10 ml of peripheral blood collected in EDTA tubes within 24 hours of blood collection. Circulating endothelial cells (CEC) were labeled with PerCP-anti-CD45, FITC-anti-CD31, PE-anti-CD146, and APC-anti-CD34 (all from BD Pharmingen). Appropriate isotype controls were included. Samples were analyzed on a FACSCalibur using CellQuest software (Bectin Dickinson, San Jose, CA). For each flow reaction, 300,000 to 500,000 events were collected. Since most mononuclear cells fell in the lymphocyte gating after Ficoll gradient purification, the number of CEC/μL was calculated as: absolute lymphocyte countμL CEC/lymphocyte gate count.
RESULTS
Patient Characteristics and Disposition
Twenty-five patients were enrolled, of whom 22 were assessable for response (3 were enrolled but received no therapy) (Table 1). The primary analysis was performed using the 22 evaluable subjects with a median follow-up of 38 months based on the Kaplan-Meier estimate. Median age was 68 years, 96% had stage III & IV disease, and 64% had elevated lactate dehydrogenase (LDH). Patients generally had unfavorable baseline international prognostic index (72% with IPI score of 3–5), as well as MIPI scores (12% with intermediate risk, and 80% with high risk). Median time from diagnosis was 2 years (range, 1–13 years), and median number of prior therapies was two (range, 1 to 7). Twenty-two (88%) patients had received a CHOP-based regimen (mostly R-CHOP), and fifteen (60%) progressed after prior bortezomib-containing therapy. Three patients had prior high dose chemotherapy and stem cell transplantation.
Table 1.
Baseline Patient and Disease Characteristics (N=25)
| Characteristic | No. of Patients | Percent | |
|---|---|---|---|
| No. of Patients | 25 | 100% | |
| Sex | |||
| Male | 19 | 76% | |
| Female | 6 | 24% | |
| Age, years | |||
| Median | 68 | ||
| Range | 52–81 | ||
| IPI risk category | |||
| 2 | 7 | 28% | |
| 3 | 13 | 52% | |
| 4–5 | 5 | 20% | |
| MIPI risk category | |||
| Low risk (score <5.7) | 2 | 8% | |
| Intermediate risk (5.7 ≤ score < 6.2) | 3 | 12% | |
| High risk (score ≥ 6.2) | 20 | 80% | |
| Stage III – IV | 24 | 96% | |
| Elevated LDH | 16 | 64% | |
| Time from Diagnosis, years | |||
| Median | 2 | ||
| Range | 1–13 | ||
| No. of prior lines of therapy | |||
| 1 | 7 | 28% | |
| 2 | 11 | 44% | |
| 3 and above | 7 | 28% | |
| Prior Treatment | |||
| CHOP-like | 22 | 88% | |
| Rituximab | 23 | 92% | |
| Bortezomib | 15 | 60% | |
| All 3 of the above | 12 | 48% | |
| Purine analog-based therapy | 3 | 12% | |
| Radioimmunotherapy | 2 | 8% | |
| Involved field radiation therapy | 4 | 16% | |
| Autologous SCT | 3 | 12% | |
| Experimental biologics | |||
| Oblimersen sodium | 4 | 16% | |
| Tumor vaccine (Id-KLH) | 1 | 4% | |
| PD0332991 (Pfizer) | 3 | 12% | |
Efficacy
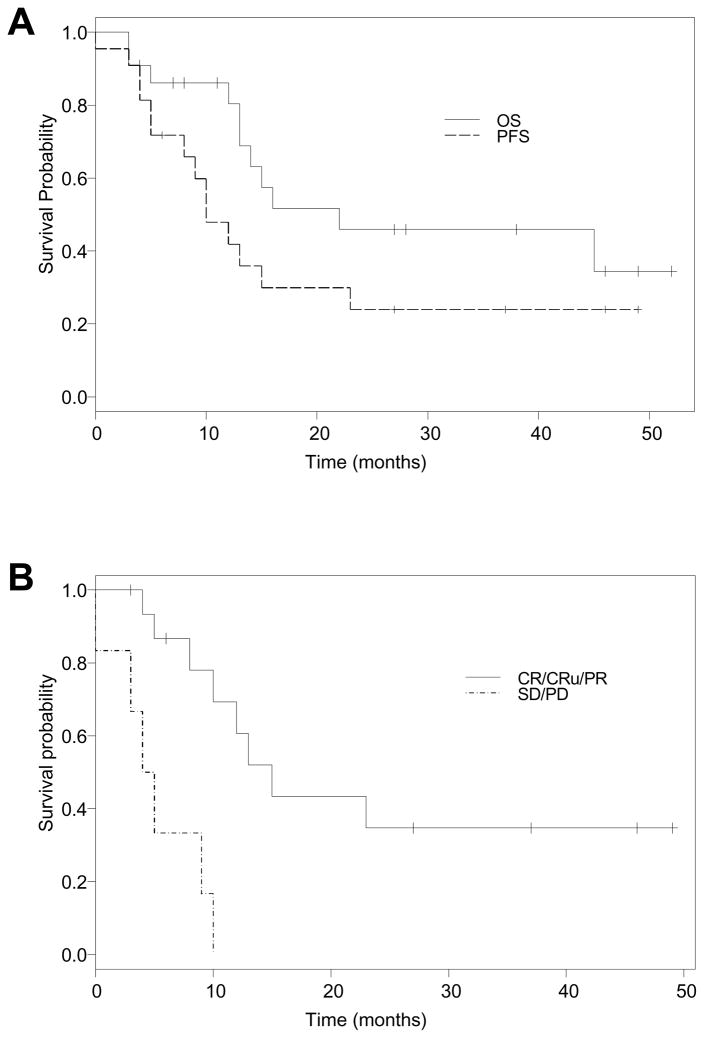
The overall response rate (ORR) was 73% (95% CI: 50%--89%), with CR/Cru of 32% and PR of 41% (Table 2). Four additional subjects (18%) achieved stable disease (SD) lasting 4, 6, 10 and 11 months, respectively. Figure 2 shows disease measurements in two responders. Median time to response was 119 days (range, 76 to 692 days). With a median follow-up time of 38 months, the 2-year OS rate was 45% (95% CI: 28–76%), 2-year PFS was 24% (95% CI: 10%--56%). The median PFS was 10 months (95% C.I. 5–23 months) (Figure 3). No IPI factors correlated with OS or PFS in a multivariate Cox regression analysis, while a majority of patients had high risk MIPI scores. Response (CR/PR) to RT-PEPC treatment favorably associated with PFS (hazard ratio 0.13, 95% CI: 0.04–0.48; p<0.002). Response to prior regimen trended with response to RT-PEPC, although not statistically significant (p=0.07).
Table 2.
RT-PEPC Response
| Response | Number (n=22) | Percentage |
|---|---|---|
| ORR (CR+PR) | 16 | 73 % |
| CR + CRu | 7 | 32 % |
| PR | 9 | 41 % |
| SD | 4 | 18 % |
| PD | 2 | 9% |
| Median time to response (days) | 119 (range 76–692) | |
| Median time to progression (months) | 12 (95% C.I. 8–23) | |
Abbreviations: ORR, overall response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
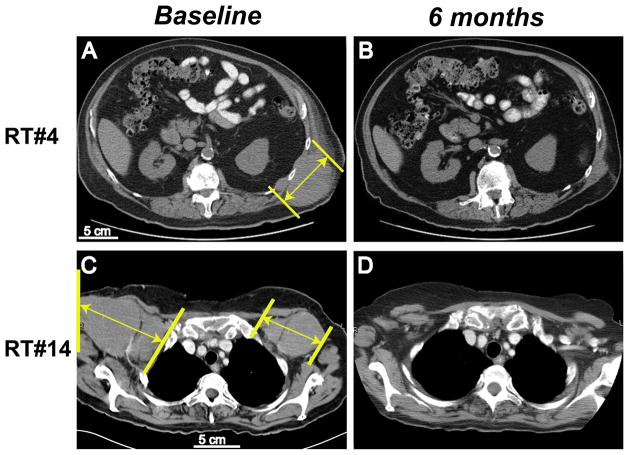
Figure 2. Response measurements in two representative patients.
CT images from baseline and at 6 months were compared for study subjects RT#4 (A, B) and RT#14 (C, D). Tumor sizes (long axis) were marked with yellow lines.
Figure 3. Kaplan-Meier survival curves.
A: Overall survival (OS) curve and progression-free survival (PFS) curve. B: PFS based on response status.
Six patients remain on study. Four patients have durable responses with 3 CRs ongoing at 50+, 48+ and 31+ months, and 1 PR at 43+ months. Of 16 who discontinued treatment, 10 have died, one went to hospice, three received additional treatment, and two patients successfully underwent stem cell mobilization and consolidative autologous stem cell transplantation while in clinical response (1 CR and 1 PR).
Toxicities
Therapy was generally well tolerated. By design, the primary toxicity was reversible myelosuppression. Grade 3 or 4 neutropenia occurred in 14 patients (64%), including 3 with febrile neutropenia. Other grade 3 or 4 hematologic toxicities included anemia (5%) and thrombocytopenia (18%) (Table 3). Nonhematologic toxicity was generally mild and mainly consisted of grade 1 or 2 events, including fatigue (100%), constipation (64%), cough (64%), nausea (59%), neuropathy (59%), dyspnea (50%) and rash (45%). Five patients (23%) developed grade 3 infections. One case each of PCP pneumonia and CMV retinitis were observed. Thrombosis developed in 3 patients with two episodes of DVT/PE and one episode of TIA prior to the institution of thromboprophylaxis with asprin. One case of early myelodysplasia (bone marrow aspirate morphology only with normal cytogenetics and blast count) occurred in a patient who had prior high-dose therapy and stem cell transplant. She subsequently underwent reduced-intensity matched related donor allogeneic stem cell transplant, and has remained in CR. No long-term hematological toxicity or bone marrow dysfunction occurred in other subjects.
Table 3.
Major Hematologic and Nonhematologic Toxicities (N=22)
| Toxicity | Any Grade | Grade 3/4 | ||
|---|---|---|---|---|
| No. | Percentage | No. | Percentage | |
| Hematologic | ||||
| Neutropenia | 20 | 91% | 14 | 64% |
| Anemia | 14 | 64% | 1 | 5% |
| Thrombocytopenia | 14 | 64% | 4 | 18% |
| Infectious | ||||
| CAP/URI | 9 | 41% | 4 | 18% |
| Fever (ANC ≥ 1.0) | 7 | 32% | 0 | 0% |
| Febrile neutropenia | 3 | 14% | 3 | 14% |
| Opportunistic Infectiona | 2 | 9% | 1 | 5% |
| Other | ||||
| Fatigue | 22 | 100% | 0 | 0% |
| Constipation | 14 | 64% | 0 | 0% |
| Cough | 14 | 64% | 0 | 0% |
| Nausea | 13 | 59% | 1 | 5% |
| Neuropathy | 13 | 59% | 0 | 0% |
| Dyspnea | 11 | 50% | 0 | 0% |
| Rash | 10 | 45% | 0 | 0% |
| Dizziness | 10 | 45% | 0 | 0% |
| Abdominal pain | 8 | 36% | 0 | 0% |
| Alopecia | 8 | 36% | 0 | 0% |
| Vomiting | 6 | 27% | 0 | 0% |
| DVT/PE | 2 | 9% | 1 | 5% |
| TIA | 1 | 5% | 1 | 5% |
Abbreviations:
CAP/URI: community-acquired pneumonia/upper respiratory illness; ANC: absolute neutrophil count; DVT/PE: deep vein thrombosis/pulmonary embolism; TIA: transient ischemic attack.
one case each of grade 3 PCP pneumonia and grade 2 CMV retinitis; both resolved with treatment.
Quality of Life Assessment
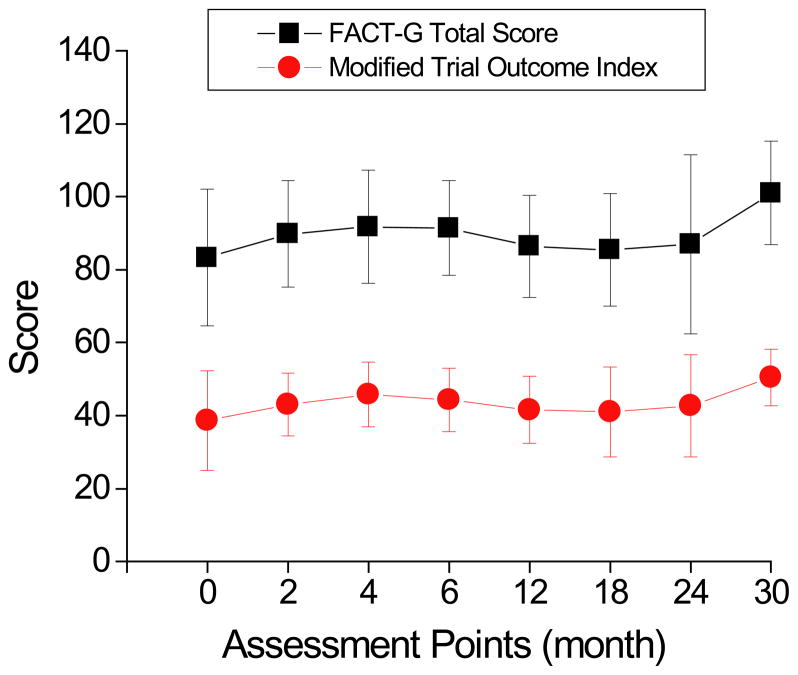
Responders generally reported sustained or improved QoL, while nonresponders reported worsening status related to progression. At baseline, the mean of FACT-G total score was 83.3 (SD 18.8), and modified Trial Outcome Index (TOI) was 38.7 (SD 13.6). Means of baseline FACT-G scores did not correlate with IPI or clinical response on RT-PEPC. In four long-term responders (PFS > 31 months and ongoing), the mean FACT-G scores remained stable or slightly improved. Importantly, the FACT-G scores on average remained unchanged (total score: 89.4±5.5, p=0.16; TOI: 43.5±3.5, p=0.27) during treatment (Figure 4).
Figure 4. FACT-G quality-of-life (QoL) assessment.
The FACT-G total score and modified trial outcome index (TOI) were measured at baseline, every 2 months until month 6, and every 6 months until disease progression to assess the impact of the metronomic therapy on QoL.
Angiogenesis Biomarker Results
Angiogenesis biomarkers were analyzed on the available pretreatment tissue specimens to assess tumor cells and the vascular microenvironment. In addition, dynamic levels of plasma VEGF and circulating endothelial cells at baseline and during therapy were measured and correlated to response in a subset of patients with available specimens.
VEGF receptor expression
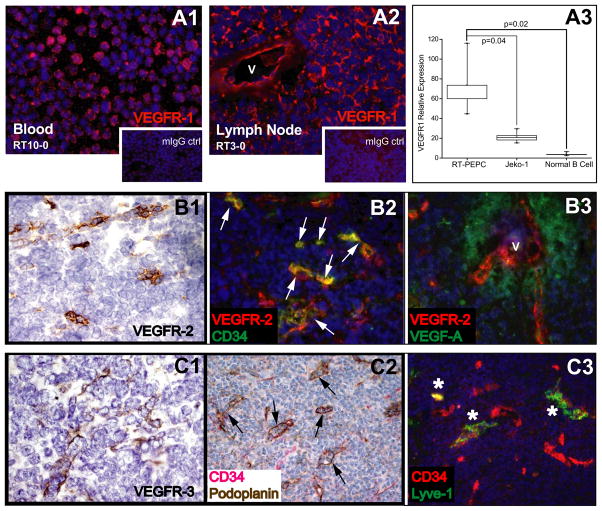
VEGFR-1 expression was detected consistently by immunohistochemistry on the surface of neoplastic B-cells, in addition to neo-vasculature (Figure 5, A1–A2). VEGFR-1 expression in primary MCL B-cells was higher than that of MCL cell line Jeko-1 and normal donor B-cells by real-time PCR (Figure 5, A3, p=0.02). VEGFR-2 and VEGFR-3 expression on MCL B-cells could not be demonstrated.
Figure 5. Angiogenesis and lymphangiogenesis characterization in MCL.
A. VEGFR-1 is expressed by MCL B-cells (A1) and neovessels (v = vessel) (A2) by immunofluorescence analysis, and appears to be overexpressed in neoplastic B-cells (n=5) compared to normal B-cells (n=5) and MCL cell line Jeko-1 by quantitative PCR analysis (A3). B. VEGFR-2 expression in neovasculature. B1: VEGFR-2 immunohistochemistry on frozen tissue section. B2: A majority of VEGFR-2+ tumor endothelial cells coexpress CD34 by immunofluorescence analysis (white arrows). B3: VEGFR-2 vessels (V) are situated in proximity to VEGF-producing tumor cells (B3). C. Lymphangiogenesis as marked by VEGFR-3+ (C1), podoplanin+ (C2) and Lyve-1+ (C3). Some lymphatic vessels appear to co-express CD34. Black arrows point to CD34+podoplanin+ vessels, and asterisks point to CD34+lyve-1+ vessels.
Vascular stroma profiling
Stromal angiogenesis was assessed using blood vascular and perivascular markers including VEGFR-1, VEGFR-2, CD34, α-SMA, as well as lymphatic vascular markers of VEGFR-3, podoplanin and lyve-1. In addition to VEGFR-1, tumor neovessels were marked by VEGFR-2 and CD34. A majority of CD34+ tumor endothelial cells coexpressed VEGFR-2. Tumor B-cells expressed VEGF-A, in a paracrine-fashion in relation to VEGFR-2+ tumor endothelia (Figure 5, B1–B3). Most CD34+ tumor microvessels were ensheathed by α-SMA+ pericytes (data not shown). Evident in the tumor-infiltrating sinus zone was significant lymphangiogenesis activity marked by VEGFR-3+, podoplanin+ and Lyve-1+ lymphatic vessels, some of which co-expressed CD34 (Figure 5, C1–C3). The heightened angiogenesis and lymphangiogenesis in MCL implicate their role in promoting lymphoma growth and spread.
Plasma VEGF
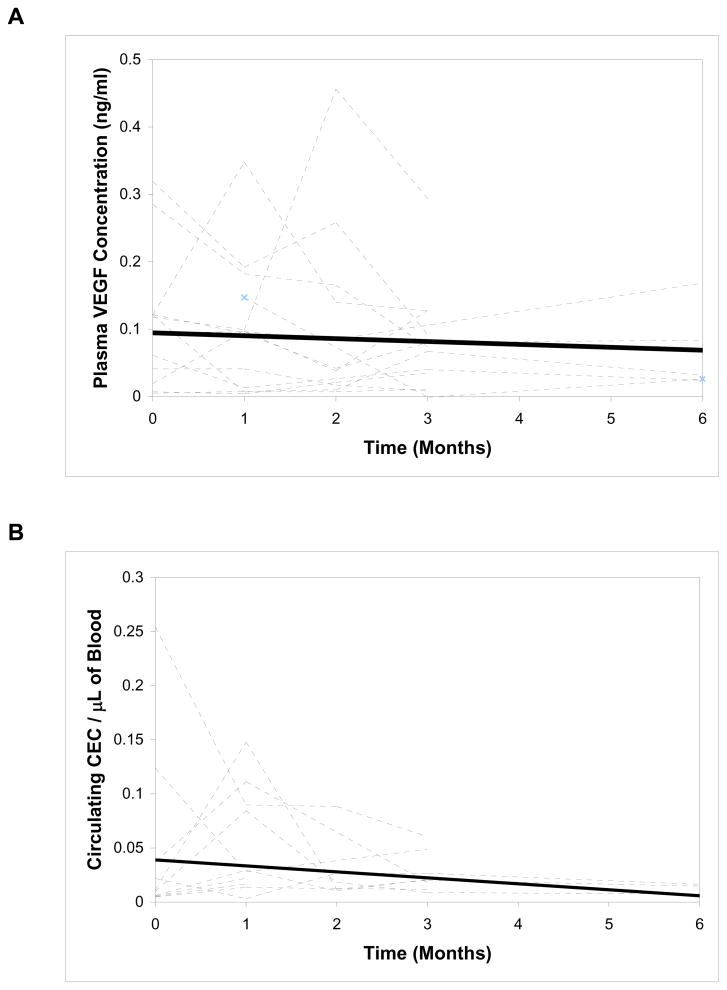
Baseline median plasma VEGF (N=20) was 109.5 pg/mL (range 7–319, n=20), compared to 68.2 pg/mL (range 8–98, n=5) in normal controls. There were no statistically significant differences between responders and nonresponders (100.5 vs. 155.9 pg/mL, p=0.44), although the median VEGF trended higher in nonresponders. Thirteen patients (11 responders, and 2 nonresponders with stable disease) had consecutive samples on therapy, and were analyzed in an exploratory fashion to correlate levels to clinical responses. Although not statistically significant, the median VEGF levels trended down with time during treatment in both responders and nonresponders (Figure 6A). At 3 months, 62% of the 13 patients had a reduction in plasma VEGF, while 38% had an increase.
Figure 6. Individual profiles of VEGF (A) and total CECs (B) over time.
Individual time course of plasma VEGF and total CECs (dotted lines) with linear regression (solid lines) in a subset of patients.
Circulating endothelial cells (CECs)
Total CECs, defined as CD45-CD146+CD31+CD34+ cells, were quantified in 10 consecutive patients (7 responders) during treatment. Median baseline CECs were 47.9 cells/mL (range 4.7–254.3 cells/mL). At month 1, 8 out of 10 patients had an increase in CECs, while 2 had a decrease. Compared to baseline CECs, median CECs at month 1 trended up, although not statistically significant, and then trended down after month 2 (Figure 6B). No significant differences in median values were detected between responders and nonresponders.
DISCUSSION
We report the first prospective study evaluating the efficacy and safety of oral low-dose metronomic chemotherapy PEPC with rituximab and thalidomide in recurrent mantle cell lymphoma. MCL has increased angiogenesis in the tumor microenvironment, and we combined two putative anti-angiogenic regimens, namely PEPC oral chemotherapy 13, and rituximab with thalidomide 14. This study is novel in several important aspects: we prospectively combined these regimens, assessed quality of life, and explored a putative mechanism of action (anti-angiogenesis). Additionally, we employed a lower, more tolerable dose of thalidomide compared to the prior RT regimen 14. The ultimate clinical goal of the study was to develop a novel low-intensity treatment regimen that affords durable response with preservation of quality of life, particularly for elderly patients. The RT-PEPC regimen compares favorably in efficacy and tolerability to data reported from alternative treatment strategies in this patient population 7–12, 15.
Subjects in this study generally had unfavorable baseline IPI (72% with IPI score of 3–5) and MIPI scores (12% with intermediate risk, and 80% with high risk), in addition to other clinical features typical for MCL including predominantly elderly patients (median age 68) with advanced stage diseases. Most had progressive disease on bortezomib, the only FDA-approved agent for this setting. Compared to immediate prior therapies, 70% of non-progressors (including CR+PR+SD) achieved longer PFS on RT-PEPC, and 45% had better response quality (i.e., from prior PD or SD to either PR or CR on RT-PEPC). Durable responses generally occurred in complete responders. Most patients would eventually be maintained on PEPC dosing of 1–2 times weekly, and thalidomide dosing of 50–100 mg daily. Treatment was convenient (at home except for rituximab) and well tolerated as reflected by quality of life.
Both metronomic therapy and thalidomide putatively target tumor microenvironment and angiogenesis 18, 19. Metronomic therapy has broad applicability in lymphoma, including refractory disease 13, 20, 21. Principal targets of metronomic therapy are thought to be the endothelial cells of the growing tumor vasculature. In addition, metronomic therapy has been shown to suppress the surge of bone marrow-derived endothelial progenitor cells (EPC) mobilization following conventional “maximal tolerated dose” therapy 22, 23. Metronomic chemotherapy can be combined with new compounds to augment anti-angiogenic effects, as in the RT-PEPC combination. In relapsed breast cancer 24 and aggressive NHL 21, responses to metronomic regimens appeared to be associated with overall declining levels of circulating endothelial cells, and increased fraction of apoptotic endothelial cells. We explored the correlation of angiogenesis biomarkers to clinical responses in a subset of our study patients. While limited by sample size, nonresponders appeared to have higher baseline VEGF levels, and plasma VEGF levels generally trended down with therapy in both responders and nonresponders. Our data also indicate that CECs declined in response to treatment in both responders and non-responders. A non-statistically significant increase in total CEC was noted at month 1 for 80% of the samples. Since we did not differentiate total CECs from apoptotic CECs, it is possible that this may represent a temporary increase in apoptotic CECs as a result of the treatment. The declining trend of both plasma VEGF and CECs levels in response to therapy may reflect potential anti-angiogenic effect of the RT-PEPC regimen with the caveat that disease in non-responders may have developed resistance via alternative mechanisms. Further investigations with larger sample sizes are needed in trials of conventional or anti-angiogenic treatment strategies to better define the prognostic significance of these angiogenesis biomarkers.
While the precise role of tumor angiogenesis in MCL pathogenesis remains under active investigation, our exploratory correlative study data indicate that MCL has increased angiogenesis in the tumor microenvironment. Consistent with the SWOG S0108 trial 25, we detected VEGFR-1 over-expression in MCL B-cells compared to normal B-cells. VEGFR-1 expression promotes leukemic proliferation via autocrine fashion and extramedullary metastasis26. Universal expression of VEGFR-1 in primary MCL cells suggests a role in MCL proliferation and extranodal metastasis. We also demonstrated that VEGFR-2 and VEGFR-3 were primarily expressed in a paracrine fashion by CD34+ neovascular blood and lymphatic vessels in relation to VEGF-A producing lymphoma cells, implicating heightened angiogenesis and lymphangiogenesis in MCL. The presence of both an autocrine loop via VEGF and VEGFR-1 expression on MCL tumor cells, and a paracrine loop between tumor VEGF and neovascular VEGFR-2 supports the strategy of targeting VEGF in MCL. As in solid tumors, single agent bevacizumab has limited activity in MCL 25, and current approaches involving bevacizumab in combination are under evaluation in MCL and other lymphomas. Other agents targeting angiogenesis and lymphangiogenesis pathways are also in development and our data indicate potential utility in MCL.
The RT-PEPC regimen offers significant and durable clinical activity in MCL while maintaining quality of life, in part by targeting lymphoma microenvironment and angiogenesis. This approach compares favorably to other regimens for recurrent MCL. Novel low-intensity anti-angiogenic approaches warrant further evaluation in MCL and other lymphomas, either as part of a chronic maintenance treatment strategy, or as initial therapy in elderly patients.
Acknowledgments
The authors would like to thank Drs. Eric J. Feldman, Michael Schuster and Tsiporah shore, and RNs Patricia Glynn and Maureen Joyce for providing patient care; Drs. Amy Chadburn, Elizabeth Hyjek and Andrea Hooper for providing advice and technical support on immunohistochemistry for paraffin and frozen tissue slides; Dr. Maureen Lane and Maureen Ward of the Translational Core Lab at Weill Cornell for flow analysis of circulating endothelial cells; Dr. Elena Resnick, Jennnifer O’Laughlin, Jeff Goodwin and Emily Shenkein for assistance in patient data collection.
Supported in part by an ASCO Young Investigator Award and an ASCO Career Development Award to Dr. Ruan, Lymphoma Research Foundation grants to Drs. Leonard and Ruan, NIH grants K08HL091517 to Dr. Ruan, a grant from Lymphoma Foundation to Dr. Leonard, and the fund for Blood and Cancer Research to Dr. Coleman.
Footnotes
Financial Disclosure
J.P.L. has received research support and/or served as a consultant for Genentech, Biogen Idec, Celgene, and Sigma-Tau.
Authorship Contributions
J.R. wrote the manuscript, participated in the study design, patient care and data analysis, and performed the correlative studies.
J.P.L. critically contributed to study design, patient care, coordination of the logistics, interpretation of the data, and writing of the manuscript.
P.M. contributed to data management and analysis, and critical review of the manuscript.
M.C. contributed to study design, patient care and critical review of the manuscript.
R.F., R.E. provided patient care.
K.C. provided biostatistical analysis and critically reviewed the manuscript.
A.F. participated in the correlative studies and data analysis.
M.L. and K.A.H. contributed to the logistic support of the study, and critically reviewed the manuscript.
References
- 1.Fisher RI, Dahlberg S, Nathwani BN, Banks PM, Miller TP, Grogan TM. A clinical analysis of two indolent lymphoma entities: mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories): a Southwest Oncology Group study. Blood. 1995;85:1075–1082. [PubMed] [Google Scholar]
- 2.Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 4.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL networks. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 7.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 8.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 9.Hess G, Romaguera JE, Verhoef G, et al. Phase III study of patients with relapsed, refractory mantle cell lymphoma treated with temsirolimus compared with investigator’s choice. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2008.20.7977. Abstract 8513. [DOI] [PubMed] [Google Scholar]
- 10.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 11.Czuczman MS, Reeder CB, Polikoff J, et al. International study of lenalidomide in relapsed/refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26 Abstract 8509. [Google Scholar]
- 12.Habermann T, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 13.Coleman M, Martin P, Ruan J, et al. Low-dose metronomic, multidrug therapy with the PEP-C oral combination chemotherapy regimen for mantle cell lymphoma. Leuk Lymphoma. 2008;49:447–450. doi: 10.1080/10428190701837330. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann H, Raderer M, Wohrer S, et al. Antitumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphoma. Blood. 2004;104:2269–2271. doi: 10.1182/blood-2004-03-1091. [DOI] [PubMed] [Google Scholar]
- 15.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG) Blood. 2006;108:4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 20.Coleman M, Martin P, Ruan J, et al. The PEP-C (prednisone, etoposide, procarbazine, and cyclophosphamide) oral combination chemotherapy regimen for relapsing/refractory lymphoma: low dose metronomic, multidrug therapy. Cancer. 2008;112:2228–2232. doi: 10.1002/cncr.23422. [DOI] [PubMed] [Google Scholar]
- 21.Buckstein R, Kerbel RS, Shaked Y, et al. High-Dose celecoxib and metronomic “low-dose” cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:5190–5198. doi: 10.1158/1078-0432.CCR-06-0474. [DOI] [PubMed] [Google Scholar]
- 22.Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 23.Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso P, Colleoni M, Calleri A, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stopeck AT, Unger JM, Rimsza LM, et al. A phase II trial of single agent bevacizumab in patients with relapsed, aggressive non-Hodgkin lymphoma: Southwest oncology group study S0108. Leuk Lymphoma. 2009;50:728–735. doi: 10.1080/10428190902856808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fragoso R, Pereira T, Wu Y, Zhu Z, Cabecadas J, Dias S. VEGFR-1 (FLT-1) activation modulates acute lymphoblastic leukemia localization and survival within the bone marrow, determining the onset of extramedullary disease. Blood. 2006;107:1608–1616. doi: 10.1182/blood-2005-06-2530. [DOI] [PubMed] [Google Scholar]