Abstract
Current influenza virus vaccines primarily elicit antibodies and can be rendered ineffective by antigenic drift and shift. Vaccines that elicit CD8+ T cell responses targeting less variable proteins may function as universal vaccines that have broad reactivity against different influenza virus strains. To generate such a universal vaccine, we encapsulated live influenza virus in a biopolymer and delivered it to mice subcutaneously. This vaccine was safe, induced potent CD8+ T cell immunity and protected mice against heterosubtypic lethal challenge. Safety of subcutaneous (SQ) vaccination was tested in Rag2−/−γc−/− double knockout mice which we show cannot control intranasal infection. Biopolymer encapsulation of live influenza virus could be used to develop universal CD8+ T cell vaccines against heterosubtypic and pandemic strains.
Keywords: influenza virus, vaccine, CD8+ T cells
1. Introduction
The increasing likelihood of an influenza virus pandemic and the potential threat of utilizing easily transmittable viruses such as influenza virus as bioterrorism agents have made the call to develop novel vaccine and adjuvant strategies urgent. The need for pandemic vaccine production and delivery is constrained by the requirement to rapidly immunize vast numbers of immunologically naive individuals in order to protect them from the impending wave of influenza and to achieve protective levels of herd immunity that would slow down or inhibit transmission and spread. In the event of a pandemic, current vaccine strategies will require 6 or more months to generate one billion monovalent doses [1]. This would be enough for the immunization of 500 million individuals with the two doses of killed vaccine that are expected to be required for protection [2, 3]. Currently there are two anti-influenza vaccines available for humans in the USA: one is a killed virus vaccine administered as intramuscular injection and the other is an attenuated live vaccine given as a nasal spray. The current vaccines target the viral surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) [4]. Both these vaccines elicit anti-influenza virus antibodies that will neutralize subsequent virus infections. However, emerging strains of virus which express variant antigenic epitopes may not be recognized by antibodies elicited by prior vaccination, therefore the need for a new vaccine to be administered yearly. If a pandemic strain were to emerge, it would take a considerable amount of time to develop a vaccine against the new strain [1], therefore highlighting the need for new techniques of vaccine development and production.
Since antigenic drift and shift result in loss of effectiveness of current influenza virus vaccines, vaccines that elicit anti-viral CD8+ T cell responses may circumvent this major drawback of commercially available ones. To overcome the problem of antigenic variability of influenza virus, vaccines that target less variable antigens of influenza virus may provide more broad protection. It should be noted that heterosubtypic immunity against avian influenza has been shown to protect both mice and birds [5–7] and that in humans, influenza immunodominant epitopes are preserved to a high degree in avian flu isolates [7–11]. In humans there is also some evidence of heterosubtypic protection [12, 13]. Vaccines that generate CD8+ T cell responses against viral molecules which are conserved among different serotypes of influenza virus could thus serve as “universal vaccines” [7]. Such a conserved molecule is the nuclear protein (NP) and several vaccine strategies have targeted NP for generation of a long-lasting homotypic and heretosubtypic immunity [14–16]. Although the subject of intense research, currently there is no CD8+ T cell inducing vaccine against influenza virus. The generation of CD8+ T cells requires replication of the virus and protein immunization is ineffective at eliciting CD8+ T cell responses. Infection of mice with live influenza virus induces high levels of cross-reactive cytotoxic T cells whereas intact inactivated vaccine induces low levels of protection against a heterosubtypic viral infection [17]. To overcome many of the difficulties related to the development of an efficient vaccine that elicits a CD8+ T cell mediated immune response against conserved molecules of influenza virus, we used biopolymer encapsulated live influenza virus to vaccinate C57Bl/6J mice using a subcutaneous route of administration. Influenza virus infection is initiated by exposure of the respiratory tract to virus. The pathogenic aspects of influenza virus infection have been associated with respiratory and systemic infection [2, 3] with the later correlating with viremia [18]. Systemic influenza disease can be induced in chickens with intravenous administration [19]. No evidence however exists that indicates that intradermal (ID) or subcutaneous (SQ) exposure to influenza virus can lead to dissemination and cause systemic disease. Indeed for many years researchers have been using large doses of influenza virus intraperitoneally (IP) to prime animals for secondary responses [20–22] with no infection occurring. As we have shown previously, even very large doses of live influenza virus administered IP do not result in infection or disease in mice [23]. IP injection of 3×106 TCID50 PR8 influenza strain induces no inflammatory cytokines in tissues and no viral replication or weight loss in animals [23]. We found that the subcutaneous route of administration of live virus did not cause overt disease and mice did not lose weight, nor appeared sick. To confirm the inability of live virus administered SQ to cause infection, we tested the SQ injection of live virus in Rag2−/−γc−/− mice because these mice cannot develop an immune response [24, 25]. Indeed as we show here these Rag2−/−γc−/− mice fail to control intranasal influenza virus infection. Here we demonstrate that subcutaneous vaccination of mice with live influenza virus is safe based on our observations that immunodeficient Rag2−/−γc−/− mice that received live influenza virus did not become sick and had no viral replication in tissues. To provide an additional layer of protection against accidental infection via aerosols containing live virus, we used alginate biopolymer to encapsulate the live virus. Subcutaneous vaccination of mice with alginate biopolymer encapsulated live H1N1 influenza virus induced a strong CD8+ T cell mediated immune response upon rechallenge of vaccinated mice with a heterosubtypic H3N2 strain of influenza virus, and this immune response was focused on a conserved epitope of influenza nucleoprotein (NP366–374). Finally, this vaccination strategy provided heterosubtypic protection of mice against lethal challenge with the highly virulent influenza H7N7 strain.
2. Materials and Methods
2.1 Animals, and influenza virus infections
Animal studies were conducted according to approved Drexel University IACUC protocols. All mice are maintained in AAALAC certified barrier facilities at Drexel University College of Medicine, Drexel University. Specific pathogen-free 8–12 week old C57BL/6J (B6) wild type animals were purchased from Jackson Laboratories (Bar Harbor, ME). Rag−/−γc−/− female mice were purchased from Taconic (Germantown, NY). For intranasal infections (IN) the following viruses were used: Influenza A/Puerto Rico/8/34 (PR8) viral strain (H1N1, generous gift of Dr. W. Gerhard, Wistar Institute, Philadelphia, PA), the X31 recombinant strain of A/Aichi/2/68 and A/Puerto Rico/8/34 (H3N2, kind gift from Dr. R. G. Webster, St Jude Children’s Research Hospital, Memphis, TN) and the A/Equine/London/1416/73 virus (H7N7 was a kind gift from Dr. Y. Kawaoka, University of Wisconsin). At the time of IN infection the mice were anesthetized with Avertin (2-2-2 Tribromoethanol, 0.025 mg/g of body weight, injected IP), as indicated by Drexel University IACUC protocol approved for these studies. Twenty microliters of virus at the appropriate virus dilution in sterile saline were used for IN infections. For vaccinations H1N1 PR8 virus was administered subcutaneously (SQ) in the rump of the mouse in 100 μl of biopolymer or sterile normal saline. Viral stocks were quantitated as tissue culture infectious doses (TCID50) units using a hemagglutinaton assay of virus-infected MDCK cell supernatants, as previously described [26]. Lethal and sublethal doses were established for each viral stock by IN infection of C57Bl/6 mice.
2.2 Quantitation of the virus-specific immune response
For quantitation of primary and secondary immune responses, mouse lungs were digested at 37°C for 90 min in 3 mg/ml Collagenase A (Roche Molecular Biochemicals, Indianapolis, IN) and 40U/ml DNAse (Sigma, St Louis, Mo) and passed through a 100μm nylon mesh and then washed with 5% fetal bovine serum supplemented RPMI. Lung lymphocytes were separated by density centrifugation (500×g, 30 min., room temperature) using Lympholyte-M (Cedarlane, Hornby, Ontario, Canada). Single cell suspensions (1×106 cells) were stained for 30 minutes on ice with fluorochrome-bound monoclonal antibodies: anti-CD8-PerCP (eBioscience, San Diego, CA), anti-CD4-PE (eBioscience) and APC-labeled MHC class I tetramers complexed with NP366–374 peptide. Following washes and fixation in paraformaldehyde, 2×105 cells were analyzed by flow cytometry using a FACS Caliburγ (BD Biosciences) and FlowJo software (Treestar, San Carlos, CA).
2.3 Biopolymer formulation
The biopolymer was obtained by dissolving sodium alginate (LVG, Pronova) at a final dilution of 5.5% (w/v) in a 0.9% (w/v) NaCl solution containing 0.9 mM CaCl2. Gelation and crosslinking was allowed to occur for 1h at 37°C. Live virus stock was added to the reaction mixture at the concentration of 104 TCID50/100μl of gel.
2.4 Viral load quantitation
Influenza virus loads were measured as previously described [27]. Briefly, tissue was frozen in 1 ml of TRIzole (TRI-Reagent, Molecular Research Center, Cincinnati, OH) at −20°C. Tissue was homogenized in TRIzole reagent, on ice, using a polytron blade homogenizer. RNA was extracted using the TRIzole protocol of the Molecular Research Center, followed by cleanup of the RNA with QIAGEN RNeasy Kit (Valencia, CA). cDNA synthesis was performed using both a specific primer (5′TCT AAC CGA GGT CGA AAC GTA 3′) and random hexamers. Real-time PCR assays were performed in triplicate with 5 μl cDNA, 12.5 μl 2X TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 900 nM influenza A virus sense primer (5′AAG ACC AAT CCT GTC ACC TCT GA 3′), 900 nM influenza A virus antisense primer (5′CAA AGC GTC TAC GCT GCA GTC C 3′), and 200 nM influenza A virus probe (FAM-5′TTT GTG TTC ACG CTC ACC GT 3′-TAMRA). All primers were specific for the influenza A virus matrix protein. Amplification and detection were performed using an ABI Prism 7900HT sequence detection system with SDS 2.2.1 software (Applied Biosystems) at the following conditions: 2 min at 50°C and 10 min at 95°C, then 45 cycles of 15 s at 95°C and 1 min at 60°C. Viral loads were calculated as TCID50U/lung based on an influenza viral stock standard curve run in every assay.
2.5 Statistical Analysis
Mann-Whitney U test, ANOVA and Shapiro-Wilk W test for normality were used for statistical analysis with the JMP statistical analysis program (SAS). P values < 0.05 were considered significant.
3. Results
3.1. Subcutaneous immunization using live influenza virus results in potent CD8+ T cell responses
Eight week old C57Bl/6J mice were immunized IP or SQ with 30 TCID50 of the H1N1 PR8 strain of influenza type A virus and heterosubtypically rechallenged IN 45 days later with 0.6 TCID50 H3N2 X31 strain of influenza virus. As a positive control, we infected IN C57Bl/6J mice with a sublethal dose of influenza virus H1N1 PR8 (3 TCID50) and rechallenged them IN 45 days later with 0.6 TCID50 H3N2 X31 influenza virus. Mice were sacrificed on day 7 at the peak of the secondary immune response [20] and the immunodominant influenza virus nuclear protein NP366–374-specific CD8+ T cell response was examined.
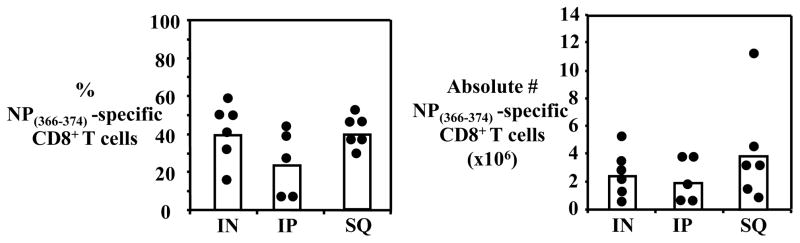
The IP and SQ routes of administration resulted in a strong secondary immune response in the lungs of the rechallenged mice, comparable to mice infected IN, based on the percentages and total numbers of NP366–374-specific CD8+ T cells recovered (Fig. 1). We recovered 3.9±3.7×106 and 1.9±1.6×106 NP366–374-specific CD8+ T cells from the lungs of mice immunized SQ (n=6), or IP (n=5), respectively, and 2.4±1.4×106 virus-specific CD8+ T cells from the lungs of mice infected IN with H1N1 PR8 influenza virus (n=6) (Fig. 1).
Figure 1. Virus-specific CD8+ T cell responses to live influenza virus delivered IN, SQ or IP.
Secondary virus-specific CD8+ T cell response in C57Bl/6J mice primed with 30 TCID50 of H1N1 PR8 influenza virus by different infection routes: IN, SQ or IP. Lungs were harvested 7 days after IN rechallenge with H3N2 X31 influenza virus and virus-specific CD8+ T cells were measured. Percentage NP366–374-specific CD8+ T cells out of total CD8+ T cells (A) and total numbers of NP366–374-specific CD8+ T cells (B) are shown.
These initial results indicated that live virus injected SQ induces a strong CD8+ T cell immune response in the lungs of rechallenged mice, directed against the immunodominant epitope NP366–374, comparable to the magnitude of an immune response induced by IN infection with virus.
3.2 Immunodeficient Rag−/−γc−/− mice cannot control intranasal infection with influenza virus
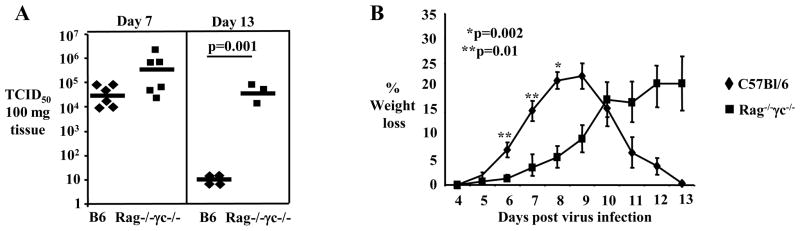
In order to establish a stringent animal model to assess the safety of a vaccine based on administration of live influenza virus, we infected IN wild type C57Bl/6J mice and immunodeficient mice that lack T, B and NK cells (strain C57Bl/10SgSnAiRag−/−γc−/−, henceforth denoted Rag−/−γc−/−) with a sublethal dose (3 TCID50) of influenza virus H1N1 PR8 strain. The Rag−/−γc−/− mice are very sensitive to virus infection since they are unable to mount an NK-mediated or an adaptive immune response to the virus [24, 25]. As expected, IN influenza virus infected Rag−/−γc−/− mice could not control viral replication. Following IN infection, the lung viral load of Rag−/−γc−/− mice was twenty fold increased compared to C57Bl/6 mice at day 7 after infection (824.8 ± 775.9 ×103 TCID50 vs. 39.2 ± 4.9 ×103 TCID50, mean ± SE for Rag−/−γc−/− and C57Bl/6 mice respectively) (Fig. 2A). At day 13 of infection C57Bl/6 mice had very low levels of virus in lungs (7.75 ± 1.5 TCID50) whereas Rag−/−γc−/− mice could not control and clear the virus and had >6,000 fold more virus in lungs (50 ± 37.8 ×103 TCID50) (Fig. 2A). Although both wild type C57Bl/6 and of Rag−/−γc−/− mice manifested weight loss, this was delayed and lower initially in Rag−/−γc−/− mice (Fig. 2B). We attribute this delay in morbidity presented by Rag−/−γc−/− mice to the lack of cells of the adaptive immune response and of NK cells which can secrete pro-inflammatory cytokines that contribute to morbidity [28–30]. However, after day 9 of infection C57Bl/6 mice started recovering and regained weight while Rag−/−γc−/− mice continued to lose weight up to day 13 when they become moribund and were euthanized (Fig. 2B). From the above, it is apparent that immunodeficient Rag−/−γc−/− mice fail to control influenza virus infection and therefore could be used as a sensitive animal model to test the safety of subcutaneous administration of live influenza virus and exclude that this route could lead to active viral infection.
Figure 2. Rag−/−γc−/− mice cannot control intranasal influenza virus infection.
(A) Real Time-PCR was used to measure viral load in lungs harvested from C57Bl/6 and Rag−/−c−/− mice at days 7 and 13 after IN infection with 3 TCID50 of H1N1 PR8 influenza virus; each symbol represents an individual mouse. (B) C57Bl/6 and Rag−/−γc−/− mice (n=3–15 mice per time point) were IN infected with 3TCID50 influenza virus PR8. Body weight was measured at the indicated days after infection and mean percentage weight loss ± SEM, is presented from two independent experiments; statistically significant differences are indicated by asterisk.
3.3. Safety of live virus administered SQ
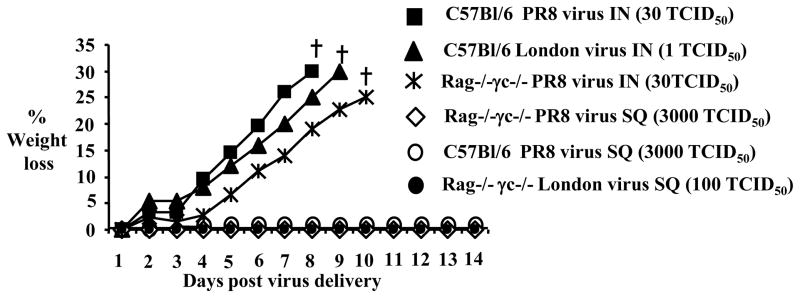
To test whether SQ administration of live virus was safe and did not result in viral infection, we immunized subcutaneously C57Bl/6 and Rag−/−γc−/− mice with a virus dose 1000 times higher (3000 TCID50) compared to the virus dose used for intranasal infection (3 TCID50). 3000 TCID50 of live H1N1 PR8 virus administered SQ failed to induce disease as manifested by weight loss in Rag−/−γc−/−mice or in C57Bl/6 mice (Fig. 3A). The lack of disease symptoms in Rag−/−γc−/− mice after SQ injection of live virus was not due to a lack of T cells, B cells or NK cells, since Rag−/−γc−/− mice can develop severe morbidity and loss of 30% of the initial body weight which required euthanasia, when infected IN with lethal (30 TCID50) doses (Fig. 3) or sublethal (3 TCID50) doses (Fig. 2B) of H1N1 PR8 influenza virus.
Figure 3. Subcutaneous injection of live H1N1 PR8 or highly virulent H7N7 London Influenza virus strains is safe.
(A) C57Bl/6J (filled squares) mice and immunodeficient Rag−/−γc−/−mice (stars) were infected IN with 30 TCID50 of PR8 influenza virus; C57Bl/6J mice were infected IN with 1 TCID50 of influenza virus strain London (H7N7) (filled triangles). Weight loss was monitored over the next 14 days after infection. Mice that lost more than 30% of their initial body weight were removed from the experiment (marked with a cross symbol). C57Bl/6 mice (open circles) and Rag−/−γc−/− mice (open diamonds) were injected SQ with live PR8 influenza virus (3000 TCID50) or with London influenza virus (100 TCID50) (filled circles) and weight loss was monitored for the next 14 days after treatment. The mean weight loss per group is shown (n=5 mice per group).
To test whether the safety of the SQ route of administration was not compromised when a more virulent strain of influenza virus was used, we performed experiments in mice with a virulent H7N7 London influenza virus strain [31]. IN infection of C57BL/6J mice with 1 TCID50 of H7N7 influenza virus strain resulted in sustained weight loss and animals had to be euthanized when they reached a 30% loss of their initial body weight (Fig. 3). However, 100 TCID50 (100 times higher than the dose used IN) of H7N7 influenza virus strain administered SQ, induced no weight loss in Rag−/−γc−/− mice (Fig. 3).
To exclude that viral replication occurs with SQ administration of live virus we examined whether virus can be detected in different tissues. As influenza virus H7N7 London strain has been described to disseminate to different organs during infection [31, 32], we examined whether this strain of virus could have replicated in different tissues of Rag−/−γc−/− mice after SQ injection, such as lungs, brain, liver, spleen and kidney, without causing overt disease. As positive control, we infected IN C57Bl/6J mice with a sublethal dose (0.02 TCID50) of influenza virus H7N7 London strain. Both Rag−/−γc−/− mice and wild type C57Bl/6J mice were sacrificed 7 days after virus administration. Viral load was quantitated by real time PCR (RT-PCR) in the lungs, brains, livers, spleens and kidneys of Rag−/−γc−/− mice and in the lungs of C57Bl/6J mice (Table 1). The amount of influenza virus RNA in the organs of SQ injected Rag−/−γc−/− mice was below the limit of detection the RT-PCR assay.
Table 1.
SQ injection of live virus is safe and does not lead to spread of the virus to different organs
| Strain | Injection/infection | Tissue Viral load/100mg tissue |
|---|---|---|
| C57Bl/6 | IN 0.02 TCID50 London | Lung: 14.5±12.8 ×103 TCID50 |
| Rag−/−γc−/− | SQ injected 100 TCID50 London |
Lung: below detection limit* |
| SQ injected 100 TCID50 London |
Brain: below detection limit* | |
| SQ injected 100 TCID50 London |
Liver: below detection limit* | |
| SQ injected 100 TCID50 London |
Spleen: below detection limit* | |
| SQ injected 100 TCID50 London |
Kidney: below detection limit* |
RT-PCR detection limit: 4×10−4 TCID50
In conclusion, SQ injection of live influenza virus does not result in productive viral infection and disease even when immunodeficient Rag−/−γc−/− mice, which we have shown above to be unable to control intranasal influenza virus infection, are infected with a strain of influenza virus known to be very virulent in mice. The above indicate that SQ injection is a safe route of immunization with live influenza virus.
3.4. Live virus encapsulated in polymer induces an efficient immune response
While subcutaneous administration of the live virus has proven to be safe in our studies, the prevention of aerosol production would add an extra level of safety to a live virus vaccine. For this purpose we used alginate based biopolymer to encapsulate the live infectious virus, thus preventing aerosol formation. This biopolymer, when cross-linked to a multivalent cation, has the feature of remaining intact without any phase separation or liquid release after extrusion through the needle of a syringe, thus preventing aerosol formation (Fig. 4). To test whether the immunogenicity is preserved with encapsulation, we encapsulated live influenza virus H1N1 PR8 strain in alginate biopolymer and immunized mice. Mice were injected SQ with 104 TCID50 of virus encapsulated in 100 μl biopolymer. Control mice were injected SQ with unencapsulated 104 TCID50 live influenza virus in 100 μl sterile normal saline or with alginate alone. Forty-five days after vaccination, mice were heterosubtipically challenged IN with H3N2 X31 influenza virus strain to examine the magnitude of the secondary immune response following priming with the vaccine. The frequency and numbers of immunodominant influenza virus NP366–374-specific CD8+ T cells infiltrating the lungs of infected mice were determined at the peak of the secondary response on day 7 by flow cytometry using H-2Db MHC class I tetramers loaded with the NP366–374 peptide. As additional controls we included a group of unvaccinated animals that were infected IN with H3N2 X31 influenza virus and were analyzed at day 7 and day 10 of the primary immune response.
Figure 4. Biopolymer releases no fluid when extruded through a 26G needle.
The alginate based biopolymer used to encapsulate live influenza virus for SQ immunizations of mice can be easily extruded from a syringe with a 26G needle, without phase separation. When extruded through the needle of a syringe, either as a stack (left) or in line (middle), there is no release of liquid from the biopolymer. For comparison, a drop of PBS is shown (right).
SQ vaccination with live H1N1 PR8 virus encapsulated in alginate biopolymer generated a vigorous secondary immune response when mice were challenged with H3N2 X31 influenza virus strain (Fig. 5A and B). On day 7 post challenge the frequency of lung NP366–374-specific CD8+ T cells was 26 ± 2.7% of CD8+ T cells (mean ± SEM; n=13) and the total number of lung NP366–374-specific CD8+ T cells was 1.07 ± 0.23 ×106 (mean ± SEM; n=13) in mice that received the polymer encapsulated vaccine (Fig. 5B). These frequencies and total numbers of NP366–374-specific CD8+ T cells in mice immunized with virus encapsulated in biopolymer were significantly higher than in mice injected with alginate alone or in non vaccinated mice IN infected with H3N2 X31 influenza virus. On day 7 post H3N2 X31 influenza virus infection, mice injected with alginate alone had frequencies and absolute numbers of NP366–374-specific CD8+ T cell of 1.4±0.07% and 0.05±0.02×106, respectively (mean ± SEM, n=7) and this was comparable to non vaccinated mice which had frequencies and absolute numbers of lung NP366–374-specific CD8+ T cell responses that were 2 ± 0.6% and 0.05 ± 0.01 ×106 (mean ± SEM, n=6) (Fig. 5B). The day 7 secondary response in vaccinated animals was higher than the peak of the primary response (day 10) to H3N2 X31 influenza virus in non vaccinated animals which had a frequency of 9 ± 0.8% and a total number of 0.5 ± 0.08 ×106 lung NP366–374-specific CD8+ T cells (mean ± SEM; n=20). In the control group of mice injected SQ with H1N1 PR8 influenza virus in normal saline and challenged IN with H3N2 X31 influenza virus strain, the frequency and total number of day 7 lung NP366–374-specific CD8+ T cells were 45.3 ± 4.1% and 5.3 ± 1.8 ×106 (mean ± SEM; n=3), respectively. The results presented above show that biopolymer encapsulation of live influenza virus delivered SQ can efficiently prime a CD8+ T cell mediated immune response in mice and the magnitude of the secondary immune response 7 days after rechallenge is 10 times higher compared to the day 7 primary immune response after IN virus infection. Alginate injected without virus did not have a non-specific immunostimulatory effect since in this experimental group the number of NP366–374-specific CD8+ T cells was equal to the one in the negative control group (unimmunized mice). Although vaccination with biopolymer encapsulated live virus resulted in a 5 fold lower secondary CD8+ T cell response compared to unencapsulated live virus, the encapsulated vaccine still preserved a potent immunostimulatory capacity.
Figure 5. A single SQ dose of biopolymer encapsulated live virus induces a potent secondary NP366–374 specific CD8+ T cell immune response.
(A) Representative FACS plots showing the frequency of lung NP366–374-specific CD8+ T cell response in mice and (B) the percentages (left panel) and the total numbers of lung NP366–374-specific CD8+ T cells (right panel), under different conditions of priming and IN challenging with heterosubtypic virus, are shown. C57Bl/6J mice were injected SQ with either 100 μl biopolymer encapsulated live influenza virus H1N1 PR8 (104 TCID50) (n=12; closed squares), 100 μl alginate biopolymer alone (n=7, closed diamonds), or with 100 μl sterile saline containing 104 TCID50 H1N1 PR8 virus (n=3; triangles). 45 days after vaccination, these mice were challenged IN with influenza virus strain H3N2 X31 (0.6 TCID50). The lung NP366–374-specific CD8+ T cell response was measured 7 days after rechallenge, at the peak of the secondary immune response. Controls include naïve mice infected IN with X31 virus for 7 days (n=6; open diamonds) or 10 days (n=20; circles). Statistically significant differences are marked by horizontal lines and p values.
3.5. Live virus encapsulated in alginate biopolymer protects against lethal heterosubtypic viral challenge
In order to determine whether the CD8+ T cell response induced by vaccination with live influenza virus encapsulated in alginate biopolymer can protect mice against a heterosubtypic infection with a lethal dose of virus, we SQ vaccinated mice with 104 TCID50 of H1N1 PR8 influenza virus encapsulated in alginate biopolymer and 45 days later, challenged mice IN with a lethal dose of the virulent in mice H7N7 London influenza virus strain [31]. Unimmunized mice and mice injected SQ with 104 TCID50 H1N1 PR8 influenza virus in normal saline served as controls. Following challenge with H7N7 London influenza virus strain, unimmunized mice suffered sustained weight loss and had to be euthanized when they reached 30% weight loss (Fig. 6). Mice vaccinated SQ with virus encapsulated in alginate biopolymer however only lost about 15% of body weight by days 8–9 post infection and then recovered. Control mice injected SQ with virus in saline lost about 10% of their initial body weight by day 6 of infection and also recovered. The above indicate that vaccination with encapsulated virus confers protection against infection with a lethal dose of a heterosubtypic strain of influenza virus. This protection is most likely to be at least in part due to cellular CD4+ and CD8+ T cell immune responses [33]. Cross-reactive NP366–374-specific CD8+ T cells are expected to play an important role in such heterosubtypic immunity [8, 11, 34–36]. The NP366–374 epitope expressed in H7N7 London virus differs by only one amino acid from the NP366–374 epitope expressed by H1N1 PR8 influenza virus (ASNENMETI vs. ASNENMETM) and crossreactive NP366–374-specific CD8+ T cell are induced with H7N7 London challenge of H1N1 PR8 virus primed mice (data not shown). Similar crossreactive CD8+ T cells expansions have been shown in mice primed with influenza A/H3N2 and challenged with A/H5N1 viruses [35]. Thus at least in part the protection provided by the H1N1 PR8 vaccination against the H7N7 London is due to the induction of large numbers of crossreactive CD8+ T cells which we demonstrated above to occur after vaccination.
Figure 6. Vaccination with biopolymer encapsulated live virus protects from lethal challenge with a heterosubtypic strain of influenza virus.
C57Bl/6J mice (n=3 mice per group) were injected SQ with biopolymer encapsulated live influenza virus PR8 (squares) or with saline containing live influenza virus PR8 (104 TCID50) (triangles). A control group of C57Bl/6J mice (n=3) were left untreated (diamonds). Forty-five days later, mice were challenged IN with a lethal dose (0.4 TCID50) of influenza virus strain H7N7 London strain and weight loss was monitored for the next 15 days. Mice losing 30% of their initial body weight were euthanized (cross symbol).
4. Discussion
The generation of a CD8+ T cell based universal vaccine against influenza virus would overcome the problems associated with predicting future antigenicity of seasonal and pandemic influenza virus strains. Although CD8+ T cell eliciting universal vaccines may not prevent infection, we anticipate they will significantly alter the course of infection by reducing viral loads and thus ameliorate morbidity and mortality [7, 37]. Previous studies have suggested that live viruses confer longer lasting immunity compared to inactivated viruses [38]. Vaccines containing inactivated viruses do not induce strong CD8+ T cell immune responses required for heterosubtypic immunity [38]. We therefore chose to utilize a live influenza virus to stimulate potent CD8+ T cell responses. To enhance its safety we encapsulated the live virus in an alginate-based biopolymer. Our studies demonstrate that live influenza virus encapsulated in an alginate-based biopolymer can provide safe and effective vaccination of mice against influenza virus infection. Biopolymer encapsulation preserved the capacity of live virus to generate potent CD8+ T cell responses against influenza virus and elicited heterosubtypic immunity which could protect against lethal challenge with a virulent strain.
Although, crossreactive CD8+ T cells are expected to play an important role [34, 35], we cannot exclude that CD4+ T cell immune responses directed against other conserved epitopes also contribute to the protection against heterosubtypic challenge in our vaccinated animals. Our study was performed using C57Bl/6 mice in which CD4+ T cell restricted immunodominant epitopes are not well characterized; however, others have shown that CD4+ T cells do participate to heterosubtypic immunity against influenza virus [10, 33, 39]. Future studies need to address the contribution of CD4+ T cells to the protection conferred by our vaccination with live virus in biopolymer.
Heterosubtypic immunity is primarily mediated by T cells directed against conserved epitopes of influenza virus [7, 40]. The role of antibodies is less well understood with some studies suggesting that antibodies are not required for viral clearance of heterosubtypic challenge [33] while others have suggested that heterosubtypic immunity can occur in the absence of functional T cells and can be mediated by crossreacting antibodies [41–46]. Indeed, administration of high doses of inactivated virus, especially in the presence of an adjuvant, may confer heterosubtypic protection in animal models by the induction of cross reacting antibodies [46–49]. How broad a spectrum of protection antibodies can yield is unclear. In our studies vaccination with H1N1 PR8 live influenza virus encapsulated in alginate induced neutralizing antibodies to H1N1 PR8 (data not shown). These neutralizing antibodies are clearly not crossreactive as H3N2 and H7N7 infections were not prevented in vaccinated animals. To what extent or if non-neutralizing crossreactive antibodies are elicited and contribute to heterosubtypic protection provided by live virus vaccination is unknown. Such antibodies if elicited would synergize with the potent CD8+ T cell response we induce and facilitate viral clearance.
The recent swine flu pandemic demonstrates that even though this virus expresses hemagglutinin and neuraminidase subtype 1 (H1N1), it maintains the capability to generate new epitope regions that have not been encountered before by the human population [50]. These new epitopes are sufficiently different from the ones expressed by H1N1 viruses used in vaccines [51]; therefore, the protection conferred by seasonal flu vaccination against swine flu infection may not be efficient. In fact, it was found that only 31% of the B cells epitopes of currently circulating H1N1 human influenza viruses are conserved in swine flu; however, 69% of CD8+ T cell epitopes are completely invariant [52]. The same study also demonstrated the existence of memory T cell immunity to swine flu in normal individuals and the similarity in magnitude to the memory T cell immunity to seasonal H1N1 2008 influenza. These findings further support the necessity of developing vaccines that target conserved T cell epitopes, which can be efficient not only for reducing the effects of seasonal influenza infections but more importantly, of pandemic infections.
To what extent heterosubtypic immunity to different subtypes of influenza virus is present in human population, is a subject of debate. Children never exposed to influenza virus were 3 fold more likely to become infected with the pandemic strain H2N2, compared to adults that were previously exposed to H1N1 influenza virus [12], clearly demonstrating that previous exposures to an influenza virus strain shaped the immune response to a totally new strain of virus. Human subjects previously exposed to seasonal influenza virus have memory CD4+ and CD8+ T cells that cross-react with epitopes derived mainly from the conserved molecules NP and M1 of H5N1 strains [40]. While there is now enough evidence that cross-protection against different subtypes of influenza virus exists and functions in the human population, it has been argued that such memory T cells that mediate heterosubtypic immunity may not be long lived. Therefore vaccines that stimulate and/or boost a long lived memory CD8+ T cell pool to conserved viral epitopes may function as universal vaccines to influenza virus that induce new or boost pre-existing heterosubtypic immunity.
No evidence exists to suggest that influenza virus delivered intradermally or subcutaneously can disseminate and cause systemic disease. Large doses of influenza virus injected IP to prime immune responses in animals have been extensively used and caused no disease or infection [20–22]. Such doses can be 100–1000 fold higher than those that result in infection when virus is delivered IN [20–22]. We previously have shown that even doses up to 3×106 TCID50 H1N1 PR8 influenza virus administered IP into mice does not result in viral replication, inflammation or weight loss in mice [23]. In the current manuscript we show that SQ delivery of even the virulent H7N7 London live virus was safe, as injection of Rag−/−γc−/− mice, which have no T cells, B cells or NK cells and are very sensitive to influenza virus infection, did not infect these animals.
Biocompatible gels have been studied and used extensively for drug delivery, cytokine delivery [53], gene therapy [54] and tissue engineering [55]. We chose alginate as the hydrogel biopolymer to encapsulate the influenza virus. Alginate is a naturally occurring linear polysaccharide extracted from brown seaweed. It is composed of 1–4 linked α-L-guluronic (G) and β-D-mannuronic (M) acid residues. Different sources of alginate have different guluronic acid content, and this in turn affects their properties. Alginate can form hydrogels by reaction with divalent cations such as Ca2+, Ba2+ Sr2+ and more, but with the exception of Mg2+. Trivalent cations such as Al3+ and Fe3+ have also been used. The counter ion affects the gel strength and rigidity, depending on the fit between the ionic radius of the cation and the architecture of the polysaccharide strands. The usual method of preparation of these hydrogels simply involves dropping a sodium alginate solution into a solution that provides the crosslinking cations, and can be performed under very mild aqueous conditions and with nontoxic reactants. This makes it a very attractive choice as a matrix for the encapsulation of biologicals, including drug-containing liposomes and cells. Liposomes encapsulated in alginate have been studied for protein delivery [56–58] and several different cell lines including pancreatic islets [59] and genetically engineered fibroblasts [60, 61] have been encapsulated in alginate for therapeutic applications. In recent years, alginate has been investigated for use as a scaffold in tissue engineering [62]. Alginate hydrogels with covalently coupled peptides have been studied as synthetic extracellular materials [63, 64] and as a tissue bulking agent [65]. Our studies have demonstrated that live virus encapsulated in alginate biopolymer preserves its immunogenicity and stimulates potent CD8+ T cell responses. We did note that encapsulation of live virus resulted in a 5-fold lower CD8+ T cell response compared to the live virus in PBS when both were administered SQ. However the CD8+ T cells response was still 20-fold higher than that of unvaccinated animals. Boosting would be expected to enhance further the efficacy of the biopolymer vaccine. Clearly the trade-off between reduced immunogenicity and the extra safety of biopolymer encapsulation is acceptable as the vaccine still elicits a potent CD8+ T cell response. The extensive use and FDA approval of alginate gels make them ideal polymers for our vaccine strategies.
Assessing the safety of the biopolymer encapsulated live virus as a vaccine approach clearly needs further studies before advancing to human studies. Using live virus as a vaccine entails the danger that aerosols of live influenza virus, if released, would lead to infections. Our study here provides a first proof-of-concept report that encapsulation preserves immunogenicity and is safe even in immunocompromised mice. Our studies with Rag−/−γc−/− mice show that for productive influenza virus infection to occur, the exposure has to be via the airways of the host while intradermal or subcutaneous administration of live virus do not cause disease even in mice lacking an NK cell, T cell and B cell responses. Although biopolymer encapsulation clearly reduces the danger of aerosolization of virus, further studies are needed to examine this carefully. Such studies would need to address how well encapsulation of live influenza virus prevents aerosolization and under what conditions. It is also unclear whether intranasal application of encapsulated live virus can result in infection something important to address accidental or intentional intranasal exposure. Future studies are clearly needed to address these important issues.
If a pandemic strain were to emerge, it would take an estimated 6 months to develop a vaccine against a new strain [1] as current vaccine strategies will require 6 or more months to generate one billion monovalent doses. This would be enough for the immunization of 500 million individuals with the two doses of killed vaccine that are expected to be required for protection against some pandemic strains [2, 3]. A pandemic, however, would spread worldwide within one month of outbreak. We have explored a radical novel vaccine strategy that utilizes live influenza virus which is encapsulated in biopolymer gels and can be delivered ID or SQ. Gel encapsulation together with the ID or SQ route of delivery makes the vaccine safe. Currently the 3×108 doses of influenza vaccine produced worldwide are generated from approximately 3×108 chicken eggs, thus a single egg generates enough material for one dose [3]. Our studies show that 104 TCID50 of polymer encapsulated live virus can protect mice and lower doses may also be efficient. This would suggest that a dose of ~106–107 TCID50 (based on weight difference of mice and humans, 20gr and 70kgr respectively) would be sufficient to immunize humans. Since each egg yields ~108 TCID50 this would suggest that a single egg could yield enough doses to immunize 10–100 individuals using the biopolymer encapsulated vaccine. Thus this approach could greatly reduce the amount of material and time needed to generate sufficient vaccine doses to rapidly immunize large parts of the population.
Here we propose a novel delivery strategy of live influenza vaccine that is safe, requires very low doses of virus, and therefore may be produced faster than the current vaccine. Safety is ensured by encapsulation of the vaccine in a polymer gel that releases the virus once in the body. Delivery at subcutaneous or intradermal sites requires reduced amounts of virus and avoids disease symptoms and pathogenicity associated with influenza virus infection, when the virus uses the natural routes of entry and infection. In our study encapsulation of virus in alginate preserved the antigenicity of the vaccine and allowed generation of robust CD8+ T cell responses. This vaccine strategy elicits strong CD8+ T cell mediated responses and can protect mice from a lethal challenge against a heterosubtypic strain. Therefore the biopolymer encapsulated vaccine may serve as a universal vaccine that confers broad protection against antigenically diverse influenza virus strains.
Acknowledgments
This work has been supported by RO1 grants AI66215 and AI46719 to PDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaiser J. Influenza: girding for disaster. Facing down pandemic flu, the world’s defenses are weak. Science. 2004 Oct 15;306(5695):394–7. doi: 10.1126/science.306.5695.394. [DOI] [PubMed] [Google Scholar]
- 2.Lipatov AS, Govorkova EA, Webby RJ, Ozaki H, Peiris M, Guan Y, et al. Influenza: emergence and control. J Virol. 2004 Sep;78(17):8951–9. doi: 10.1128/JVI.78.17.8951-8959.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterholm MT. Preparing for the next pandemic. N Engl J Med. 2005 May 5;352(18):1839–42. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- 4.Palese P. Making better influenza virus vaccines? Emerg Infect Dis. 2006 Jan;12(1):61–5. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill E, Krauss SL, Riberdy JM, Webster RG, Woodland DL. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J Gen Virol. 2000 Nov;81(Pt 11):2689–96. doi: 10.1099/0022-1317-81-11-2689. [DOI] [PubMed] [Google Scholar]
- 6.Seo SH, Peiris M, Webster RG. Protective cross-reactive cellular immunity to lethal A/Goose/Guangdong/1/96-like H5N1 influenza virus is correlated with the proportion of pulmonary CD8(+) T cells expressing gamma interferon. J Virol. 2002 May;76(10):4886–90. doi: 10.1128/JVI.76.10.4886-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006 Jan;12(1):48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bui HH, Peters B, Assarsson E, Mbawuike I, Sette A. Ab and T cell epitopes of influenza A virus, knowledge and opportunities. Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):246–51. doi: 10.1073/pnas.0609330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianfrani C, Oseroff C, Sidney J, Chesnut RW, Sette A. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum Immunol. 2000 May;61(5):438–52. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 10.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007 Jan 15;178(2):1030–8. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 11.Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008 Jun;82(11):5161–6. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis. 2006 Jan 1;193(1):49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 13.Slepushkin AN. The effect of a previous attack of A1 influenza on susceptibility to A2 virus during the 1957 outbreak. Bull World Health Organ. 1959;20(2–3):297–301. [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005 Nov 16;23(46–47):5404–10. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 15.Epstein SL, Stack A, Misplon JA, Lo CY, Mostowski H, Bennink J, et al. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8(+) cytotoxic T lymphocytes: either CD4(+) or CD8(+) T cells can promote survival and recovery after challenge. Int Immunol. 2000 Jan;12(1):91–101. doi: 10.1093/intimm/12.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 17.Webster RG, Askonas BA. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 18.Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999 Jul;73(7):5903–11. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swayne DE, Slemons RD. Comparative pathology of a chicken-origin and two duck-origin influenza virus isolates in chickens: the effect of route of inoculation. Vet Pathol. 1994 Mar;31(2):237–45. doi: 10.1177/030098589403100211. [DOI] [PubMed] [Google Scholar]
- 20.Belz GT, Xie W, Altman JD, Doherty PC. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J Virol. 2000;74(8):3486–93. doi: 10.1128/jvi.74.8.3486-3493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683–91. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 22.Turner SJ, Cross R, Xie W, Doherty PC. Concurrent naive and memory CD8(+) T cell responses to an influenza A virus. J Immunol. 2001 Sep 1;167(5):2753–8. doi: 10.4049/jimmunol.167.5.2753. [DOI] [PubMed] [Google Scholar]
- 23.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009 Jun 1;182(11):6697–708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005 Feb 1;174(3):1213–21. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 25.Yates F, Malassis-Seris M, Stockholm D, Bouneaud C, Larousserie F, Noguiez-Hellin P, et al. Gene therapy of RAG-2−/− mice: sustained correction of the immunodeficiency. Blood. 2002 Dec 1;100(12):3942–9. doi: 10.1182/blood-2002-03-0782. [DOI] [PubMed] [Google Scholar]
- 26.Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998 Sep;74(1):57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 27.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007 Nov 15;179(10):6494–503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 28.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006 Oct 5;443(7111):578–81. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007 Jan 18;445(7125):319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 30.Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004 Oct 7;431(7009):703–7. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 31.Kawaoka Y. Equine H7N7 influenza A viruses are highly pathogenic in mice without adaptation: potential use as an animal model. J Virol. 1991 Jul;65(7):3891–4. doi: 10.1128/jvi.65.7.3891-3894.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinya K, Suto A, Kawakami M, Sakamoto H, Umemura T, Kawaoka Y, et al. Neurovirulence of H7N7 influenza A virus: brain stem encephalitis accompanied with aspiration pneumonia in mice. Arch Virol. 2005 Aug;150(8):1653–60. doi: 10.1007/s00705-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 33.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001 Jun 15;166(12):7437–45. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 34.Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007 Jan 8;25(4):612–20. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Kreijtz JH, Bodewes R, van den Brand JM, de Mutsert G, Baas C, van Amerongen G, et al. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine. 2009 Aug 6;27(36):4983–9. doi: 10.1016/j.vaccine.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 36.Sette A, Peters B. Immune epitope mapping in the post-genomic era: lessons for vaccine development. Curr Opin Immunol. 2007 Feb;19(1):106–10. doi: 10.1016/j.coi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006 Oct;12(10):1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark A, Potter CW, Jennings R, Nicholl JP, Langrick AF, Schild GC, et al. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunity. J Hyg (Lond) 1983 Jun;90(3):361–70. doi: 10.1017/s0022172400028990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T Cells Direct Protective Responses to Influenza Virus in the Lungs through Helper-Independent Mechanisms. J Virol. 2010 Sep;84(18):9217–26. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008 Oct;118(10):3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008 Sep 15;181(6):4168–76. doi: 10.4049/jimmunol.181.6.4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein SL, Lo CY, Misplon JA, Lawson CM, Hendrickson BA, Max EE, et al. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, beta2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997 Feb 1;158(3):1222–30. [PubMed] [Google Scholar]
- 43.Nguyen HH, Moldoveanu Z, Novak MJ, van Ginkel FW, Ban E, Kiyono H, et al. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999 Feb 1;254(1):50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen HH, van Ginkel FW, Vu HL, McGhee JR, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 2001 Feb 1;183(3):368–76. doi: 10.1086/318084. [DOI] [PubMed] [Google Scholar]
- 45.Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, et al. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol. 2008 Jan 1;180(1):454–63. doi: 10.4049/jimmunol.180.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001 Jun;75(11):5141–50. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008 Feb;82(3):1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song SK, Moldoveanu Z, Nguyen HH, Kim EH, Choi KY, Kim JB, et al. Intranasal immunization with influenza virus and Korean mistletoe lectin C (KML-C) induces heterosubtypic immunity in mice. Vaccine. 2007 Aug 21;25(34):6359–66. doi: 10.1016/j.vaccine.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 49.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine. 2003 Jul 4;21(23):3212–8. doi: 10.1016/s0264-410x(03)00234-2. [DOI] [PubMed] [Google Scholar]
- 50.Maurer-Stroh S, Ma J, Lee RT, Sirota FL, Eisenhaber F. Mapping the sequence mutations of the 2009 H1N1 influenza A virus neuraminidase relative to drug and antibody binding sites. Biol Direct. 2009;4:18. doi: 10.1186/1745-6150-4-18. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deem MW, Pan K. The epitope regions of H1-subtype influenza A, with application to vaccine efficacy. Protein Eng Des Sel. 2009 Sep;22(9):543–6. doi: 10.1093/protein/gzp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenbaum JA, Kotturi MF, Kim Y, Oseroff C, Vaughan K, Salimi N, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20365–70. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Sakaguchi T, Kanda T, Hitomi J, Tabata Y, Hatakeyama K. Delivery of interleukin-12 in gelatin hydrogels effectively suppresses development of transplanted colonal carcinoma in mice. Cancer Chemother Pharmacol. 2003 Jan;51(1):53–7. doi: 10.1007/s00280-002-0547-y. [DOI] [PubMed] [Google Scholar]
- 54.Schek RM, Hollister SJ, Krebsbach PH. Delivery and Protection of Adenoviruses Using Biocompatible Hydrogels for Localized Gene Therapy. Molecular Therapy. 2004;9(1):130–8. doi: 10.1016/j.ymthe.2003.10.002. 2004/1. [DOI] [PubMed] [Google Scholar]
- 55.Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliv Rev. 2004 Sep 22;56(11):1635–47. doi: 10.1016/j.addr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Dhoot NO, Wheatley MA. Microencapsulated liposomes in controlled drug delivery: Strategies to modulate drug release and eliminate the burst effect. Journal of Pharmaceutical Sciences. 2003;92(3):679–89. doi: 10.1002/jps.19104. [DOI] [PubMed] [Google Scholar]
- 57.Wheatley MA, Chang M, Park E, Langer R. Coated alginate microspheres: factors influencing the controlled delivery of macromolecules. Journal of Applied Polymer Science. 1991;43(11):2123–35. [Google Scholar]
- 58.Wheatley MA, Langer RS, Eisen HN, inventors. Massachusetts Institute of Technology, USA, assignee. System for delayed and pulsed release of biologically active substances. 88–161198 4900556. Application: US US patent. 1990:19880223.
- 59.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science (Washington, DC, United States) 1980;210(4472):908–10. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 60.Cheng WTK, Chen B-C, Chiou S-T, Chen C-M. Use of nonautologous microencapsulated fibroblasts in growth hormone gene therapy to improve growth of midget swine. Human Gene Therapy. 1998;9(14):1995–2003. doi: 10.1089/hum.1998.9.14-1995. [DOI] [PubMed] [Google Scholar]
- 61.Tobias CA, Dhoot NO, Wheatley MA, Tessler A, Murray M, Fischer I. Grafting of encapsulated BDNF-producing fibroblasts into the injured spinal cord without immune suppression in adult rats. Journal of neurotrauma. 2001;18(3):287–301. doi: 10.1089/08977150151070937. [DOI] [PubMed] [Google Scholar]
- 62.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6):511–21. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 63.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. Journal of Biomedical Materials Research. 2000;50(3):405–9. doi: 10.1002/(sici)1097-4636(20000605)50:3<405::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 65.Loebsack A, Greene K, Wyatt S, Culberson C, Austin C, Beiler R, et al. In vivo characterization of a porous hydrogel material for use as a tissue bulking agent. Journal of Biomedical Materials Research. 2001;57(4):575–81. doi: 10.1002/1097-4636(20011215)57:4<575::aid-jbm1204>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]