Abstract
Diabetes mellitus and hypertension commonly coexist, but the nature of this link is not well understood. The authors tested whether diabetes and higher concentrations of fasting serum glucose and insulin are associated with increased risk of developing incident hypertension in the community-based Multi-Ethnic Study of Atherosclerosis. At baseline, 3,513 participants were free of hypertension, defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications to treat high blood pressure. Of these, 965 participants (27%) developed incident hypertension over 4.7 years’ median follow-up between 2002 and 2007. Compared with participants with normal baseline fasting glucose, those with impaired fasting glucose and diabetes had adjusted relative risks of hypertension of 1.16 (95% confidence interval (CI): 0.96, 1.40) and 1.41 (95% CI: 1.17, 1.71), respectively (P = 0.0015). The adjusted relative risk of incident hypertension was 1.08 (95% CI: 1.04, 1.13) for each mmol/L higher glucose (P < 0.0001) and 1.15 (95% CI: 1.05, 1.25) for each doubling of insulin (P = 0.0016). Further adjustment for serum cystatin C, urinary albumin/creatinine ratio, and arterial elasticity measured by tonometry substantially reduced the magnitudes of these associations. In conclusion, diabetes and higher concentrations of glucose and insulin may contribute to the development of hypertension, in part through kidney disease and arterial stiffness.
Keywords: diabetes mellitus, glucose, hypertension, insulin, kidney, nephrology
Diabetes mellitus and hypertension are common, morbid health conditions that frequently coexist (1, 2). Diabetes and hypertension may be linked by underlying obesity, which is a well-established cause of both insulin resistance and elevated blood pressure (3). In addition, cohort studies and clinical trials suggest that higher levels of glucose and insulin may contribute to the development of hypertension independent of obesity and other established hypertension risk factors (4–13). However, the relations of glucose and insulin across their measured ranges with incident hypertension have not been reported in a multiethnic community-based population, and pathways mediating these potential relations are not well understood.
Higher levels of glucose and insulin may contribute to the pathogenesis of hypertension by promoting kidney disease and vascular stiffness. Hyperglycemia can damage both the kidney and the arterial wall through deposition of advanced glycation end products, generation of reactive oxygen species, and activation of protein kinase C (14, 15). Hyperinsulinemia stimulates the sympathetic nervous system and the renin-angiotensin-aldosterone system (RAAS), which can cause kidney and vascular damage through both hemodynamic and nonhemodynamic pathways (16–18). In turn, kidney disease may lead to hypertension through impaired sodium excretion and resultant volume expansion or through further activation of the RAAS, while impaired arterial elasticity leads to hypertension by increasing systemic vascular resistance (19, 20).
We tested whether diabetes and higher fasting serum concentrations of glucose and insulin are associated with increased risk of incident hypertension in the Multiethnic Study of Atherosclerosis, a diverse community-based population of adults free of clinical cardiovascular disease at baseline. In addition, we explored whether subtle kidney dysfunction (elevated levels of serum cystatin C and the urinary albumin/creatinine ratio) and impaired arterial elasticity (measured by tonometry) may mediate this link.
MATERIALS AND METHODS
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a community-based cohort study designed to assess subclinical cardiovascular disease (21). The MESA cohort comprised 6,814 adults who were recruited between 2000 and 2002 from 1 of 6 US regions: Forsyth County, North Carolina; northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California. Participants ranged between 45 and 84 years of age and were free of clinical cardiovascular disease at baseline. The diverse cohort is 38% Caucasian, 28% African American, 22% Hispanic, and 12% Chinese. MESA included 3 follow-up examinations spaced approximately 18 months apart, between 2002 and 2007, along with periodic telephone contacts every 6–12 months. All MESA procedures were approved by the institutional review board of each clinical site, and all participants granted informed consent.
For our analyses of incident hypertension (n = 3,513), we excluded individuals whose glucose status was not defined (n = 24), who reported use of oral corticosteroids (n = 105), or who ate or drank in the 8 hours prior to the baseline examination (n = 5). We further excluded participants who had prevalent hypertension at baseline (n = 2,982) or did not attend any follow-up examination (n = 185).
Glucose, insulin, and diabetes
Fasting (>8 hours) serum glucose and insulin concentrations were measured and used to define glucose status at the baseline MESA examination. Serum glucose was measured by using the Vitros 950 analyzer (Johnson & Johnson Ortho-Clinical Diagnostics, Rochester, New York). Serum insulin was measured by using the Linco Human Insulin Specific Radioimmunoassay kit (Linco Research, Inc., St. Charles, Missouri). We defined glucose status as normal (fasting glucose, <5.6 mmol/L (100 mg/dL)), impaired fasting glucose (fasting glucose, 5.6–6.9 mmol/L (100–125 mg/dL) without hypoglycemic medications), or diabetes (fasting glucose, ≥7 mmol/L (126 mg/dL) or use of any hypoglycemic medication) (1). The homeostasis model of assessment-insulin resistance (HOMA-IR) score, an estimate of insulin resistance, was calculated as (fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5) (22).
Hypertension
Blood pressure and use of antihypertensive medications were assessed at each MESA examination. MESA investigators conducted 3 resting blood pressure readings 5 minutes apart, after 5 minutes in the seated position, using a Dinamap Pro 100 automated oscillometric sphygmomanometer (GE Medical Systems Information Technologies, Inc., Milwaukee, Wisconsin), with appropriate cuff size. The average of the second and third measurements was used for all analyses. Participants brought all medications to each study visit, and inventories were performed by MESA investigators. Hypertension was defined as diastolic blood pressure of ≥90 mm Hg, systolic blood pressure of ≥140 mm Hg, or use of an antihypertensive medication in combination with a self-report of hypertension (2).
Covariates
All covariates were assessed at the baseline MESA examination. Race/ethnicity, smoking, alcohol use, physical activity, and attained education were assessed by questionnaire (21). Moderate and vigorous physical activity was quantitated from questions assessing household, work-related, and leisure activities (23). Time spent on each activity was multiplied by its standard metabolic equivalent of the task (MET) prior to summing, with physical activity reported in MET-minutes/week (24). The highest level of attained education was categorized as some high school or less, completed high school, or completed college or more. Smoking and alcohol consumption were assessed by dichotomous indicators of current use. Body mass index was calculated as weight (kg)/height (m)2. Waist circumference was measured at the umbilicus by using a steel measuring tape with standard 4-ounce (113.4-g) tension.
Serum cystatin C was measured by using the BN II nephelometer (25). Urine was collected from single voided specimens, with the urinary albumin concentration measured by nephelometry, the urinary creatinine concentration measured by the Jaffe reaction, and the urinary albumin/creatinine ratio (ACR) expressed in units of mg/g. Serum cystatin C and urinary ACR were log transformed for all analyses. Pulse-wave measurements on the radial artery were taken by using the HDI PulseWave CR-2000 Research CardioVascular Profiling Instrument (Hypertension Diagnostics, Inc., Eagan, Minnesota). Small and large artery elasticity indices were computed on the basis of a formula using large artery (capacitive) and small artery (oscillatory) compliances (components of total systemic arterial compliance), respectively, along with other patients’ characteristics (age, heart rate, ejection time, weight, and height) (26).
Statistical analyses
For incident hypertension, the time at risk was calculated from the baseline examination through the examination at which incident hypertension was diagnosed, the fourth MESA examination, or the last examination before loss to follow-up. Poisson regression with robust standard errors and an offset for follow-up time was used to estimate incident hypertension rate ratios across exposure groups (27). Serial models were adjusted for demographic data and known hypertension risk factors. We confined adjustment for baseline blood pressure to sensitivity analyses, because 1) blood pressure is in the causal pathway leading to hypertension, and 2) baseline glucose and blood pressure were correlated, and adjusting analyses of incident hypertension for baseline blood pressure could introduce bias in this setting (28). We used a 2-parameter Wald test to calculate a P value for associations between glucose status and incident hypertension.
Fasting glucose and insulin concentrations were assessed in quartiles and as continuous exposures, and participants with treated diabetes were excluded from these analyses. We reported the estimated hypertension incidence rate ratios associated with a 1-mmol/L (18 mg/dL) higher glucose concentration and a doubling of insulin concentration, because these incremental changes roughly corresponded to a comparison of the 25th and 75th percentiles. Insulin was log transformed because a multiplicative association between insulin and hypertension incidence was expected, because of the highly skewed distribution of insulin levels in a normal adult population. We explored whether associations of glucose concentration with incident hypertension differed by racial/ethnic group or insulin level by testing for multiplicative interactions (using Wald tests).
To assess potential mediators of glucose/insulin–hypertension associations, we added to the fully adjusted model sets of covariates assessing kidney disease (serum cystatin C, urinary ACR), arterial elasticity (small and large arterial elasticity), or both. We conducted sensitivity analyses 1) excluding patients with incident hypertension diagnosed solely by incident use of antihypertensive medications, 2) restricting the study population to participants with systolic blood pressure <130 mm Hg and diastolic blood pressure <85 mm Hg at baseline, 3) adjusting for baseline systolic and diastolic blood pressures, and 4) restricting the study population to nonobese participants (body mass index, <30 kg/m2). For all models, we conducted a complete case analysis to address missing data. All P values are 2 sided, and all analyses were completed by using STATA, version 10.1, software (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics and incident hypertension
For analyses of incident hypertension, 2,982 of 6,680 participants (45%) were excluded for prevalent hypertension, and 185 were excluded because of lack of follow-up. The remaining cohort of 3,513 participants was 49% male with a mean age of 59 years. Within this cohort without baseline hypertension, participants with impaired fasting glucose and diabetes were more likely to be older, male, non-Caucasian, larger, less educated, and less physically active compared with participants with normal fasting glucose (Table 1). Participants with impaired fasting glucose and diabetes also had higher geometric mean urinary ACR and lower mean small artery elasticities. Glucose concentrations tended to be slightly higher in non-Caucasian participants (mean levels of 5.2, 5.0, and 5.2 mmol/L for Chinese Americans, African Americans, and Hispanics, respectively) than Caucasian participants (4.9 mmol/L).
Table 1.
Baseline Characteristics of 3,513 Participants Without Prevalent Hypertension Recruited Between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosisa
| Glucose Status |
|||||||||
| Characteristic | Normal (n = 2,879) |
Impaired Fasting Glucose (n = 376) |
Diabetes (n = 258) |
||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Demographic data and medical history | |||||||||
| Age, years | 58.7 (9.9) | 60.8 (9.6) | 61.6 (9.4) | ||||||
| Gender (male) | 1,340 | 47 | 231 | 61 | 149 | 58 | |||
| Race | |||||||||
| White | 1,348 | 47 | 123 | 33 | 59 | 23 | |||
| Chinese American | 358 | 12 | 65 | 17 | 48 | 19 | |||
| African American | 567 | 20 | 79 | 21 | 60 | 23 | |||
| Hispanic | 606 | 21 | 109 | 29 | 91 | 35 | |||
| Highest level of attained education | |||||||||
| Some high school or less | 375 | 13 | 82 | 22 | 80 | 31 | |||
| Completed high school | 1,121 | 39 | 139 | 37 | 87 | 34 | |||
| Completed college or more | 1,378 | 48 | 153 | 41 | 91 | 35 | |||
| Current alcohol user | 1,767 | 62 | 230 | 62 | 116 | 45 | |||
| Current cigarette user | 416 | 15 | 58 | 16 | 40 | 16 | |||
| Physical activity, MET-minutes/week | 6,263 (6,019) | 5,555 (5,078) | 5,845 (6,191) | ||||||
| Physical examination | |||||||||
| Body mass index, kg/m2 | 27.0 (4.9) | 29.5 (5.5) | 29.8 (5.7) | ||||||
| Waist circumference, cm | 93.9 (13.5) | 101.6 (13.7) | 102.6 (13.9) | ||||||
| Systolic blood pressure, mm Hg | 124.4 (21.0) | 132.0 (21.2) | 133.1 (21.9) | ||||||
| Diastolic blood pressure, mm Hg | 71.4 (10.1) | 74.2 (10.7) | 72.0 (10.3) | ||||||
| Laboratory data | |||||||||
| Glucose, mmol/Lb,c | 4.7 (0.4) | 5.9 (0.4) | 10.5 (4.0) | ||||||
| Insulin, pmol/Lb,c | 4.56 (1.80) | 7.38 (1.80) | 7.36 (2.01) | ||||||
| HOMA-IR scoreb,c | 0.96 (1.86) | 1.94 (1.83) | 3.24 (1.92) | ||||||
| Serum cystatin C, mg/Lb | 0.83 (1.21) | 0.86 (1.18) | 0.83 (1.24) | ||||||
| Urinary albumin/creatinine ratio, mg/gb | 4.71 (2.19) | 6.01 (2.45) | 10.33 (3.40) | ||||||
| Small artery elasticity, mL/mm Hg × 100b | 4.50 (1.79) | 4.26 (1.76) | 3.87 (1.76) | ||||||
| Large artery elasticity, mL/mm Hg × 100b | 13.93 (1.44) | 13.45 (1.42) | 12.49 (1.43) | ||||||
Abbreviations: HOMA-IR, homeostasis model of assessment-insulin resistance; MET, metabolic equivalent of the task; SD, standard deviation.
Summary measures are frequency and percent for categorical variables or mean (standard deviation) for continuous variables unless otherwise noted.
Summary measures are geometric mean (geometric standard deviation).
Excludes participants with treated diabetes.
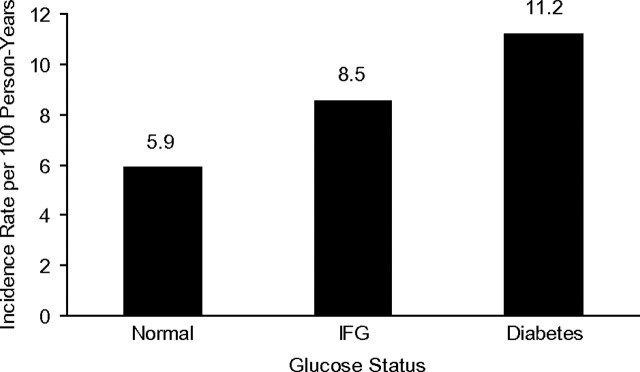
Retention rates were high; 97%, 94%, and 90% of study participants had their hypertension status ascertained at examinations 2, 3, and 4, respectively. During a median follow-up of 4.7 years, 965 participants (27%) developed hypertension, 596 participants (17%) were diagnosed with incident hypertension by elevated blood pressure, and 462 (13%) were diagnosed by medication use (Table 2). The unadjusted incidence rate of hypertension was 6.5 events per 100 person-years. Incident hypertension was more common among African Americans than among participants of Caucasian, Hispanic, and Chinese-American race/ethnicity (9.4 vs. 5.6, 6.5, and 5.6 events per 100 person-years, respectively). Rates of incident hypertension were also higher with older age, but they were similar when comparing men and women.
Table 2.
Incident Hypertension Over 4.7 Years’ Median Follow-up Among 3,513 Participants Recruited Between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosis
| Glucose Status |
||||||||
| Basis of Incident Hypertension Diagnosis | All Participants (N = 3,513) |
Normal (n = 2,879) |
Impaired Fasting Glucose (n = 376) |
Diabetes (n = 258) |
||||
| No. | % | No. | % | No. | % | No. | % | |
| Any diagnosisa | 965 | 28 | 727 | 25 | 125 | 33 | 113 | 44 |
| Diagnosed by elevated blood pressurea | 596 | 17 | 464 | 16 | 70 | 19 | 62 | 24 |
| Diagnosed by use of antihypertensive medicationa | 462 | 13 | 316 | 11 | 70 | 19 | 76 | 29 |
| ACEI and/or ARBb | 207 | 45 | 114 | 36 | 36 | 51 | 57 | 75 |
| Diureticb | 129 | 28 | 95 | 30 | 19 | 27 | 15 | 20 |
| Beta-blockerb | 137 | 30 | 105 | 33 | 20 | 29 | 12 | 16 |
| Calcium channel blockerb | 50 | 11 | 39 | 12 | 9 | 13 | 2 | 3 |
| Otherb | 29 | 6 | 18 | 6 | 5 | 7 | 6 | 8 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
“Percent” refers to column percentage of total at risk.
“Percent” refers to column percentage of participants diagnosed by incident use of antihypertensive medication. Medication use within categories is alone or in combination with other medications.
Glucose and incident hypertension
The unadjusted incidence rate of hypertension was higher with less favorable baseline glucose status (Figure 1). This was true whether hypertension was diagnosed by elevated blood pressure or by use of antihypertensive medications (Table 2). When participants with impaired fasting glucose or diabetes were newly treated with medications, they were more likely to be treated with angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, consistent with current practice guidelines (Table 2) (1). Associations of impaired fasting glucose and diabetes with incident hypertension were attenuated by adjustment for demographic variables and adiposity (Table 3). However, the association of diabetes with incident hypertension (incidence rate ratio = 1.41, 95% confidence interval (CI): 1.17, 1.71) and the trend across glucose categories (P = 0.0015) remained significant with full adjustment.
Figure 1.
Unadjusted incidence rates of hypertension by baseline glucose status for 3,513 participants recruited between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosis. IFG, impaired fasting glucose.
Table 3.
Hypertension Incidence Rate Ratios and 95% Confidence Intervals by Glucose Status Among 3,513 Participants Recruited Between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosisa
| Glucose Status |
|||||||
| Normal | Impaired Fasting Glucose |
Diabetes |
P Valueb | % Completec | |||
| IRR | 95% CI | IRR | 95% CI | ||||
| Unadjusted | Referent | 1.44 | 1.20, 1.73 | 1.90 | 1.59, 2.27 | <0.0001 | 100 |
| Model 1d | Referent | 1.32 | 1.10, 1.59 | 1.66 | 1.39, 2.00 | <0.0001 | 100 |
| Model 2e | Referent | 1.33 | 1.11, 1.60 | 1.65 | 1.37, 1.98 | <0.0001 | 99.5 |
| Model 3f | Referent | 1.16 | 0.96, 1.40 | 1.41 | 1.17, 1.71 | 0.0015 | 99.5 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
Incident hypertension cases (n = 965).
Two-sided P values are computed by using 2-parameter Wald tests.
“Percent complete” describes the proportion of participants with complete data included in each model.
Adjusted for age, gender, and race/ethnicity.
Additionally adjusted for attained education, moderate/vigorous physical activity, smoking, and alcohol use.
Additionally adjusted for body mass index and waist circumference.
Excluding participants with treated diabetes, the fasting glucose concentration and risk of incident hypertension were positively associated across the full range of fasting glucose, including within the normal range (Table 4). On average, each mmol/L (18 mg/dL) higher fasting glucose concentration was associated with an 8% (95% CI: 4, 13) higher adjusted incidence rate of hypertension. Associations were reasonably consistent across racial/ethnic groups; each mmol/L higher glucose level was associated with an 11% (95% CI: 6, 16), 17% (95% CI: 11, 23), 6% (95% CI: −1, 14), and 1% (95% CI: −9, 11) higher risk of hypertension in Caucasians, Chinese Americans, African Americans, and Hispanics, respectively (Pinteraction = 0.07).
Table 4.
Hypertension Incidence Rate Ratios and 95% Confidence Intervals by Baseline Concentrations of Fasting Serum Glucose and Insulin and Homeostasis Model of Assessment-Insulin Resistance Score Among 3,330 Participants Without Treated Diabetes Recruited Between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosisa
| Glucose |
P valueb | ||||
| Median (range), mmol/L | Median (range), mg/dL | IRRc | 95% CI | ||
| Quartile | |||||
| 1 | 4.3 (<4.5) | 78 (<82) | 1.0 | Referent | |
| 2 | 4.7 (4.5–4.8) | 85 (82–87) | 1.21 | 0.99, 1.48 | |
| 3 | 5.1 (4.9–5.2) | 91 (88–94) | 1.38 | 1.13, 1.69 | |
| 4 | 5.6 (>5.2) | 101 (>94) | 1.55 | 1.26, 1.91 | |
| Continuous exposure | 1.08d | 1.04, 1.13 | <0.0001 | ||
| Insulin |
|||||
| Median (range), pmol /L |
IRRc |
95% CI |
|||
| Quartile | |||||
| 1 | 2.4 (<3.3) | 1.0 | Referent | ||
| 2 | 3.9 (3.3–4.7) | 1.08 | 0.89, 1.31 | ||
| 3 | 5.8 (4.8–7.3) | 1.14 | 0.93, 1.40 | ||
| 4 | 10.05 (>7.3) | 1.25 | 1.01, 1.55 | ||
| Continuous exposure | 1.15e | 1.05, 1.25 | 0.0016 | ||
| HOMA-IR |
|||||
| Median (range), score |
IRRc |
95% CI |
|||
| Quartile | |||||
| 1 | 0.5 (<0.7) | 1.0 | Referent | ||
| 2 | 0.8 (0.7–1.0) | 1.10 | 0.90, 1.35 | ||
| 3 | 1.3 (1.1–1.7) | 1.33 | 1.08, 1.63 | ||
| 4 | 2.3 (>1.7) | 1.44 | 1.16, 1.80 | ||
| Continuous exposure | 1.16e | 1.08, 1.26 | 0.001 | ||
Abbreviations: CI, confidence interval; HOMA-IR, homeostasis model of assessment-insulin resistance; IRR, incidence rate ratio.
Incident hypertension cases (n = 881).
Two-sided P values are computed by using continuous exposures and Wald tests.
Models fully adjusted for age, gender, race/ethnicity, attained education, moderate/vigorous physical activity, smoking, alcohol use, body mass index, and waist circumference.
Per mmol/L increase.
Per doubling.
Insulin and incident hypertension
Serum insulin concentrations were monotonically associated with risk of incident hypertension (Table 4). When insulin was evaluated on a log scale, it was nearly linearly associated with hypertension risk across its full range. A 2-fold higher baseline fasting insulin concentration was associated with a 27% greater unadjusted incidence rate of hypertension. Adjustment for adiposity attenuated but did not extinguish this association. In the fully adjusted model, a doubling of insulin was associated with an estimated 15% (95% CI: 5, 25) higher incidence rate. This association was largely consistent across race/ethnicities; estimated incidence rate ratios were 1.19 (95% CI: 1.04, 1.37), 1.24 (95% CI: 0.93, 1.65), 1.08 (95% CI: 0.93, 1.27), and 1.15 (95% CI: 0.96, 1.39) in Caucasians, Chinese Americans, African Americans, and Hispanics, respectively (Pinteraction = 0.74). Results were similar for HOMA-IR (Table 4).
Glucose, insulin, and incident hypertension
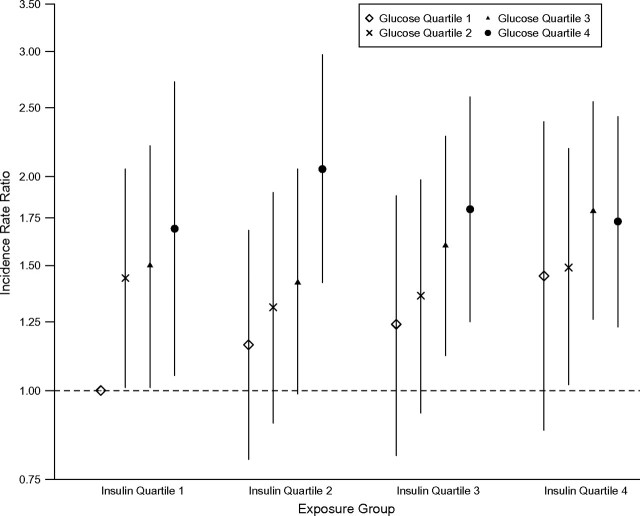
Baseline glucose and insulin concentrations were moderately correlated (r = 0.21) after excluding participants with treated diabetes. Higher baseline glucose and insulin levels were independently associated with elevated risks of hypertension, with no evidence of a multiplicative interaction between the 2 exposures. Estimates of the joint association between glucose and insulin levels and incident hypertension are displayed in Figure 2, where the relation between glucose quartile and hypertension risk is allowed to differ by insulin quartile (Pinteraction = 0.8). In a model including continuous glucose and insulin levels (but no interaction), the fully adjusted incidence rate ratios were 1.08 (95% CI: 1.04, 1.13) per mmol/L higher glucose and 1.13 (95% CI: 1.04, 1.23) per doubling of insulin.
Figure 2.
Adjusted hypertension incidence rate ratios and 95% confidence intervals by quartiles of baseline fasting glucose and insulin concentrations for 3,330 participants without baseline treated diabetes mellitus recruited between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosis. All models are adjusted for age, gender, race/ethnicity, attained education, moderate/vigorous physical activity, smoking, alcohol use, body mass index, and waist circumference. Error bars indicate 95% confidence intervals. The number of incident hypertension cases/number of participants initially at risk in each exposure group, proceeding from left to right, was as follows: 55/360, 59/256, 42/159, 22/82, 48/264, 52/228, 58/228, 51/138, 34/152, 51/213, 67/215, 70/210, 21/86, 50/170, 80/225, and 121/343.
Potential mediators of the glucose–hypertension link
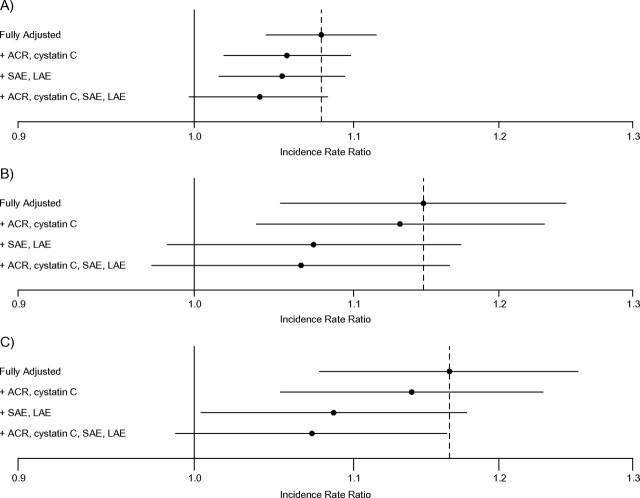
Adding serum cystatin C and urinary ACR levels to a fully adjusted model attenuated associations of impaired fasting glucose and diabetes with incident hypertension (Table 5). Adjustment for arterial elasticities instead resulted in a similar degree of attenuation. Similar patterns were observed when these covariates were added to fully adjusted models assessing the associations of glucose, insulin, and HOMA-IR (continuous variables) with incident hypertension (Figure 3).
Table 5.
Hypertension Incidence Rate Ratios and 95% Confidence Intervals by Glucose Status, With Adjustment for Potential Mediators, Among 3,513 Participants Recruited Between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosisa
| Glucose Status |
||||||
| Normal | Impaired Fasting Glucose |
Treated or Untreated Diabetes |
% Completeb | |||
| IRR | 95% CI | IRR | 95% CI | |||
| Fully adjustedc | Referent | 1.16 | 0.96, 1.40 | 1.41 | 1.17, 1.71 | 99.5 |
| + ACR, cystatin C | Referent | 1.11 | 0.91, 1.34 | 1.28 | 1.05, 157 | 98.5 |
| + SAE, LAE | Referent | 1.10 | 0.91, 1.33 | 1.25 | 1.03, 1.53 | 93.1 |
| + ACR, cystatin C, SAE, LAE | Referent | 1.08 | 0.89, 1.31 | 1.19 | 0.97, 1.47 | 92.2 |
Abbreviations: ACR, albumin/creatinine ratio; CI, confidence interval; IRR, incidence rate ratio; LAE, large artery elasticity; SAE, small artery elasticity.
Incident hypertension cases (n = 965).
“Percent complete” describes the proportion of participants with complete data included in each model.
Adjusted for age, gender, race/ethnicity, attained education, moderate/vigorous physical activity, smoking, alcohol use, body mass index, and waist circumference.
Figure 3.
Associations of baseline fasting glucose, insulin, and HOMA-IR score (continuous variables) with incident hypertension, adjusted for potential mediators of these associations, among 3,330 participants without baseline treated diabetes mellitus recruited between 2000 and 2002 in the Multi-Ethnic Study of Atherosclerosis. All models are adjusted for age, gender, race/ethnicity, attained education, moderate/vigorous physical activity, smoking, alcohol use, body mass index, and waist circumference. Error bars indicate 95% confidence intervals. ACR, albumin/creatinine ratio; HOMA-IR, homeostasis model of assessment-insulin resistance; LAE, large artery elasticity; SAE, small artery elasticity.
Sensitivity analyses
We conducted a series of sensitivity analyses. In the first, we excluded 570 participants with baseline systolic or diastolic blood pressures of ≥130 or 85 mm Hg, respectively. Fully adjusted hypertension incidence rate ratios were 1.11 (95% CI: 0.87, 1.42) and 1.51 (95% CI: 1.18, 1.92) for impaired fasting glucose and diabetes, respectively, compared with normal fasting glucose. Further excluding participants with treated diabetes, each mmol/L higher fasting glucose and 2-fold higher fasting insulin was associated with a 7% (95% CI: 1, 13) and 14% (95% CI: 2, 27) higher adjusted hypertension rate, respectively. In a second sensitivity analysis, in which we excluded 336 participants who were diagnosed with incident hypertension by antihypertensive medication alone, impaired fasting glucose and diabetes were associated with fully adjusted estimated incidence rate ratios of 1.04 (95% CI: 0.81, 1.34) and 1.29 (95% CI: 0.99, 1.67), respectively. Further excluding participants with treated diabetes, each mmol/L higher fasting glucose and doubling of fasting insulin was associated with a 9% (95% CI: 4, 14) and 15% (95% CI: 3, 28) higher adjusted rate of incident hypertension, respectively. In a third sensitivity analysis, baseline systolic and diastolic blood pressures were added as covariates to fully adjusted models. In these analyses, impaired fasting glucose and diabetes were associated with estimated hypertension incidence rate ratios of 1.03 (95% CI: 0.86, 1.24) and 1.47 (95% CI: 1.22, 1.76), respectively, and each mmol/L higher fasting glucose and doubling of fasting insulin was associated with a 6% (95% CI: 2, 9) and 3% (95% CI: −5, 12) higher rate of incident hypertension, respectively. In an additional sensitivity analysis removing 906 obese participants, impaired fasting glucose and diabetes were associated with fully adjusted estimated incidence rate ratios of 1.23 (95% CI: 0.97, 1.58) and 1.55 (95% CI: 1.21, 1.98), respectively. Further excluding participants with treated diabetes, each mmol/L higher fasting glucose and doubling of fasting insulin was associated with a 12% (95% CI: 9, 15) and 14% (95% CI: 2, 27) higher adjusted rate of incident hypertension, respectively.
DISCUSSION
Diabetes and higher fasting concentrations of serum glucose and insulin were associated with increased risks of incident hypertension in a community-based, multiethnic population. These associations were independent of known risk factors for hypertension, including adiposity measured as body mass index and waist circumference. Diabetes was associated with a 41% (95% CI: 17, 71) increased risk of incident hypertension after full adjustment. Among participants without treated diabetes, fasting glucose concentrations of 4.5–4.8 mmol/L (82–87 mg/dL), 4.9–5.2 mmol/L (88–94 mg/dL), and >5.2 mmol/L (>94 mg/dL) were associated with 21%, 38%, and 55% increased risks of hypertension, respectively, compared with glucose <4.5 mmol/L (<82 mg/dL), P < 0.0001. These independent temporal relations suggest that hyperglycemia and/or hyperinsulinemia may contribute to the development of hypertension, particularly when viewed in concert with randomized clinical trials demonstrating that glucose-lowering therapies reduce risk of hypertension (12, 13), and that relatively modest hyperglycemia and hyperinsulinemia may contribute to hypertension in the absence of clinical diabetes.
Our data build upon prior studies assessing the relations of glucose and insulin with hypertension. Specifically, elevated fasting glucose (4–6), hyperinsulinemia (6), elevated 2-hour postload glucose concentrations (6–8), and the insulin sensitivity index (9) were associated with increased risks of incident hypertension in disparate populations. Overt type 2 diabetes has been associated with increased risk of hypertension among women (7) and among American Indians (10, 11). Although diabetes and hypertension are highly likely to have shared origins, such as genetic predispositions and obesity (3, 29, 30), these observational studies generate a hypothesis that impaired glucose metabolism may contribute to the pathogenesis of hypertension. This hypothesis is supported by 2 clinical trials. Specifically, acarbose in the setting of impaired glucose tolerance and intensive insulin therapy in type 1 diabetes reduced rates of incident hypertension (12, 13).
Building on these observational and interventional studies, our results demonstrate temporal relations with hypertension across broad ranges of fasting glucose and insulin concentrations in a community-based population. We find largely consistent associations across racial/ethnic groups in a diverse population. Our study was also able to investigate the joint association of glucose and insulin with incident hypertension, and results provide evidence of independent links between higher levels of both glucose and insulin and risk of hypertension.
In addition, our results suggest potential mechanisms through which higher levels of glucose and insulin may lead to hypertension. Magnitudes of association were attenuated by approximately 50% after adjustment for serum cystatin C concentration, urinary ACR, and arterial elasticity measured by tonometry. This suggests that subtle kidney dysfunction and arterial stiffness may mediate, in part, associations of diabetes, glucose, and insulin with incident hypertension. Hyperglycemia may damage both the kidney and the arterial wall through deposition of advanced glycation end products, generation of reactive oxygen species, and activation of protein kinase C (14, 15). Hyperinsulinemia stimulates the sympathetic nervous system and the RAAS, which can cause kidney and vascular damage through both hemodynamic and nonhemodynamic pathways (3, 16–18).
Subtle kidney damage may contribute to hypertension through impaired sodium excretion and resultant volume expansion or through activation of the RAAS (19, 20). Kidney damage may manifest as a reduced glomerular filtration rate or albuminuria (31). An elevated serum concentration of cystatin C, an endogenously produced protein which is filtered and catabolized by the kidney, detects mild impairment of the glomerular filtration rate with greater sensitivity than serum creatinine-based estimates (32–34). Urinary ACR is a complementary sign of kidney disease that reflects glomerular and/or tubular damage. In a previous MESA study, serum cystatin C and urinary ACR were independently and additively associated with increased risk of incident hypertension (20).
Reduced arterial elasticity is one result of long-term exposure of the vascular wall to elevated glucose and insulin levels. Reduced elasticity leads to hypertension by increasing peripheral vascular resistance. In MESA, arterial elasticity was measured by using the HDI PulseWave CR-2000 Research CardioVascular Profiling Instrument. Elasticities calculated by this noninvasive method have been validated against direct brachial artery cannulation, and impaired arterial elasticity using this method is associated with increased risk of incident hypertension (26).
It has been hypothesized that the link between diabetes and hypertension is largely due to underlying obesity (3). Indeed, associations of diabetes, glucose, and insulin with prevalent and incident hypertension were attenuated by adjustment for adiposity. However, substantial associations persisted in our data after adjustment for both body mass index and waist circumference. Although residual confounding could still exist, it is likely that factors other than obesity also contribute to the glucose–hypertension link, as discussed above.
Strengths of this study include the diverse, community-based population; absence of clinical cardiovascular disease as a potential confounder, by MESA study design; standardized longitudinal assessment of blood pressure and antihypertensive medications; and well-measured covariates, including critical variables related to adiposity, kidney disease, and arterial elasticity. There are several potential limitations to our study. First, initiation of antihypertensive medications could vary by baseline glucose status. This is particularly true for patients with diagnosed and treated diabetes, whose blood pressure may be checked more frequently because of more frequent health-care visits, who may be started on antihypertensive medications for reasons other than elevated blood pressure (e.g., albuminuria), and who may be held to tighter standards of blood pressure control. However, participants with treated diabetes were excluded from analyses assessing associations of glucose and insulin (continuous variables) with incident hypertension, for which results were strongly positive, and additional sensitivity analyses suggest a relation between glucose status and hypertension diagnosed by MESA examination blood pressure alone. Second, follow-up time was relatively short (median, 4.7 years). In type 1 diabetes, the effects of tight glycemic control on blood pressure were evident only after 7 years of follow-up, suggesting that longer follow-up may be required to ascertain the full effect of impaired glucose metabolism on hypertension (12). Third, the substantial attenuation of observed associations does not definitely establish mediation. Alternative explanations include the possibility that subtle kidney disease and diminished arterial elasticity may predate impaired glucose metabolism and independently increase the risk of hypertension, and thus the observed attenuation could be due to classic confounding. In addition, our ability to assess the full impact of potential mediators may be limited by measurement error and lack of longitudinal data. Finally, as with most longitudinal cohort studies, loss to follow-up could bias results if censoring was related to the risk of hypertension. However, retention in MESA was excellent, reducing the potential impact of this problem.
In conclusion, diabetes and higher concentrations of serum glucose and insulin were associated with increased risk of incident hypertension in a diverse, community-based cohort. These associations were independent of adiposity and other established hypertension risk factors and were attenuated by adjustment for serum cystatin C, urinary ACR, and arterial elasticity. These findings support the hypothesis that higher levels of glucose and/or insulin contribute to elevated blood pressure, in part by damaging the kidney and arterial wall.
Acknowledgments
Author affiliations: Cedars-Sinai Medical Center, Los Angeles, California (Yii-Der Ida Chen, Jerome I. Rotter); Department of Biostatistics, University of Washington, Seattle, Washington (Gregory Levin); Department of Epidemiology, University of Washington, Seattle, Washington (Bruce M. Psaty, David S. Siscovick, Bryan Kestenbaum); Division of Epidemiology, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Division of Nephrology, University of Washington, Seattle, Washington (Bryan Kestenbaum, Ian H. de Boer); and Kidney Research Institute, Seattle, Washington (Bryan Kestenbaum, Ian H. de Boer).
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by National Institutes of Health grants 1KL2RR025015-01, K23 DK63274-01, and HL071205.
The authors thank the other investigators and staff of MESA for their valuable contributions.
A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Conflict of interest: none declared.
Glossary
Abbreviations
- ACR
albumin/creatinine ratio
- CI
confidence interval
- HOMA-IR
homeostasis model of assessment-insulin resistance
- MESA
Multi-Ethnic Study of Atherosclerosis
- MET
metabolic equivalent of the task
- RAAS
renin-angiotensin-aldosterone system
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Seventh Report of the Joint National Committee in Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: US National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services; 2004. [PubMed] [Google Scholar]
- 3.Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension. 1995;26(6 pt 1):869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 4.Suematsu C, Hayashi T, Fujii S, et al. Impaired fasting glucose and the risk of hypertension in Japanese men between the 1980s and the 1990s. The Osaka Health Survey. Diabetes Care. 1999;22(2):228–232. doi: 10.2337/diacare.22.2.228. [DOI] [PubMed] [Google Scholar]
- 5.Fagot-Campagna A, Balkau B, Simon D, et al. Is insulin an independent risk factor for hypertension? The Paris Prospective Study. Int J Epidemiol. 1997;26(3):542–550. doi: 10.1093/ije/26.3.542. [DOI] [PubMed] [Google Scholar]
- 6.Bjørnholt JV, Erikssen G, Kjeldsen SE, et al. Fasting blood glucose is independently associated with resting and exercise blood pressures and development of elevated blood pressure. J Hypertens. 2003;21(7):1383–1389. doi: 10.1097/00004872-200307000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, Valdez R, Morales PA, et al. Greater effect of glycemia on incidence of hypertension in women than in men. Diabetes Care. 1992;15(10):1277–1284. doi: 10.2337/diacare.15.10.1277. [DOI] [PubMed] [Google Scholar]
- 8.Boyko EJ, Barr EL, Zimmet PZ, et al. Two-hour glucose predicts the development of hypertension over 5 years: the AusDiab Study. J Hum Hypertens. 2008;22(3):168–176. doi: 10.1038/sj.jhh.1002316. [DOI] [PubMed] [Google Scholar]
- 9.Arnlöv J, Pencina MJ, Nam BH, et al. Relations of insulin sensitivity to longitudinal blood pressure tracking: variations with baseline age, body mass index, and blood pressure. Circulation. 2005;112(12):1719–1727. doi: 10.1161/CIRCULATIONAHA.105.535039. [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Devereux RB, Chinali M, et al. Risk factors for arterial hypertension in adults with initial optimal blood pressure: the Strong Heart Study. Hypertension. 2006;47(2):162–167. doi: 10.1161/01.HYP.0000199103.40105.b5. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Lee ET, Fabsitz RR, et al. A longitudinal study of hypertension risk factors and their relation to cardiovascular disease: the Strong Heart Study. Hypertension. 2006;47(3):403–409. doi: 10.1161/01.HYP.0000200710.29498.80. [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Kestenbaum B, Rue TC, et al. Insulin therapy, hyperglycemia, and hypertension in type 1 diabetes mellitus. Arch Intern Med. 2008;168(17):1867–1873. doi: 10.1001/archinternmed.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A. Controlling oxidative stress as a novel molecular approach to protecting the vascular wall in diabetes. Curr Opin Lipidol. 2006;17(5):510–518. doi: 10.1097/01.mol.0000245256.17764.fb. [DOI] [PubMed] [Google Scholar]
- 16.Anderson EA, Hoffman RP, Balon TW, et al. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87(6):2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perlstein TS, Gerhard-Herman M, Hollenberg NK, et al. Insulin induces renal vasodilation, increases plasma renin activity, and sensitizes the renal vasculature to angiotensin receptor blockade in healthy subjects. J Am Soc Nephrol. 2007;18(3):944–951. doi: 10.1681/ASN.2006091026. [DOI] [PubMed] [Google Scholar]
- 18.Durvasula RV, Shankland SJ. The renin-angiotensin system in glomerular podocytes: mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep. 2006;8(2):132–138. doi: 10.1007/s11906-006-0009-8. [DOI] [PubMed] [Google Scholar]
- 19.Guyton AC, Coleman TG, Cowley AV, Jr, et al. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52(5):584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 20.Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the Multi-Ethnic Study of Atherosclerosis. Ann Intern Med. 2008;148(7):501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Irwin ML, Addy CL, et al. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 26.McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33(6):1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 27.McCullagh P. Generalized Linear Models. London, United Kingdom: Chapman and Hall; 2002. pp. 193–244. [Google Scholar]
- 28.Glymour MM, Weuve J, Berkman LF, et al. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 29.Cheng LS, Davis RC, Raffel LJ, et al. Coincident linkage of fasting plasma insulin and blood pressure to chromosome 7q in hypertensive Hispanic families. Circulation. 2001;104(11):1255–1260. doi: 10.1161/hc3601.096729. [DOI] [PubMed] [Google Scholar]
- 30.Xiang AH, Azen SP, Raffel LJ, et al. Evidence for joint genetic control of insulin sensitivity and systolic blood pressure in Hispanic families with a hypertensive proband. Circulation. 2001;103(1):78–83. doi: 10.1161/01.cir.103.1.78. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl1):S1–S266. [PubMed] [Google Scholar]
- 32.Newman DJ, Thakkar H, Edwards RG, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47(1):312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 33.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 34.O'Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648–655. doi: 10.1258/000456303770367243. [DOI] [PubMed] [Google Scholar]