Abstract
This study examined prepregnancy cardiometabolic risk factors and gestational diabetes mellitus (GDM) in subsequent pregnancies. The authors selected 1,164 women without diabetes before pregnancy who delivered 1,809 livebirths between 5 consecutive examinations from 1985 to 2006 in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The authors measured prepregnancy cardiometabolic risk factors and performed multivariate repeated-measures logistic regression to compute the odds of GDM adjusted for race, age, parity, birth order, and other covariates. Impaired fasting glucose (100–125 vs. <90 mg/dL), elevated fasting insulin (>15–20 and >20 vs. <10 μU/mL), and low levels of high-density lipoprotein cholesterol (<40 vs. >50 mg/dL) before pregnancy were directly associated with GDM: The odds ratios = 4.74 (95% confidence interval (CI): 2.14, 10.51) for fasting glucose, 2.19 (95% CI: 1.15, 4.17) for middle insulin levels and 2.36 (95% CI: 1.20, 4.63) for highest insulin levels, and 3.07 (95% CI: 1.62, 5.84) for low levels of high-density lipoprotein cholesterol among women with a negative family history of diabetes; all P < 0.01. Among overweight women, 26.7% with 1 or more cardiometabolic risk factors developed GDM versus 7.4% with none. Metabolic impairment exists before GDM pregnancy in nondiabetic women. Interconceptual metabolic screening could be included in routine health assessments to identify high-risk women for GDM in a subsequent pregnancy and to potentially minimize fetal exposure to metabolic abnormalities that program future disease.
Keywords: cohort studies; diabetes, gestational; lipids; longitudinal studies; obesity; preconception care; pregnancy; risk factors
Women who develop gestational diabetes mellitus (GDM) are more likely to experience antepartum and peripartum complications (1, 2) and, for those free of diabetes before pregnancy, are 4 times more likely to develop type 2 diabetes several years later (3). Their offspring are often macrosomic at birth and more likely to become overweight and develop the metabolic syndrome or type 2 diabetes later in life (4–6). Better prepregnancy prediction of GDM may enable a preventive approach that could improve long-term health outcomes for women and avoid adverse intrauterine metabolic programming of the offspring.
Established clinical predictors of GDM pregnancies include older maternal age, higher body mass index before pregnancy, family history of diabetes, and weight gain in early adulthood (7, 8). Obesity before pregnancy (9), weight gain since the age of 18 years (10), interpregnancy weight gain, and gain within 5 years before pregnancy have been directly associated with GDM pregnancy (8, 11–13). Gestational weight gain that precedes glucose tolerance screening during pregnancy has been directly associated with risk of GDM (14, 15) or mild glucose intolerance during pregnancy (16, 17) but, in 2 studies, these associations were found only among nonobese women (15) or overweight women (17).
Biomarkers measured during early pregnancy, such as elevated plasma triglycerides and tumor necrosis factor, hypoadiponectinemia, and hyperandrogenicity have been directly associated with risk of GDM (18–23). Yet, metabolic alterations in the first trimester produce fasting plasma glucose and fatty acid levels below preconception levels (24). Thus, prepregnancy metabolic measurements might be more advantageous to prediction than early pregnancy values, because they could provide more accurate profiles of subsequent GDM risk. Although preconception metabolic profiles are rarely obtained in clinical care or epidemiologic studies, health assessments during the interconceptual period are routine. Metabolic screening could be included in these routine health assessments and steps taken toward preventing GDM in a subsequent pregnancy.
Previous studies of prepregnancy biochemical measures have retrospectively examined women by history of GDM. Women with a history of GDM were more likely to have had dyslipidemia, obesity, and/or hypertension before pregnancy than those without GDM (25, 26). A single longitudinal study reported higher prepregnancy plasma free fatty acid levels among 5 women who later developed GDM versus 4 women who did not (27). The Coronary Artery Risk Development in Young Adults (CARDIA) Study measured risk factors before pregnancy (1985–1992) and found that the waist/hip ratio, but not fasting insulin or body mass index, was directly associated with subsequent risk of GDM (28). Although these prospective studies excluded women with overt diabetes, neither study examined prepregnancy fasting glucose and lipid profiles as independent predictors of GDM, even though the CARDIA Study (28) had measured these indices at each examination.
This study examines the independent associations of prepregnancy cardiometabolic risk factors in relation to subsequent risk of GDM pregnancy. We utilized biochemical analyses and other clinical data obtained prospectively from the US population-based CARDIA Study to assess whether unfavorable levels of prepregnancy cardiometabolic risk factors increase the risk of GDM.
MATERIALS AND METHODS
Study participants
The CARDIA Study is a multicenter, longitudinal, observational study designed to describe the development of risk factors for coronary heart disease in young adults. In 1985–1986, 2,787 women (52% black, 48% white) aged 18–30 years were enrolled from 4 geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California (29, 30). Retention was 81%, 79%, 74%, and 72% of the survivors 7, 10, 15, and 20 years later (31, 32).
Of the 2,787 women enrolled at baseline (1985–1986), we excluded women with no births during follow-up (n = 1,364) or lost to follow-up (n = 96). We also excluded women with diabetes (fasting glucose, ≥126 mg/dL; 2 hours after a 75-g oral glucose tolerance test, ≥200 mg/dL; diabetes medication use; and/or self-report) at examinations preceding all of their pregnancies since baseline (n = 14), unknown delivery dates (n = 1), or fasting triglyceride levels above 400 mg/dL (n = 1), as well as women who were missing all prepregnancy measurements (n = 147; 89 were pregnant or lactating), for examinations at years 0, 7, 10, or 15. We selected 1,164 women who delivered 1,809 livebirths during 20 years (Figure 1); 1,655 pregnancies were classified as non-GDM and 154 as GDM. The analytical sample tended to be white, college educated, nonsmoking, younger, nulliparous, and less centrally obese and to have lower body mass index and fasting glucose levels than those excluded. Institutional review boards at each participating study center approved the study. Written, informed consent was obtained from subjects for all study procedures.
Figure 1.
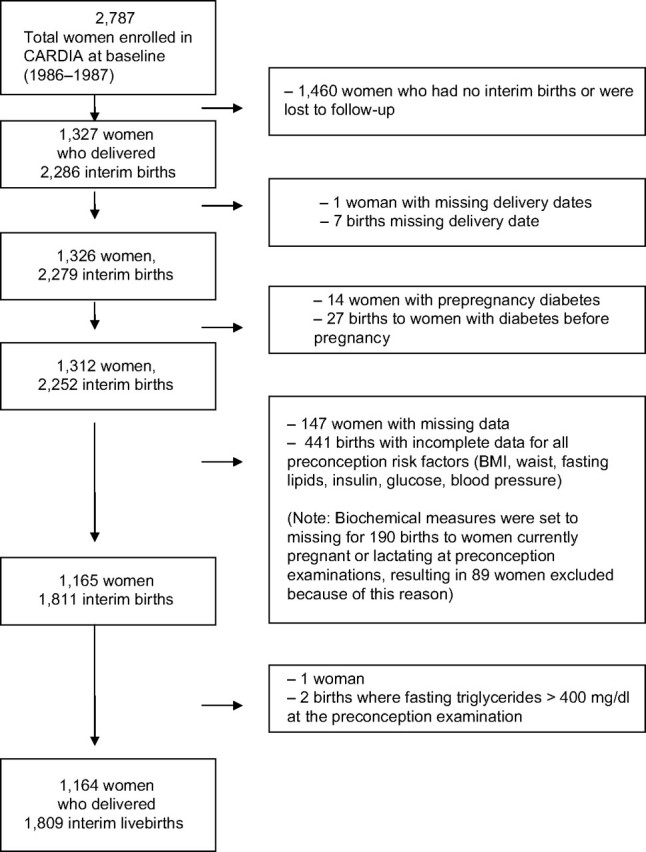
Flow diagram of analytical sample selection criteria, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2006. BMI, body mass index.
Data collection
Characteristics of participants, including lifestyle, sociodemographic and medical conditions, medication use, family history of diabetes, reproductive events (pregnancies and births), and GDM status, as well as clinical assessments, anthropometric measurements, and blood specimens, were obtained at baseline and follow-up examinations by standardized research methodologies that included self- and interviewer-administered questionnaires (29, 30).
Pregnancies and GDM status
Reproductive events were assessed since the previous examination: current pregnancy or lactation status and number of pregnancies ending in abortion, miscarriage, and live- or stillbirths, along with length(s) of gestation, multifetal gestation, dates of delivery(ies), and diabetes only during pregnancy. Livebirths were defined as delivery of a live infant of >20 weeks’ gestation that was conceived after the baseline CARDIA Study examination and delivered within the subsequent intervals (0–7, >7–10, >10–15, and >15–20 years) between 2 consecutive examinations. We calculated time to conception from the prepregnancy examination for the first pregnancy within each interval. We validated self-report of GDM among 165 women for whom oral glucose tolerance test results were available for GDM diagnosis (33, 34) for their 200 births between baseline and year 10. Sensitivity for classification by self-report as ever having GDM was 100% (20 of 20), and specificity was 92% (134 of 145) (3).
Prepregnancy cardiometabolic risk factor measurements
Venous blood samples were drawn in the morning after an overnight fast of 8 or more hours. Procedures for blood specimen collection and methodologies to assay concentrations of triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, glucose, and insulin are reported elsewhere (35, 36). The homeostasis model assessment of insulin resistance (HOMA-IR) evaluated insulin resistance (fasting glucose (mmol/L) × fasting insulin (mU/L))/22.5, because of its strong correlation with physiologic measures of insulin sensitivity across a range of glucose levels (37–39). The metabolic syndrome before pregnancy was defined by National Cholesterol Education Program Adult Treatment Panel III criteria (40). All biochemical measurements were obtained in the nonpregnant and nonlactating state in years 0, 7, 10, and 15 prior to subsequent pregnancies.
Blood pressure was measured, after an initial 5-minute rest, 3 times at 1-minute intervals by using a Hawksley random-zero sphygmomanometer (Hawksley, Lancing, United Kingdom) from baseline through year 15; the first and fifth phase Korotkoff sounds were recorded with second and third measurements averaged (30). The Omron HEM907XL oscillometer (Omron Corporation, Schaumburg, Illinois) was used at year 20. The appropriate cuff size (small, medium, large, extra large) was based on the upper arm circumference, as the midpoint between the acromion and the olecranon. Systolic and diastolic blood pressure measurements in year 20 were calibrated to random-zero sphygmomanometers from prior CARDIA Study examinations (41).
Certified technicians obtained weight, height, and waist circumference measurements using a standardized protocol to the nearest 0.1 kg or 0.5 cm in participants wearing light clothing and without shoes (42). Waist circumference was measured midway between the iliac crest and bottom of the rib cage (43). Body mass index was computed as weight (kg)/height (m)2.
Other covariates
Sociodemographic, medical treatment, and behavioral characteristics assessed before pregnancy included age, race, education, parity, medication use (antihypertensives, diabetes), cigarette smoking, and oral contraceptive use. Categorical variables were smoking (never, current, or past), years of education (≤12, 13–15, ≥16), and oral contraceptive use (never, past, or current). Family history of diabetes was based on report of one or more first-degree relatives (father, mother, or siblings) with diabetes at examination years 0, 5, and 10.
Statistical methods
We examined prepregnancy cardiometabolic risk factors (body mass index, fasting glucose, insulin, triglycerides, total and lipoprotein cholesterol, waist circumference, diastolic blood pressure, systolic blood pressure), hypertension status, sociodemographics, and time from examination to conception for subsequent pregnancies by GDM and non-GDM status utilizing repeated measures regression models. P values were obtained from 2-sided tests of significance (P < 0.05).
We calculated the proportion of women with GDM across categories of the prepregnancy cardiometabolic risk factors, sociodemographics, and clinical characteristics. We used logistic regression to estimate of the odds ratios for GDM and 95% confidence intervals for time-dependent prepregnancy risk factors. Because 211 women contributed births within more than one time interval, generalized estimating equation methods accounted for correlations within individuals (PROC GENMOD 9.1; SAS Institute, Inc., Cary, North Carolina) for estimation of logistic regression parameters, assuming compound symmetry as the working correlation structure.
We examined each cardiometabolic risk factor separately in unadjusted and adjusted models that included race, age at delivery, education level, time from prepregnancy measurement to conception, time-dependent parity, number and order of births during the interval, and family history of diabetes. Cardiometabolic risk factors that reached statistical significance in separate models (P < 0.05) formed a single multivariate model to identify independent predictors of GDM. First, we obtained estimates unadjusted for covariates and then adjusted for the same covariates used in the separate risk factor models. Models were adjusted for time-dependent body mass index or waist circumference, but not both, because of collinearity for these measures. We examined race, body mass index, family history of diabetes, and parity as effect modifiers of prepregnancy risk factor associations with risk of GDM and found no evidence for interaction (P > 0.10), except for family history of diabetes by high-density lipoprotein cholesterol levels (P = 0.0475).
To describe the distribution of risk factors within our sample, we compared the percentage of women having a subsequent GDM pregnancy versus those with a non-GDM pregnancy across combinations of 3 prepregnancy risk factors that remained significant in multivariate models. We also calculated the incidence of GDM as a percentage for all women, women with one or more prepregnancy risk factors, and women with no risk factors (none) within each prepregnancy body mass index group.
RESULTS
Among 1,164 women, 141 (12.1%) had one or more births with a range of 6.6%–12.9% across 4 time intervals (Table 1). GDM was more likely to occur in women who had a positive family history of diabetes, the metabolic syndrome, or higher education level; who were older, a current smoker, heavier, more centrally obese, and insulin resistant; or who had higher prepregnancy diastolic blood pressure, fasting triglycerides, low-density lipoprotein cholesterol, insulin, glucose, and HOMA-IR, as well as lower levels of high-density lipoprotein cholesterol before pregnancy (Table 2). For 73% of pregnancies, all cardiometabolic risk factors were measured less than 4 years before conception (range, 1–74 months). The median time from prepregnancy measure to conception was 33.6 (interquartile range, 41) months for GDM and 31.0 (interquartile range, 39) months for non-GDM pregnancies.
Table 1.
Number and Percentage of Livebirths Within Each Time Intervala Between 2 Consecutive Examinations by GDM Pregnancy Status and Number of Women by GDM Status Across Births, the CARDIA Study, 1985–2006
| Livebirths by Consecutive CARDIA Examination Yearsb |
||||||||||
| GDM Pregnancy Status | Examination Years 0–7 (1985–1986, 1992–1993) |
Examination Years >7–10 (>1992–1993, 1995–1996) |
Examination Years >10–15 (>1995–1996, 2000–2001) |
Examination Years >15–20 (>2000–2001, 2005–2006) |
Total All 4 Intervals (1985–2006) |
|||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Non-GDM | 1,105 | 228 | 268 | 54 | 1,655 | |||||
| GDM | 103 | 8.5 | 24 | 9.5 | 19 | 6.6 | 8 | 12.9 | 154 | 8.5 |
| Total pregnancies | 1,208 | 252 | 287 | 62 | 1,809 | |||||
| No. of Women Who Delivered 1,809 Livebirths by No. of Births per Woman |
||||||||||
| 1 Birth |
2 Births |
3 Births |
4 Births |
5 Births |
Total |
|||||
| Non-GDM births only | 594 | 325 | 91 | 9 | 4 | 1,023 | ||||
| GDM births only | 67 | 8 | 0 | 0 | 0 | 75 | ||||
| Non-GDM and 1 GDM | 47 | 13 | 1 | 0 | 61 | |||||
| Non-GDM and 2 GDM | 4 | 1 | 0 | 5 | ||||||
| Total no. of women | 661 | 380 | 108 | 11 | 4 | 1,164 | ||||
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; GDM, gestational diabetes mellitus.
An examination at the beginning of the time interval (range in years) serves as the prepregnancy measurement.
Prepregnancy measurements were obtained at CARDIA examinations in years 0, 7, 10, and 15 since baseline.
Table 2.
Sociodemographic and Prepregnancy Characteristics by Subsequent GDM Pregnancy Status, the CARDIA Study, 1985–2006
| Prepregnancy Characteristics | GDM Pregnancies (n = 154) |
Non-GDM Pregnancies (n = 1,655) |
P Value | ||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Race (black) | 75 | 49 | 782 | 47 | 0.75 | ||
| Education (high school or less)a | 39 | 25 | 509 | 31 | 0.23 | ||
| Smoker (past/current)a | 78 | 51 | 670 | 41 | 0.02 | ||
| Oral contraceptive use (current)a | 28 | 18 | 239 | 15 | 0.28 | ||
| Family history of diabetes (yes) | 66 | 43 | 439 | 27 | <0.001 | ||
| Nulliparous | 81 | 53 | 986 | 60 | 0.07 | ||
| Multifetal pregnanciesb | 5 | 3 | 33 | 2 | 0.37 | ||
| Hypertensionc | 5 | 4 | 40 | 2 | 0.23 | ||
| Metabolic syndrome (NCEP ATP III) | 5 | 3 | 6 | 0.4 | <0.001 | ||
| Age, years | 27.4 (5.2) | 27 (5.2) | 0.17 | ||||
| Body mass index, kg/m2 | 26.7 (7.5) | 24.1 (5.3) | <0.001 | ||||
| Waist circumference, cm | 79.5 (15.5) | 73.7 (10.9) | <0.001 | ||||
| Fasting | |||||||
| Glucose, mg/dL | 83.9 (10.9) | 81.6 (8.0) | 0.01 | ||||
| Insulin, μU/mL | 14.0 (10.1) | 10.8 (6.9) | <0.001 | ||||
| Triglycerides, mg/dL | 79.0 (36.6) | 65.6 (32.1) | <0.001 | ||||
| HDL-C, mg/dL | 52.1 (14.3) | 56.1 (13.1) | 0.002 | ||||
| LDL-C, mg/dL | 110.9 (27.7) | 105.1 (29.4) | 0.008 | ||||
| Total cholesterol, mg/dL | 179.0 (30.0) | 174.4 (30.7) | 0.05 | ||||
| HOMA-IR | 3.0 (2.4) | 2.2 (1.6) | <0.001 | ||||
| Systolic BP, mm Hg | 106.2 (10.6) | 105 (9.5) | 0.12 | ||||
| Diastolic BP, mm Hg | 68.0 (7.8) | 66.4 (8.9) | 0.02 | ||||
| Mean arterial pressure, mm Hg | 80.7 (8.0) | 79.3 (8.2) | 0.02 | ||||
Abbreviations: BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; SD, standard deviation.
Missing variables: education (n = 1); smoking (n = 3); and oral contraceptive use (n = 6).
Multifetal pregnancies during follow-up.
Hypertension before pregnancy (BP, ≥140/90 or taking antihypertensive medication).
In separate risk factor models (Table 3) adjusted for covariates, prepregnancy risk factors directly associated with risk of GDM included fasting triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, insulin and glucose, homeostasis model assessment of insulin resistance (HOMA-IR), body mass index, and waist circumference (all P < 0.05). Prepregnancy systolic blood pressure and diastolic blood pressure were not associated with GDM. The metabolic syndrome before pregnancy conferred a 7-fold higher risk of GDM pregnancy but occurred in less than 0.1% of women (8 of 1,164) overall.
Table 3.
Prepregnancy Cardiometabolic Risk Factors for 1,809 Pregnancies in 1,164 Women by Subsequent GDM Pregnancies and Risk of GDM from Separate Logistic Regression Models, the CARDIA Study, 1985–2006
| Prepregnancy Risk Factor Measures | GDM Pregnancies (n = 154) |
Non-GDM Pregnancies (n = 1,655) |
Unadjusted Separate Models |
Ptrend | Adjusted Separate Modelsa |
Ptrend | ||||
| No. | % | No. | % | OR | 95% CI | OR | 95% CI | |||
| Anthropometric | ||||||||||
| Body mass index, kg/m2b | <0.001 | <0.001 | ||||||||
| <25 | 85 | 6.8 | 1,161 | 93.2 | 1.00 | Referent | 1.00 | Referent | ||
| 25–29.9 | 26 | 8.4 | 283 | 91.6 | 1.31 | 0.82, 2.10 | 1.34 | 0.81, 2.21 | ||
| ≥30 | 43 | 16.9 | 211 | 83.1 | 3.01 | 1.96, 4.61 | 2.78 | 1.74, 4.44 | ||
| Waist circumference, cm | <0.001 | <0.001 | ||||||||
| ≤80 | 98 | 6.9 | 1,317 | 93.1 | 1.00 | Referent | 1.00 | Referent | ||
| 80.1–92 | 26 | 11.0 | 211 | 89.0 | 1.78 | 1.12, 2.84 | 1.64 | 1.00, 2.70 | ||
| >92 | 30 | 19.1 | 127 | 90.9 | 3.53 | 2.14, 5.83 | 3.29 | 1.95, 5.55 | ||
| Biochemical, fasting | ||||||||||
| Glucose, mg/dL | <0.001 | <0.001 | ||||||||
| <90 | 109 | 7.2 | 1,407 | 92.8 | 1.00 | Referent | 1.00 | Referent | ||
| 90–99 | 33 | 12.8 | 225 | 87.2 | 2.01 | 1.30, 3.11 | 1.78 | 1.14, 2.80 | ||
| 100–125 | 12 | 34.3 | 23 | 65.7 | 7.62 | 3.45, 16.8 | 7.32 | 3.30, 16.26 | ||
| Insulin, μU/mL | <0.001 | <0.001 | ||||||||
| <10 | 64 | 6.1 | 987 | 93.9 | 1.00 | Referent | 1.00 | Referent | ||
| 10–15 | 40 | 9.2 | 396 | 90.8 | 1.57 | 1.02, 2.41 | 1.81 | 1.17, 2.80 | ||
| >15–20 | 22 | 12.3 | 157 | 87.7 | 2.30 | 1.34, 3.94 | 2.88 | 1.65, 5.03 | ||
| >20 | 28 | 19.6 | 115 | 80.4 | 3.85 | 2.30, 6.42 | 4.33 | 2.43, 7.71 | ||
| Triglycerides, mg/dL | <0.001 | <0.001 | ||||||||
| <70 | 80 | 6.8 | 1,095 | 93.2 | 1.00 | Referent | 1.00 | Referent | ||
| 70–119.9 | 54 | 10.7 | 453 | 89.3 | 1.74 | 1.21, 2.49 | 1.67 | 1.15, 2.43 | ||
| ≥120 | 20 | 15.7 | 107 | 84.3 | 2.58 | 1.48, 4.50 | 2.45 | 1.39, 4.32 | ||
| LDL-C, mg/dL | 0.06 | 0.07 | ||||||||
| <100 | 57 | 7.2 | 730 | 92.9 | 1.00 | Referent | 1.00 | Referent | ||
| 100–129 | 64 | 9.1 | 636 | 90.9 | 1.35 | 0.92, 1.98 | 1.39 | 0.94, 2.05 | ||
| ≥130 | 33 | 10.2 | 289 | 89.8 | 1.51 | 0.93, 2.44 | 1.48 | 0.91, 2.42 | ||
| HDL-C, mg/dL | <0.001 | <0.001 | ||||||||
| <40 | 31 | 19.0 | 132 | 81.0 | 3.41 | 2.09, 5.58 | 3.02 | 1.79, 5.11 | ||
| 40–50 | 47 | 9.6 | 444 | 90.4 | 1.51 | 1.03, 2.23 | 1.47 | 1.00, 2.17 | ||
| >50 | 76 | 6.6 | 1,079 | 93.4 | 1.00 | Referent | 1.00 | Referent | ||
| Total cholesterol, mg/dL | 0.02 | 0.04 | ||||||||
| <150 | 22 | 5.9 | 354 | 94.1 | 1.00 | Referent | 1.00 | Referent | ||
| 150–180 | 63 | 8.6 | 671 | 91.4 | 1.53 | 0.92, 2.54 | 1.51 | 0.90, 2.53 | ||
| >180 | 69 | 9.9 | 630 | 90.1 | 1.80 | 1.09, 2.99 | 1.73 | 1.04, 2.89 | ||
| HOMA-IR | <0.001 | <0.001 | ||||||||
| ≤4.0 | 120 | 7.4 | 1,509 | 92.6 | 1.00 | Referent | 1.00 | Referent | ||
| >4.0 | 34 | 18.9 | 146 | 81.1 | 2.93 | 1.89, 4.54 | 2.91 | 1.79, 4.73 | ||
| Clinical and anthropometric | ||||||||||
| Systolic BP, mm Hg | 0.18 | 0.20 | ||||||||
| <100 | 39 | 7.5 | 479 | 92.5 | 1.00 | Referent | 1.00 | Referent | ||
| 100–115 | 92 | 8.8 | 953 | 91.2 | 1.25 | 0.82, 1.89 | 1.29 | 0.85, 1.97 | ||
| 116–130 | 19 | 8.6 | 202 | 91.4 | 1.21 | 0.67, 2.15 | 1.24 | 0.68, 2.25 | ||
| >130 | 4 | 2.6 | 21 | 1.3 | 2.68 | 0.90, 7.97 | 1.93 | 0.70, 5.36 | ||
| Diastolic BP, mm Hg | 0.05 | 0.08 | ||||||||
| <65 | 50 | 6.7 | 700 | 93.3 | 1.00 | Referent | 1.00 | Referent | ||
| 65–75 | 77 | 9.6 | 727 | 90.4 | 1.45 | 0.99, 2.14 | 1.45 | 0.97, 2.16 | ||
| 76–85 | 23 | 10.9 | 188 | 89.1 | 1.65 | 0.95, 2.88 | 1.71 | 0.97, 3.00 | ||
| >85 | 4 | 9.0 | 40 | 91.0 | 1.36 | 0.43, 4.25 | 1.05 | 0.35, 3.17 | ||
| Hypertensionc | 0.23 | 0.49 | ||||||||
| No | 148 | 8.4 | 1,615 | 91.6 | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 6 | 13.0 | 40 | 87.0 | 1.75 | 0.70, 4.41 | 1.38 | 0.55, 3.45 | ||
| Metabolic syndrome | <0.001 | 0.004 | ||||||||
| No | 149 | 8.3 | 1,649 | 91.7 | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 5 | 45.4 | 6 | 54.6 | 9.50 | 2.77, 32.59 | 8.18 | 1.97, 33.94 | ||
| Family history of diabetes | <0.001 | <0.001 | ||||||||
| No | 88 | 6.7 | 1,216 | 93.3 | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 66 | 13.1 | 439 | 86.9 | 2.16 | 1.51, 3.10 | 2.24 | 1.56, 3.20 | ||
| Sociodemographics | ||||||||||
| Age, years | 0.57 | 0.83 | ||||||||
| <30 | 106 | 8.3 | 1,171 | 91.7 | 1.00 | Referent | 1.00 | Referent | ||
| ≥30 | 48 | 9.0 | 484 | 91.0 | 1.17 | 0.69, 1.98 | 1.06 | 0.61,1.84 | ||
| Race | 0.75 | 0.62 | ||||||||
| White | 79 | 8.3 | 873 | 91.7 | 1.00 | Referent | 1.00 | Referent | ||
| Black | 75 | 8.8 | 782 | 91.2 | 1.06 | 0.74, 1.53 | 0.90 | 0.59, 1.36 | ||
| Center | 0.43 | 0.40 | ||||||||
| Oakland, California | 42 | 8.6 | 447 | 91.4 | 1.00 | Referent | 1.00 | Referent | ||
| Birmingham, Alabama | 43 | 10.0 | 386 | 90.0 | 1.18 | 0.74, 1.89 | 1.25 | 0.78, 2.01 | ||
| Chicago, Illinois | 33 | 6.7 | 459 | 93.3 | 0.79 | 0.48, 1.30 | 0.79 | 0.47, 1.32 | ||
| Minneapolis, Minnesota | 36 | 9.0 | 363 | 90.4 | 1.07 | 0.65, 1.75 | 1.01 | 0.61, 1.69 | ||
| Time to conception, months | 0.09 | 0.37 | ||||||||
| <12 | 30 | 7.7 | 360 | 92.3 | 1.00 | Referent | 1.00 | Referent | ||
| 12–24 | 19 | 5.8 | 310 | 94.2 | 0.78 | 0.44, 1.37 | 0.79 | 0.44, 1.43 | ||
| >24 | 105 | 9.6 | 985 | 90.4 | 1.40 | 0.89, 2.20 | 1.39 | 0.71, 2.70 | ||
| Order of birth | 0.41 | 0.01 | ||||||||
| First birth | 116 | 8.3 | 1,277 | 91.7 | 1.13d | 0.85, 1.50 | 1.51d | 1.09, 2.10 | ||
| Second birth | 34 | 9.4 | 326 | 90.6 | ||||||
| Third birth | 4 | 8.0 | 46 | 92.0 | ||||||
| Fourth birth | 0 | 0.0 | 6 | 100 | ||||||
Abbreviations: BP, blood pressure; CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
Adjusted for family history of diabetes, parity at conception (time dependent), births during the interval, time to the first conception, smoking, age at preconception examination (continuous), and race; Ptrend test.
The numbers of women with body mass index ≥30 and waist girth >88 cm include the following: 36 (83.7%) in the GDM group and 156 (73.9%) in the non-GDM group.
Hypertension prior to pregnancy is defined as blood pressure ≥140/90 or taking antihypertensive medication.
Ordinal variable expressed as increase in risk of GDM per additional birth.
We evaluated all significant prepregnancy risk factors simultaneously in multivariate models to identify independent predictors of GDM accounting for all others (Table 4). Initial models were unadjusted for sociodemographic covariates and included either body mass index or waist circumference before pregnancy. Prepregnancy cardiometabolic risk factors were strongly associated with GDM pregnancy, with minimal influence of overall or central adiposity. In fact, body mass index and waist circumference were no longer independently associated with risk of GDM in multivariate models that included fasting glucose, insulin, and lipids.
Table 4.
Multivariate Adjusted Odds Ratios of GDM From a Single Model With All Prepregnancy Cardiometabolic Risk Factors for 1,809 Pregnancies in 1,164 Women, the CARDIA Study, 1985–2006
| Prepregnancy Risk Factors | All Risk Factors |
Ptrend | All Risk Factors Covariate Adjusteda |
Ptrend | ||
| OR | 95% CI | OR | 95% CI | |||
| Fasting | ||||||
| Glucose, mg/dL | <0.001 | <0.001 | ||||
| <90 | 1.00 | Referent | 1.00 | Referent | ||
| 90–99 | 1.53 | 0.96, 2.45 | 1.42 | 0.88, 2.29 | ||
| 100–125 | 4.54 | 2.16, 9.55 | 4.74 | 2.14, 10.51 | ||
| Insulin, μU/mL | 0.03 | 0.005 | ||||
| <10.0 | 1.00 | Referent | 1.00 | Referent | ||
| 10–15 | 1.38 | 0.87, 2.20 | 1.56 | 0.98, 2.48 | ||
| >15–20 | 1.71 | 0.91, 3.22 | 2.19 | 1.15, 4.17 | ||
| >20 | 1.90 | 1.02, 3.53 | 2.36 | 1.20, 4.63 | ||
| HDL-C, mg/dLb | ||||||
| Negative family history of diabetes | 0.002 | 0.005 | ||||
| <40 | 3.38 | 1.80, 6.34 | 3.07 | 1.62, 5.84 | ||
| 40–50 | 1.22 | 0.70, 2.14 | 1.18 | 0.67, 2.07 | ||
| >50 | 1.00 | Referent | 1.00 | Referent | ||
| Positive family history of diabetes | 0.87 | 0.99 | ||||
| <40 | 0.97 | 0.43, 2.19 | 0.90 | 0.39, 2.07 | ||
| 40–50 | 1.17 | 0.66, 2.07 | 1.19 | 0.66, 2.14 | ||
| >50 | 1.00 | Referent | 1.00 | Referent | ||
| Total cholesterol, mg/dL | 0.08 | 0.09 | ||||
| <150 | 1.00 | Referent | 1.00 | Referent | ||
| 150–180 | 1.40 | 0.83, 2.37 | 1.41 | 0.84, 2.39 | ||
| >180 | 1.59 | 0.94, 2.69 | 1.56 | 0.92, 2.64 | ||
| Triglycerides, mg/dL | 0.14 | 0.31 | ||||
| <70 | 1.00 | Referent | 1.00 | Referent | ||
| 70–119 | 1.26 | 0.86, 1.84 | 1.21 | 0.82, 1.79 | ||
| ≥120 | 1.37 | 0.77, 2.45 | 1.21 | 0.67, 2.17 | ||
| Body mass index, kg/m2 | 0.55 | 0.56 | ||||
| <25 | 1.00 | Referent | 1.00 | Referent | ||
| 25–29.9 | 0.92 | 0.57, 1.51 | 0.95 | 0.58, 1.57 | ||
| ≥30 | 1.22 | 0.70, 2.15 | 1.21 | 0.68, 2.17 | ||
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; OR, odds ratio.
Adjusted for race, family history of diabetes, parity at conception (time dependent), number of births during the interval, time to the first conception, smoking, and age at preconception examination. Prepregnancy risk factors are repeated measurements.
Pinteraction = 0.0475 for the test between family history of diabetes and HDL-C categories.
Multivariate risk factor models adjusted for body mass index and potential confounders showed that prepregnancy fasting glucose (100–125 vs. <90 mg/dL), middle and highest insulin levels (>15–20 and >20 vs. <10 μU/mL), and low high-density lipoprotein cholesterol (<40 vs. >50 mg/dL) plus negative family history of diabetes were each independently associated with increased odds of GDM: odds ratios = 4.74 (95% confidence interval (CI): 2.14, 10.51), 2.19 (95% CI: 1.15, 4.17), 2.36 (95% CI: 1.20, 4.63), and 3.07 (95% CI: 1.62, 5.84), respectively (all Ptrend < 0.01). Total cholesterol above 180 mg/dL showed a borderline 1.6 times higher odds of GDM independent of other risk factors (P = 0.09). Low levels of high-density lipoprotein cholesterol were associated with greater risk of GDM, but only for those with negative family history of diabetes (2-way interaction P = 0.0475). Adjustment for waist circumference instead of body mass index had little impact on the odds ratios (data not shown).
Fasting glucose and insulin showed stronger associations in the multivariate model as individual risk factors than as their product (HOMA-IR; data not shown). Covariates including race, parity, smoking, number and order of births in the interval, and age at delivery also had minimal impact on risk of GDM. In a sensitivity analysis, removal of repeat pregnancies within the same interval and multifetal pregnancies did not alter the findings (data not shown).
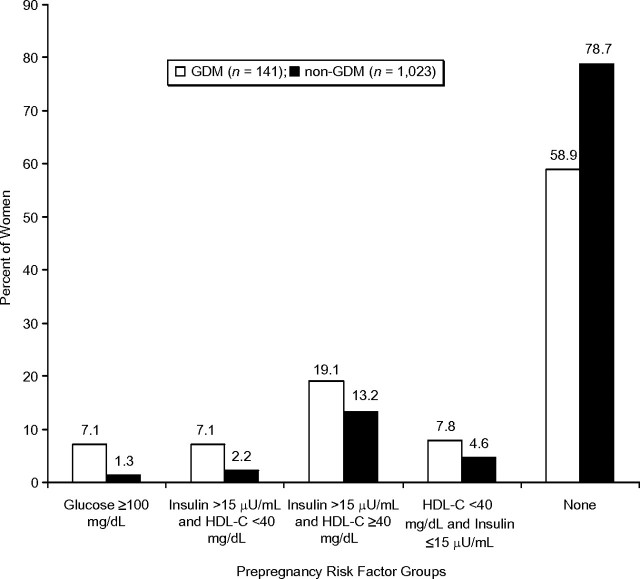
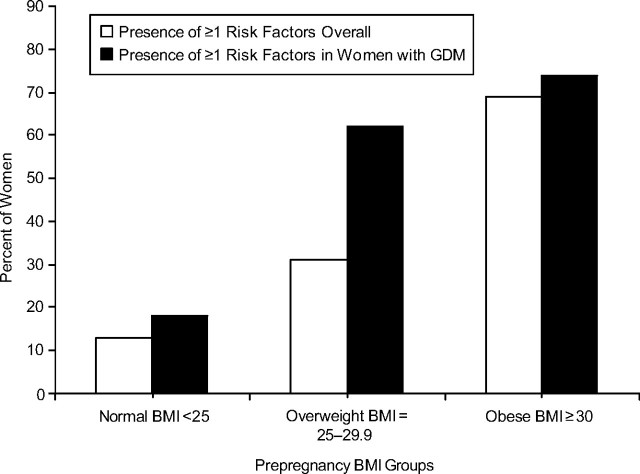
The prevalence of prepregnancy risk factors is shown in Figure 2 for 1,164 women. Impaired fasting glucose (100–125 mg/dL), with or without elevated fasting insulin (>15 μU/mL) or low high-density lipoprotein cholesterol levels (<40 mg/dL), was found in 7.1% of women who later developed GDM versus only 1.3% of women without GDM. Among women with normal fasting glucose (<100 mg/dL), prepregnancy fasting hyperinsulinemia combined with a low level of high-density lipoprotein cholesterol occurred in 7.1% of GDM women versus 2.2% of non-GDM women. Overall, 41.1% who developed GDM exhibited one or more cardiometabolic risk factors versus 21.3% with no GDM. The prevalence of prepregnancy risk factors (one or more) also varied by body mass index group (Figure 3). Only 12% of the normal body mass index group had one or more risk factors compared with 31% and 69% in overweight and obese groups, respectively. Among women who developed GDM, 62% of overweight women and 74% of obese women had one or more risk factors.
Figure 2.
Percentage of women by subsequent gestational diabetes mellitus status groups among prepregnancy cardiometabolic risk factor groups, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2006. Cardiometabolic risk factors include fasting glucose ≥100 mg/dL, fasting insulin >15 μU/mL, and/or high-density lipoprotein cholesterol <40 mg/dL. Cardiometabolic groupings are hierarchical. The category of fasting glucose ≥100 mg/dL includes women regardless of insulin or HDL-C. For all other risk factor groups, fasting glucose <100 mg/dL is combined with hyperinsulinemia (fasting insulin >15 μU/mL), with or without low HDL-C (defined as <40 mg/dL), or with low HDL-C alone. GDM, gestational diabetes mellitus; HDL-C, high-density lipoprotein cholesterol.
Figure 3.
Prevalence of 1 or more cardiometabolic risk factors (fasting glucose ≥100 mg/dL, fasting insulin >15 μU/mL, and/or high-density lipoprotein cholesterol <40 mg/dL) in women overall and among women with gestational diabetes mellitus across prepregnancy body mass index groups, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2006. BMI, body mass index; GDM, gestational diabetes mellitus.
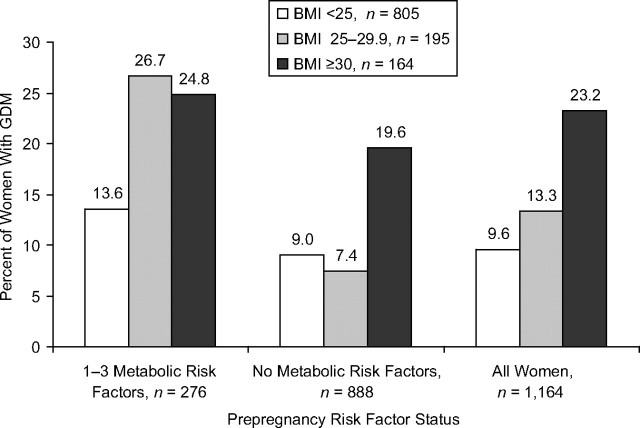
The rates of GDM varied by risk factors and body mass index groups (Figure 4). Among overweight women with one or more risk factors, the rate of GDM was 26.7% versus 7.4% in those with none. Differences in GDM rates across the risk factor groups (one or more vs. none) were smaller for other body mass index groups: 24.8% versus 19.6% in obese and 13.6% versus 9.0% in normal weight.
Figure 4.
Unadjusted risk of subsequent GDM as the percentage of women with GDM among prepregnancy BMI categories according to prepregnancy cardiometabolic risk factor status, the Coronary Artery Risk Development in Young Adults (CARDIA) Study, 1985–2006. The 1–3 metabolic risk factor category includes the following: fasting glucose ≥100 mg/dL, fasting insulin >15 μU/mL, and/or high-density lipoprotein cholesterol <40 mg/dL. Note: The group of 1–3 metabolic risk factors combines the 4 risk factor groups shown in Figure 2. BMI, body mass index; GDM, gestational diabetes mellitus.
DISCUSSION
In our study, the strongest predictors of GDM in a subsequent pregnancy were impaired fasting glucose (100–125 mg/dL), fasting hyperinsulinemia (>15 μU/mL), and low high-density lipoprotein cholesterol (<40 mg/dL) with negative family history of diabetes, and lastly, fasting insulin above 15 μU/mL before pregnancy; relative risks for GDM were 4.7, 3.1, and 2.4, respectively (all P < 0.01). These findings were adjusted for race, parity, family history of diabetes, body mass index or waist circumference, time from prepregnancy measurement to conception, age, and other confounders. Although overall adiposity and abdominal adiposity are antecedents to insulin resistance, prepregnancy obesity was no longer independently predictive of GDM after taking into account cardiometabolic risk profiles. We also found no association between prepregnancy blood pressure or hypertension and risk of GDM, possibly because of the low prevalence of these disorders in healthy women of reproductive age. By combining all prepregnancy risk factors into a single multivariate, adjusted model, we identified the respective independent associations with development of glucose intolerance during pregnancy (i.e., GDM).
Among 141 nondiabetic CARDIA Study women who subsequently developed GDM, impaired fasting glucose, elevated fasting insulin levels, and/or low high-density lipoprotein cholesterol were present before pregnancy in 41% of women (n = 58). Normoglycemia with at least one risk factor (low plasma high-density lipoprotein cholesterol and/or hyperinsulinemia) was present before pregnancy in 34% of women who developed GDM. Among overweight women, the presence of any cardiometabolic risk factors was associated with almost 4-fold higher GDM rates (26.7% for any vs. 7.4% for none).
Our analysis extends findings from previous studies that examined only prepregnancy clinical risk factors such as body size, weight gain, hypertensive conditions, or health behaviors retrospectively in relation to risk of GDM pregnancy. Two earlier prospective studies (27, 28) examined only 1 or 2 metabolic risk factors but did not report blood glucose data, and one had a very small sample (n < 10) (27). A study of first trimester plasma triglyceride levels reported a direct association with risk of GDM but did not measure other cardiometabolic risk factors (23). Plasma triglyceride levels steadily increase during gestation to a zenith of 300% above nonpregnant levels by the second to third trimester, but previous studies never assessed triglycerides as a predictor of GDM (44). Plasma high-density lipoprotein cholesterol below 40 mg/dL, a known strong correlate of type 2 diabetes mellitus in women after pregnancy, was also strongly associated with higher risk of GDM. The link between low prepregnancy high-density lipoprotein cholesterol and risk of GDM, to our knowledge, had not been previously explored.
Strengths of our study include the longitudinal cohort design and measurements before all pregnancies, no preexisting diabetes before pregnancy based on glycemia and/or diagnosis of diabetes, and prepregnancy cardiometabolic and clinical risk factor measurements in a large, population-based sample of women of reproductive age, of whom only 7% had ever taken lipid-lowering medications in the 20-year follow-up.
Limitations of our study include the variable time intervals for risk factor measurements before pregnancy and recurrent pregnancies for 20% of women within a single interval. However, we controlled for time from prepregnancy examination to conception and for number and order of births as covariates, and we utilized statistical methods that accounted for correlations between repeated pregnancies and prepregnancy risk factor measurements over multiple intervals for the same woman. Self-report of GDM by CARDIA Study women proved to be extremely reliable on the basis of our validation study (3). Because GDM is a heterogeneous disorder in which elevated glucose and insulin resistance are not evident before pregnancy, our study could not determine how well preconception risk factors predicted severity of GDM (i.e., post-meal defect vs. fasting hyperglycemia) because oral glucose tolerance test results during pregnancy were unavailable in the CARDIA Study. Future studies are needed that assess the rate of gestational weight gain before screening and diagnosis of GDM to examine whether prepregnancy cardiometabolic risk factors predict GDM independent of gestational weight gain.
Maternal overweight or obesity is the most common high-risk obstetric condition in the United States, affecting 45% of pregnant women (45). High prepregnancy weight has clinical importance for obstetricians primarily to identify women at risk for fetal macrosomia and delivery complications, as well as those likely to develop maternal metabolic abnormalities. In 1988–1997, about 39% of US women were overweight or obese (body mass index, ≥25) before pregnancy (45), which is comparable to 34% of CARDIA Study women who were classified as overweight or obese. In our study, 14% of overweight or obese women developed GDM, which is consistent with 5%–15% reported by others (46). Previous studies have reported a 2-fold to 6-fold higher risk of GDM for prepregnancy overweight and obesity (47–49). Our study found a 3-fold higher risk of GDM associated with obesity (body mass index, ≥30 vs. <25), but it was largely explained by metabolic risk factors, which were present in about 70% of the women. However, being overweight (body mass index, 25–29.9), along with unfavorable cardiometabolic risk factors, was related to higher risk of GDM.
In conclusion, prepregnancy impaired fasting glucose and hyperinsulinemia, individually or in combination with low high-density lipoprotein cholesterol in nondiabetic women, were strong predictors of GDM. At least one of these risk factors was present in over 40% of all women who later developed GDM.
For prevention of GDM before pregnancy, our findings suggest that interventions should be provided to all obese women and that overweight and normal weight women should be screened for cardiometabolic risk factors. Among 1,164 CARDIA Study women, 141 subsequently developed GDM, with a ratio of 8.2 women screened to 1 case of GDM.
Prevention of GDM is important for minimizing intrauterine exposure of the fetus to maternal metabolic abnormalities that program the fetus for future disease. The US Centers for Disease Control and Prevention (CDC) and the March of Dimes have recognized the importance of preconception health care for prevention of adverse pregnancy outcomes (50, 51). Among the recommendations, the CDC 2006 report stated that all women of child-bearing age should receive preconception care services, including “evidence-based risk screening, health promotion, and interventions” (50, p. 1). Screening for cardiometabolic risk is important for all young women. Measurement of fasting insulin, glucose, and high-density lipoprotein cholesterol levels during the postpartum and interconceptual periods (particularly for 30% of US women aged 20–39 years who are overweight) is feasible within the current health-care system and could identify a high-risk group for subsequent GDM pregnancy. All women with metabolic risk factors could benefit from interventions before and/or during early pregnancy to prevent GDM and from early screening for GDM during pregnancy. Metabolic risk factor screening during the preconception or interconceptual period may also motivate women to modify lifestyle behaviors, and it provides an opportunity not only to prevent GDM before pregnancy but also to reduce weight retention and central obesity that lead to type 2 diabetes and cardiovascular disease in midlife.
Acknowledgments
Author affiliations: Division of Research, Epidemiology, and Prevention, Kaiser Permanente Northern California, Oakland, California (Erica P. Gunderson, Charles P. Quesenberry, Jr., Juanran Feng, Stephen Sidney); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Department of Nutrition, University of Oslo, Oslo, Norway (David R. Jacobs, Jr.); and Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, Alabama (Cora E. Lewis).
This work was supported by the US National Institutes of Health (contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095 from the National Heart, Lung, and Blood Institute); the National Institute of Diabetes and Digestive and Kidney Diseases (career development award, grant K01 DK059944); and the American Diabetes Association (research award).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Glossary
Abbreviations
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- GDM
gestational diabetes mellitus
- HOMA-IR
homeostasis model assessment of insulin resistance
References
- 1.Sepe SJ, Connell FA, Geiss LS, et al. Gestational diabetes. Incidence, maternal characteristics, and perinatal outcome. Diabetes. 1985;34(suppl 2):13–16. doi: 10.2337/diab.34.2.s13. [DOI] [PubMed] [Google Scholar]
- 2.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson EP, Lewis CE, Tsai AL, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007;56(12):2990–2996. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer-Graf UM, Pawliczak J, Passow D, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. 2005;28(7):1745–1750. doi: 10.2337/diacare.28.7.1745. [DOI] [PubMed] [Google Scholar]
- 5.Malcolm JC, Lawson ML, Gaboury I, et al. Glucose tolerance of offspring of mother with gestational diabetes mellitus in a low-risk population. Diabet Med. 2006;23(5):565–570. doi: 10.1111/j.1464-5491.2006.01840.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers I EURO-BLCS Study Group. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27(7):755–777. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 7.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. [PubMed] [Google Scholar]
- 8.Di Cianni G, Volpe L, Lencioni C, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract. 2003;62(2):131–137. doi: 10.1016/j.diabres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Radesky JS, Oken E, Rifas-Shiman SL, et al. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol. 2008;22(1):47–59. doi: 10.1111/j.1365-3016.2007.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudra CB, Sorensen TK, Leisenring WM, et al. Weight characteristics and height in relation to risk of gestational diabetes mellitus. Am J Epidemiol. 2007;165(3):302–308. doi: 10.1093/aje/kwk007. [DOI] [PubMed] [Google Scholar]
- 11.Glazer NL, Hendrickson AF, Schellenbaum GD, et al. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733–737. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- 12.Hedderson MM, Williams MA, Holt VL, et al. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus [electronic article] Am J Obstet Gynecol. 2008;198(4):409.e1–409.e7. doi: 10.1016/j.ajog.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moses RG. The recurrence rate of gestational diabetes in subsequent pregnancies. Diabetes Care. 1996;19(12):1348–1350. doi: 10.2337/diacare.19.12.1348. [DOI] [PubMed] [Google Scholar]
- 14.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604. doi: 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratner RE, Hamner LH, III, Isada NB. Effects of gestational weight gain in morbidly obese women. I. Maternal morbidity. Am J Perinatol. 1991;8(1):21–24. doi: 10.1055/s-2007-999331. [DOI] [PubMed] [Google Scholar]
- 16.Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia [electronic article] Am J Obstet Gynecol. 2009;201(1):61.e1–61.e7. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saldana TM, Siega-Riz AM, Adair LS, et al. The relationship between pregnancy weight gain and glucose tolerance status among black and white women in central North Carolina. Am J Obstet Gynecol. 2006;195(6):1629–1635. doi: 10.1016/j.ajog.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Williams MA, Qiu C, Muy-Rivera M, et al. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(5):2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 19.Thadhani R, Wolf M, Hsu-Blatman K, et al. First-trimester sex hormone binding globulin and subsequent gestational diabetes mellitus. Am J Obstet Gynecol. 2003;189(1):171–176. doi: 10.1067/mob.2003.343. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Sauk J, Shah A, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27(1):21–27. doi: 10.2337/diacare.27.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 22.McLachlan KA, O'Neal D, Jenkins A, et al. Do adiponectin, TNF-alpha, leptin, and CRP relate to insulin resistance in pregnancy? Studies in women with and without gestational diabetes, during and after pregnancy. Diabetes Metab Res Rev. 2006;22(2):131–138. doi: 10.1002/dmrr.591. [DOI] [PubMed] [Google Scholar]
- 23.Enquobahrie DA, Williams MA, Qiu C, et al. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70(2):134–142. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Kitzmiller JL, Gavin LA, Gin GD, et al. Preconception care of diabetes. Glycemic control prevents congenital anomalies. JAMA. 1991;265(6):731–736. [PubMed] [Google Scholar]
- 25.Noussitou P, Monbaron D, Vial Y, et al. Gestational diabetes mellitus and the risk of metabolic syndrome: a population-based study in Lausanne, Switzerland. Diabetes Metab. 2005;31(4 pt 1):361–369. doi: 10.1016/s1262-3636(07)70205-7. [DOI] [PubMed] [Google Scholar]
- 26.Hedderson MM, Ferrara A. High blood pressure before and during early pregnancy is associated with an increased risk of gestational diabetes mellitus. Diabetes Care. 2008;31(12):2362–2367. doi: 10.2337/dc08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalano PM, Nizielski SE, Shao J, et al. Downregulated IRS-1 and PPARγ in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282(3):E522–E533. doi: 10.1152/ajpendo.00124.2001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Folsom AR, Flack JM, et al. Body fat distribution before pregnancy and gestational diabetes: findings from Coronary Artery Risk Development in Young Adults (CARDIA) Study. BMJ. 1995;311(7013):1139–1140. doi: 10.1136/bmj.311.7013.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12(1 suppl):1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 30.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 31.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151(12):1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 32.Steffen LM, Kroenke CH, Yu X, et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2005;82(6):1169–1177. doi: 10.1093/ajcn/82.6.1169. quiz 1363–1364. [DOI] [PubMed] [Google Scholar]
- 33.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 35.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6(3):235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 36.Lewis CE, Funkhouser E, Raczynski JM, et al. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144(3):247–254. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 37.Hanley AJ, Williams K, Gonzalez C, et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52(2):463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 38.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20(7):1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 40.American Heart Association, National Heart, Lung, and Blood Institute. Grundy SM, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Executive summary. Cardiol Rev. 2005;13(6):322–327. [PubMed] [Google Scholar]
- 41.Gordon-Larsen P, Boone-Heinonen J, Sidney S, et al. Active commuting and cardiovascular disease risk: the CARDIA Study. Arch Intern Med. 2009;169(13):1216–1223. doi: 10.1001/archinternmed.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis CE, Smith DE, Wallace DD, et al. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA Study. Am J Public Health. 1997;87(4):635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith DE, Lewis CE, Caveny JL, et al. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271(22):1747–1751. [PubMed] [Google Scholar]
- 44.Koukkou E, Watts GF, Lowy C. Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. J Clin Pathol. 1996;49(8):634–637. doi: 10.1136/jcp.49.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Table 2D. 2008 Pregnancy nutrition surveillance. Summary of health indicators. Pediatric and Pregnancy Nutrition Surveillance System. Atlanta, GA: Centers for Disease Control and Prevention; 2010. ( http://www.cdc.gov/pednss/pnss_tables/pdf/national_table2.pdf). (Accessed July 17, 2010) [Google Scholar]
- 46.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 47.Cnattingius S, Bergström R, Lipworth L, et al. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338(3):147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 48.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109(2 pt 1):419–433. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 49.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1–23. [PubMed] [Google Scholar]
- 51.The March of Dimes. Preconception health care can improve the lives of mothers and babies. White Plains, NY: March of Dimes Foundation; 2010. ( http://www.marchofdimes.com/professionals/14332_1156.asp). (Accessed July 17, 2010) [Google Scholar]