Abstract
Objectives
To determine whether nitrogen-containing bisphosphonate (NCBP) therapy is associated with the prevalence of cardiovascular calcification.
Background
Cardiovascular calcification correlates with atherosclerotic disease burden. Experimental data suggest that NCBP may limit cardiovascular calcification, which has implications for disease prevention.
Methods
The relationship of NCBP use to the prevalence of aortic valve, aortic valve ring, mitral annulus, thoracic aorta, and coronary artery calcification (AVC, AVRC, MAC, TAC, and CAC, respectively) detected by computed tomography was assessed in 3,636 women within the Multi-Ethnic Study of Atherosclerosis (MESA) using regression modeling.
Results
Analyses were age-stratified because of a significant interaction between age and NCBP use (interaction p-values: AVC p<0.0001; AVRC p<0.0001; MAC p=0.002; TAC p<0.0001; CAC p=0.046). After adjusting for age, body mass index, demographics, diabetes, smoking, blood pressure, cholesterol levels, and statin, hormone replacement, and renin-angiotensin inhibitor therapy, NCBP use was associated with a lower prevalence of cardiovascular calcification in women ≥65 years old (prevalence ratio [95% confidence interval]: AVC 0.68 [0.41, 1.13]; AVRC 0.65 [0.51, 0.84]; MAC 0.54 [0.33, 0.93]; TAC 0.69 [0.54, 0.88]; CAC 0.89 [0.78, 1.02]), whereas calcification was more prevalent in NCBP users among the 2,181 women <65 years old (AVC 4.00 [2.33, 6.89]; AVRC 1.92 [1.42, 2.61]; MAC 2.35 [1.12, 4.84]; TAC 2.17 [1.49, 3.15]; CAC 1.23 [0.97, 1.57]).
Conclusions
Among women in the diverse MESA cohort, NCBPs were associated with decreased prevalence of cardiovascular calcification in older subjects, but more prevalent cardiovascular calcification in younger ones. Further study is warranted to clarify these age-dependent NCBP effects.
Keywords: bisphosphonate, calcification, coronary artery, valve, vascular
INTRODUCTION
Atherosclerosis is a systemic disease frequently associated with calcification of the aorta, coronary and peripheral arteries and left heart valves through an active process that resembles bone formation.(1-5) Calcification at each anatomic site is a marker of atherosclerotic disease burden that is independently associated with increased morbidity and mortality.(6-9)
Experimental data from animal models and patients on chronic hemodialysis suggest that nitrogen-containing bisphosphonates (NCBPs; e.g., ibandronate, alendronate, risedronate, and zoledronate) may limit vascular and valvular calcification.(10-12) Bisphosphonates are primarily used in the management of osteoporosis to prevent osteoclast-mediated bone resorption by binding to hydroxyapatite.(13) Nitrogen-containing bisphosphonates inhibit farnesylpyrophosphate synthase, an enzyme in the mevalonate pathway distal to HMG-CoA reductase, the site of statin action.(14) Consequently, several pharmacologic effects are common to both NCBPs and statins. NCBPs decrease serum LDL-cholesterol levels by approximately 5%, raise HDL-cholesterol by 10-18%,(15,16) and reduce inflammation by inhibiting the secretion of several inflammatory cytokines.(17,18) However, NCBP inhibition of vascular and valvular calcification may alternatively be secondary to prevention of bone resorption and the subsequent release of calcium phosphate particles from bone.(11) Recent data suggesting that NCBPs and other osteoporosis therapies may slow the progression of aortic stenosis support this hypothesis.(19) Thus, NCBPs may provide a unique and novel means to slow cardiovascular calcification.
Despite experimental evidence that NCBPs may modulate cardiovascular calcification, clinical efficacy in this regard has not been assessed in patients with subclinical cardiovascular disease. We present the first evaluation of the relationship between NCBPs and the prevalence of cardiovascular calcification in women without recognized cardiovascular disease within a multiethnic, community-based cohort.
METHODS
Study Population and Data Collection
The Multi-Ethnic Study of Atherosclerosis (MESA) is a National Heart, Lung, and Blood Institute –sponsored longitudinal cohort study of 6,814 community-dwelling, men and women aged 45-84 years without evidence of clinical cardiovascular disease recruited from 6 U.S. communities (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA). Eligible subjects were sampled by self-reported race to generate an ethnically diverse cohort that was 38% white, 22% African American, 22% Hispanic, and 12% Asian. Participants were excluded if they carried a previous diagnosis of cardiovascular disease. Participants attended study visits that include physical examination, prescription medication review, and assessment of subclinical cardiovascular disease by trained study staff using a variety of noninvasive modalities according to standardized protocols. A complete description of the design of MESA has been published elsewhere.(20)
Data for the present study were taken from the first examination of the cohort (July 2000 to August 2002). This analysis was confined to the 3,710 women enrolled in MESA because the overwhelming majority of subjects (>93%) receiving NCBP therapy in MESA are women.
Measurement of Vascular and Valvular Calcification
Cardiovascular calcification was assessed by electron-beam CT at 3 centers and multi-detector row helical CT at 3 centers. All studies were interpreted at a central reading center (Harbour-UCLA Research and Education Institute, Los Angeles, CA). Aortic valve, aortic valve ring, mitral annulus, thoracic aorta, and coronary artery calcification (AVC, AVRC, MAC, TAC, and CAC, respectively) was quantified by the Agatston scoring method.(21) Detectable calcium was defined as a score >0 Agatston units (AU); a minimum focus of calcification was based on at least 4 contiguous voxels, resulting in identification of calcium of 1.15 mm3 with the multi-detector CT scanners (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 with the electron-beam CT scanners (0.68 × 0.68 × 3.00 mm). Details of the image acquisition and interpretation protocols, quality control measures and interobserver reliability characteristics have been reported.(22,23)
Aortic valve calcification was defined as any calcified lesion within the aortic valve leaflets. Aortic valve ring calcium was measured at the level of the aortic ring. Mitral annulus calcification was differentiated from that in the circumflex artery. Thoracic aorta calcification was quantified in the segment of the descending aorta imaged during cardiac CT. Coronary calcification was measured along the anatomic course of the coronary arteries.
Nitrogen-Containing Bisphosphonate Therapy
A validated medication inventory was used to assess medication use.(24) Subjects were asked to bring all prescribed and over-the-counter medication packages to each MESA visit, where trained study personnel recorded the names and dosages. NCBP therapy was defined by the use of any oral or intravenous NCBP, such as ibandronate, alendronate, risedronate, and zoledronate on the date of their cardiac CT scan.
Covariate Measurements
Standardized questionnaires were used to collect data on age, sex, ethnicity, and medical history. Information regarding physical activity was collected using a combination of self–administered and interviewer–administered questionnaires. Smoking status was defined as current, former, or never with current smoking defined as having smoked a cigarette in the last 30 days. Diabetes was defined as a fasting glucose ≥126 mg/dL or by the use of a hypoglycemic medication. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or by the use of medication prescribed for hypertension. Blood pressure was measured 3 times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon; General Electric, Madison, WI). The average of the second and third readings was recorded. Serum lipid levels were measured from blood samples obtained after a 12–hour fast. LDL cholesterol was calculated with the Friedewald equation.(25)
Statistical Analysis
Characteristics of the participants, including demographics, medical history, and serologic test results were evaluated according to NCBP use. Differences in characteristics across NCBP use were determined using the Student t-test for normally distributed continuous variables, the Wilcoxon-Mann-Whitney two-sample test for continuous variables not normally distributed, and chi-square test for categorical variables.
The primary outcomes for this analysis were the prevalences of AVC, AVRC, and MAC, TAC, and CAC >0 AU at initial CT scan evaluation. Relative risk regression was used to model prevalence ratios (PR) assessing the relationships between NCBP use and baseline prevalence of AVC, AVRC, MAC, TAC, and CAC, adjusting for identified confounders using robust variance estimation.(26) To incorporate severity of calcification into our analyses, we logarithmically transformed calcification scores (natural log [Agatston units + 1]) to normalize their distribution. Linear regression models using log (Agatston units + 1) as the dependent variable were then used to assess associations with NCBP use while adjusting for potential confounders. Covariates potentially related to vascular and valvular calcification were selected for inclusion in multivariable models a priori. Interactions were tested for as multiplicative terms in the statistical models only for the main effects (NCBP use and calcification). When an interaction was detected, the results were reported as a stratified analysis. We used multiple imputation techniques to adjust for missing data in 2% of subjects. Statistical analyses were performed using SAS (version 9.1.3, SAS institute, Inc., Cary, NC) with significance accepted at p<0.05. Prevalence ratios are reported with 95% confidence intervals.
RESULTS
Subject Characteristics
A total of 3,710 female MESA participants were included. The mean age was 63 years (range, 45-84 years) and mean body mass index was 29 ± 6. Of this cohort, 1,411 (38%) were white, 1,025 (28%) black, 814 (22%) Hispanic, and 460 (12%) Chinese. The medical history included diabetes in 481 (13%), hypertension in 1,529 (41%), hyperlipidemia in 1,532 (41%), current tobacco use in 415 (11%) and past tobacco use in 1086 (29%). Five hundred ninety-eight were taking lipid-lowering medication (16%) and 1,253 (34%) antihypertensive medication. The mean systolic blood pressure was 127 ± 23 mmHg and diastolic blood pressure 68 ± 10 mmHg. The mean fasting total serum cholesterol was 200 ± 36, LDL-cholesterol 117 ± 32, HDL-cholesterol 57 ± 15, and triglycerides 128 ± 71 mg/dl.
Two hundred fourteen subjects (6%) were receiving NCBP therapy at baseline (Table 1). NCBP users were on average older (67 ± 8 vs 62 ± 10 years; p<0.0001) and had lower BMI (25 ± 5 vs 28 ± 6; p<0.0001) than non-users (n=3,423). A greater proportion of NCBP users were receiving lipid-lowering medications compared to non-users (21 vs 16% p=0.06), whereas a smaller proportion were taking a reninangiotensin system inhibitor (7 vs 12%, p=0.02). Serum LDL-cholesterol levels were lower (113 ± 33 vs 118 ± 32 mg/dl; p=0.04) and HDL-cholesterol levels higher (61 ± 17 vs 57 ± 15 mg/dl; p<0.0001) in NCBP-users than in non-users.
Table 1.
Baseline characteristics stratified by NCBP use.
| Patient Characteristics | NCBP Users (N = 214) | Non-Users (N = 3,496) | P-value |
|---|---|---|---|
| Age, mean (SD), yrs | 67 (8) | 62 (10) | <0.0001 |
| Race, No. (%) | |||
| White | 126 (59) | 1285 (37) | <0.0001 |
| Black | 33 (15) | 992 (28) | |
| Hispanic | 24 (11) | 790 (23) | |
| Chinese | 31 (14) | 429 (12) | |
| Serum cholesterol, mean (SD), mg/dl | |||
| Total Cholesterol | 198 (37) | 200 (36) | 0.55 |
| LDL-cholesterol | 113 (33) | 118 (32) | 0.04 |
| HDL-cholesterol | 59 (17) | 56 (15) | <0.0001 |
| Triglycerides | 120 (56) | 128 (71) | 0.04 |
| Diabetes Mellitus, N (%) | 20 (9) | 461 (13) | 0.12 |
| Hyperlipidemia, N (%) | 100 (47) | 1432 (41) | 0.12 |
| Hypertension, N (%) | 80 (37) | 1449 (41) | 0.41 |
| Smoking Status, N (%) | |||
| Former | 78 (36) | 1008 (29) | 0.02 |
| Current | 17 (8) | 398 (11) | 0.12 |
| Concurrent Medication, N (%) | |||
| RAS inhibitor | 14 (7) | 421 (12) | 0.02 |
| B-Blocker | 20 (9) | 360 (10) | 0.73 |
| Calcium Channel Blocker | 33 (15) | 471 (13) | 0.41 |
| Diuretic | 35 (16) | 633 (18) | 0.58 |
| Statin | 42 (20) | 527 (15) | 0.08 |
| Blood Pressure, mean (SD), mmHg | |||
| Diastolic | 68 (10) | 69 (10) | 0.09 |
| Systolic | 125 (23) | 127 (23) | 0.40 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; RAS, renin-angiotensin system.
Relationship of Bisphosphonate Use to Cardiovascular Calcification
The prevalences of AVC, AVRC, MAC, TAC, and CAC, defined as calcification scores >0 AU, were 11, 34, 11, 29, and 40%, respectively. The prevalences of AVRC and MAC were similar in NCBP users and non-users (AVRC 38 vs 34%; MAC 10 vs 11%; respectively), but NCBP use was associated with more prevalent aortic valve and vascular calcification (AVC 15 vs 10%; TAC 35 vs 28%; CAC 47 vs 40%).
Significant interactions between age and NCBP use were observed for each measure of cardiovascular calcification (interaction p-values: AVC p<0.0001; AVRC p<0.0001; MAC p=0.002; TAC p<0.0001; CAC p=0.046). Consequently, we age-stratified subsequent analyses of calcification using the midpoint of the MESA age range. Because of the established differences in the prevalence and severity of osteoporosis across ethnic groups,(27) we also tested for interactions between NCBP use and ethnicity for each measure of calcification. No significant interactions with race were detected for any of the calcification measures in the entire cohort (interaction p-values: AVC p=0.13; AVRC p=0.56; MAC p=0.95; TAC p=0.14; CAC p=0.21), in women <65 years old (AVC p=0.75; AVRC p=0.66; MAC p=0.57; TAC p=0.24; CAC p=0.56), or in women ≥65 years old (AVC p=0.14; AVRC p=0.66; MAC p=0.86; TAC p=0.74; CAC p=0.43)
Across all measures of cardiovascular calcification, the association with NCBP use (Table 2) varied with age. In female subjects ≥65 years old, NCBP use was associated with less prevalent cardiovascular calcification (NCBP users vs non-users: AVC 13 vs 20%, p=0.11; AVRC 38 vs 59%, p<0.0001; MAC 11 vs 21%, p=0.01; TAC 38 vs 54%, p<0.0001; CAC 57 vs 63%, p=0.27). In contrast, cardiovascular calcification was more prevalent in NCBP users younger than 65 years (AVC 18 vs 4%, p<0.0001; AVRC 38 vs 17%, p=0.0001; MAC 9 vs 3%, p=0.009; TAC 31 vs 11%, p<0.0001; CAC 36 vs 25%, p=0.02).
Table 2.
Prevalence of valvular or vascular calcification stratified by bisphosphonate use.
| Calcification Site | Bisphosphonate Users (N = 214) | Non-Users (N = 3496) | P-value |
|---|---|---|---|
| Aortic Valve, N (%) | |||
| Age <65 years | 18 (18) | 78 (4) | <0.0001 |
| Age ≥65 years | 15 (13) | 279 (20) | 0.11 |
| Aortic Valve Ring, N (%) | |||
| Age <65 years | 38 (38) | 348 (17) | <0.0001 |
| Age ≥65 years | 43 (38) | 832 (59) | <0.0001 |
| Mitral Annulus, N (%) | |||
| Age <65 years | 9 (9) | 70 (3) | 0.009 |
| Age ≥65 years | 13 (11) | 303 (21) | 0.01 |
| Thoracic Aorta, N (%) | |||
| Age <65 years | 31 (31) | 224 (11) | <0.0001 |
| Age ≥65 years | 43 (38) | 766 (54) | <0.0001 |
| Coronary Arteries, N (%) | |||
| Age <65 years | 36 (36) | 516 (25) | 0.02 |
| Age ≥65 years | 65 (57) | 885 (63) | 0.27 |
Prevalent calcification defined as a calcification score >0 Agatston units.
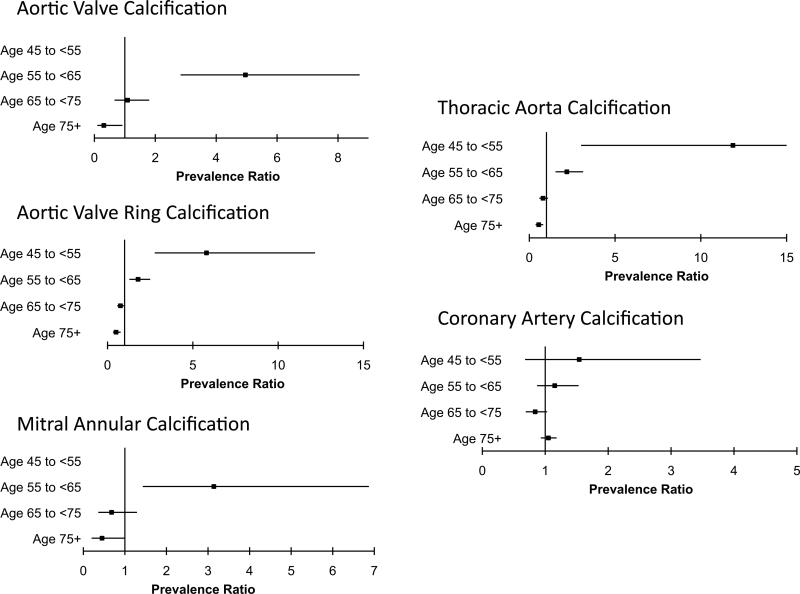
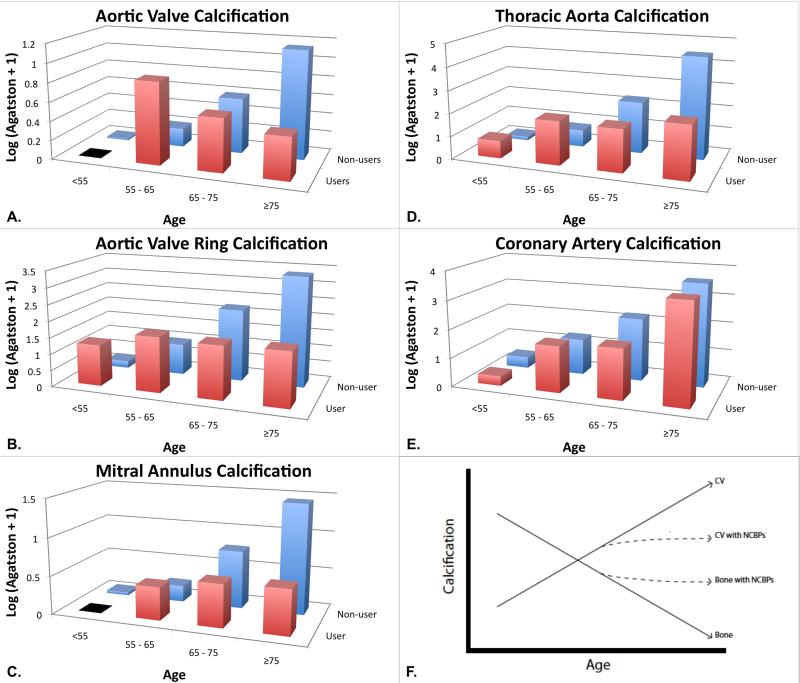
Multivariate regression models adjusting for age, body mass index, ethnicity, education, income, health insurance status, physical activity, study site, smoking, systolic and diastolic blood pressure, LDL- and HDL-cholesterol levels, diabetes mellitus, hypertension, and statin, hormone replacement, and renin-angiotensin inhibitor therapy similarly demonstrated that NCBP correlates were age-dependent. Among 1,498 women ≥65 years old, NCBP use was associated with a lower prevalence ratio of cardiovascular calcification (Table 3; AVC PR=0.68 [95% CI 0.41, 1.13], p=0.14; AVRC 0.65 [95% CI 0.51, 0.84], p=0.0008; MAC 0.54 [95% CI 0.33, 0.93], p=0.02; TAC 0.69 [95% CI 0.54, 0.88], p=0.003; CAC 0.89 [95% CI 0.78, 1.02], p=0.09). In contrast, NCBP use was associated with a higher prevalence ratio of calcification at each anatomical site in the 2,181 female subjects <65 years of age (Table 3; AVC PR=4.00 [95% CI 2.33, 6.89], p<0.0001; AVRC 1.92 [95% CI 1.42, 2.61], p<0.0001; MAC 2.35 [95% CI 1.12, 4.84], p=0.02; TAC 2.17 [95% CI 1.49, 3.15], p<0.0001; CAC 1.23 [95% CI 0.97, 1.57], p=0.09). Finer grouping by 10-year age strata demonstrated a gradual reduction in NCBP-associated calcification with increasing age despite adjusting for the aforementioned confounders (Figure 1). The relationship of NCBP therapy to prevalent CAC followed a similar pattern but did not reach statistical significance. Similar age-dependent relationships were noted between the natural logarithm of calcification score plus 1 and NCBP use at each anatomic site such that calcification was more significant in younger NCBP users compared to non-users, but less significant in older NCBP users compared to non-users (Figure 2A-E). We observed no consistent relationship between NCBP use and the severity of cardiovascular calcification alone. To eliminate the possibility of residual confounding by statin use, we performed parallel analyses of the prevalence of cardiovascular calcification stratified by statin use. Although power was diminished by this added stratification, effect measures were consistent.
Table 3.
Prevalence ratio for vascular and valvular calcification with bisphosphonate use stratified by age.
| Calcification site | Prevalence ratio | 95% Confidence Interval | P-value | Interaction with Age P-value |
|---|---|---|---|---|
| Aortic Valve | <0.0001 | |||
| Age <65 years | 4.00 | 2.33 – 6.89 | <0.0001 | |
| Age ≥65 years | 0.68 | 0.41 – 1.13 | 0.14 | |
| Aortic Valve Ring | <0.0001 | |||
| Age <65 years | 1.92 | 1.42 – 2.61 | <0.0001 | |
| Age ≥65 years | 0.65 | 0.51 – 0.84 | 0.003 | |
| Mitral Annulus | 0.002 | |||
| Age <65 years | 2.35 | 1.12 – 4.84 | 0.02 | |
| Age ≥65 years | 0.54 | 0.33 – 0.93 | 0.02 | |
| Thoracic Aorta | <0.0001 | |||
| Age <65 years | 2.17 | 1.49 – 3.15 | <0.0001 | |
| Age ≥65 years | 0.69 | 0.54 – 0.88 | 0.003 | |
| Coronary Arteries | 0.046 | |||
| Age <65 years | 1.23 | 0.97 – 1.57 | 0.09 | |
| Age ≥65 years | 0.89 | 0.78 – 1.02 | 0.09 |
Models adjust for age, body mass index, ethnicity, study site, education, income, health insurance status, diabetes, hypertension, smoking, physical actively, blood pressure, serum cholesterol, statin therapy, and use of renin-angiotensin system inhibitors and hormone replacement therapy.
Figure 1. Bisphosphonate-associated prevalence ratio of prevalent cardiovascular calcification stratified by age.
The prevalence ratio of cardiovascular calcification associated with NCBP use is increased in young patients, but decreases with age and becomes significantly reduced in the elderly. Models adjust for age, body mass index, ethnicity, study site, education, income, health insurance, diabetes, hypertension, smoking, physical activity, blood pressure, serum cholesterol levels, and statin, hormone replacement, and renin-angiotensin inhibitor therapy.
Figure 2. (A-E) Relationship of NCBP use to log (Agatston scores + 1) at each anatomic site is age-dependent.
NCBP use appears to be associated with higher log (Agatston score + 1) in younger subjects but lower log (Agatston score + 1) in older subjects when compared to NCBP non-users. Thus NCBP use appears to be associated with an attenuated age-dependent increase in log (Agatston score + 1). (F) Theoretical schematic demonstrating relationship of calcification in bone and cardiovascular tissues and the proposed effects of NCBPs. Bone and cardiovascular calcification are inversely related. Cardiovascular calcification increases with advancing age in concert with lessening BMD. NCBPs may attenuate both of these processes. As NCBPs are prescribed predominantly for patients with osteoporosis, their use serves as a marker for those with low BMD and the associated increased risk of cardiovascular calcification seen in younger subjects.
DISCUSSION
In this ethnically diverse cohort of women, we demonstrated robust and consistent relationships between the prevalence of cardiovascular calcification and NCBP use. We found a clear interaction of NCBP use with age such that there was reduced prevalence of vascular and valvular calcification in women ≥65 years of age and increased prevalence of cardiovascular calcification in younger women on treatment. Finer age stratification supported a gradual transition of risk with increasing age.
Independent data from MESA and other observational studies have associated osteoporosis with cardiovascular calcification.(28-30) However, the mechanisms responsible for the inverse relationship between cardiovascular calcification and bone mineral density (BMD) are not known. In cell culture models, valve and bone cell types respond in opposite ways to statins, with reduced calcification in aortic valve myofibroblasts and augmented calcification in a pre-osteoblast cell line.(31) Similar effects have been demonstrated in response to inflammatory cytokines and oxidized LDL in other models of vascular and bone calcification.(30,32) Among the proposed explanations for this paradox is that chronic inflammation in response to oxidized LDL modulates calcification differently in vascular tissue and in bone, resulting in tandem calcification of soft tissue and softening of calcified tissues.(30) These observations suggest that NCBPs, which lead to increases in BMD, may be inversely associated with cardiovascular calcification.
NCBPs have the potential to influence calcium homeostasis in cardiovascular tissue by several mechanisms. NCBPs exert pleiotropic, statin-like effects that include inhibition of protein prenylation,(14) various inflammatory processes,(14,17,33) and vitamin K metabolism,(11) each of which has been associated with atherosclerotic disease.(3,11,31,34-36) Inhibition of isoprenoid synthesis, which is required for vitamin K metabolism, affects several vitamin K-dependent bone regulatory proteins, such as osteocalcin and matrix Gla protein.(11,37) NCBPs inhibit secretion of interleukin (IL)-1β, IL-6, TNF-α, and several matrix metalloproteinases in a variety of cell types and reduce numbers of circulating monocytes and tissue macrophages at the time of vascular injury.(17,18,33) NCBPs may also reduce serum lipids, which accumulate in vessel walls and valve leaflets triggering calcification.(15,16) Alternatively, cardiovascular effects of NCBPs may be related to selective inhibition of bone resorption by which calcium-phosphate mineral complexes released into the bloodstream are deposited in vascular and valvular tissues.(11) Hence, though the effects of NCBPs may be similar to statins in some respects, the two drug classes may have disparate actions mediated by effects on bone metabolism.
In a recent retrospective analysis of echocardiographic data, Skolnick and colleagues demonstrated in patients with a mean baseline aortic valve area of 1.33 cm2, that the use of bisphosphonates, calcitonin, or estrogen receptor modulators for osteoporosis was associated with a mean annual change in aortic valve area of -0.10±0.18 cm2 compared to -0.22±0.22cm2 in those not receiving these medications (p<0.03).(19) Our findings in subjects ≥ 65 years of age lend support to this previous report and suggest that the observed effects on progression of aortic stenosis may be due to modulation of calcification within the cardiovascular system, broaden the potential impact of bisphosphonate drugs to include direct or indirect effects on aortic, coronary artery, and mitral valve calcification.
We found a surprising association of NCBP use with increased prevalence of cardiovascular calcification in those subjects younger than 65 years of age. While the reasons for this relationship are elusive, there are several possible explanations. First, low BMD has been associated with increased risk of cardiovascular calcification.(28-30) Given that NCBP therapy is prescribed predominantly for patients with osteoporosis, treatment may identify a group of patients in whom an increased risk of cardiovascular calcification is associated with low BMD (Figure 2F). With age, and presumably longer duration of NCBP use the putative protective effect of these agents may overcome BMD-associated cardiovascular risk thereby reversing the association. Previous data from MESA demonstrated that compared to those women in the highest quartile of bone density, those in the lowest quartile displayed a modestly increased risk of prevalent CAC (adjusted PR 1.16 [95%CI 0.96, 1.41]) but no difference in risk of prevalent aortic calcification (adjusted PR 1.02 [95%CI 0.93, 1.12]).(28) These effects were smaller in magnitude than those that we observed with NCBP use, suggesting that NCBPs may exert a direct cardiovascular effect beyond modulating BMD. Alternatively, bisphosphonate use in younger women may be a marker for another disease. The U.S. Preventive Services Task Force recommends screening postmenopausal women beginning at age 60 years for osteoporosis if they are at increased risk and routinely for those over 65 years.(38) As such, women who warrant therapy at a young age possess a more aggressive form of osteoporosis, secondary osteoporosis, or other diseases that places them at higher risk of cardiovascular calcification. Lastly, a true age-dependent mechanism of NCBP action or of the relationship between osteoporosis and atherosclerosis remains. Further clarification of the observed age-dependent associations between NCBP use and cardiovascular calcification requires knowledge of indication for NCBP use and duration of use, neither of which were available for this analysis. Future evaluations of NCBP effects on cardiovascular calcification are warranted with particular attention to age, indication for NCBP, and duration of use.
Our findings suggest that NCBPs may modulate cardiovascular calcification, a surrogate marker for atherosclerosis and cardiovascular outcomes. Whether attenuation of cardiovascular calcification alone, without interruption of the underlying atherosclerotic process, improves cardiovascular morbidity and mortality remains controversial.(39) NCBPs may attenuate elements of the atherosclerotic process through several pleiotropic effects,(11,15-18,33) but further study is needed to elucidate the clinical impact of these effects. Unfortunately, randomized trials evaluating NCBPs, until recently, have not reported cardiovascular events. The HORIZON Recurrent Fracture Trial, a randomized, trial evaluating the benefits and safety of zoledronate therapy after low-trauma hip fracture, provides the most substantial supportive evidence.(40) Annual intravenous administration of zoledronate was associated with a trend towards reduced cardiovascular mortality compared to placebo (3.4 vs 4.9%; p=0.10). No such effect was suggested among patients enrolled in the HORIZON Pivotal Fracture Trial who had not experienced prior hip fracture.(41) Additional studies are needed to specifically address the effects of NCBP use on cardiovascular morbidity and mortality.
Given our cross-sectional study design, lack of serological data on levels of calcium, phosphate, parathyroid hormone, vitamin D, and various inflammatory markers, and unavailability of data on duration of NCBP use, the effects of NCBP use on cardiovascular calcification cannot be defined mechanistically. Moreover, the relatively good health of the MESA cohort limits our ability to evaluate the development or progression of cardiovascular calcification over time. Despite these limitations, the robust and consistent associations between NCBPs and vascular and valvular calcification within a cohort of relatively healthy women highlight the need for further study of the effect of NCBPs on cardiovascular calcification and to explore their therapeutic potential. Additional studies are needed to elucidate the mechanisms involved and to evaluate whether NCBP's may exert harmful effects in younger women.
In summary, this study suggests an association between nitrogen-containing bisphosphonate drug therapy and valvular, aortic, and coronary arterial calcification in women with subclinical cardiovascular disease. Given similarities between the effects of NCBP and statin agents on lipid metabolism and protein prenylation and the beneficial effects of NCBPs on bone metabolism, these drugs warrant further investigation as potential therapeutic agents in elderly women prone to calcific cardiovascular disease. It is also important to determine whether an apparently detrimental increase in the prevalence of cardiovascular calcification in younger women reflects heightened cardiovascular risk associated with osteoporosis, a toxic effect of this type of medication, or another age-dependent processes.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: MESA was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. Dr. Elmariah is supported by The Glorney-Raisbeck Fellowship Program, Corlette Glorney Foundation and The New York Academy of Medicine, by the GlaxoSmithKline Research and Education Foundation for Cardiovascular Disease, and by a grant from the National Heart Lung, and Blood Institute (T32 HL007824).
ABBREVIATIONS LIST
- AU
Agatston units
- AVC
aortic valve calcification
- AVRC
aortic valve ring calcification
- BMD
bone mineral density
- CAC
coronary artery calcification
- CT
computed tomography
- NCBP
nitrogen-containing bisphosphonate
- MAC
mitral annulus calcification
- MESA
Multi-Ethnic Study of Atherosclerosis
- TAC
thoracic aorta calcification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Elmariah receives grant support from the New York Academy of Medicine, the National Heart, Blood, and Lung Institute, and GlaxoSmithKline. Dr. O'Brien possesses grants from Abbott Laboratories, the National Heart, Blood and Lung Institute, and the National Institute of Diabetes and Kidney Diseases, receives speaker honoraria from AstraZeneca, Merck, and Sanofi-Aventis, and is a consultant for Boehringer-Ingelheim, Merck, and Novartis. Dr. Budoff has a modest consulting agreement with the General Electric Company. Dr. Fuster receives grant support from the National Heart, Blood, and Lung Institute and chairs the HRP study with is funded by BG Medicine. Dr. Halperin receives consulting fees from Astellas Pharma, Bayer AG Healthcare, the Bristol-Myers Squibb/Sanofi Partnership, Boehringer-Ingelheim, Daiichi Sankyo, Johnson & Johnson, Sanofi-Aventis and honoraria from Genzyme and Portola Pharmaceuticals and is co-chairman of the IMPACT trial, which is sponsored by Biotronik.
REFERENCES
- 1.Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation. 2006;113:861–6. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto H, Shavelle D, Takasu J, et al. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am Heart J. 2003;146:153–9. doi: 10.1016/S0002-8703(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 3.Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–13. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Subramaniam M, Springett M, et al. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–5. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–7. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 7.Eisen A, Tenenbaum A, Koren-Morag N, et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: association with cardiovascular events and death. Circulation. 2008;118:1328–34. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 8.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–6. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 9.Kohsaka S, Jin Z, Rundek T, et al. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc Imaging. 2008;1:617–23. doi: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariyoshi T, Eishi K, Sakamoto I, Matsukuma S, Odate T. Effect of etidronic acid on arterial calcification in dialysis patients. Clin Drug Investig. 2006;26:215–22. doi: 10.2165/00044011-200626040-00006. [DOI] [PubMed] [Google Scholar]
- 11.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–24. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 12.Nitta K, Akiba T, Suzuki K, et al. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44:680–8. [PubMed] [Google Scholar]
- 13.Russell RG, Rogers MJ, Frith JC, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14(Suppl 2):53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 14.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 15.Adami S, Braga V, Guidi G, Gatti D, Gerardi D, Fracassi E. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res. 2000;15:599–604. doi: 10.1359/jbmr.2000.15.3.599. [DOI] [PubMed] [Google Scholar]
- 16.Montagnani A, Gonnelli S, Cepollaro C, et al. Changes in serum HDL and LDL cholesterol in patients with Paget's bone disease treated with pamidronate. Bone. 2003;32:15–9. doi: 10.1016/s8756-3282(02)00924-9. [DOI] [PubMed] [Google Scholar]
- 17.Pennanen N, Lapinjoki S, Urtti A, Monkkonen J. Effect of liposomal and free bisphosphonates on the IL-1 beta, IL-6 and TNF alpha secretion from RAW 264 cells in vitro. Pharm Res. 1995;12:916–22. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- 18.Corrado A, Santoro N, Cantatore FP. Extra-skeletal effects of bisphosphonates. Joint Bone Spine. 2007;74:32–8. doi: 10.1016/j.jbspin.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol. 2009;104:122–4. doi: 10.1016/j.amjcard.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 20.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Budoff MJ, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–72. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 23.Detrano RC, Anderson M, Nelson J, et al. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–84. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 27.Thomas PA. Racial and ethnic differences in osteoporosis. J Am Acad Orthop Surg. 2007;15(Suppl 1):S26–30. doi: 10.5435/00124635-200700001-00008. [DOI] [PubMed] [Google Scholar]
- 28.Hyder JA, Allison MA, Wong N, et al. Association of Coronary Artery and Aortic Calcium With Lumbar Bone Density: The MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennisi P, Signorelli SS, Riccobene S, et al. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int. 2004;15:389–95. doi: 10.1007/s00198-003-1550-9. [DOI] [PubMed] [Google Scholar]
- 30.Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20:2346–8. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Elmariah S, Kaplan FS, Cheng G, Mohler ER., 3rd Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts: implications for end-stage valvular heart disease. Arterioscler Thromb Vasc Biol. 2005;25:592–7. doi: 10.1161/01.ATV.0000154278.01871.64. [DOI] [PubMed] [Google Scholar]
- 32.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 33.Danenberg HD, Fishbein I, Gao J, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 34.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Lee JS, Ko YJ, et al. Inhibitory effect of simvastatin on the TNF-alpha-and angiotensin II-induced monocyte adhesion to endothelial cells is mediated through the suppression of geranylgeranyl isoprenoid-dependent ROS generation. Arch Pharm Res. 2008;31:195–204. doi: 10.1007/s12272-001-1141-2. [DOI] [PubMed] [Google Scholar]
- 36.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–7. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 37.Berkner KL. The vitamin K-dependent carboxylase. J Nutr. 2000;130:1877–80. doi: 10.1093/jn/130.8.1877. [DOI] [PubMed] [Google Scholar]
- 38.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–41. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol. 2009;6:681–8. doi: 10.1038/nrcardio.2009.165. [DOI] [PubMed] [Google Scholar]
- 40.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. N Engl J Med. 2007;357:nihpa40967. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]