Abstract
Heightened perception of facial cues is at the core of many theories of social behavior and its disorders. In the present study, we continuously measured electrocortical dynamics in human visual cortex, as evoked by happy, neutral, fearful, and angry faces. Thirty-seven participants endorsing high versus low generalized social anxiety (upper and lower tertiles of 2,104 screened undergraduates) viewed naturalistic faces flickering at 17.5 Hz to evoke steady-state visual evoked potentials (ssVEPs), recorded from 129 scalp electrodes. Electrophysiological data were evaluated in the time-frequency domain after linear source space projection using the minimum norm method. Source estimation indicated an early visual cortical origin of the face-evoked ssVEP, which showed sustained amplitude enhancement for emotional expressions specifically in individuals with pervasive social anxiety. Participants in the low symptom group showed no such sensitivity, and a correlational analysis across the entire sample revealed a strong relationship between self-reported interpersonal anxiety/avoidance and enhanced visual cortical response amplitude for emotional, versus neutral expressions. This pattern was maintained across the 3500 ms viewing epoch, suggesting that temporally sustained, heightened perceptual bias towards affective facial cues is associated with generalized social anxiety.
Keywords: steady-state, EEG, social anxiety, faces, KDEF, facial expressions, social phobia
Introduction
Attentional biases that subserve an intense apprehension of interpersonal scrutiny and negative evaluation have been proposed to underlie social phobia, in particular the generalized subtype (Hofmann et al., 2004; Rosenberg et al., 2010). To test perceptual hyper-sensitivity to social threat cues in socially anxious individuals, static facial expressions have often been presented and much of the data has been interpreted in accordance with the vigilance-avoidance hypothesis—that perception of threat-relevant stimuli in anxious individuals is characterized by initial hypervigilance and consequent defensive avoidance (Mogg et al., 1997). For example, the dot-probe paradigm entails a spatial cueing procedure in which participants make a speeded response to a probe (e.g., a letter) when it replaces a cue in the same visual hemifield (e.g., angry face) after approximately 500 to 1000 ms. Typically, response latencies to hemifield probes are reduced when preceded by fear-relevant (angry or disgusted faces) as opposed to neutral cues and this pattern is enhanced with social anxiety and interpreted as evidence of early hypervigilance (Amir et al., 2009; Klumpp & Amir, 2009; Mogg & Bradley, 2002; Mogg et al., 2004; Pishyar et al., 2004). Although the dot-probe task has yielded compelling results implicating altered spatial attention to facial displays in social anxiety it is difficult to disentangle attentional effects such as facilitation to versus impaired disengagement from fear-relevant stimuli (Bogels & Mansell, 2004; Mogg et al., 1997). Furthermore, discrete reaction times represent the outcome of a cascade of perceptual processes as well as explicit decision-making and speeded motor responses.
More temporal information about orienting in social anxiety has been revealed via eye-tracking methodology. When pairs of computer-generated faces are presented, high compared to low socially anxious individuals more often fixate initially on the angry or happy relative to neutral face (Wieser, Pauli, Weyers, et al., 2009) and show impairments when directed to inhibit reflexive orienting towards emotional as well as neutral expressions (Wieser, Pauli, Mühlberger, 2009). Horley and colleagues (2003, 2004) found that over the course of prolonged presentations (i.e., 10 seconds) of single facial expressions social phobia patients relative to controls demonstrated increased scanning over the face coupled with reduced fixations on the eyes, a pattern accentuated in response to aversive facial displays. Overall, emerging oculomotor data suggests that social phobia is associated with biased orienting to facial displays, especially those connoting interpersonal threat. However, it should be noted that these findings do not inform on covert shifts of attention or other aspects of the fear response (e.g., physiological arousal). Furthermore, disengagement or perceptual avoidance of viewed stimuli is difficult to quantify due to the involvement of several saccades (Garner et al., 2006; Mogg et al., 1997).
As electrophysiological techniques are sensitive to covert attention processes and provide continuous measures of attention fluctuations easily related to behavioral (Ihssen et al., 2007; Keil & Heim, 2009), oculomotor (Müller et al., 2008), probe reflex (Löw et al., 2008; Schupp et al., 1997) and autonomic (Keil et al., 2008; Moratti et al., 2006) measures they can productively complement the aforementioned studies of social anxiety. Primarily utilizing event-related potentials (ERPs) obtained by means of time-domain averaging multiple segments of electrocortical activity, investigators have assessed attentional allocation to facial expressions over a wide range of exposure durations (500 ms – 10 s). Overall, the findings have been mixed (Kolassa & Miltner, 2006; Rossignol et al., 2007) but suggest that social anxiety is associated with enhanced visual occipito-temporal P1 to faces irrespective of expression (Helfinstein et al., 2008; Kolassa et al., 2007; Kolassa et al., 2009; Mueller et al., 2009; Mühlberger et al., 2009; Wieser et al., 2010) and right temporo-parietal N170 (Kolassa & Miltner, 2006; Wieser et al., 2010) to angry and happy faces. There is also some evidence in later centro-parietal P3/LPP responses that aversive facial displays are more significant in high socially anxiety (Moser et al., 2008; Sewell et al., 2008; but see Wieser et al., 2010) although correspondent enhancements to neutral faces have also been observed as a function of elevated interpersonal apprehension (Mühlberger et al., 2009)1. In the few studies (Kolassa et al., 2009; Mühlberger et al., 2009) that assessed relative electrocortical responses to expression-types within hedonic valence (pleasant, aversive), socially anxious participants showed commensurate amplitude enhancements across aversive contents (i.e., angry, sad, disgusted, fearful faces).
Concerning neurophysiological underpinnings, hemodynamic imaging studies have revealed that relative to controls socially anxious individuals and patients show exaggerated activation of limbic circuitry, particularly amygdala (Birbaumer et al., 1998; Evans et al., 2008; Phan et al., 2006; Schneider et al., 1999; Stein et al., 2002; Straube et al., 2004, 2005; Veit et al., 2002), in response to emotional facial expressions, again presented for a wide range of durations (i.e., 100 ms – 2500 ms). Less frequently an a priori area of interest, concurrent hyper-reactivity has also been observed in the extrastriate visual cortex (Evans et al., 2008; Goldin et al., 2009; Pujol et al., 2009; Straube et al., 2004; 2005). This covariation accords with functional data from unselected (Sabatinelli et al., 2009) as well as specific phobia populations during viewing of fear-relevant pictures (Ahs et al., 2009; Sabatinelli et al., 2005; Schienle et al., 2005) and with structural data from non-human primates revealing such reciprocal cortico-amygdaloid connections (Amaral, Behniea, & Kelly 2003).
Although the spatial resolution is superior to ERP methods, functional MRI findings lack the temporal specificity necessary for characterizing proposed attention dynamics in social anxiety. Here, we used steady-state visual evoked potentials (ssVEPs) as a continuous measure of visual cortical engagement in processing a visual stimulus (Müller, et al., 1998). The ssVEP is an oscillatory response to stimuli modulated in luminance (i.e., flickered), in which the frequency of the electrocortical response recorded from the scalp equals that of the driving stimulus (Müller, Teder-Salejarvi, et al., 1998; Regan, 1989). Of significant advantage, the oscillatory cortical response is of known frequency and can thus be reliably separated from noise and quantified in the frequency domain (Wang et al., 2007). Generators of the ssVEP have been localized to extended visual cortex (Müller et al., 1997), with strong contributions from V1 and higher-order cortices (Di Russo et al., 2007). Importantly, ssVEPs reflect multiple excitations of the visual system with the same stimulus over a brief epoch. Thus, changes in driven neural mass activity can be affected both by initial sensory processing and by subsequent re-entrant, top-down modulation of sensory activity by higher order processes (Keil et al., 2001; Silberstein et al., 1995).
Enhanced sensory responding as indexed with ssVEP amplitude covaries with resource allocation of attention to the driving stimulus. Signal energy increases are reliably observed to visual stimuli as a function of instructed attention (Müller et al., 2003), fear conditioning (Moratti et al., 2006, Moratti & Keil, 2005), and emotional arousal (Keil et al., 2003; 2008), showing high sensitivity to both intrinsic and extrinsic motivation. Viewing pleasant and unpleasant relative to neutral pictures at 10 Hz has consistently shown amplitude enhancements in occipito-parietal recording sites (Keil et al., 2003; Moratti et al., 2004). The affect-modulation of the ssVEP has been proposed to reflect sensory gain enhancement for motivationally significant processing via re-entrant modulatory activity (Keil et al., 2009) from connections such as the parieto-frontal cortex and amygdaloid complex (Baizer et al., 1993; Iwai & Yukie, 1987). As noted, the fMRI findings on social anxiety suggest that pervasive interpersonal apprehension may be characterized by a similar tuning of the sensory gain to emotional facial expressions.
Accounting for time course information, ssVEPs show a near sinusoidal waveform mirroring the stimulus frequency, often accompanied by higher harmonics (Riemslag et al., 1985). Because of their known frequency, ssVEPs can be analyzed not only in the frequency domain (Regan, 1989) but also the time-frequency domain (Müller et al., 2008). These latter analyses allow researchers to follow the stimulus-evoked contour of the ssVEP at the frequency of interest and thus obtain a continuous measure of visual cortical facilitation related to the eliciting stimulus at near-optimum time resolution. For instance Müller and colleagues (Müller, Teder-Salejarvi, et al., 1998) found that the time varying amplitude of the ssVEP during shifts of spatial attention corresponded with near-simultaneous behavioral performance. The ssVEP is especially suited to detecting fluctuations in selective attention over extended time periods, as the recorded signal remains stationary in its spatial distribution: Unlike transient event-related potentials that consist of characteristic spatio-temporal electrocortical dynamics moving over the scalp, ssVEPs represent a stable visual cortical signal, with stable topography, sensitive to temporal changes in visual processing (Hillyard, et al., 1997). Essentially ssVEP amplitude provides a productive index of time-varying engagement of neural masses in sensory cortex to a continuously presented stimulus (Regan & Spekreijse, 1977) and hence, informs on the procession of stimulus salience and of top-down regulation of attention.
In the present study, we were interested in the visual cortical dynamics during prolonged (i.e., multiple seconds) exposure to face stimuli varying in emotional expression, for which ssVEP is a judicious dependent variable. Naturalistic facial expressions were presented at 17.5 Hz for 3500 ms to individuals varying in generalized social anxiety and the oscillatory cortical response was recorded with a high-density electrode array (129 sensors). Neutral, happy, fearful, and angry expressions were included as all four conditions have rarely been assessed simultaneously (Mühlberger et al., 2009), thereby precluding conclusions regarding the specificity of enhanced attention in social anxiety. Electrocortical responses were analyzed using the minimum norm estimate (MNE), a distributed source projection algorithm (see methods). Although not providing precise neuroanatomical localization, the MNE as implemented here allows one to infer the gross location of the origin of the surface-recorded signal. In addition, generators that are tangential with respect to the scalp tend to be represented as one active area in the MNE, as opposed to two areas, which is the case with voltage or Laplacian maps (Hauk et al., 2002). This is particularly important in situations where sources are located in lower-tier visual cortex, often oriented tangentially to the scalp. Continuous MNE was in turn submitted to time-frequency-domain transformation, enabling analysis of changes on the level of modeled sources rather than on the level of voltage maps. The resulting time-varying ssVEP amplitude was averaged into three epochs corresponding to two early windows (100-500 ms, 500-1000 ms) for assessing the possibility of group differences in initial vigilance and a later window to assess sustained processing of the stimulus (1500-3500 ms). These early segments were selected on the basis of preceding work with ssVEP during selective attention tasks, suggesting that the early ramp and the early plateau of the ssVEP (100-500 and 500-1000 ms post-stimulus) may be differentially sensitive to task instructions (Müller & Hillyard, 2000) or stimulus properties (Keil et al., 2006). Thus, with the three segments chosen, we intended to cover aspects of early and late sustained processing, compromising between high time resolution (small time windows) and signal-to-noise (long time windows), where appropriate.
As briefly reviewed, the accumulating literature on attentional biases to facial expressions in social anxiety results from the implementation of a wide range of recording methodologies and stimulus types (naturalistic, schematic, computer-generated) presented for highly variable time intervals (100 ms – 10 s). Taken together, the findings remain tentative but generally implicate a hypersensitivity to emotional (particularly angry) expressions evident at multiple time windows. Thus, in the current study high social anxiety was expected to be characterized by electrocortical hyperreactivity primarily in occipito-parietal regions to emotional (most pronounced to angry) expressions that would be sustained throughout the 3500 ms viewing epoch. An alternative hypothesis, although not strongly supported by empirical data, is also considered here based on patient reports of interpersonal behavior (Trower & Gilbert, 1989) and prominent cognitive-behavioral models of social anxiety (Clark & Wells, 1995; Roth & Heimberg, 2001; Schultz & Heimberg, 2008). That is a pattern also consistent with the vigilance-avoidance hypothesis (Mogg et al., 1997) in which high social anxiety is associated with an amplified cortical response to threatening faces early in the viewing epoch followed by a reduction, reflecting initial hypersensitivity followed by covert disengagement.
Methods
Participants
Thirty-seven (62% female; 76% Caucasian) students from University of Florida undergraduate psychology courses participated for course credit (mean age=19.65; SD=1.69). All participants reported corrected to normal vision and a negative personal and family history of seizure disorder. Participants were recruited based on responses to the self-report form of the Liebowitz Social Anxiety Scale (LSAS-SR; Fresco et al., 2001) collected during an online screening procedure. The LSAS-SR is a 24-item scale that indexes the extent of experienced anxiety (0=none; 3=severe) and frequency of avoidance (0=never; 3=usually) of 24 social interaction and performance situations during the preceding week. The LSAS total, a composite score of social anxiety and avoidance shown to correlate highly with interview-based measures of social anxiety severity (Heimberg et al., 1999) was utilized for screening 2,104 individuals (M=38.92; SD=21.03; MD=36). To help ensure the participation of individuals high and low in social anxiety, those endorsing LSAS-SR total scores in the upper and lower tertiles were invited to participate.
The 37 participants who attended the laboratory session were re-administered the LSAS-SR (M=49; SD=22.37; MD=50). Participants endorsing below 40 (n=14) were identified as “low socially anxious”, and those above 60 (n=14) as “high socially anxious”. The gender ratios were equivalent in the high (50% male) and low (42.9% male) symptom groups, X2 (1) = 0.14, ns. Dichotomous groups were distinguished to enhance generalizability to clinical samples (Borkovec & Rachman, 1979) and, as shown in Table 1, the resulting mean total (M=72.5) and subscale scores for the high socially anxious group are similar to those observed for treatment-seeking individuals diagnosed with generalized social anxiety disorder (LSAS-SR: Baker et al., 2002; Fresco et al., 2001; Rytwinski et al., 2009; LSAS-I: Cox et al., 1998; Heimberg & Holaway, 2007). Furthermore, the normative subscale scores for the Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995) for the same participants indicate that rather than reflecting broad dysphoria, the elevated distress in this group is specific to social anxiety (Table 1).
Table 1.
Questionnaire subscale scores (means and standard deviations) for high and low social anxiety groups.
| Measure/Subscale | Low Social Anxiety (N=14) | High Social Anxiety (N=14) | Group Effect |
|---|---|---|---|
| Liebowitz Social Anxiety Scale (LSAS-SR) | |||
| Total social fear & avoidance | 25.07 (9.06) | 72.50 (8.64) | F(1,26) = 201.04, p < .001 |
| Interaction fear | 5.71 (3.07) | 18.79 (3.29) | F(1,26) = 118.14, p < .001 |
| Interaction avoidance | 5.93 (4.07) | 17.93 (3.29) | F(1,26) = 73.65, p < .001 |
| Performance fear | 7.57 (2.24) | 19.21 (3.58) | F(1,26) = 106.44, p < .001 |
| Performance avoidance | 5.93 (2.21) | 17.93 (4.20) | F(1,26) = 71.28, p < .001 |
| Mood & Anxiety Symptom Questionnaire (MASQ) | |||
| Non-specific distress | 30.43 (10.71) | 34.71 (8.56) | F(1,26) = 1.37, ns |
| Non-specific anxiety | 18.29 (5.59) | 21.0 (5.35) | F(1,26) = 1.72, ns |
| Non-specific depression | 19.64 (7.23) | 25.14 (8.64) | F(1,26) = 3.34, ns |
| Anhedonia | 52.29 (7.93) | 55.36 (10.14) | F(1,26) = 0.80, ns |
| Anxious arousal | 25.57 (8.87) | 23.64 (5.39) | F(1,26) = 0.49, ns |
Note. Liebowitz Social Anxiety Scale Self-report Version (LSAS-SR; Fresco et al., 2001); Mood & Anxiety Symptom Questionnaire (MASQ; Watson & Clark, 1991; 1995).
Stimuli
Ninety-six pictures were selected from the Karolinska Directed Emotional Faces (KDEF; Lundqvist, Flykt, & Ohman (1998); http://www.facialstimuli.com/) of 24 actors gazing directly at the viewer (12 female, 12 male actors) posing 4 different expressions (neutral, happy, fearful, angry). Stimuli were pre-processed by means of the MATLAB image processing toolbox to have equal overall lightness and color composition.
Pilot Study: Normative Ratings of Facial Expressions
For comparison with prior face-processing studies (e.g., Kolassa & Miltner, 2006) as well as ssVEP investigations of emotional scenes (e.g., Keil et al., 2003), an online pilot study was conducted to gather normative affective ratings of the 96 stimuli selected for the laboratory session. This study allowed us to collect complementary evidence as to whether or not the face stimuli were effective in conveying the intended expression and, in turn, elicited a reliable affective response in viewers. The rating study also aimed to inform the present study regarding potential stimulus differences in emotional intensity, known to affect the ssVEP (Moratti et al., 2004). For course credit 140 students (mean age=19.65; SD=0.99; 67.9% female) from undergraduate psychology courses provided informed consent and then viewed and rated pseudorandom presentations of each facial expression with the Self-Assessment Manikin (SAM; Bradley & Lang, 1994) to assess three dimensions: experienced pleasure, emotional arousal, and dominance. See Table 2 for means and standard deviations. Repeated measures ANOVAs were performed separately on these dimensions with facial expression as the within-subjects factor. Arousal varied across expressions, F(3,137)=52.78, p<.001, ηp 2=0.54, with the most intense ratings for angry, followed by fearful, happy, and lastly neutral. The mean arousal for all expressions reliably differed, contrasts Fs=6.92-154.54, ps<.01, including the two most arousing conditions, fearful versus angry, F(1,139)=6.92, p<.01. Expression also influenced hedonic valence, F(3,137)=258.92, p<.001, ηp 2=0.85, with the most aversion reported for angry, followed by fearful, and the most pleasure for happy expressions. Similar to rated arousal, the mean pleasure for all expressions reliably differed, contrasts Fs=48.64-782.44, ps<.001. Participants endorsed intermediate dominance ratings across all expressions, F(3,137)=1.93, ns, indicating feeling neither in control or dominated.
Table 2.
Normative pleasure, arousal and dominance ratings (N=140) of KDEF stimuli on Self-Assessment Manikin (SAM) by expression
| Facial Expression | Pleasure | Arousal | Dominance |
|---|---|---|---|
| Neutral | 4.62 (0.54) | 3.61 (1.36) | 5.28 (1.85) |
| Happy | 7.12 (0.98) | 4.47 (1.76) | 5.29 (2.21) |
| Fearful | 3.75 (1.02) | 4.93 (1.46) | 4.81 (2.16) |
| Angry | 3.41 (1.09) | 5.07 (1.49) | 5.21 (2.03) |
Note. Pleasure rated on SAM (Bradley & Lang, 1994; Lang, 1980): 1=Completely unhappy, 9=Completely happy; Arousal: 1=Completely relaxed, 9=Completely aroused; Dominance 1=Completely dominant, 9=Completely submissive.
Laboratory Procedure
Upon providing informed consent in the laboratory, participants were seated in a sound-attenuated, dimly lit room and the EEG sensor net was attached. Participants were instructed that a series of pictures would be displayed and that they should view each picture for the duration of presentation, keeping their eyes comfortably focused on the center of the screen.
Controlled by an IBM-compatible computer running MATLAB and functions from the Psychtoolbox suite (Brainard, 1997) faces were presented 116 cm from the participant on a 51 cm monitor with a vertical refresh rate of 70 Hz, subtending a visual angle of 5° horizontally and 6.9° vertically. Pictures were presented in a random order, each presentation flickering at a rate of 17.5 Hz (i.e., 1 cycle=28.57 ms picture + 28.57 ms of black screen) for 3428 ms (60 cycles) followed by a randomly variable 2-4 second inter-trial interval.
At the completion of the EEG recording participants were administered the LSAS-SR and the MASQ (Watson et al., 1995) and then debriefed.
EEG Recording & Data Collection
Electroencephalogram (EEG) was continuously recorded from 129 electrodes using an Electrical Geodesics (EGI) HydroCel high-density EEG system with NetStation software on a Macintosh computer. The EEG recording was digitized at a rate of 250 Hz, using Cz as a recording reference. As suggested for the EGI high input impedance amplifier by the manufacturer, and supported by empirical studies of signal-to-noise ratios under varying impedance levels (Ferree et al., 2001), electrode impedances were kept below 50 kΩ. This procedure is standard in studies using the EGI dense-array system and has been validated in a plethora of published studies of EEG and event-related potentials (Junghöfer et al., 2000). A subset of electrodes located at the outer canthi and below the right eye was used to determine the horizontal and vertical electrooculogram (EOG). All channels were preprocessed online by means of 0.1-Hz high-pass and 100 Hz low-pass filtering. Epochs were extracted from the continuously recorded EEG relative to the onset of each picture, using 300 ms pre- and 4400 ms post-picture onset, data were low-pass filtered at a frequency of 30 Hz (48 dB/octave, 18th order Butterworth filter) and then submitted to the procedure proposed by Junghöfer and colleagues (2000), as implemented in the EMEGS software suite provided by Peyk and Junghöfer (www.emegs.org). This procedure used statistical parameters of the data to exclude channels and trials that were contaminated with artifacts. Recording artifacts were first detected using the recording reference (i.e., Cz), and then global artifacts were detected using the average reference, which was used for all analyses. Cz was used for recording only, and the average reference was calculated after artifact rejection and used for all subsequent analyses. The average reference is considered an acceptable solution to the problem of reference-dependent topographies (Junghöfer et al., 1997) and is particularly suitable in multi-electrode studies with sufficient coverage of the volume conductor, i.e., the head (Bertrand, Perrin, & Pernier, 1985). Subsequently, distinct sensors from particular trials were removed based on the distribution of their amplitude, standard deviation, and gradient. Data at eliminated electrodes were replaced with a statistically weighted spherical spline interpolation from the full channel set (Junghöfer et al., 2000). After artifact correction, an average of 72% of trials/condition was retained in the analyses.
Steady-state VEP Analyses
Grand mean time-locked averages of the voltages recorded during the angry expressions for all 37 participants are shown in Figure 1 to demonstrate that the expression conditions evoked reliable and pronounced 17.5-Hz oscillation, clearly time-locked across conditions and separable from noise.
Figure 1.
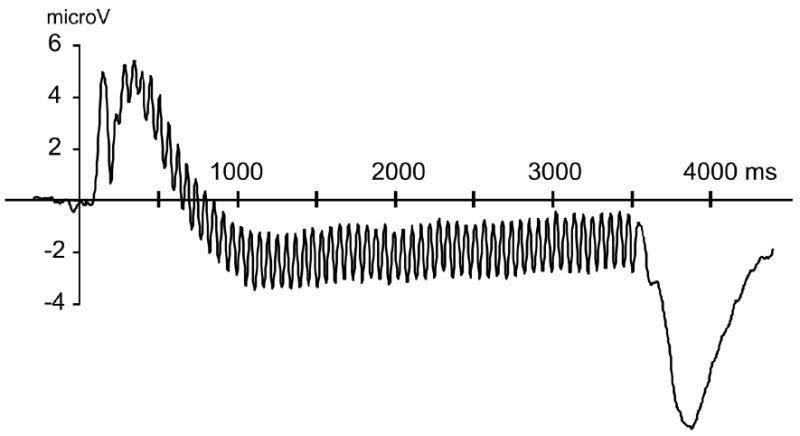
Grand mean time-locked averages of the voltages over eight occipital sensors recorded during angry expressions for all 37 participants are shown to demonstrate that the expression conditions evoked reliable and pronounced 17.5-Hz oscillation, clearly time-locked across conditions and separable from noise.
Artifact free epochs of the voltage data were averaged by condition and projected to an estimated source space using the minimum norm estimation (MNE) method proposed by Hauk and collaborators (Hauk, 2004; Hauk et al., 2002). Among other advantages, this source projection method reduces the amount of topographical variability between individual participants, because it tends to veridically represent the origin of tangential dipolar electric fields: The ssVEP elicited by rapid flicker has been shown to have deep, mostly calcarine sources, associated with tangential dipolar fields. Deep sources tangential to the scalp surface may result in very different voltage maps, depending on the orientation of the generator structure in a given participant. The MNE reduces this variability dramatically (Hauk et al. 2002) by indicating a spatially smeared maximum near the true signal origin instead of remote positive and negative voltage gradients. This is especially beneficial for studies of interindividual differences.
On a source space consisting of four concentric spheres, dipoles were placed equidistantly to approximate the brain volume. This source space contained 655 source locations (i.e., the model sources). A high number of model sources is needed for the initial calculation of the MNE to capture the scalp-recorded potential at sufficient spatial resolution, to avoid mislocalization (Hauk, 2004). To capture voltage gradients in all possible directions currents were modeled for three spatial orientations orthogonal to each other (1 radial, two tangential relative to the scalp surface) at each source location. The four shells had the radii 0.8, 0.6, 0.4, and 0.2 relative to the electrode radius of 1. For regularization the Tikhonov–Philips approach was used to suppress uncorrelated noise (Hauk, 2004). From the source space, the shell at 0.6 of the radius was selected as a compromise between depth sensitivity and spatial resolution (Hauk et al., 2002). After calculation of the solutions, the 655 model sources were reduced by selecting the 129 sources located closest to the electrode positions, for mapping purposes and statistical analyses. This procedure leaves the originally estimated source distribution intact (Hauk et al., 2002). At each of the resulting 129 source locations the time-varying amplitude at the stimulation frequency of 17.5 Hz was extracted by means of complex demodulation (Regan, 1989), separately for the three orientations of the MNE. The averaged condition data were multiplied with a sine and cosine function at the stimulation frequency. The resulting time series were then digitally low-pass filtered using a third-order Butterworth filter, set at a cutoff of 1.5 Hz. This led to sensitivity of the resulting waveforms to amplitude changes between 16 Hz and 19 Hz, with a center frequency of 17.5 Hz. The time resolution of the resulting metric was 120 ms (full width at half maximum). Next, the three dipole orientations at each source location were combined by means of the modulus (Euclidean distance) for a measure of current density (nanoamperes/mm2). Subsequently, the time course of the ssVEP was baseline corrected for each channel by subtracting the mean time-varying ssVEP current density in a segment between -240 to -120 ms, prior to stimulus onset. This baseline segment ended well before stimulus onset, to avoid contamination of the baseline estimate with post-stimulus activity due to the temporal smearing of the complex demodulation.
For each participant and condition the ssVEP source strength was averaged into three epochs corresponding to two early windows (100-500 ms, 500-1000 ms) for assessing the possibility of group differences in initial vigilance and a later window to assess more sustained processing (1500-3500 ms, see the preceding text for the rationale underlying these temporal segments). Repeated measures ANOVA was performed with group status (high versus low social anxiety) as the between-subjects factor and facial expression and time as the within-subjects factor. ssVEPs have been shown to strongly co-vary with rated emotional arousal (Keil et al., 2003; 2009), thus contents were entered according to the linear increase in arousal for KDEF stimuli demonstrated in the normative sample (i.e., neutral, happy, fearful, angry) as well as previous samples (Mühlberger et al., 2009). To provide a quantitative means of assessing significant interactions of expression condition and group, within-group repeated measures analyses and simple planned contrasts of ssVEPS to emotional (happy, fearful, angry) relative to neutral displays were utilized. Effects of time were similarly analyzed. Wilks’ lambda addressed sphericity issues (Vasey & Thayer, 1987).
As observed in previous steady-state examinations (e.g., Müller et al., 2008) dipole source strength was most pronounced over the occipital pole, near electrode Oz. Thus, mean time-varying amplitudes in the three time windows (100-500 ms, 500-1000 ms, 1500-3500 ms) were averaged across an occipital electrode cluster comprising Oz as well as 7 neighboring sensors (70 73 74 75 81 82 83 88) for each participant.
Results
High versus Low Social Anxiety: ssVEP Dipole Source Strength to Facial Expressions
In figure 2 the time-varying ssVEP amplitude for the four expression conditions is shown for participants high and low social in anxiety defined according to the total symptom score of the LSAS-SR.
Figure 2.
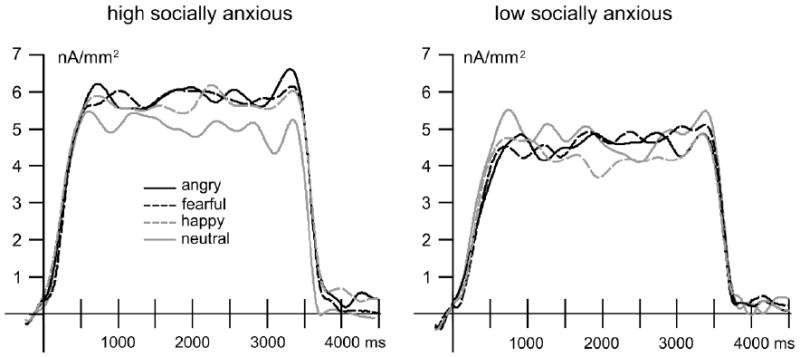
The time-varying ssVEP amplitude (nA/mm2) averaged over eight occipital sensors for the angry, fearful, happy and neutral facial expression conditions for participants high and low social in anxiety.
Repeated-measures ANOVA with group status (high versus low social anxiety) as the between-subjects factor and facial expression (neutral, happy, fearful, angry) and time (100-500 ms, 500-1000 ms, 1500-3500 ms) as the within-subjects factors revealed that mean ssVEP amplitudes varied over the presentation epoch, F(2,25)=18.15, p<.001, ηp 2=0.59, in a pattern similar across groups, Time × Group, F(2,25)=0.13, ns, and expression, Time × Expression, F(6,21)=2.56, p=.05, ηp 2=0.42; Time × Expression × Group, F(6,21)=1.13, ns. A significant interaction emerged between high and low socially anxious participants in relative sensitivities to expressions, Group × Expression, F(3,24)=5.08, p<.01, ηp 2=0.39, whereas ssVEP amplitudes did not differ by expression when considered irrespective of group status, F(3,24)=0.37, ns, and no overall group difference emerged in the mean amplitude of evoked responses, F(1,26)=0.51, ns.2
Follow-up tests of the main effect of time reflected that as shown in Figure 2 the mean ssVEP amplitude reliably increased from the first (100-500 ms) to second stimulation epoch (500-1000), F(1,27)=38.67, p<.001, ηp 2=0.59, and then maintained this level for the duration of the presentation, F(1,27)=0.34, ns.3
To explore the omnibus group by expression interaction, repeated measures analyses were performed separately for the groups (averaging across epochs). While low socially anxious individuals demonstrated no modulation of the ssVEP amplitude as a function of expression (Figure 2), F(3,11)=2.16, ns, high socially anxious participants tended to show greater sensitivity to emotional displays, F(3,11)=3.55, p=.05, ηp 2=0.49: happy, fearful, and angry expressions each elicited larger mean amplitude responses than neutral presentations, Fs=5.03-6.91, ps<.05. Follow-up univariate ANOVAs on the difference scores demonstrated that this augmentation in the high socially anxious group for happy, F(1,26)=7.16, p<.05, ηp 2=0.22, fearful, F(1,26)=8.33, p<.01, ηp 2=0.24, and angry, F(1,26)=12.86, p<.01, ηp 2=0.33, relative to neutral expressions, all reliably exceeded the respective source strength differences in the low socially anxious group. Notably, as shown in Figure 2 the increase in amplitude relative to neutral for the high socially anxious group was similar across happy, fearful, and angry expressions, indicating a broad sensitivity to emotional facial displays.
The difference in the topographical distribution of the ssVEP source strength for emotional relative to neutral expressions (averaged across epochs) by symptom group is illustrated in Figure 3. As high socially anxious participants showed enhanced sensitivity to emotional faces regardless of specific expression, the mean amplitude increases during happy, fearful, and angry expressions were also averaged for this analysis. Notably, the increased amplitude to emotional versus neutral contents in the high symptom group was distinctly circumscribed to the visual cortex.
Figure 3.
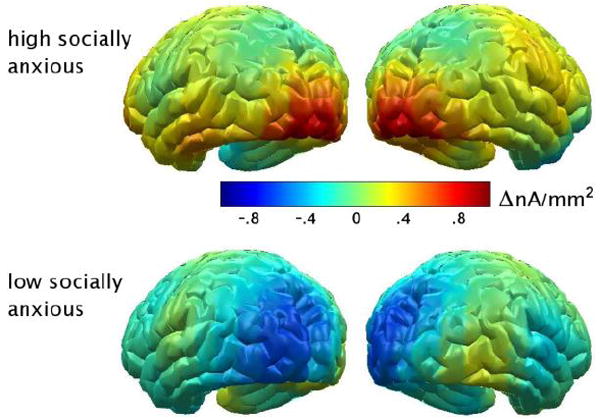
The difference in the topographical distribution of the ssVEP source strength (nA/mm2) for emotional relative to neutral facial expressions (averaged across epochs) for participants high and low social in anxiety.
Dimensional Social Anxiety, Broad Dysphoria, & Sensitivity to Emotional Expressions
To extend the findings for the dichotomous comparison of 14 high versus 14 low socially anxious participants, the LSAS-SR total severity score was correlated with the mean amplitude difference during emotional relative to neutral expressions (averaged across epochs) for the total sample of 37 participants. As illustrated in figure 4, there was a significant bivariate correlation between LSAS-SR total score and increase in amplitude during emotional relative to neutral expressions, Spearman’s rho: rs(37)=0.62, p<.001, such that as social anxiety symptom severity increased, the difference in mean ssVEP amplitude during emotional relative to neutral expressions increased. To assess this association in relation to broad negative affectivity, hierarchical multiple linear regression was employed with MASQ subscale scores (non-specific distress, non-specific anxiety, non-specific depression, anhedonia, anxious arousal) entered as the first block and LSAS-SR total score as the second block4. MASQ subscale scores did not predict ssVEP amplitude during emotional relative to neutral expressions, ΔR2=.17, ΔF(5,31)=1.29, ns, whereas even after partialing out variance attributable to broad anxious and depressive symptomatology, social anxiety accounted for 27% of the total variance in amplitude difference during emotional versus neutral expressions, ΔF(1,30)=14.37, p<.001. Specificity of the positive association between social anxiety and expression differentiation was underscored in the standardized coefficients for the simultaneous model: social anxiety, β=0.62, p<.01; non-specific distress, β =-0.19, ns, non-specific anxiety, β =0.15, ns, non-specific depression, β =- 0.06, ns, anhedonia, β=0.09, ns, anxious arousal, β =-0.28, ns.
Figure 4.
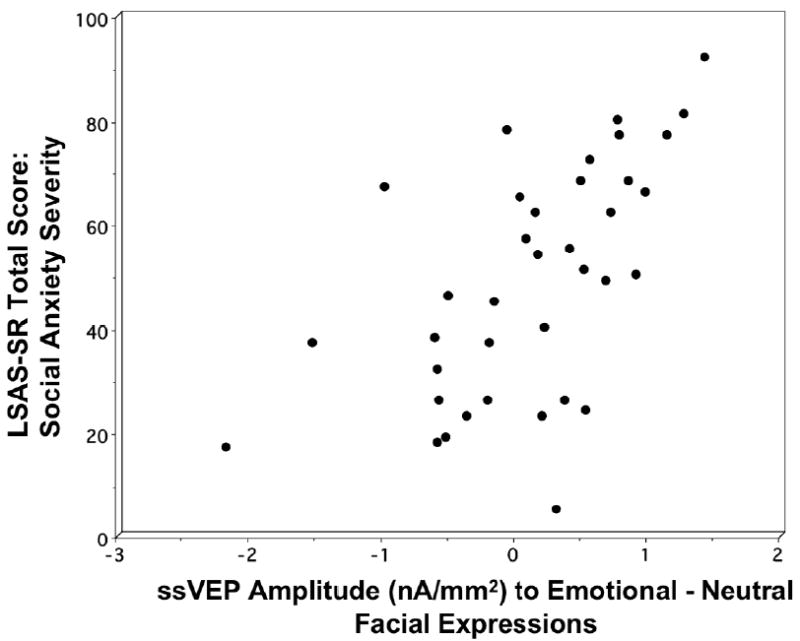
Association between LSAS-SR total score on the y-axis, and increase in source strength amplitude (nA/mm2) during emotional relative to neutral facial expressions on the x-axis for all 37 participants.
Discussion
In the current study continuous steady-state visual evoked potentials to naturalistic emotional and neutral facial expressions were assessed in individuals varying in generalized social anxiety. Evoked responses were recorded with a high-density EEG montage and distributed source modeling (minimum norm estimate) was used to infer electric source distribution and strength, and thus to elucidate the neural mechanisms underlying putative perceptual processing differences in social anxiety. Similar to a subset of preceding studies and consistent with predictions based on the clinical nosology, high social anxiety was characterized by exaggerated visual responses to angry relative to neutral facial expressions. However, enhanced neural mass activity in visual cortex was not limited to stimuli connoting interpersonal challenge, disapproval and/or rejection. Instead, commensurate occipitocortical facilitation was observed in response to other aversive (fearful) as well as appetitive (happy) expressions, implicating a broad visual sensitivity to emotional facial displays in social anxiety. In contrast, individuals endorsing low interpersonal apprehension demonstrated no modulation of the ssVEP amplitude as a function of expression—all facial displays were seemingly innocuous. Furthermore, the signature of enhanced perceptual salience of emotional displays at higher symptom levels was demonstrated in both categorical and dimensional analyses, the latter suggesting a strong linear relationship.
Although heightened sensitivity specific to angry expressions would seem most consistent with the nosology of social anxiety, vigilance to other expressions has frequently been revealed in both ERP (Kolassa et al., 2007; 2009; Wieser et al., 2010) and hemodynamic imaging (Amir et al., 2005, Birbaumer et al., 1998; Phan et al., 2006; Stein et al., 2002) studies—even to happy faces (Yoon et al., 2007; Straube et al., 2005) and aversive non-social scenes (Shah et al., 2009). Much of the evidence for exaggerated reactivity to “harsh” facial expressions has been demonstrated as the mean response to heterogeneous aversive displays (angry, fearful, disgusted) (e.g., Moser et al., 2008; Phan et al., 2006; Stein et al., 2002). In post hoc tests of the BOLD response to individual aversive contents, Phan and colleagues revealed that the overall effect was attributable to reliable, similar magnitude amygdala activity to angry, fearful and disgusted faces.
Whereas early and later event-related components have shown sensitivity to emotional versus neutral facial expressions in unselected samples (Eimer & Holmes, 2007; Schupp et al., 2004), the absence here of an expression differentiation among individuals low in social anxiety is consistent with a subset of prior studies that specifically isolated a minimal symptom group. For example, no differences in early (Mueller et al., 2009) and later event-related positivities (Kolassa et al., 2007) have been observed. These data, notably, are not directly comparable to the present findings as ERPs reflect spatio-temporal transient responses throughout the entire cortex, whereas the ssVEP indexes fluctuations in sustained sensory processing. Nonetheless, in a recent study ssVEP amplitudes to emotional and neutral expressions also did not differ (Wieser, McTeague, & Keil, 2010) among participants who endorsed minimal interpersonal apprehension. As a further complement, Bar-Haim and colleagues (2007) concluded on the basis of a meta-analysis that attention biases towards threat stimuli are most reliably seen in high anxious and patient groups, but are less reliable in low anxious samples. Additionally, the affective ratings collected for this as well as other investigations (Goeleven et al., 2008) suggest that in an unselected sample, naturalistic facial expressions evoke at most, moderate levels of subjective arousal coupled with moderate unpleasantness or pleasantness—ratings much less extreme than typically observed for emotional scenes that evoke robust physiological differentiation irrespective of individual differences (Bradley et al., 2001; Keil et al., 2003).
The time-varying ssVEP amplitude is a continuous measure of sensory activation at the stimulation frequency. Thus, it is sensitive to potential differences between groups or experimental conditions in terms of temporal dynamics (Müller et al. 2008). In the present study, we did not find evidence of a dynamic change during the viewing epoch, but a sustained enhancement of neural mass activity in visual cortex for emotional expression, specific to participants with high social anxiety. The minimum norm estimate was utilized here to enhance the resolution of steady-state responses without constraining the inferred electric activity to point sources or single dipoles (Hämäläinen & Ilmoniemi, 1984). The resulting topographical distribution of the source strength to the facial displays suggests that the ssVEP signal was generated predominantly in the medial occipital cortex and the bilateral occipital poles in both groups, throughout the 3500 ms viewing epoch. Furthermore, the increased amplitude to emotional relative to neutral pictures in the high social anxiety group was strictly localized to the same regions. Whereas in previous studies affective modulation of the ssVEP more broadly extended from striate and extrastriate to higher order visual cortical areas such as occipito-temporal, parietal, and fronto-parietal regions (Keil et al., 2003; Moratti et al., 2004) no such concordance characterized the emotional sensitivity demonstrated by the high symptom group. The focused occipital activation in the current study is not wholly unexpected as the stimulation frequency (17.5 Hz) appreciably exceeded that of the aforementioned investigations, and dipole sensitivity to the driving frequency (Müller et al., 1997) has been demonstrated in selective attention paradigms with higher frequencies (≥20 Hz) associated with very narrow and lower level occipital sources (Müller, Picton et al., 1998). Presumably, the prominent affective modulation of the primary visual response elicited here in the high socially anxious is the result of re-entrant signals from anterior cortical areas, ultimately originating in higher visual, deep, and/or subcortical structures (Keil et al., 2009), that tune visual cortical neurons, altering thresholds and/or enhancing gain in the networks representing interpersonal evaluative cues.
The possible reorganization of the sensory visual cortex correspondent to alterations in motivational thresholds associated with excessive social apprehension is supported by animal and human work revealing that the functional neuroarchitecture of the adult visual cortex is subject to changes related to behavioral contingencies (Karmarkar & Dan, 2006; Li et al., 2004) and re-entrant modulation from higher visual areas including temporal cortex and deep structures such as the amygdala (Damasio, 1998; Leppänen & Nelson, 2009; Sabatinelli et al., 2005; 2009)—areas consistently shown during functional neuroimaging to be hyper-reactive to facial stimuli in social phobia patients (Birbaumer et al., 1998; Evans et al., 2008; Phan et al., 2006; Schneider et al., 1999; Stein et al., 2002; Straube et al., 2004, Veit et al., 2002). Although speculative, the heightened attention to emotional faces may be the result of visual system plasticity secondary to chronic expectations of interpersonal failure typical of social anxiety (Andrews et al., 1994; Foa et al., 1996; Poulton & Andrews, 1996; Rapee & Heimberg, 1997). Complementing the broad attentional enhancement to aversive and pleasant facial displays observed here, recent findings from other laboratories indicate that social anxiety is marked by fear not only of negative (Clark & Wells, 1995; Rapee & Heimberg, 1997) but also positive (Fergus et al., 2009; Weeks, Heimberg, & Rodebaugh, 2008; Weeks, Heimberg, Rodebaugh & Norton, 2008) evaluation. Others have postulated that either pleasant or unpleasant relative to neutral interactions increase perceived interpersonal performance demands in social anxiety, and hence, the perceived inevitability of failure (Fergus et al., 2009; Weeks et al., 2008a, 2008b).
Guided by compelling accounts of cognitive processing stages in clinical social anxiety (Trower & Gilbert, 1989), the literature is replete with speculation concerning the temporal dynamics of visual processing biases in the disorder, most of which promotes the vigilance-avoidance hypothesis—that in pathological anxiety perception of threat-relevant stimuli is characterized by initial hypervigilance and consequent defensive avoidance (Mogg et al., 1997). To date, however, as briefly reviewed, the dot-probe, oculomotor, ERP, and fMRI data with socially anxious individuals and patients lack the temporal resolution for addressing the proposed unfolding of attentional engagement and disengagement to facial expressions. In the current study, employing a continuous measure of perceptual processing capable of quantifying covert attention shifts revealed broad, sustained sensitivity to emotional expressions evident over the entire 3500 ms stimulation epoch in social anxiety. Future studies are warranted to disentangle effects specific to the onset of the stimulus train from those secondary to shifting the facial expression amidst an ongoing train (i.e., flickering neutral to flickering angry face), but these preliminary results showed facilitated attention among socially anxious individuals to emotional expressions as early as the first 500 ms with no evidence of subsequent perceptual avoidance.
Although the severity of generalized social anxiety reported in the current high symptom sample was commensurate to patients in prior clinical investigations, the implications of these results for explicating disorder-level dysfunction in social anxiety is necessarily limited by the use of an analogue sample. For example, the limited comorbid symptomatology and the mean age of 20.6 years in the high socially anxious group caution that the visual sensitivities observed here may be indicative only of the early stages of social anxiety, prior to the development of the broad negative affectivity characteristic of older treatment-seeking samples (Brown et al., 1998; Kessler et al., 1999). Future investigations are warranted that consider the developmental course of social anxiety and the role of co-occurring conditions in these affective dispositions. At the same time, the specific nature of the social fearfulness in the current sample was advantageous. Recent evidence has shown that concurrent depression may abolish attentional biases (Musa et al., 2003) and reduce defensive responding (McTeague et al., 2009) in social anxiety, and as such symptom profiles indicative of limited comorbidity are necessary for investigating attentional processes putatively unique to the disorder.
In summary, as assessed here, during passive viewing generalized social anxiety was associated with sustained and amplified visual processing of affective social cues, suggesting that enduring, extreme fear may tune visual sensory gains parallel to mechanisms of phasic selective attention (Hillyard & Anllo-Vento, 1998). Future studies are warranted to determine whether this enhanced sensitivity to emotional faces is characteristic not only of generalized social anxiety, but also of circumscribed performance phobia and/or other anxiety disorders. Additionally, it will be essential to assess whether other indices of motivated attention (Bradley, 2009; Lang & Bradley, 2009) such as skin conductance, heart rate, facial electromyography, event-related potentials, subjective judgments, and performance converge with the findings for ssVEP amplitudes to facial expressions. Furthermore, with the growing implementation of attentional modification interventions for social anxiety utilizing facial stimuli (e.g., Amir et al., 2008, 2009, Schmidt et al., 2009), the continuous measure of perceptual processing provided by the steady-state procedure may help to elucidate covert, temporal dynamics underlying the reported symptom reductions.
Research highligts.
Faces flickering at 17.5 Hz evoke reliable visual cortical steady-state potentials
Stronger visual cortical response to emotional then neutral faces in social anxiety
Visual sensitivity to emotional faces increases linearly with social anxiety severity
Visual hypersensitivity to emotional faces in social anxiety is temporally sustained
Social anxiety is marked by hypersensitivity to both aversive and pleasant faces
Acknowledgments
This work was supported in part by National Institute of Mental Health grants (P50 MH 72850) to the Center for the Study of Emotion and Attention (CSEA) and (R01 MH084932) Andreas Keil at the University of Florida, Gainesville, FL. Special thanks to the Esther Jean-Baptiste, Ilana Seff, and Michael Gray for assistance in data collection.
Footnotes
The observed attentional biases in social anxiety differ when participants anticipate giving a speech following the experimental procedure. High relative to low socially anxious individuals respond faster to probes proceeding household objects than emotional faces (Chen et al., 2002), show faster eye movements but reduced fixation time to emotional than neutral faces (Garner et al., 2006), and demonstrate larger N1 and associated component responses to angry than neutral faces (Wieser et al., 2010).
In follow-up analyses gender exerted neither a main effect or interaction on ssVEP amplitude to emotional and neutral facial expressions: Gender, F(1,24)=0.02, ns, Gender × Time, F(2,23)=2.44, ns, Gender × Expression, F(3,22)=1.78, ns, Gender × Time × Expression, F(6,19)=0.81, ns, Gender × Expression × Group, F(3,22)=1.20, ns, Gender × Expression × Time × Group, F(6,19)=0.40, ns.
Guided by preceding work with selective attention tasks ssVEP amplitude was averaged into three epochs corresponding to two early (100-500 ms, 500-1000ms) and one later window (1500-3500 ms). For completeness, analyses were also performed on the ramp (100-500ms) and two equal-length subsequent windows (500-2000 ms, 2000-3500 ms). The results underscored the pattern differences in the symptom groups: Group × Expression, F(3,24)=5.83, p<.01, ηp 2=0.42: Time, F(2,25)=15.61 p<.001, ηp 2=0.56, Expression, F(3,24)=0.43, ns, Group, F(1,26)=0.56, ns, Time × Group, F(2,25)=0.05, ns, Time × Expression, F(6,21)=2.19, ns, Time × Expression × Group, F(6,21)=0.83, ns.
Tolerance (0.3 - 0.8) values for each predictor indicated non-redundancy of the symptom measures in predicting ssVEP amplitude.
Financial Disclosures All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahs F, Pissiota A, Michelgård A, Frans O, Furmark T, Appel L, Fredrikson M. Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Res. 2009;172:103–8. doi: 10.1016/j.pscychresns.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. J Consult Clin Psychol. 2009;77:961–73. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 1. 2005;57:975–81. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. J Abnorm Psychol. 2008;117:860–8. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Freed S, Teesson M. Proximity and anticipation of a negative outcome in phobias. Behav Res Ther 1994. 1994;32:643–5. doi: 10.1016/0005-7967(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- Baker SL, Heinrichs N, Kim HJ, Hofmann SG. The liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behav Res Ther. 2002;40:701–15. doi: 10.1016/s0005-7967(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bertrand O, Perrin F, Pernier J. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalogr Clin Neurophysiol. 1985;626:462–464. doi: 10.1016/0168-5597(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24:827–56. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Borkovec T, Rachman S. The utility of analogue research. Behav Res Ther. 1979;17:253–61. doi: 10.1016/0005-7967(79)90040-8. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–98. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. 55. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585–99. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Chen YP, Ehlers A, Clark DM, Mansell W. Patients with generalized social phobia direct their attention away from faces. Behav Res Ther. 2002;40:677–87. doi: 10.1016/s0005-7967(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz M, Hope D, Schneider F, editors. Social Phobia: Diagnosis, Assessment, and Treatment. Guilford; New York: 1995. pp. 66–93. [Google Scholar]
- Cox BJ, Ross L, Swinson RP, Direnfeld DM. A comparison of social phobia outcome measures in cognitive-behavioral group therapy. Behav Modif. 1998;22:285–97. doi: 10.1177/01454455980223004. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Emotion in the perspective of an integrated nervous system. Brain Res Brain Res Rev. 1998;26:83–6. doi: 10.1016/s0165-0173(97)00064-7. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum Brain Mapp. 2007;28:323–334. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol Med. 2001;31:1025–35. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112:536–44. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fergus TA, Valentiner DP, McGrath PB, Stephenson K, Gier S, Jencius S. The Fear of Positive Evaluation Scale: psychometric properties in a clinical sample. J Anxiety Disord. 2009;23:1177–83. doi: 10.1016/j.janxdis.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Foa EB, Franklin ME, Perry KJ, Herbert JD. Cognitive biases in generalized social phobia. J Abnorm Psychol. 1996;105:433–9. [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. J Abnorm Psychol. 2006;115:760–70. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska directed emotional faces: A validation study. Cognition and Emotion. 2008;22:1094–1118. [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M, Ilmoniemi R. Interpreting measured magnetic fields of the brain: Estimates of current distributions (Tech Rep No TKK-F-A559) Helsinki: Helsinki University of Technology; 1984. [Google Scholar]
- Hauk O. Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. Neuroimage. 2004;21:1612–21. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Hauk O, Keil A, Elbert T, Müller MM. Comparison of data transformation procedures to enhance topographical accuracy in time-series analysis of the human EEG. J Neurosci Methods. 2002;113:111–22. doi: 10.1016/s0165-0270(01)00484-8. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Holaway RM. Examination of the known-groups validity of the Liebowitz Social Anxiety Scale. Depress Anxiety. 2007;24:447–54. doi: 10.1002/da.20277. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, White LK, Bar-Haim Y, Fox NA. Affective primes suppress attention bias to threat in socially anxious individuals. Behav Res Ther. 2008;46:799–810. doi: 10.1016/j.brat.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hinrichs H, Tempelmann C, Morgan ST, Hansen JC, Scheich H, et al. Combining steady-state visual evoked potentials and fMRI to localize brain activity during selective attention. Human Brain Mapping. 1997;5:287–292. doi: 10.1002/(SICI)1097-0193(1997)5:4<287::AID-HBM14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Heinrichs N, Moscovitch DA. The nature and expression of social phobia: toward a new classification. Clin Psychol Rev. 2004;24:769–97. doi: 10.1016/j.cpr.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. J Anxiety Disord. 2003;17:33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Res. 2004;127:43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Heim S, Keil A. The costs of emotional attention: affective processing inhibits subsequent lexico-semantic analysis. J Cogn Neurosci. 2007;19:1932–49. doi: 10.1162/jocn.2007.19.12.1932. [DOI] [PubMed] [Google Scholar]
- Iwai E, Yukie M. Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in maca ques (Macaca fuscata, M. mulatta, and M fascicularis) J Comp Neurol. 1987;261:362–387. doi: 10.1002/cne.902610304. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Leiderer P, Berg P, Rockstroh B. Mapping EEG-potentials on the surface of the brain: a strategy for uncovering cortical sources. Brain Topogr. 1997;9:203–217. doi: 10.1007/BF01190389. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Karmarkar UR, Dan Y. Experience-dependent plasticity in adult visual cortex. Neuron. 2006;52:577–85. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neurosci Biobehav Rev. 2001;25:527–34. doi: 10.1016/s0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM, Moratti S, Stolarova M, Bradley MM, Lang PJ. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cogn Affect Behav Neurosci. 2003;3:195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- Keil A, Heim S. Prolonged reduction of electrocortical activity predicts correct performance during rapid serial visual processing. Psychophysiology. 2009;46:718–25. doi: 10.1111/j.1469-8986.2009.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Ihssen N, Heim S. Early cortical facilitation for emotionally arousing targets during the attentional blink. BMC Biol. 2006;4:23. doi: 10.1186/1741-7007-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Re-entrant projections modulate visual cortex in affective perception: Evidence from Granger causality analysis. Hum Brain Mapp. 2009;30:532–540. doi: 10.1002/hbm.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Smith JC, Wangelin BC, Sabatinelli D, Bradley MM, Lang PJ. Electrocortical and electrodermal responses covary as a function of emotional arousal: a single-trial analysis. Psychophysiology. 2008;45:516–23. doi: 10.1111/j.1469-8986.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Stang P, Wittchen HU, Stein M, Walters EE. Lifetime co-morbidities between social phobia and mood disorders in the US National Comorbidity Survey. Psychol Med. 1999;29(3):555–67. doi: 10.1017/s0033291799008375. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Amir N. Examination of vigilance and disengagement of threat in social anxiety with a probe detection task. Anxiety Stress Coping. 2009;22:283–96. doi: 10.1080/10615800802449602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I-T, Kolassa S, Musial F, Miltner WHR. Event-related potentials to schematic faces in social phobia. Cogn Emot. 2007;21:1721–1744. [Google Scholar]
- Kolassa I-T, Kolassa S, Bergmann S, Lauche R, Dilger S, Miltner WHR, Musial F. Interpretive bias in social phobia: An ERP study with morphed emotional schematic faces. Cogn Emot. 2009;23:63–95. [Google Scholar]
- Kolassa I-T, Miltner WH. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118:130–41. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. doi: 10.1016/j.biopsycho.2009.10.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nat Rev Neurosci. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–7. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw A, Lang PJ, Smith JC, Bradley MM. Both predator and prey: emotional arousal in threat and reward. Psychol Sci. 2008;19:865–73. doi: 10.1111/j.1467-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. Karolinska directed emotional faces (KDEF) Stockholm: Karolinska Institutet; 1998. [Google Scholar]
- McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biol Psychiatry. 2009;65:374–82. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, De Bono J, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behav Res Ther. 1997;35:297–303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. J Abnorm Psychol. 2004;113:160–5. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A. Cortical activation during Pavlovian fear conditioning depends on heart rate response patterns: An MEG study. Brain Res Cogn Brain Res. 2005;25:459–471. doi: 10.1016/j.cogbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Miller GA. Fear but not awareness predicts enhanced sensory processing in fear conditioning. Psychophysiology. 2006;43:216–26. doi: 10.1111/j.1464-8986.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Stolarova M. Motivated attention in emotional picture processing is reflected by activity modulation in cortical attention networks. Neuroimage. 2004;21:954–964. doi: 10.1016/j.neuroimage.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: An electrophysiological study. Biol Psychol. 2008;78:93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychol Med. 2009;39:1141–52. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlberger A, Wieser MJ, Hermann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. J Neural Transm. 2009;116:735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Müller MM, Andersen SK, Keil A. Time course of competition for visual processing resources between emotional pictures and foreground task. Cereb Cortex. 2008;18:1892–9. doi: 10.1093/cercor/bhm215. [DOI] [PubMed] [Google Scholar]
- Müller MM, Hillyard S. Concurrent recording of steady-state and transient event-related potentials as indices of visual-spatial selective attention. Clin Neurophysiol. 2000;111:1544–1552. doi: 10.1016/s1388-2457(00)00371-0. [DOI] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of the attentional spotlight. Nature. 2003;424:309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Müller MM, Picton TW, Valdes-Sosa P, Riera J, Teder-Sälejärvi WA, Hillyard SA. Effects of spatial selective attention on the steady-state visual evoked potential in the 20-28 Hz range. Brain Res Cogn Brain Res. 1998;6:249–61. doi: 10.1016/s0926-6410(97)00036-0. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder W, Hillyard SA. Magnetoencephalographic recording of steady-state visual evoked cortical activity. Brain Topogr. 1997;9:163–168. doi: 10.1007/BF01190385. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Salejarvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nat Neurosci, 1. 1998;7:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Musa C, Lépine JP, Clark DM, Mansell W, Ehlers A. Selective attention in social phobia and the moderating effect of a concurrent depressive disorder. Behav Res Ther. 2003;41:1043–54. doi: 10.1016/s0005-7967(02)00212-7. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pishyar R, Harris LM, Menzies RG. Attentional bias for words and faces in social anxiety. Anxiety Stress Coping. 2004;17:23–36. [Google Scholar]
- Poulton RG, Andrews G. Change in danger cognitions in agoraphobia and social phobia during treatment. Behav Res Ther. 1996;4:413–21. doi: 10.1016/0005-7967(96)00009-5. [DOI] [PubMed] [Google Scholar]
- Pujol J, Harrison BJ, Ortiz H, Deus J, Soriano-Mas C, López-Solà M, Yücel M, Perich X, Cardoner N. Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychol Med. 2009;9:1177–87. doi: 10.1017/S003329170800500X. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. Elsevier; New York: 1989. [Google Scholar]
- Regan D, Spekreijse H. Auditory-visual interactions and the correspondence between perceived auditory space and perceived visual space. Perception, 6. 1977;2:133–138. doi: 10.1068/p060133. [DOI] [PubMed] [Google Scholar]
- Riemslag FC, Ringo JL, Spekreijse H, Verduyn Lunel HF. The luminance origin of the pattern electroretinogram in man. J Physiol. 1985;363:191–209. doi: 10.1113/jphysiol.1985.sp015704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Ledley DR, Heimberg RG. Treating refractory cases in specific diagnostic populations and clinical problems: Social Anxiety Disorder. In: McKay D, Abramowitz J, Taylor S, editors. The expanded scope of cognitive-behavior therapy: Lessons learned from refractory cases. American Psychological Association; Washington, DC: in press. [Google Scholar]
- Rossignol M, Anselme C, Vermeulen N, Philippot P, Campanella S. Categorical perception of anger and disgust facial expression is affected by non-clinical social anxiety: an ERP study. Brain Res. 2007;1132:166–76. doi: 10.1016/j.brainres.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Roth DA, Heimberg RG. Cognitive-behavioral models of social anxiety disorder. Psychiatr Clin North Am. 2001;24:753–71. doi: 10.1016/s0193-953x(05)70261-6. [DOI] [PubMed] [Google Scholar]
- Rytwinski NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, Stein MB, Hofmann SG. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depress Anxiety. 2009;26:34–8. doi: 10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Costa VD, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. J Neurosci. 2009;29:14864–8. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Müller-Gärtner HW, Posse S, Salloum JB, Grodd W, Himmelmann F, Gaebel W, Birbaumer N. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 1999;4:863–71. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. J Abnorm Psychol. 2009;118:5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: potential for interactive processes. Clin Psychol Rev. 2008;28:1206–21. doi: 10.1016/j.cpr.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Sewell C, Palermo R, Atkinson C, McArthur G. Anxiety and the neural processing of threat in faces. Neuroreport. 2008;19:1339–1343. doi: 10.1097/WNR.0b013e32830baadf. [DOI] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- Silberstein RB, Ciorciari J, Pipingas A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalogr Clin Neurophysiol. 1995;96:24–35. doi: 10.1016/0013-4694(94)00189-r. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa I-T, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Trower P, Gilbert P. New theoretical conceptions of social anxiety and social phobia. Clin Psychol Rev. 1989;9:19–35. [Google Scholar]
- Vasey MW, Thayer JF. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology. 1987;24:479–86. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45:1393–9. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The Mood and Anxiety Symptom Questionnaire. Unpublished manuscript. University of Iowa, Department of Psychology; Iowa City: 1991. [Google Scholar]
- Weeks JW, Heimberg RG, Rodebaugh TL. The Fear of Positive Evaluation Scale: assessing a proposed cognitive component of social anxiety. J Anxiety Disord. 2008a;22:44–55. doi: 10.1016/j.janxdis.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Rodebaugh TL, Norton PJ. Exploring the relationship between fear of positive evaluation and social anxiety. J Anxiety Disord. 2008b;22:386–400. doi: 10.1016/j.janxdis.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, McTeague LM, Keil A. Under review Sustained preferential processing of social threat cues—bias without competition? Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2010.21566. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Mühlberger A. Probing the attentional control theory in social anxiety: An emotional saccade task. Cognitive, Affective, & Behavioral Neuroscience. 2009;9:314–322. doi: 10.3758/CABN.9.3.314. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Reicherts P, Mühlberger A. Don’t look at me in anger! - Enhanced processing of angry faces in anticipation of public speaking. Psychophysiology. 2010;47(2):271–280. doi: 10.1111/j.1469-8986.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Weyers P, Alpers GW, Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: An eye-tracking study. Journal of Neural Transmisson. 2009;116:717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res 15. 2007;154:93–8. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]


