Abstract
Background
We have developed a culture system using reconstituted basement membrane components in which normal human mammary epithelial cells exhibit several aspects of the development and differentiation process, including formation of acinar-like structures, production and basal deposition of basement membrane components, and production and apical secretion of sialomucins. Cell lines and cultures from human breast carcinomas failed to recapitulate this process. The data indicate the importance of cellular interactions with the basement membrane in the regulation of normal breast differentiation and, potentially, its loss in neoplasia.
Purpose
Our purpose was to use this assay to investigate the role of the putative metastasis suppressor gene nm23-H1 in mammary development and differentiation.
Methods
The metastatic human breast carcinoma cell line MDA-MB-435, clones transfected with a control pCMVBamneo vector, and clones transfected with pCMVBamneo vector containing nm23-H1 complementary DNA (the latter of which exhibited a substantial reduction in spontaneous metastatic potential in vivo) were cultured within a reconstituted basement membrane. Clones were examined for formation of acinus-like spheres, deposition of basement membrane components, production of sialomucin, polarization, and growth arrest.
Results
In contrast to the parental cell line and control transfectants, MDA-MB-435 breast carcinoma cells overexpressing Nm23-H1 protein regained several aspects of the normal phenotype within reconstituted basement membrane. Nm23-H1 protein-positive cells formed organized acinus-like spheres, deposited the basement membrane components type IV collagen and, to some extent, laminin to the outside of the spheres, expressed sialomucin, and growth arrested. Growth arrest of Nm23-H1 protein-positive cells was preceded by and correlated with formation of a basement membrane, suggesting a causal relationship.
Conclusion
The data indicate a previously unidentified cause-and-effect relationship between nm23-H1 gene expression and morphological–biosynthetic–growth aspects of breast differentiation in this model system.
Implications
While the basement membrane microenvironment is capable of directing the differentiation of normal human breast cells, neoplastic transformation abrogates this relationship, suggesting that intrinsic cellular events are also critical to this process. The data identify nm23-H1 gene expression as one of these events, suggesting an important role in the modulation of cellular responsiveness to the microenvironment. The data also identify previously unknown growth inhibitory effects of nm23-H1 gene overexpression.
While inappropriate changes in cell growth have been considered the hallmark of cancer, alterations in other characteristics such as cell–cell and cell–extracellular matrix interactions and apoptosis have also been recognized as important determinants of the malignant phenotype. We have recently developed a model system for the morphological and functional differentiation of human breast cells, based on their interaction with a basement membrane-containing microenvironment. When cells from 12 reduction mammoplasties and two normal cell lines were cultured within reconstituted basement membrane (Engelbreth–Holm–Swarm [EHS] matrix), they formed organized acinus-like structures, frequently deposited a basement membrane to the outside of the acinus, secreted sialomucin to the inside of the acinus, and growth arrested (1). In contrast, two primary human breast carcinoma cultures as well as six established breast carcinoma cell lines failed to recapitulate this process. Gross distinctions in growth rates and culture morphology were not apparent between normal cells and malignant cell lines on tissue culture plastic. The data indicate dedifferentiation as an important correlate of malignant progression in human breast epithelial cells and confirm the importance of cell–basement membrane interactions in this process (1).
Decreased expression of the nm23 family of genes has been associated with histopathologic and/or clinical course correlates of aggressive breast carcinoma in several cohort studies (2-8). Functional analysis of nm23-H1 gene overexpression was reported in the human MDA-MB-435 breast carcinoma cell line. Both bulk transfectants as well as stable, high expression clones exhibited a significant reduction in metastatic potential in vivo (9). In addition, clonal nm23-H1 transfectants exhibited a reduced responsiveness to transforming growth factor-β (TGF-β) in soft agar colonization assays in culture (9) and to insulinlike growth factor, platelet-derived growth factor, and serum in motility assays in culture (10). The biochemical mechanism of Nm23 protein action is not known; however, the recent identification of an Nm23 serine phosphorylation that correlated with its metastasis-suppressive activity suggests this phosphorylation may lie on its functional pathway (11).
A role for the nm23 gene in the development and differentiation process has been suggested by studies of its Drosophila homologue abnormal wing discs (awd), in which reductions in awd gene expression or mutation resulted in lethal abnormalities in cell morphology and differentiation postmetamorphosis (12). In mammals, increased Nm23 protein was detected immunohistochemically in virtually all epithelial tissues during mouse embryogenesis concurrent with their functional differentiation, although Nm23 protein was not maintained in all adult differentiated epithelia (13). Direct evidence of a role for Nm23 protein in mammalian differentiation is lacking to date. We report the first functional evidence of a role for nm23-H 1 gene overexpression in the morphological and biosynthetic differentiation of a human breast carcinoma cell line in response to culture within a reconstituted basement membrane. The data implicate a novel role for the nm23 gene in breast physiology and strengthen the hypothesis that tumor metastasis and embryogenesis may use similar or identical genetic pathways.
Materials and Methods
Cell culture
Human metastatic MDA-MB-435 breast carcinoma cells, derivatives transfected with the control vector pCMVBamneo (C-100 and C-103), and derivatives transfected with pCMVBamneo-nm23-H1 complementary DNA (cDNA) [HI-170 and H1-177, expressing fourfold and eightfold greater Nm23 protein than the control vector cell lines, respectively, (9)] and normal HMT-3522 breast epithelial cells were cultured as previously described (1,9). EHS matrix was prepared from EHS ascites tumors passaged in C57BL mice at a concentration of 7-10 mg/mL and stored at 0 °C for up to 4 weeks as described (1). The care of the C57BL mice was in accord with institutional guidelines. In some experiments, commercially prepared EHS-matrix (Matrigel; Collaborative Research Inc., Bedford, Mass.) was used. Prior to seeding into EHS matrices or Matrigel, MDA-MB-435 cells and transfectants were cultured for 48 hours on collagen type I-coated plates in serum-free CDM3 medium as described previously (1). The cell lines were trypsinized and replated either into monolayer culture or into 300 μL of EHS matrix or Matrigel (7-10 mg/mL) as single cells at a concentration of approximately 2.5 × 105 cells per well of a 24-well plate and cultured as described previously (1).
Immunochemistry
Frozen and formalin-fixed, paraffin-embedded sections (5 μm) were prepared from EHS cultures. The Nm23 protein was localized in paraffin sections and Bouins-fixed monolayer cultures using affinity-purified anti-Nm23 peptide 11 antibody (12). Frozen sections were used for localization of 1) milk-fat globule membrane antigen (MGFGM-A; sialomucin) using monoclonal antibody 115D8 (San Bio, Am Uden, The Netherlands) as described (1); 2) cadherins with anti–P- and anti–E-cadherin antibodies (gifts of Drs. S. Hirohashi and C. Damsky); 3) type IV collagen with antibody PHM-12 (AMD; Armaton, New South Wales, Australia) and COP (Medac, Hamburg, Federal Republic of Germany); and 4) laminin with antibody M638 (Dakopatts, Glostrup, Denmark). Control sections were stained with secondary antibodies only.
Growth analysis
Colony cell content was determined microscopically as described (1). Thymidine labeling indices were determined by 24-hour incorporation of [3H]thymidine (20 Ci/mmol: Du Pont NEN Research Products, Boston, Mass.) as described (1). To ensure that cells were at comparable densities at the time of thymidine labeling index determination, Nm23 protein-positive clones were seeded within EHS at five times the concentration of control or parental cultures. For monolayer culture, the cells were plated at 2 × 104 cells/cm2 and labeling indices were determined on day 12 of culture at sub-confluent densities.
Statistical analyses
The two-tailed Student's t test was used to compare cells-per-colony data and sialomucin production. Analysis of the time course of basement membrane formation/[3H]thymidine labeling was performed using the nonparametric Wilcoxon signed rank test.
Results
Analysis of Morphology of MDA-MB-435 Breast Carcinoma Cells Overexpressing the nm23-H1 Gene in a Three-Dimensional Matrix
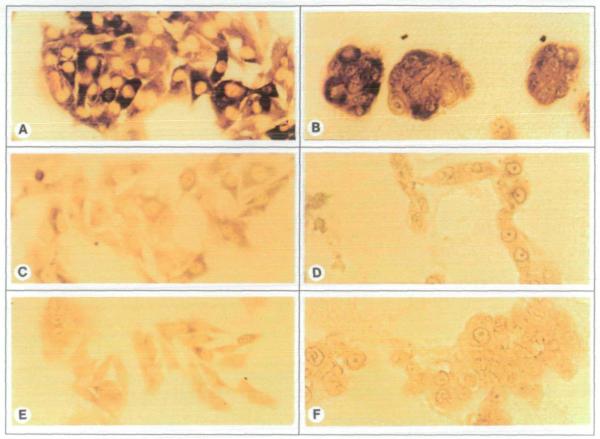
The normal breast culture HMT-3522 was previously reported to form organized acinar structures when cultured within an EHS matrix, while CAMA-1, T47D, BT-20, ZR-75, HMT-3909, and MCF-7 breast carcinoma cell lines failed to recapitulate this process (1). To determine the effect of nm23-H1 gene expression on the morphological differentiation of breast cells, MDA-MB-435 breast carcinoma clonal cell lines transfected with pCMVBamneo vectors (C-100 and C-103) or the same vector containing the full length nm23-H1 cDNA (H1-170 and H1-177) were cultured within an EHS matrix for 12 days. Cultures were examined for Nm23 protein expression by immunohistochemistry and for the presence of acinus-like structures by microscopy. Both the parent MDA-MB-435 cell line as well as the control clones expressed little Nm23 protein and produced large disorganized colonies (Fig. 1, D and F). In contrast, the H1-177 transfectant expressed significant Nm23 protein and produced small spheres with occasional lumens or remained as single cells (Fig. 1, B). The morphology of two different passages of the H1-170 cell line was heterogeneous, containing nm23-negative colonies of unorganized morphology as well as nm23-positive spherical colonies (not shown). The morphology of the H1-177 cells and Nm23 protein-positive H1-170 cells differed from that of the previously characterized HMT-3522 cells (1) only in the proportion of spheres with a central lumen. Less than 1 % of the Nm23 protein-positive spheres contained a lumen.
Fig. 1.
Immunohistochemical staining of Nm23 protein to show morphology and Nm23 protein expression in 12-day cultures of MDA-MB-435 cells in monolayer (A, C, and E) and in EHS matrix (B, D, and F). Panels A and B show nm23-H1 gene-transfected clone H1-177; Panels C and D show control-transfected clone C-100; panels E and F show untransfected parent MDA-MB-435 cells. Note the intense cytoplasmic staining for exogenous Nm23 protein in H1-177 cells (A and B) and the capacity of these cells to form spheres in EHS (B). Panels A, C,and E (original magnification ×440; panels B, D, and F original magnification ×550).
Analysis of Basement Membrane Deposition
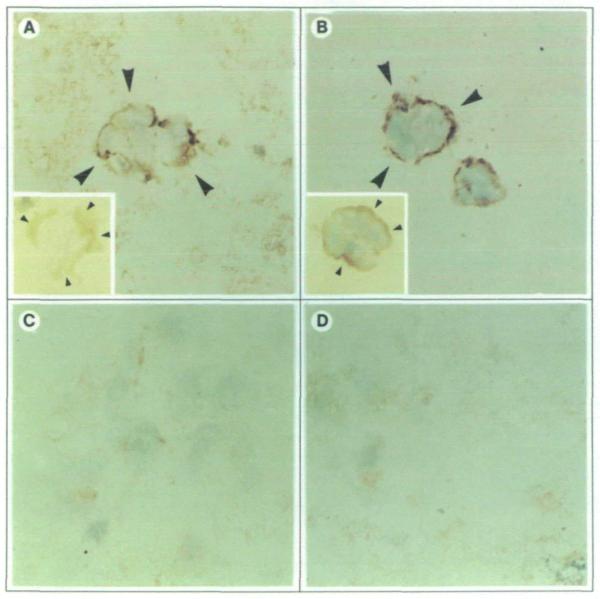
In addition to morphological evidence of differentiation, culture of normal HMT-3522 cells within an EHS matrix induced the expression of basement membrane proteins and their deposition to the outside of the acinus-like spheres (1). Analysis of six breast carcinoma cell lines and two primary carcinoma cultures failed to show similar trends (1). Immunohistochemical staining of type IV collagen and laminin was conducted on parental MDA-MB-435 cells, control transfectants, and nm23-H1 gene transfectants cultured within an EHS matrix (Fig. 2 and data not shown). By day 6 of culture, 87.8% ± 3.8% (means ± SE) of Nm23 protein-positive transfectants deposited type IV collagen; this percentage increased to 97.1% ± 1.9% by day 12 of culture. These latter data compared closely with the normal HMT-3522 cultures, in which 100% of spheres deposited a basement membrane by day 12 of culture. However, the basement membrane of HMT-3522 cells differed from nm23 gene transfectants in that it was detected with a broader range of anti-type IV collagen antibodies (data not shown), suggesting differences in basement membrane immunoreactivity. Deposition of laminin, although occasional, weak, and fragmented compared with type IV collagen, confirmed the presence of an elaborate basement membrane in nm23 gene transfectants (Fig. 2, A, inset). Basement membrane proteins were localized to the outside of the acinus-like spheres. In contrast, none of the parental cells, control transfectants, or Nm23 protein-negative H1-170 transfectants expressed type TV collagen or laminin.
Fig. 2.
Immunohistochemical staining of type IV collagen and laminin expressed by nm23-H1 gene-transfected MDA-MB-435 clone H1-177 cells (A) and normal HMT-3522 breast epithelial cells (B). Arrows show localization of type IV collagen at the basal surface of spheres formed by nm23 gene-transfected cells and normal breast cells. Insets show similar localization but less intense staining of laminin for H1-177 cells (inset of panel A) and for reference HMT-3522 cells (inset of panel B). Note the absence of collagen IV deposition by the untransfected parental MDA-MB-435 cells (C) and control transfectants clone C-100 (D) (original magnification ×400; inset original magnification ×320).
Other Biosynthetic Activities
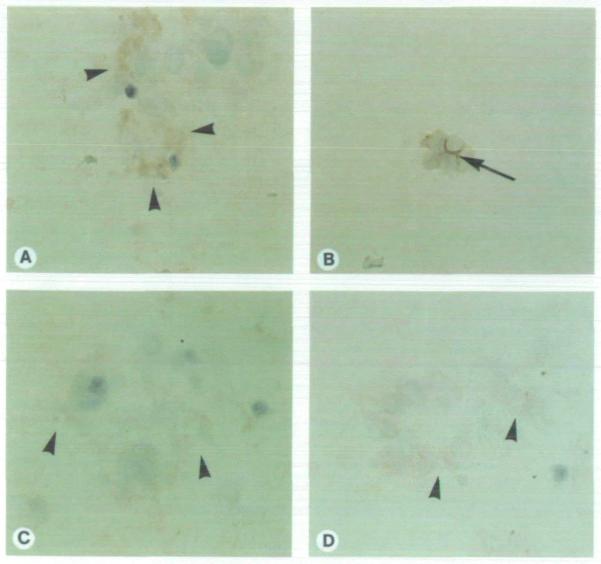
Immunostaining was conducted for sialomucin, a glycoprotein synthesized by epithelial cells that accumulates at the apical cell surface of polarized mammary epithelia and in milk-fat droplets. Increased staining of sialomucin was observed in Nm23 protein-positive cells compared with control transfectants (Fig. 3). By day 9 of culture, 49.5% ± 4.5% of Nm23 protein-positive transfectants expressed sialomucin compared with 5.5% ± 1.5% of controls (P = .011). Deposition of sialomucin was apical and lateral in normal HMT-3522 cultures and nonpolar in Nm23 protein-positive transfectants. The frequency of sialomucin expression was lower than that of basement membrane deposition for both Nm23 protein-positive MDA-MB-435 and HMT-3522 cells, where 12% of spheres expressed sialomucin and 100% expressed basement membrane (1).
Fig. 3.
Immunohistochemical localization of sialomucin (arrows) expressed by MDA-MB-435 clone H1-177 cells (A), normal control HMT-3522 cells (B), control MDA-MB-435 clone C-100 cells (C), and control untransfected parental MDA-MB-435 cells (D). Note the apical and lateral accumulation of sialomucin by normal HMT-3522 spheres (B) and the nonpolar expression of sialomucin by the MDA-MB-435 cells (A, C, and D) (original magnification ×400).
The potential role of cell–cell adherence in acinar differentiation in culture was determined. While HMT-3522 cells exhibited basolateral deposition of E- and P-cadherins, the parental MDA-MB-435 cells and all transfectants failed to express detectable protein at the cell membrane (data not shown). Thus, the morphological and biosynthetic evidence of differentiation occurred independently of E- and P-cadherins.
Formation of Acinar Structures by the nm23-H1 Gene Over-expressing MDA-MB-435 Breast Carcinoma Cells: Association With Growth Inhibition
Monolayer culture of the control- and nm23-H1–transfected MDA-MB-435 breast carcinoma cell lines on tissue culture plastic revealed no significant differences in growth rate. Thymidine labeling indices conducted in the passages of these cell lines used in the present series of experiments were 98.2 ± 0.9, 96.8 ± 0.8, 96.9 ± 0.8, and 98.5 ± 1.2 for the C-100, C-103, H1-170, and H1-177 cell lines, respectively. We initially noted a difference in both the morphology and size of colonies produced by control and nm23-H1 gene transfectants when cultured within an EHS matrix. Table 1 lists the number of cells per colony in parental, control, and nm23-H1 gene-transfected MDA-MB-435 cells on day 12 of culture within an EHS matrix. The parent line and control transfectants produced colonies ranging from 16-26 cells per colony. H1-177 cells produced colonies containing an average of 9.6 cells (P<.001), which compared closely with the 8.0 cells per sphere exhibited by normal HMT-3522 cells. Analysis of H1-170 cells further strengthened this trend; Nm23 protein-negative colonies contained a mean of 27 cells, while Nm23 protein-positive colonies contained a mean of 8.2 cells (P<.001). Similar trends were observed on culture of the control- and nm23-H1–transfected cell lines within Matrigel (data not shown).
Table 1.
Overexpression of the nm23-H1 gene by MDA-MB-435 human breast carcinoma cells: association with the formation of smaller colonies upon culture within EHS matrix on day 12
| Cell line | Mean ± SE cells per colony* | P† |
|---|---|---|
| HMT-3522 | 8.0 ± 0.3 | |
| MDA-MD-435 parental | 19.1 ± 0.9 | |
| C-100 | 16.5 ± 0.8 | |
| C-103 | 25.8 ± 1.4 | |
| H1-170(nm23 negative) | 27.3 ± 2.1 | |
| H1-170 (nm23 positive) | 8.2 ± 0.6 | <.001 |
| H1-177 | 9.6 ± 0.2 | <.001 |
The number of cells per colony profile were counted in sections of EHS gels as described previously (I) (n = 20 profiles per point).
Student's t test versus smallest control clone (C-100).
The relationship of biosynthetic and growth inhibition aspects of breast cell differentiation have been evaluated in the H1-177 cell line. Table 2 shows the time course of basement membrane deposition and thymidine labeling in the H1-177 cell line. Deposition of basement membrane was prevalent among colonies by day 6 of culture (87.8%) and virtually homogeneous by day 12 of culture (97.1%). In contrast, the percentage of thymidine labeled cells remained high at day 6 of culture (92%) but was reduced to 29.9% by day 12. Evaluation of both parameters simultaneously for H1-177 cells is also shown. The percentage of spheres that were basement membrane positive-[3H]thymidine negative rose from 7.6% on day 6 of culture to 70.1% on day 12 (P = .001). A concurrent decrease in the percentage of basement membrane-positive-[3H]thymidine-positive spheres was observed, from 80.2% on day 6 to 27.0% on day 12 (P = .002). All the remaining basement membrane-negative spheres were [3H]thymidine positive on day 12 of culture. The data suggest the hypothesis that basement membrane synthesis and secretion, an early event in this system, may signal an inhibition of cell growth. Taken together, the data provide evidence of an antiproliferative effect of nm23-H1 gene expression in breast epithelial cells.
Table 2.
Relationship between cell growth and basement membrane deposition in culture of the nm23-H1 gene transfectant H1-177 cell*
| % colonies ± SE |
||
|---|---|---|
| Phenotype | Day 6 of culture | Day 12 of culture |
| [3H]Thymidine positive | 92.0 ± 2.5 | 29.9 ± 8.9 |
| Basement membrane positive | 87.8 ± 3.8 | 97.1 ± 1.9 |
| Basement membrane positive/[3H]thymidine positive | 80.2 ± 4.2 | 27.0 ± 7.4† |
| Basement membrane positive/[3H]thymidine negative | 7.6 ± 2.6 | 70.1 ± 8.9‡ |
| Basement membrane negative/[3H]thymidine positive | 11.7 ± 3.9 | 2.8 ± 1.9 |
| Basement membrane negative/[3H]thymidine negative | 1.0 ± 0.9 | 0 |
Basement membrane deposition was visualized by immunoperoxidase immunostaining, and DNA synthesis was determined by [3H]thymidine autoradiography in sections of EHS cultures. Colonies were simultaneously scored for the presence of basement membrane and thymidine-labeled nuclei on the days of culture noted.
Comparison of day 6 and day 12 using Wilcoxon signed rank, P = .002.
Comparison of day 6 and day 12 using Wilcoxon signed rank, P<.001.
Discussion
The importance of lactogenic hormones and basement membrane for morphological, biosynthetic, and growth regulatory aspects of mammary differentiation is well established for cells of both rodent and human origin. Culture of dispersed rodent mammary epithelial cells [(14,15), reviewed in (16)] or human reduction mammoplasty cultures (1) on or within basement membrane components has resulted in the formation of polarized acinar structures, production and vectorial secretion of milk proteins, and limited growth rates. Yet, under similar conditions, evidence of differentiation was lacking when primary cultures of human breast carcinomas or human breast carcinoma cell lines were used (1). These observations indicate the importance of additional, unknown factors intrinsic to breast cells in the differentiation process.
The present article has evaluated the potential contribution of nm23-H1 gene expression on morphological, growth regulatory, and biosynthetic aspects of breast differentiation, using the metastatic human MDA-MB-435 breast carcinoma cell line cultured within a physiologically relevant microenvironment of reconstituted basement membrane. Two lines derived from the metastatic human MDA-MB-435 breast carcinoma cell line, which overexpress the nm23-H1 gene, recapitulate portions of the breast differentiation process in reconstituted basement membrane culture, including the formation of acinus-like structures, directional deposition of basement membrane components, expression of sialomucin, and limitation of growth. These observations are in contrast to the behavior of the parental MDA-MB-435 cell line, the C-100 and C-103 control transfectants, and revertants of the H1-170 cell line that do not overexpress the nm23-H1 gene. The results represent the first cause and effect data concerning nm23-H1 genes and mammalian differentiation. The data do not imply that nm23 gene expression universally controls breast differentiation; the inability of tumor cell lines such as MCF-7 to fully differentiate in this system, despite relatively high expression of the nm23 gene (17), confirms the existence of additional important regulatory events. However, the statistically significant correlation of high Nm23 protein expression with a high grade of differentiation in several infiltrating ductal breast carcinoma cohorts (3,5) suggests that data generated in this culture system may be relevant in vivo.
In previous experiments, the differentiated phenotype of reduction mammoplasty cultures was correlated with full polarization of sialomucin secretion and correct cadherin mediated expression [(1) and Bissell MJ and Petersen OW: unpublished observations]. Our data indicate that MDA-MB-435 cells can recapitulate certain portions of the differentiation process in the absence of detectable E- and P-cadherin expression at the cell membrane and without the polarized luminal expression of sialomucin.
Our data also indicate that nm23-H1 gene expressing colonies were composed of twofold to threefold fewer cells than control colonies by day 12 of culture (Table 1). Similar trends were observed using thymidine labeling and cell per culture measurements (data not shown). These data indicate a suppressive effect of nm23-H1 gene expression on cell growth in this system. These data stand in agreement with the inhibition of soft agar colonization in response to TGF-β among nm23 gene-transfected breast carcinoma and murine melanoma cells (9,18). However, they contrast the lack of significant differences between the control and the nm23 gene transfectants in primary tumor size for these lines in vivo (9,18) as well as their growth rates (9,18) and thymidine labeling indices (data presented herein) on tissue culture plastic. In addition, the expression of the nm23 gene in cohorts of infiltrating ductal carcinomas has not been significantly correlated with primary tumor size, where analyzed (3,5,6). There are several possible reasons for these apparently conflicting data. First, we have noted that the directional deposition of the basement membrane component type IV collagen preceded growth arrest in the nm23-H1 gene-positive H1-177 cell line (Table 2). These data permit the hypothesis that cellular contact with basement membrane in nm23-H1 gene-positive cells may constitute part of the signal transduction pathway involved in growth suppression. It is possible that, in primary tumors, the wealth of tumor and stromal cells may produce sufficient proteases to disrupt this interaction; in developing metastases where single tumor cells or small emboli are present, sufficient intact basement membrane may be formed to inhibit growth, thus contributing to the apparent lack of metastases. Second, growth stimuli overriding basement membrane-induced control of nm23-H1 gene-positive breast cell proliferation may exist in the mammary environment, such as locally produced growth factors and/or tumor cell-stromal interactions. The observations presented here, linking endogenous basement membrane deposition to the reversion of the higH1y metastatic MD-MB-435 cell line to a more normally differentiated phenotype, suggest that the presence of an intact basement membrane may be one critical point of regulation that is lost in malignancy. The present model promises to allow the elucidation of aspects of the molecular mechanisms involved in basement membrane formation and its relationship to growth arrest and will help to establish the significance of Nm23 protein in these processes.
Acknowledgments
Supported by contract DE-ACO3-76SF00098 from the Health and Effects Research Division, Office of Health and Environmental Research, Department of Energy, the START program of the Danish Research Council, The Danish Cancer Society, The Novo Foundation, The Meyer Foundation, The Thaysen Foundation, the Gangsted Foundation, and the National Institutes of Health, Department of Health and Human Services.
We thank Marianne Lund and Torill Rignes for expert technical assistance and Dr. Calvin Roskelley for critical review of the manuscript.
References
- 1.Petersen OW, Rønnov-Jessen L, Howlett AR, et al. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [published erratum appears in Proc Natl Acad Sci U S A 90:2556, 1993] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevilacqua G, Sobel ME, Liotta LA, et al. Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res. 1989;49:5185–5190. [PubMed] [Google Scholar]
- 3.Royds JA, Stephenson TJ, Rees RC, et al. Nm23 protein expression in ductal in situ and invasive human breast carcinoma. J Natl Cancer Inst. 1993;85:727–731. doi: 10.1093/jnci/85.9.727. [DOI] [PubMed] [Google Scholar]
- 4.Hirayama R, Sawai S, Takagi Y, et al. Positive relationship between expression of anti-metastatic factor (nm23 gene product or nucleoside diphosphate kinase) and good prognosis in human breast cancer. J Natl Cancer Inst. 1991;83:1249–1250. doi: 10.1093/jnci/83.17.1249. [DOI] [PubMed] [Google Scholar]
- 5.Hennessy C, Henry JA, May FE, et al. Expression of the antimetastatic gene nm23 in human breast cancer: an association with good prognosis. J Natl Cancer Inst. 1991;83:281–285. doi: 10.1093/jnci/83.4.281. [DOI] [PubMed] [Google Scholar]
- 6.Barnes R, Masood S, Barker E, et al. Low nm23 protein expression in infiltrating ductal breast carcinomas correlates with reduced patient survival. Am J Pathol. 1991;139:245–250. [PMC free article] [PubMed] [Google Scholar]
- 7.Tokunaga Y, Urano T, Furukawa K, et al. Reduced expression of nm23-H1, but not of nm23-H2, is concordant with the frequency of lymph-node metastasis of breast cancer. Int J Cancer. 1993;55:66–71. doi: 10.1002/ijc.2910550113. [DOI] [PubMed] [Google Scholar]
- 8.Toulas C, Marek E, Cheutin F, et al. Expression of NM23 HI protein is related to metastasis free survival, tumor size and estrogen receptor level in human breast cancer. Proc Am Assoc Cancer Res. 1994;35:224. [Google Scholar]
- 9.Leone A, Flatow U, VanHoutte K, et al. Transfection of human nm23-Hl into the human MDA-MB-435 breast carcinoma cell line: effects on tumor metastatic potential, colonization, and enzymatic activity. Oncogene. 1993;8:2325–2333. [PubMed] [Google Scholar]
- 10.Kantor JD, McCormick B, Steeg PS, et al. Inhibition of cell motility after nm23 transfection of human and murine tumor cells. Cancer Res. 1993;53:1971–1973. [PubMed] [Google Scholar]
- 11.MacDonald NJ, De la Rosa A, Benedict MA, et al. A serine phosphorylation of Nm23, and not its nucleoside diphosphate kinase activity, correlates with suppression of tumor metastatic potential. J Biol Chem. 1993;268:25780–25789. [PubMed] [Google Scholar]
- 12.Rosengard AM, Krutzsch HC, Sheam A, et al. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989;342:177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- 13.Lakso M, Steeg PS, Westphal H. Embryonic expression of nm23 during mouse organogenesis. Cell Growth Diff. 1992;3:873–879. [PubMed] [Google Scholar]
- 14.Barcellos-Hoff MH, Aggeler J, Ram TG, et al. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlett AR, Bissell MJ. Influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- 17.Stahl JA, Leone A, Rosengard AM, et al. Identification of a second nm23 gene, nm23-H2. Cancer Res. 1991;51:445–449. [PubMed] [Google Scholar]
- 18.Leone A, Flatow U, King CR, et al. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991;65:25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]