Abstract
In most adult tissues there reside pools of stem and progenitor cells inside specialized microenvironments referred to as niches. The niche protects the stem cells from inappropriate expansion and directs their critical functions. Thus guided, stem cells are able to maintain tissue homeostasis throughout the ebb and flow of metabolic and physical demands encountered over a lifetime. Indeed, a pool of stem cells maintains mammary gland structure throughout development, and responds to the physiological demands associated with pregnancy. This review discusses how stem cells were identified in both human and mouse mammary glands; each requiring different techniques that were determined by differing biological needs and ethical constraints. These studies together create a robust portrait of mammary gland biology and identify the location of the stem cell niche, elucidate a developmental hierarchy, and suggest how the niche might be manipulated for therapeutic benefit.
Keywords: Niche, Stem cells, Mammary gland, Microenvironment
Making A Case for A Mammary Stem Cell
The mammary gland is a hormone sensitive, branching, and bilayered epithelial organ; it consists of an inner luminal epithelial layer and an outer myoepithelial layer surrounded by a basement membrane in a stromal fat pad. Cells from the two epidermal layers express a number of different proteins that can be used to unambiguously identify them. Among the most common of these markers are keratin (K) 8, K18, K19, and sialomucin-1 (Muc-1) expressed by luminal cell; and K5, K14, and α-smooth muscle actin expressed by myoepithelial cells. The arbor-like gland is organized into lobules that are inter-connected by a system of ducts; the basic functional unit of the breast is termed the terminal ductal lobular unit (TDLU). The gland is considered to have differentiated functionally and completely during lactation when the alveoli are swollen, producing milk, and secreting it into the lumen. Because the mammary gland has the ability to undergo repeated cycles of expansion (as much as tenfold at pregnancy) followed by involution, back to a state nearly indistinguishable from the virgin glands, it is clear that it is endowed with cells with remarkable regenerative capacity.
Elegant limiting dilution transplantation experiments [1] and retroviral integration studies in mice [2] suggest that single cells can give rise to all of the major cell types of the mammary gland. Similarly, analysis of Xchromosome inactivation in human mammary gland suggested that entire lobules could be derived from one cell [3]. In mass cultures of human cells derived from reduction mammoplasty, subpopulations from the primary breast luminal epithelial compartment have been shown to give rise to both luminal epithelial and myoepithelial cells [4-7]. Thus, luminal and myoepithelial cells, and in fact entire lobules of the gland, are derived from a common primitive stem cell.
Narrowing Down the Search: Where to Look for Human Stem Cells
Strong evidence that stem cells exist in mouse mammary gland was provided using flow cytometry sorting-based stem cell enrichment and transplantation into cleared mammary fat pads. CD49f+/CD24med/Lineage− [8] and CD49f+/CD29hi/Lineage− [9] subpopulations were enriched for cells that clonally generated entire functional mammary glands. However, the FACS profiles did not translate into a location in situ in mice. A number of reports have suggested that the cap region of the terminal end buds in mice is the location of the stem cells, but those observations were made in immature mice. The mammary glands of young prepubertal mice have not completely invaded their fat pads, whereas most human reduction mammoplasty tissues are isolated from post-pubertal women with completely developed glands in which terminal end buds are not prominent structures [10]. Therefore, a cap region would be an unlikely candidate for the mammary stem cell niche in adult humans.
Older literature had suggested that the mammary ducts of rodents may harbour stem cells. In virgin mice, candidate stem cells stained with antibodies JB6 and JsE3 were found in ducts rather than in alveoli, and it was postulated that these served to regenerate ductal epithelium as well as to form new alveolar buds [11]. Sca-1 was a proposed marker of mammary stem cells in mice, and its expression was observed in ducts as well as in invading terminal end buds [12]. The postulate that ducts were the location of the stem cells was born out also in an experiment when rudimentary ducts from post-gestational mice were transplanted to cleared fat pads of transforming growth factor-β1 transgenic mice. Those ducts retained the capacity to reactivate lobular structures at late pregnancy [13]. In addition, the mouse mammary gland ductal niche responds specifically to the MMTV-c-myc transgene by amplification of the stem cell compartment [14], which supported the previous findings in humans [3] that an entire terminal ductal lobular unit represents the progeny from a single early ductal progenitor. Taken together, one can narrow down the ducts as the most likely places where stem cell activity is stored in the human mammary gland.
Identifying Human Mammary Stem Cells by their Niche
Stem cells from any animal are rare constituents of their tissues, difficult to isolate, and in many cases intractable to expansion in culture. Rodents and other animal models, in addition to providing a source of tissues with nearly identical genetic content, also provide a physiological microenvironment in which to test for stem cell function. The study of human stem cells, however, presents challenges not encountered with most model organisms. Access to fresh tissue specimens from humans can be difficult to obtain, often the detailed genetic heritage and life history of the specimen is lacking, and innate differences among people further increases the variability between the data sets and between experiments. Most importantly, it is not possible to test the function of putatively isolated stem cells from most human tissues in their native micro-environments. The latter is particularly important for stem cell research because irrespective of the biochemical identity of the entity, a cell is not considered a stem cell until it meets basic functional criteria. A stem cell must self-renew, differentiate into at least one other cell type, and participate in the generation and maintenance of its tissue. To facilitate the study of human mammary gland stem cell biology, investigators have used physiologically relevant surrogate assays. Colony-forming assays on two-dimensional (2D) substrata are commonly used to identify cells with innate proliferative potential as well as to look for unior multi-potent ability determined by lineage marker expression. Both mouse-and human-derived mammary epithelial cells displayed functional properties of stem cells in three-dimensional (3D) ECM gel matrices, composed of either collagens or of laminin-rich ECM gels (lrECM), by recapitulating their behavior in vivo in terms of a number of morphogenetic criteria [15-19]. The 3D gel assays are useful for testing the ability of cells to generate bilayered, branching, gland-like structures from single cells, thus fulfilling the requirements of stem cells to give rise to at least one other cell type and to generate TDLU-like structures. Analogous to the non-adherent “neurosphere” cultures that were developed to propagate human neural progenitor cells, a “mammosphere” culture method was described to test for the property of self-renewal in primary mammary epithelial cells [16]. Because it is not plausible for human mammary cells to be tested by transplantation experiments in their native microenvironment, these surrogate assays were developed that collectively serve to generate an adequate portrait of a cell's putative stem cell activity.
From studies in multiple species it is known that adult stem cells are generally focal in their distribution and not necessarily co-localized with the bulk of transiently amplifying cells (for review see [21]). By combining micro-dissection with colony forming ability, candidate stem cells in the human hair follicle were identified from the bulge region more than a decade before the bulge was proven to be the epithelial stem cell niche in the skin [22-24]. In a similar manner, stem cells were shown to reside in proximal ducts of the prostate [25]. In their original paper, DeOme et al. [1] observed that any portion of the mouse mammary gland contained cells that could give rise to another and growth, and a similar conclusion was drawn also for human breast [26]. However, through careful microscope-directed collection of mammary organoids from reduction mammoplasty followed by multiple culture assays we recently demonstrated that the terminal ducts are one of the major repositories of stem cells in adults [19]. Dissected terminal ducts gave rise to cells capable of self-renewing as mammospheres, multipotent colony-forming in 2D culture, and forming bi-layered TDLU-like structures in 3D lrECM. Cells from dissected lobules did not display any of those functional characteristics. In situ analysis of ducts identified a narrow region of quiescent cells (Ki67−) that stained for chondroitin sulfate, K6a, K15 and SSEA-4, which are putative markers of stem cells in the breast in addition to other epithelial tissues [27-30]. Ducts were also the only location where cells double stained for keratins K14 and K19 were observed in situ. K14 is a typical marker of myoepithelial cells, and K19 is expressed in the luminal cells, but has been hypothesized also to serve as a switch keratin that permits the changeover of one type of cytoskeleton to the other [31]. The two markers are known to be expressed in the stem cell zone of prostate [32], and we hypothesize that the coincidence of the markers from two different lineages in one cell may identify stem or progenitor cells in the breast. Therefore, functional analysis of carefully dissected ducts and lobules suggests that the majority of stem cell activity indeed resides in the ducts, among quiescent cells of the phenotype K14+/K19+ that also express chondroitin sulfate, K6a, K15, and SSEA-4 [19].
The first approaches used to clonally identify a human mammary stem cell was to FACS sort or to immuno-magnetically enrich for subpopulations of cells with specific markers, which were then used for colony forming assays [33, 17]. Even prior to those reports, a number of investigators, including us, had observed that a subpopulation of luminal epithelial cells isolated by magnetic sorting were capable of giving rise to myoepithelial cells [4-7]. Initially, the makers used to characterize the human cells were EpCAM (epithelial cell adhesion molecule or epithelial specific antigen, ESA), CD49f (α6 integrin), and Muc-1 (sialomucin-1 or CD227). EpCAM+/CD49f+/Muc-1− cells were cloned by FACS from reduction mammoplasty and cultured in 96-well plates where many of the resulting colonies were composed of a ring of K14+ cells surrounding a core of K18+ cells; in collagen gels the clones formed branching structures [33]. EpCAM+/Muc-1− cells, isolated by two rounds of negative selection over a Muc-1 column followed by positive selection for EpCAM, also generated multilineage colonies and were shown to form bilayered TDLU-like structures in lrECM and when implanted into cleared fat pads of NOD/SCID mice [17]. Alternatively, the EpCAM+/CD49f+/Muc-1+ population are thought to represent a luminal-restricted progenitor and gave rise only to K18+ colonies of cells [33, 34]. The EpCam+/CD49f+ phenotype in cells flow-sorted from reduction mammoplasty coincides also with SSEA-4+/K15+/K6+ expression, as well as with the K14+/K19+ double staining phenotype [19]. Only cells from the EpCam+/CD49f+ population contained cells enriched for mammosphere-forming ability and the ability to generate TDLU-like structures in lrECM. Moreover, in 2D culture they gave rise to K14+ myoepithelial cells, K19+ luminal cells, K14+/K19+ progenitors, and to K14−/K19− cells related to the luminal lineage. Subpopulations identified by the other combinations of EpCam and CD49f did not generate colonies in 2D culture and produced spherical cellular aggregates in 3D that stained with lineage-restricted markers [19], findings that parallel the results described for mouse mammary gland [8]. Thus a link between a cell-surface phenotype, stem cell function, and a location in situ has been established in the human breast. The most likely location for the niche in the mature gland is in the ducts; however, the data published so far do not rule out the possible existence of other niches. For instance, when the gland begins to develop from the anlage in puberty and invade through the fat pad, it is reasonable that the majority of stem cells could be at the invading edge of the branching structures. Later when the gland is poised to undergo extensive proliferation and differentiation during pregnancy, it could make sense to have stem cells dispersed throughout the ductal system ready to produce alveoli throughout the fat pad.
Elucidating the Developmental Hierarchy in Breast
A developmental hierarchy has begun to emerge within the breast, which is similar to that described in the hematopoietic system and in the skin. Using mouse models, the cells that were shown to give rise to mammary gland also gave rise to lineage-restricted progenitors [8]. Quantitative RTPCR was used to show that cells of the phenotype CD24+/CD49flo/Lineage−, designated Ma-CFC, expressed luminal genes; CD24lo/CD49fmed/Lineage− cells, designated MYO, expressed myoepithelial markers (Lineage− in this case refers to non-CD45 and -CD31 expressing cells to rule out blood or endothelial cell contamination) [8]. The luminal-specific arm of the differentiation pathway is described in more detail with mouse models that lack GATA-3 in mammary gland at distinct developmental time points [35]. GATA-3 is a transcription factor that appears to be required for formation of the luminal layer of the acini [36]. GATA-3 deficiency in the stem cells, or in downstream multipotent progenitors, resulted in a build up of luminal-restricted progenitors, identified by the surface marker β3 integrin, that ultimately could not differentiate into mature cells [35]. The hierarchy of the luminal lineage was further defined in mice with the Notch pathway specifically knocked out in mammary glands. Development occurred normally through puberty, but failed to maintain the luminal layer in alveoli during pregnancy [37], which suggests that ductal and lobular luminal cells derive from separate lineage-restricted progenitors in mice. Indeed, in limiting dilution transplantation assays of postparous mammary tissue from WAP-Cre/flox-βGal mice, a population of parity-induced mammary epithelial cells (PI-MECS), which are enriched for CD24+/CD49f+ cells [38], proliferated to produce both luminal and myoepithelial cells in duct-restricted and lobule-restricted epithelial outgrowths [13]. Using primary cell culture and immortalized human cells, a similar hierarchy has been revealed.
In culture, the EpCAM+/CD49f+/Muc-1− cells give rise to uniformly myoepithelial cells over time [33], demonstrating one of the difficulties encountered in lineage tracing experiments in cultured human mammary tissue; selection. One successful approach to overcome this barrier has been to immortalize the progenitor cells with herpes virus proteins E6 and E7, which are known to affect multiple pathways and by-pass senescence (reviewed in [39, 40]). Whereas some changes in gene expression relative to primary mammary cells do occur [41], the human bipotent mammary progenitor cells harboring E6/E7 that were described by Gudjonsson et al. [34] do not form tumors in NOD/SCID mice and can form rudimentary glands in cleared fat pads. After transduction of ESA+/Muc1− cells with E6/E7, they were carried in culture for over 50 passages, consistently giving rise to cells that were primarily K14+/K19− myoepithelial cells, K14−/K19+ luminal epithelial cells, and renewing a small population of K14+/K19+ bipotent progenitor cells. They rarely give rise to K14−/K19− cells. Starting with the same material that the K14+/K19+ cell line was isolated from, but modifying the media composition, uniformly K14+/K19− myoepthelial-restricted or K14−/K19+ luminal-restricted cell lines were also isolated [19]. Clones of the K14−/K19+ luminal epithelial cell line gave rise to progeny that produced either keratin 6A or BCA-225; in situ these proteins were observed exclusively in ductal or lobular luminal epithelial cells, respectively [19]. Clones of the K14+/K19− myoepithelial cell line gave rise to progeny that produced either keratin 17 or WT-1; proteins that in situ were exclusively expressed in ductal or lobular myoepithelial cells, respectively [19]. The combined evidence from human tissue thus suggests that there is a developmental hierarchy in the mammary gland, as well (Fig. 1).
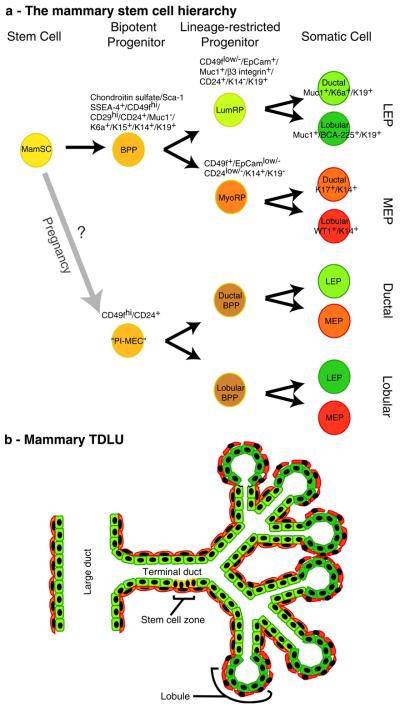
Fig. 1.
Developmental and functional hierarchies within the mammary gland. As described in the text, there is evidence of at least two potential stem cell hierarchies in the mammary gland. a Shows diagrams of the two putative mammary stem cell hierarchies. In one hierarchy, the quiescent mammary stem cells (MamSC) are thought to give rise to their nearest descendants, the bipotent progenitors (BPP), which subsequently give rise to luminal and myoepithelial line-age-restricted progenitors (LumRP and MyoRP, respectively). The lineage restricted progenitors subsequently generate differentiated ductal and luminal cells of the luminal epithelial (LEP) or myoepithelial (MEP) lineages. The second hierarchy originates from cells designated “parity-induced mammary epithelial cells” (PIMECs), which give rise to bipotent progenitors that generate both LEP and MEP, but are either duct- (ductal BPP) or lobule-specific (lobular BPP). Markers that have been used to describe or to isolate the different cell types are listed. Notably, CD24 and CD29 have so far only been used to identify murine mammary stem cells and have not yet been tested in human. b A cartoon of a terminal ductal-lobular unit is shown with color coding that corresponds to the stem cell hierarchy diagram. While the stem cell zone was reported in terminal ducts, the location of the BPP and lineage-restricted progenitors relative to the stem cells has yet to be described
Mouse experiments revealed a pathway from stem cell to differentiated luminal or myoepithelial cells, where the GATA-3 and Notch pathways play prominent roles in the evolution of the luminal lineage. The role for GATA-3 in human mammary development is not yet described, but Notch activity has been linked to self-renewal of the human mammary progenitor cells [20]. Interestingly, during pregnancy in mice there appear duct-restricted and lobule-restricted bi-potent progenitors. Culture experiments of human mammary cells suggest that stem cells give rise to luminal-restricted and myoepithelial-restricted progenitors that contribute to both ducts and lobules. Thus, there appear to be differences in the organization of the hierarchies described for mice and women (Fig. 1). However, it is still unclear whether these experiments have revealed species-specific organizational schemes or whether there is more than one cellular pathway to the same endpoint.
Are there Stem Cells Outside their Niche?
A number of the stem cell studies discussed here, particularly those in human, by necessity incorporate culture experiments or short periods of culturing before transplantation into animal hosts. It is worth considering how even a short culture period may influence the outcome of which stem cell, or descendant thereof, may grow. The protein Sca-1 and the Hoechst3342 exclusion assay, which identifies the so-called “side population”, were first used in studies of murine hematopoiesis to isolate hematopoeitic stem cells [40, 43]. Because side populations can be found in most tissues and cell types by making subtle changes to the staining conditions, and because Sca-1 is expressed by a number of different tissue-specific stem cells in mice, when other suitable marker combinations for isolating tissue-specific stem cells are not immediately obvious, investigators sometimes begin their search using these two markers. Welm and colleagues reported that murine mammary epithelial cells expressing Sca-1 and mammary side population were enriched for the ability to repopulate cleared mammary fat pads [12]. However, two groups more recently reported that the non-side population and the Sca-1-negative population contained all of the stem cell activity in mice [8, 9]. This has created some confusion. Potential differences in the experimental designs may point to properties of the mammary developmental hierarchy that should be further investigated. Welm et al. [12] used both immuno-magnetically enriched Sca-1+ cells from wild-type mice, and GFP+ cells FACS sorted from Sca1-GFP transgenic mice, which had GFP knocked in downstream from one copy of the Sca-1 promoter. The reports to the contrary used FACS sorting of antibody stained cells isolated from wild-type mice [8, 9]. First, in the Welm et al. experiments it was not shown how the expression of GFP correlated with Sca-1 expression in the Sca1-GFP transgenic. The GFP+ Sca1-GFP cells isolated directly from the glands may not have been expressing Sca-1 protein yet, but the promoter was active, which suggests that more primitive stem or progenitor cells may express Sca-1 transcripts prior to differentiating into more mature bipotent progenitors that express Sca-1 protein. Indeed, both sorting for GFP expression or Sca-1 immuno-magnetic enrichment used by Welm et al., yielded populations of cells that generated mammary glands in cleared fat pads, but the cells from GFP transgenic mice were five to tenfold more potent than the magnetically enriched, based on limiting dilution. Second, Welm et al. [12] used CD45 to exclude potential hematopoietic contaminants, but not CD31 to exclude endothelial cells [8, 9]. This raises the enticing possibility that an endothelial niche for stem cells exists also for mammary gland, as was described for the brain and hematopoietic systems [44, 45]. Finally, the magnetically enriched Sca-1+ cells were from “whole primary culture cells” and the side population cells with repopulating ability were in culture at least 72 hours prior to staining [12]. In contrast, CD49f+/Sca-1hi cells directly sorted from mam-mary glands did not have stem cell activity, but CD49f+/Sca-1lo cells did [8, 9]. Interestingly, all CD49f+ primary epithelial cells once in culture were shown to express Sca-1 overtime [8, 9]. Side population isolated from uncultured mammary gland also does not appear to correlate with stem cell activity [8, 9]. Therefore, in addition to potential differences resulting from the ages and strains of the mice, post-transcriptional or post-translational control of Sca-1, the exclusion of endothelial cells, or the setting of FACS sorting gates, it is clear that culture environments drive expression of the Sca-1 protein and of the side population phenotype and may have selected for an immature progenitor cell, which led to the discrepancies.
Indefinite self-renewal is a theoretical, but widely applied property of stem cells. In order to increase the likelihood that they were culturing human mammary stem cells, Dontu et al. [16] isolated the side population from human reduction mammoplasty tissue and demonstrated that the population contains cells with increased mammosphere forming ability. That mammospheres derived from normal reduction mammoplasty reportedly passaged (self-renew) only four to six times [46], suggests that mammosphere cultures maintain progenitors that do not indefinitely self-renew. Indeed, the side population in human breast appears to consist of a relatively less potent subset of progenitor cells. Clayton et al. [47] proposed that CALLA+/EMA+ cells (CALLA is a marker of myoepithelium, and EMA (epithelial membrane antigen) is a marker of luminal epithelium) from reduction mammoplasty were stem or multipotent progenitor cells because clones in 2D culture gave rise to five different colony types: K14+/K18+ double positive cells, K18+ luminal cells, K14+ myoepithelial cells, mixed K14+ and K18+ cells, or K14−/K18− cells. The side population did not contain CALLA+/EMA+ cells, and gave rise to only three colony types: K18+ cells, K14+ cells, or K14+/K18+ double positive cells. Thus the most potent cells were not in the side population. Taken together, these data suggest that manipulation of mammary epithelial cells in culture selects for immature progenitors or transit amplifying cells with extensive proliferative capacity, not stem cells. Moreover, the mammary side population from resting mammary gland or from cultured primary cells does not contain stem cells, but instead may enrich for bipotent progenitors at various stages of maturity. In fairness, it should also be pointed out that so far in vivo serial transplantation assays of bone marrow and of mammary tissue fails the indefinite self-renewal criteria. Perhaps indefinite self-renewal need not be a property of stem cells, or we do not yet know how to passage these cells in exactly the right context.
Because stem cells are thought to regenerate or maintain a tissue for the life of an organism, they are attributed with the ability to proliferate extensively. In hindsight, this logic seems counterintuitive as stem cells in vivo exhibit the opposite behavior, they are usually quiescent—indeed they have to be. That quiescence should be a defining property is underscored by experiments in which clones of hematopoietic stem cells from mice were cultured in bioengineered 3D niches. The majority of the cells were highly proliferative, but a small fraction of cells that were quiescent in the bioengineered matrices were shown to be the most potent in subsequent bone marrow transplant assays (Helen M. Blau, Keystone Symposia on Stem Cells, 2007, Department of Microbiology and Immunology, Stanford University, Stanford, CA, personal communication). These data imply that proliferation in culture is not an intrinsic property of stem cells; moreover adjusting culture conditions to better mimic the in vivo niche may serve to truly maintain stem cells ex vivo. In mouse and human mammary glands, it was noted that the majority of the prospective stem cells were in G1 phase of the cell cycle [8, 9], by extension of the data on hematopoietic stem cells, one might also speculate that the most potent of the mammary stem cells resided among a small quiescent fraction. Nevertheless, irrespective of potential caveats associated with any of the culture methods described, we think a number of basic mechanisms, such as quiescence and differentiation, are being successfully studied with culture models, and thus remain important tools in the biologist'skit.
The Potent Stem Cell Niche
If the supposition that cells cultured ex vivo are progenitors is correct, it would suggest that stem cells will not exist outside of their niche; that stem cells are defined by their niche. Accordingly, the dominance of stem cell niches has been demonstrated in several tissues where progenitor cells can occupy a vacated stem cell niche and reacquire stem cell traits. The melanocyte stem cell niche in mice is in the base of the hair bulb of the transient portion of the hair follicle. Stem cells occupy the very bottom of the follicle and as they divide their progeny ascend and line the sides of the follicle, becoming increasingly differentiated with increasing distance from the niche. When the melanocyte stem cell niche was experimentally vacated, committed progenitor cells from a neighboring follicle traversed the interstitial space, occupied the vacated niche, and subsequently functioned like stem cells [48]. Skeletal muscle stem cells which were first described by their unique anatomical location, residing underneath the basal lamina and juxtaposed to the muscle fiber, were designated “satellite cells” [49]. Single muscle fibers can be explanted into culture, where quiescent satellite cells become activated and generate proliferative progenitor cells, referred to as myoblasts. After myoblasts are injected back into skeletal muscle, they fuse with existing muscle fibers in response to local damage cues. It was shown that upon injection into muscle, which was first irradiated to first kill the native stem cells, myoblasts derived from a single satellite cell participated in muscle regeneration over successive rounds of damage by injection of the snake venom Notexin [50]. This experiment demonstrates that mature muscle progenitors can reoccupy vacated muscle stem cell niches and function as stem cells for the life-time of the animal. That such mechanisms are at work also in the mammary gland was suggested when glands resulting from transplantation of epithelial cells into cleared fat pads underwent multiple rounds of pregnancy [13].
Several reports of cells crossing tissue lineages to regenerate non-self tissues create fertile ground for speculation about the extent of dominance of stem cell niches over cell function and phenotype. Mice that received bone marrow transplants from syngeneic donors, using GFP or βgal transgenes to facilitate identification of donor versus host tissue, revealed that bone marrow-derived cells could participate in repair of muscle damage [51-55]. Marrow-derived cells were found to occupy the satellite cell position on muscle fibers in irradiated muscles [51, 53, 54], as well as respond appropriately to physiological damage cues such as exercise [54, 56]. Although the in vivo data are enticing, ex vivo culture experiments demonstrated that the transition from blood to muscle was not complete. In contrast to muscle-derived myoblasts that can easily undergo myo-genesis in culture by reducing or removing serum, marrrow-derived cells cultured from transplant recipient muscles would undergo myogenesis in culture only in myoblast-conditioned medium [54] or cocultured with a myoblast cell-line [55]. Those experiments underscore the importance of the muscle microenvironment in maintaining the ability of non-muscle-derived cells to exhibit myogenic traits. In an intriguing report Boulanger et al. [57] recently demonstrated a similar dominance of the mammary microenvironment over testicular stem cells. Germinal stem cells from male WAP-CRE/flox-βGal mouse testis were enriched based on α6-integrin (CD49f) expression, mixed with unlabeled mammary epithelial cells, then injected together into cleared fat pads of mice that were subsequently made to undergo a round of pregnancy and involution. Transgenic mouse-derived testicular germinal stem cells in the mammary gland microenvironment were able to self-renew and give rise to cells that expressed either markers of myoepithelial or of luminal epithelial cells, and perhaps most importantly, they showed evidence of milk production [57]. Together, these experiments suggest an important role played by the stem cell niche in establishing the identity and function of tissue-specific stem cells.
Stem Cell Niches and Breast Cancer: Causes or Cure?
It has been observed that the behavior of non-stem cells inside stem cell niches in lower organisms may yield clues as to how niches might be involved in disease states such as cancer [58]. Specifically, occupation of an experimentally vacated germinal stem cell niche in Drosophila by a somatic cell resulted in ectopic proliferation [59]. Furthermore, ectopic expression of the niche protein, DPP, induced mature cystocytes to revert to a stem cell phenotype [60]. Because cancers are often described as having phenotypes and functions in common with tissue-specific stem and progenitor cells, these basic mechanisms described for lower organisms raise interesting etiological possibilities for cancers in the humans. In addition to the possibility that cancers may result from errant stem or progenitor cells that were misguided by their microenvironment [61], or from mutated somatic cells that take on stem cell phenotypes, a third possibility has emerged that when inappropriate cells enter the stem cell niche, they could receive the wrong signal leading to tumors.
Combining the concept that stem cells are controlled by their microenvironment together with the concept that cancers are driven by a small population of cells with stem cell properties [62] raises some possibilities also for treatment. Malignant mammary epithelial cells grown in 3D lrECM form colonies that do not exhibit growth control or apical-basal polarity. However, when their communication with the microenvironment is down-modulated by manipulation of growth factor receptor or integrin receptor pathways, the malignant cells revert to a growth-arrested normal phenotype, exhibiting proper apical–basal polarity [63]. The use of retinoic acid to treat promyelomonocytic leukemia suggests that this type of approach will work clinically [64]. In fact, recent experiments in mice in which β1 integrin inhibitory antibodies were used to selectively inhibit growth of malignant human breast epithelial cells suggest that breast cancer could be treated also by “reversion” therapy [65]. Finally, modulating the signals received by the “cancer stem cells” from their niche may abort their march towards malignancy. Cells that cause bone marrow residual disease in leukemia were hypothesized to bind fibronectin through VLA4, where they become quiescent, avoiding the cytotoxic effects of drugs such as Arabinoside C (AraC). In mouse models, when anti-VLA4 was administered together with AraC the disease was completely eradicated, suggesting that failure of the leukemia cells to associate with their protective niche made them vulnerable to killing [66]. Perhaps the use of a combination of agents that will prevent “cancer stem cells” in solid tumors from associating with a protective niche together with cytotoxic agents will yield a therapeutically useful combination.
Ackowledgments
This work was supported by the National Institute of Health (CA-64786 to MJB and OWP, CA-57621 to MJB), and by grants from the OBER office of the US Department of Energy (DE-AC03-76SF00098 and a Distinguished Fellow Award to MJB), and the US Department of Defense (an Innovator Award to MJB). MAL is supported by a postdoctoral fellowship from the American Cancer Society.
References
- 1.Deome KB, Faulkin LJ, Jr., Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Research. 1959;19:515–520. [PubMed] [Google Scholar]
- 2.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS. Contiguous patches of normal human mammary epithelium derived from a single stem cell: Implications for breast carcinogenesis. Cancer Research. 1996;56:402–404. [PubMed] [Google Scholar]
- 4.Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Developmental Biology. 2005a;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Kang KS, Morita I, Cruz A, Jeon YJ, Trosko JE, Chang CC. Expression of estrogen receptors in a normal human breast epithelial cell type with luminal and stem cell characteristics and its neoplastically transformed cell lines. Carcinogenesis. 1997;18:251–257. doi: 10.1093/carcin/18.2.251. [DOI] [PubMed] [Google Scholar]
- 6.Kao CY, Nomata K, Oakley CS, Welsch CW, Chang CC. Two types of normal human breast epithelial cells derived from reduction mammoplasty: Phenotypic characterization and response to SV40 transfection. Carcinogenesis. 1995;16:531–538. doi: 10.1093/carcin/16.3.531. [DOI] [PubMed] [Google Scholar]
- 7.Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Developmental Biology. 1999;206:88–99. doi: 10.1006/dbio.1998.9133. [DOI] [PubMed] [Google Scholar]
- 8.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006a;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 9.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 10.Howard BA, Gusterson BA. Human breast development. Journal of Mammary Gland Biology and Neoplasia. 2000;5:119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg A, Daams H, van der Valk MA, Hilkens J, Hilgers J. Development of mouse mammary gland: Identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. Journal of Histochemistry and Cytochemistry. 1986;34:1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- 12.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Developmental Biology. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 13.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 14.Chepko G, Slack R, Carbott D, Khan S, Steadman L, Dickson RB. Differential alteration of stem and other cell populations in ducts and lobules of TGFalpha and c-Myc transgenic mouse mammary epithelium. Tissue and Cell. 2005;37:393–412. doi: 10.1016/j.tice.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Developmental Biology. 2005b;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & Development. 2003a;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes and Development. 2002a;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006b;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 19.Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, et al. Evidence for a stem cell hierarchy in the adult human breast. Journal of Cell Biology. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Wissam MA, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Research. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 22.Moll I. Proliferative potential of different keratinocytes of plucked human hair follicles. Journal of Investigative Dermatology. 1995;105:14–21. doi: 10.1111/1523-1747.ep12312406. [DOI] [PubMed] [Google Scholar]
- 23.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. Journal of Investigative Dermatology. 1993;101:652–659. doi: 10.1111/1523-1747.ep12371671. [DOI] [PubMed] [Google Scholar]
- 25.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, et al. Proximal location of mouse prostate epithelial stem cells: A model of prostatic homeostasis. Journal of Cell Biology. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Hare MJ, Ormerod MG, Monaghan P, Lane EB, Gusterson BA. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation. 1991;46:209–221. doi: 10.1111/j.1432-0436.1991.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 27.Bocker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: A new cell biological concept. Laboratory Investigation. 2002;82:737–746. doi: 10.1097/01.lab.0000017371.72714.c5. [DOI] [PubMed] [Google Scholar]
- 28.Dravida S, Pal R, Khanna A, Tipnis SP, Ravindran G, Khan F. The transdifferentiation potential of limbal fibroblast-like cells. Brain Research. Developmental Brain Research. 2005;160:239–251. doi: 10.1016/j.devbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Ohyama M, Terunuma A, Tock CL, Radonovich MF, PiseMasison CA, Hopping, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. Journal of Clinical Investigation. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmelz M, Moll R, Hesse U, Prasad AR, Gandolfi JA, Hasan SR, et al. Identification of a stem cell candidate in the normal human prostate gland. European Journal of Cell Biology. 2005;84:341–354. doi: 10.1016/j.ejcb.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stasiak PC, Purkis PE, Leigh IM, Lane EB. Keratin 19: Predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. Journal of Investigative Dermatology. 1989;92:707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- 32.Hudson DL, Guy AT, Fry P, O'Hare MJ, Watt FM, Masters JR. Epithelial cell differentiation pathways in the human prostate: Identification of intermediate phenotypes by keratin expression. Journal of Histochemistry and Cytochemistry. 2001;49:271–278. doi: 10.1177/002215540104900214. [DOI] [PubMed] [Google Scholar]
- 33.Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Research and Treatment. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 34.Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes and Development. 2002b;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature Cell Biology. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 36.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, et al. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Developmental Biology. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Developmental Biology. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Dimri G, Band H, Band V. Mammary epithelial cell transformation: insights from cell culture and mouse models. Breast Cancer Research. 2005;7:171–179. doi: 10.1186/bcr1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratsch SB, Gao Q, Srinivasan S, Wazer DE, Band V. Multiple genetic changes are required for efficient immortalization of different subtypes of normal human mammary epithelial cells. Radiation Research. 2001;155:143–150. doi: 10.1667/0033-7587(2001)155[0143:mgcarf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Pan J, Li JL, Lee JH, Tunkey C, Saraf K, et al. Transcriptional changes associated with breast cancer occur as normal human mammary epithelial cells overcome senescence barriers and become immortalized. Molecular Cancer. 2007;6:7. doi: 10.1186/1476-4598-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. Journal of Experimental Medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spangrude GJ, Klein J, Heimfeld S, Aihara Y, Weissman IL. Two monoclonal antibodies identify thymic-repopulating cells in mouse bone marrow. Journal of Immunology. 1989;142:425–430. [PubMed] [Google Scholar]
- 44.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 46.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. Journal of the National Cancer Institute. 2007;99:616–627. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 47.Clayton H, Titley I, Vivanco M. Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Experimental Cell Research. 2004;297:444–460. doi: 10.1016/j.yexcr.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 49.Mauro A. Satellite cell of skeletal muscle fibers. Journal of Biophysical and Biochemical Cytology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Dreyfus PA, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, et al. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. American Journal of Pathology. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 53.Fukada S, Miyagoe-Suzuki Y, Tsukihara H, Yuasa K, Higuchi S, Ono S, et al. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. Journal of Cell Science. 2002;115((Pt) 6):1285–1293. doi: 10.1242/jcs.115.6.1285. Department of Immunology, G. S. o. P. S. O. U. S. O. J. [DOI] [PubMed] [Google Scholar]
- 54.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111(4):589–601. doi: 10.1016/s0092-8674(02)01078-4. Baxter Laboratory for Genetic Pharmacology, D. o. M., and Immunology, D. o. M. P. S. U. S. o. M. C. S. C. A. USA. [DOI] [PubMed] [Google Scholar]
- 55.Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, et al. Isolation of adult mouse myogenic progenitors: Functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 56.Palermo AT, LaBarge MA, Doyonnas R, Pomerantz J, Blau HM. Bone marrow contribution to skeletal muscle: A physiological response to stress. Developmental Biology. 2005;279:336–344. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 59.Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 61.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumor-igenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: Evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 65.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Research. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Natural Medicines. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]