Abstract
High-density lipoprotein (HDL) plays a fundamental role in the Reverse Cholesterol Transport pathway. Prior to maturation, nascent HDL exist as disk-shaped phospholipid bilayers whose perimeter is stabilized by amphipathic apolipoproteins. Methods have been developed to generate reconstituted (rHDL) in vitro and these particles have been used in a variety of novel ways. To differentiate between physiological HDL particles and non-natural rHDL that have been engineered to possess additional components/functions, the term nanodisk (ND) is used. In this review, various applications of ND technology are described, such as their use as miniature membranes for solubilization and characterization of integral membrane proteins in a native like conformation. In other work, ND harboring hydrophobic biomolecules/drugs have been generated and used as transport/delivery vehicles. In vitro and in vivo studies show that drug loaded ND are stable and possess potent biological activity. A third application of ND is their use as a platform for incorporation of amphiphilic chelators of contrast agents, such as gadolinium, used in magnetic resonance imaging. Thus, it is demonstrated that the basic building block of plasma HDL can be repurposed for alternate functions.
Background
The term high-density lipoprotein (HDL) describes a continuum of plasma lipoprotein particles that possess a multitude of different proteins and a range of lipid constituents [1]. The major physiological function of HDL is in Reverse Cholesterol Transport [RCT; [2]]. The well-documented inverse relationship between plasma HDL concentration and incidence of cardiovascular disease has generated considerable interest in development of strategies to increase HDL levels. Aside from exercise, moderate consumption of alcohol and a healthy lifestyle, pharmacological approaches are being pursued with the goal of enhancing athero-protection [3]. In addition to these strategies, direct infusion of reconstituted HDL (rHDL) into subjects has been performed [4]. The idea is that parenteral administration of rHDL will promote RCT, facilitating regression of atheroma. Indeed, Nissen et al. [5] reported Phase II clinical trial results showing a decrease in intimal thickness in patients treated with rHDL harboring a variant apolipoprotein A-I.
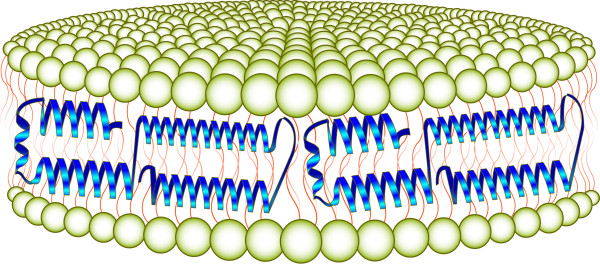
While its structural properties and composition can be rather complex, in its most basic form, HDL are relatively simple, containing only phospholipid and apolipoprotein (apo). The most abundant and primary apolipoprotein component of plasma HDL is apoA-I. Human apoA-I (243 amino acids) is well characterized in terms of its structural and functional properties. When incubated with certain phospholipid vesicles in vitro, apoA-I induces formation of rHDL. The key structural element of apoA-I required for rHDL assembly is amphipathic α-helix. Indeed, other apolipoproteins, apolipoprotein fragments or peptides that possess this secondary structure, can also combine with phospholipid to form rHDL. In general, the product particle is a nanometer scale disk-shaped phospholipid bilayer whose periphery is circumscribed by two or more apolipoprotein molecules (Figure 1). Indeed, a defining characteristic of members of the class of exchangeable apolipoprotein is an ability to form rHDL. For the purpose of this review, the protein/peptide component of discoidal rHDL is termed the "scaffold" in recognition of its function in stabilization of the otherwise unstable edge of the bilayer.
Figure 1.
Schematic diagram of rHDL structural organization. The complex depicted is comprised of a disk-shaped phospholipid bilayer that is circumscribed by an amphipathic "scaffold" protein. Note: The exact structural organization of rHDL remains controversial. Recently, evidence consistent with an ellipsoidal shape has been presented [59-61].
Production of rHDL
Detailed structure-function studies of exchangeable apolipoproteins have given rise to two general methods for discoidal rHDL formation: detergent dialysis and direct conversion. Whereas the detergent dialysis method [6] has the advantage that a broad spectrum of bilayer forming phospholipids can be employed, a disadvantage relates to the potentially problematic detergent removal step, which can be achieved by specific absorption or exhaustive dialysis. On the other hand, while limited to fewer phospholipid substrates, the direct conversion method does not employ detergents. The types of phospholipids commonly used in the direct conversion method are synthetic, saturated acyl chain glycerophospholipids such as dimyristoylphosphatidylcholine (DMPC) or dimyristoylphosphatidylglycerol. These lipids undergo a gel to liquid crystalline phase transition in the range of 23°C. Normally, the phospholipid substrate is hydrated and induced to form vesicles, either by membrane extrusion or sonication. Incubation of the phospholipid vesicle substrate with an appropriate scaffold protein (e.g. apoA-I) induces self-assembly of rHDL. It is likely that the reaction proceeds most efficiently in this temperature range because defects created in the vesicle bilayer surface serve as sites for apolipoprotein penetration, bilayer disruption and transformation to rHDL. Among the apolipoproteins that have been examined for their ability to transform phospholipid bilayer vesicles into rHDL and function as a scaffold are apoA-I, apoE, apoA-IV, apoA-V and apolipophorin III. In addition, it is known that fragments of apolipoproteins [7] or designer peptides [8] can substitute for full-length apolipoproteins in this reaction. Based on this description, it is evident that myriad combinations of phospholipid and scaffold can be employed to formulate unique rHDL. These particles are readily characterized in terms of size by non-denaturing polyacrylamide gel electrophoresis and morphology by electron or atomic force microscopy (AFM).
Over the past decade, discoidal rHDL have been repurposed for applications beyond its physiological role in lipoprotein metabolism. This review describes active areas of research that have evolved from our basic understanding of rHDL structure and assembly. Whereas rHDL has been modified to re-task it for alternate purposes, its basic structural elements, including disk shape, nanometer scale size and a planar bilayer whose periphery is stabilized by a scaffold, are preserved. In this manner, rHDL serve as a platform capable of packaging transmembrane proteins in a native-like membrane environment, solubilization and delivery of hydrophobic drugs/biomolecules and presentation of contrast agents for magnetic resonance imaging of atherosclerotic lesions. In an effort to distinguish engineered rHDL from classical rHDL, the term nanodisk (ND) is used to describe rHDL formulated to possess a transmembrane protein, drug or non-natural hydrophobic moiety.
ND as a miniature membrane environment for solubilization of transmembrane proteins
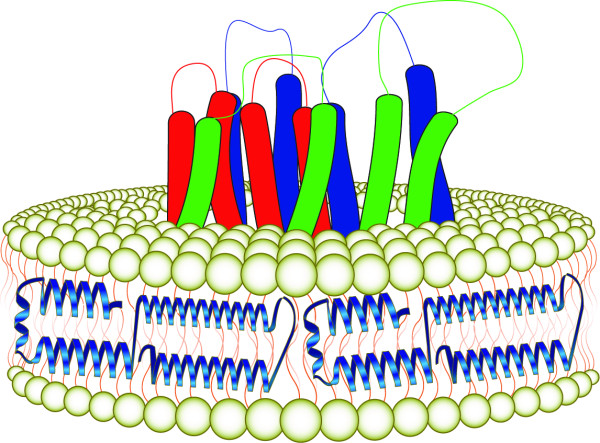
The bilayer component of ND provides a native-like environment for study of transmembrane proteins in isolation. The concepts being developed on this research front are that Type 1, Type 2 or Type 3 membrane proteins can be inserted into ND with retention of their native conformation/biological activity. As with a cell membrane, the inserted protein would align such that its transmembrane segment(s) spans the bilayer while their soluble, extra-membranous portions, exist in the aqueous environment (Figure 2). If correctly inserted, it is anticipated that specific biological or enzymatic properties of the protein will be preserved. The surface area of a 20 nm diameter ND particle is ~300 nm2, ample area to accommodate several molecules of a multiple pass transmembrane protein. Advantages of ND versus detergent micelles include a more natural environment and the absence of detergent related effects on conformation or activity of the subject protein. While liposomes are amenable to study of transmembrane proteins, these complexes suffer from having an inaccessible inner aqueous space, protein orientation issues, size variability and lack of complete solubility.
Figure 2.
Diagram of a ND particle with an embedded transmembrane protein. The bilayer component of the ND provides a miniature bilayer membrane that can accommodate one or more transmembrane proteins in a native-like conformation.
Several groups have successfully generated membrane protein-containing ND, including cytochrome P450 s, seven-transmembrane proteins, bacterial chemoreceptors and others [[9-12] for reviews]. Advantages of ND for this purpose include particle size homogeneity, access to both sides of the membrane and greater control over the oligomerization state of the inserted protein. The power and potential of this technology is illustrated by the following specific examples:
a. Bacteriorhodopsin
Bacteriorhodopsin (bR) from the purple membrane of halobacterium halobium is a prototype integral membrane protein. This 247 amino acid, light-driven proton pump possesses a covalently bound molecule of retinal. Elegant electron crystallography methods were developed and employed by Henderson and Unwin to decipher the structure of bacteriorhodopsin at near atomic resolution [13]. The protein is comprised of a bundle of seven ~25 residue α-helical rods that span the bilayer while charged residues at the surface of the membrane contact the aqueous solvent. In its native form bR exists as trimers that organize into a two-dimensional hexagonal array in the plane of the membrane. In 2006, Bayburt et al. [14] assembled bR into ND. Under the conditions employed each ND contained three bR molecules. Small angle X-ray scattering analysis provided evidence that bR embeds in the ND bilayer while evidence of trimer formation was obtained by near UV circular dichroism spectroscopy of the retinal absorbance bands. In further study of this system, Blanchette et al. [15] used atomic force microscopy to image and analyze bR-ND. The self-assembly process employed by these authors generated two distinct ND populations, bR-ND and empty-ND, as distinguished by an average particle height increment of 1.0 nm for bR-ND. When bR is present during assembly, ND diameters are larger suggesting the inserted protein influences the dimensions of the product ND.
b. Cytochrome P450
Baas and coworker [16] reported on structural and functional characterization of cytochrome P450 3A4 (CYP 3A4)-ND. Solution small angle X-ray scattering of CYP 3A4-ND provided evidence that CYP 3A4 retains hydroxylation activity. In other work, Das and Sligar [17] incorporated cytochrome P450 reductase (CPR) into ND and investigated its ability to transfer electrons from NADPH to microsomal P450 s. The redox potential of CPR's FMN and FAD cofactors shifted to more positive values in ND compared to a solubilized version of the reductase in which the N-terminal membrane spanning domain was cleaved. Moreover, when anionic lipids were used to alter the membrane composition of CPR-ND, the redox potential of both flavins became more negative, favoring electron transfer from CPR to cytochrome P450.
c. ß2-adrenergic receptor
Leitz et al. [18] reported on ND harboring ß2-adrenergic receptor. Evidence that the receptor adopts a native like conformation within the ND milieu was obtained from study of its G-protein coupling activity.
d. Hydrogenase
Baker et al. [19] reported the physical characterization and hydrogen-evolving activity of ND assembled with hydrogenase obtained from the thermophilic Archea, Pyrococcus furiosus. Insofar as this class of membrane bound enzyme is capable of ex vivo hydrogen production from starch or glucose, this work may impact development of bioengineered hydrogen generation methods for renewable energy production.
e. SecYEG
In bacteria, protein transit across the cytoplasmic membrane is mediated by translocase [20]. Translocase consists of the transmembrane protein conducting channel, SecYEG, a soluble motor protein, SecA, and the chaperone, SecB. Nascent proteins destined for secretion are bound by SecB and directed to SecYEG- associated SecA. Protein translocation is subsequently driven by SecA through repeated cycles of ATP binding and hydrolysis wherein the target protein is threaded through the SecYEG pore. Alami et al. [21] successfully reconstituted SecYEG into ND and used these particles to study the interaction of SecYEG and its cytosolic partner, SecA. SecYEG-ND were able to trigger dissociation of SecA dimers and associate with the SecA monomer, leading to activation of SecA ATPase. Thus, SecYEG-ND represent a novel means to investigate the role of bacterial protein transport via translocase.
f. Anthrax toxin
Katayama et al. [22] obtained structural insight into the mechanism whereby protective antigen (PA) pore formation mediates translocation of the enzymatic components of anthrax toxin across membranes. Two populations of PA pores, in vesicles and ND, were reconstructed from electron microscopic images at 22 Å resolution. Fitting the X-ray crystallographic coordinates of the PA pre-pore revealed a prominent flange, formed by convergence of mobile loops that function in protein translocation. Identification of the location of functional elements of the PA pore from electron microscopic characterization of ND embedded PA represents an innovative use of ND technology.
g. VDAC-1
The voltage-dependent anion channel (VDAC) is an essential protein in the eukaryotic outer mitochondrial membrane, providing a pore for substrate diffusion. High-resolution structures of VDAC-1 in detergent micelles and bicelles have been reported using solution NMR and X-ray crystallography. These studies have resolved longstanding issues related to VDAC membrane topology and provide the first eukaryotic ß-barrel membrane protein structure. At the same time, the structure - function basis for the voltage gating mechanism of VDAC-1 or its modulation by NADH remain unresolved. To address these issues Raschle et al. [23] conducted electron microscopy and solution NMR spectroscopy on VDAC-1-ND. Electron microscopy provided evidence for formation of VDAC-1 multimers, while high-resolution NMR spectroscopy revealed that VDAC-1 is properly folded and manifests NADH binding activity. Thus, ND offer a new approach for study of the biophysical properties of VDAC-1 under native-like conditions.
h. Hemagglutinin
Influenza virus infection causes significant mortality and morbidity in human populations. Hemagglutinin (HA) is the major protein target of the protective antibody response induced by influenza viral infection. The influenza virion grows by budding from the plasma membrane of an infected cell. The outer envelope of influenza virus consists of a lipid bilayer into which the integral membrane glycoprotein, HA, inserts. Whereas recombinant HA is relatively easy to produce, its efficacy as a vaccine is limited by an inability to retain a native, membrane-bound conformation. Bhattacharya et al. [24] generated recombinant HA-ND (influenza virus strain A/New Caledonia/20/99; H1N1) and investigated its ability to confer immunity upon influenza virus challenge. HA-ND vaccination induced a robust antibody response with a high hemagglutination inhibition titer. The finding that HA-ND vaccination conferred a level of protection comparable to Fluzone® and FluMist® following H1N1 challenge, suggests this approach is worth pursuing in greater detail.
Vehicle for solubilization and delivery of hydrophobic biomolecules
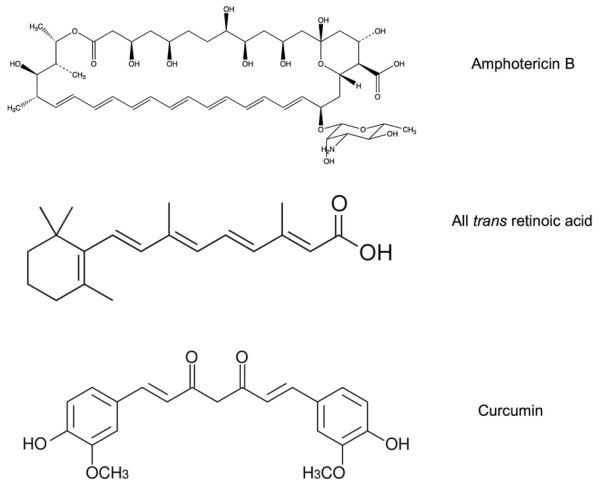
Aside from study of membrane bound proteins, another application of ND technology is as a vehicle for transport/delivery of small hydrophobic biomolecules/drugs [25]. To date, several bioactive compounds, including the macrolide polyene antibiotic, amphotericin B (AMB), the isoprenoid, all trans retinoic acid (ATRA) and the polyphenol, curcumin, have been successfully integrated into the ND milieu (Figure 3). On the basis of studies characterizing drug incorporation efficiency, retention of biological activity and ease of formulation, it is apparent that ND constitute a platform for solubilization, transport and delivery of hydrophobic bioactive molecules. Recent success in the design and production of targeted-ND offer a means to expand the capability of this approach [26].
Figure 3.
Structure of small hydrophobic molecules. The water insoluble molecules shown, including amphotericin B, all trans retinoic acid and curcumin, have been successfully incorporated in ND with retention of biological activity.
Amphotericin B
AMB has been used clinically for nearly half a century. It is an amphoteric molecule that interacts with membrane sterols (preferably 24 substituted sterols such as ergosterol), forming pores that facilitate leakage of cell contents. Clinical application of this potent antifungal is limited by poor oral bioavailablility, infusion-related toxicity and nephrotoxicity [27]. Using the direct solubilization method, AMB-ND have been formulated with high incorporation efficiency [28,29]. AMB-ND inhibited growth of Saccharomyces cerevisiae as well as several pathogenic fungal species [28]. Furthermore, compared to AMB-deoxycholate, AMB-ND display attenuated red blood cell hemolytic activity and decreased toxicity toward cultured hepatoma cells. In vivo studies in immunocompetent mice revealed that AMB-ND are nontoxic at concentrations up to 10 mg/kg AMB, and show efficacy in a mouse model of candidiasis at concentrations as low as 0.25 mg/kg [28]. Taken together, these results indicate that AMB-ND constitute a novel formulation that effectively solubilizes the antibiotic and elicits strong in vitro and in vivo antifungal activity, with no observed toxicity at therapeutic doses.
AMB-ND have also been examined for efficacy in Leishmania major infected mice [30,31]. Membranes of these protozoal parasites contain episterol and, as such, are susceptible to AMB. When L. major-infected mice were treated with AMB-ND, enhanced efficacy was observed. Mice administered AMB-ND at 1 or 5 mg/kg displayed decreased lesion size and parasite burden. At 5 mg/kg AMB-ND induced complete clearance of the infection, with no lesions remaining and no parasites isolated from infected animals. By contrast, liposomal AMB, at the same dose, was far less effective. The ability of AMB-ND to induce clearance of L. major parasites from a susceptible strain of mice without an appreciable change in cytokine response suggests AMB-ND represent a potentially useful formulation for treatment of intrahistiocytic organisms.
All trans retinoic acid
Retinoids, such as ATRA, are useful agents in cancer therapy as they exhibit a central role in cell growth, differentiation, and apoptosis [32,33]. Its beneficial actions have been well documented for treatment of acute promyelocytic leukemia [34]. ATRA binding to nuclear hormone receptors transactivates target genes, leading to cell growth arrest or apoptosis [35-37]. At the same time, ATRA is insoluble in water, toxic at higher doses and has limited bioavailability [38]. Pharmacological levels can cause retinoic acid syndrome and neurotoxicity, particularly in children [39]. Redmond et al. [40] formulated ATRA into ND. Subsequently, Singh et al. [41] evaluated effects of ATRA-ND on Mantle cell lymphoma (MCL), a subtype of non-Hodgkin's lymphoma that arises from uncontrolled proliferation of a subset of pregerminal center cells located in the mantle region of secondary follicles [42]. In cell culture studies, compared to free ATRA, ATRA-ND more effectively induced reactive oxygen species generation and led to a greater degree of cell death. Mechanistic studies revealed that ATRA-ND enhanced G1 growth arrest, up-regulated p21and p27 and down-regulated cyclin D1. At ATRA concentrations that induce apoptosis, expression levels of retinoic acid receptor-α and retinoid X receptor-γ increased. Taken together, evidence indicates that incorporation of ATRA into ND enhances the biological activity of this retinoid.
Curcumin
Known chemically as diferuloylmethane, curcumin is a hydrophobic polyphenol derived from rhizome of turmeric (Curcuma longa), an East Indian plant. Curcumin possesses diverse pharmacologic effects including anti-inflammatory, anti-oxidant and anti-proliferative activities [43,44]. Furthermore, curcumin is non-toxic, even at relatively high doses [45]. Despite this, clinical advancement of curcumin has been hindered by poor water solubility, short biological half-life and low bioavailability following oral administration. Ghosh et al. [46] formulated curcumin-ND at a 6:1 phospholipid:curcumin molar ratio. When formulated in ND, curcumin is water-soluble and gives rise to a characteristic absorbance spectrum. AFM analysis revealed curcumin-ND are disk-shaped particles with a diameter < 50 nm. In cell culture studies, curcumin-ND induced enhanced HepG2 cell growth inhibition compared to free curcumin. Moreover, curcumin-ND were a more potent inducer of apoptosis in cultured MCL cells than free curcumin.
Contrast agent enriched ND for medical imaging
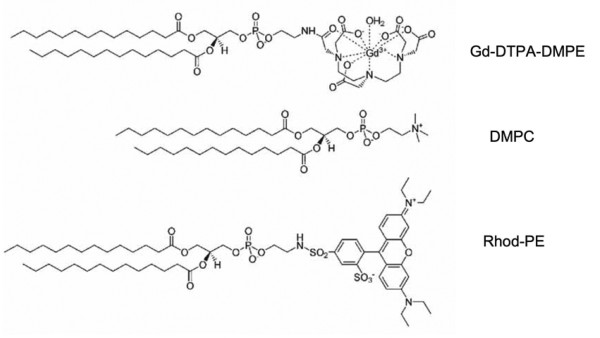
Given that cardiovascular disease is the major cause of mortality in North America, there is a pressing need for noninvasive imaging of atherosclerotic lesions. One of the most promising techniques currently available is magnetic resonance imaging (MRI). In the case of cardiovascular disease, MRI can be used to identify and characterize plaque deposits. In this way it facilitates diagnosis, choice of therapy as well as assessment of the effectiveness of a given intervention. The utility of MRI is significantly enhanced by the use of paramagnetic ions [47]. A popular paramagnetic ion used as a contrast agent for MRI is the chemical element gadolinium (Gd; atomic number 64). Gd3+ chelates are widely used because they provide positive contrast (imaging brightening) in anatomical images rather than negative contrast. Furthermore, Gd has no known biological role and Gd3+-chelates are generally considered nontoxic. An example of an amphiphilic Gd3+ chelator is diethylenetriaminepentaacetate-dimyristoylphosphatidylethanolamine (Gd3+-DTPA-DMPE) (Figure 4). The lipophilic DMPE moiety of this chelator provides a means to tether Gd3+ to ND. In addition to amphiphilic Gd3+ chelates, ND have also been modified with lipophilic fluorophores, extending their use to fluorescence imaging techniques.
Figure 4.
Structures of specialized lipids. Gd3+-DTPA-DMPE (diethylenetriaminepentaacetate-dimyristoylphosphatidylethanolamine) is an amphiphilic Gd3+ chelator useful in magnetic resonance imaging; DMPC (dimyristoylphosphatidylcholine) is a glycerophospholipid commonly employed as a structural lipid in ND; Rhod-PE (1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)ammonium salt) is a lipophilic fluorophore useful in fluorescence imaging techniques.
Skajaa et al. [48] have summarized progress toward establishing ND as a vehicle for delivery of diagnostic agents to vulnerable atherosclerotic plaques in mouse models of atherosclerosis. For example, Frias et al. [49] injected Gd3+-ND into mice with atherosclerotic lesions. Subsequent MRI analysis revealed a clear enhancement of plaque contrast. Likewise, Cormode et al. [50] used Gd3+-ND to enhance contrast in macrophage-rich areas of plaque in a mouse model of atherosclerosis. Cormode et al. [51] incorporated gold, iron oxide, or quantum dot nanocrystals into ND for computed tomography, magnetic resonance, and fluorescence imaging, respectively. By including additional probes in these particles, unique functionalities were introduced. Importantly, the in vitro and in vivo behavior of such ND mimicked the behavior of native HDL.
Chen et al. [52] introduced a targeting moiety into Gd3+-ND in an effort to improve macrophage uptake. A carboxyfluorescein-labeled apoE-derived peptide, termed P2fA2, was used as scaffold in Gd3+-ND. Macrophage uptake was studied in J774A.1 macrophages and MRI studies were performed in apoE (-/-) mice. In vivo studies showed a more pronounced and significantly higher signal enhancement with the apoE peptide while confocal microscopy studies revealed that P2fA2 Gd3+-ND co-localize with intraplaque macrophages. In another application, Chen et al. [53] functionalized Gd3+-ND with an α,ß 3-integrin-specific pentapeptide as a means to target ND to angiogenic endothelial cells. Subsequent studies revealed preferential uptake of the targeted ND by endothelial cells.
Other applications
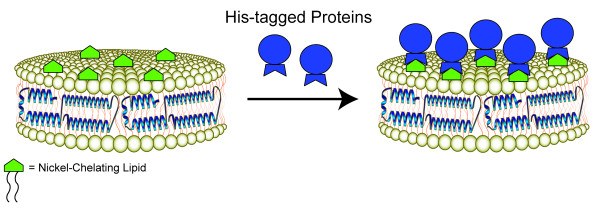
As the applications described above continue to be developed and improved, additional new uses of ND technology have emerged recently. For example, Fischer et al. [54] incorporated synthetic nickel-chelating lipids into ND and examined their ability to bind His-tagged proteins (Figure 5). The nickel-chelating lipid, DOGS-NTA-Ni (1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl) iminodiacetic acid] succinyl}(nickel salt), was incorporated into ND at varying amounts. Gel filtration chromatography, native PAGE and AFM analysis revealed that His-tagged proteins bind to these modified ND in a nickel-dependent manner. In an example of the utility of this approach, DOGS-NTA-Ni-ND were employed as a substrate for binding His-tagged West Nile virus envelope protein [55]. The observation that envelope protein immunogenicity increased upon conjugation to ND suggests they may be useful as a vaccine to prevent West Nile encephalitis. In a modification of this general approach Borch et al. [56] generated ND harboring ganglioside GM1. Subsequent studies with GM1-ND showed they possess the capacity to recognize and bind its soluble interaction partner, cholera toxin B subunit. Finally, sphingosine-1-phosphate (S1P) is a naturally occurring bioactive lipid that elicits effects on mitogenesis, endothelial cell motility, cell survival and differentiation. Matsuo et al. [57] examined the effect of S1P-ND on tube formation in endothelial cells. The effect of S1P-ND on endothelial cells observed in this study vividly illustrates the utility of incorporating bioactive lipids into the ND platform.
Figure 5.
Capturing His-tagged proteins on the surface of ND. Incorporation of the nickel-chelating lipid, DOGS-NTA-Ni (1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxypentyl) iminodiacetic acid] succinyl}(nickel salt) into ND confers the ability to stably and specifically bind His tagged proteins.
Conclusions and Future Directions
Emerging from basic studies of HDL metabolism is new technology built around the basic structure of nascent HDL particles. A variety of applications, ranging from membrane protein insertion, drug delivery to functionalized lipid incorporation, have led to significant new advances. The utility of ND technology is intimately linked to the ease with which these particles are generated, the water solubility and nanoscale size of the product particles, together with the imagination of the investigator. An example of the latter is the synthesis of bio-mimetic nanoparticles wherein a gold core serves as a template for assembly of a mixed phospholipid bilayer and association of apoA-I [58]. As the examples described in this review document, the future is very bright for ND technology.
Abbreviations
HDL: high density lipoprotein; rHDL: reconstituted HDL; RCT: reverse cholesterol transport; Apo: apolipoprotein; DMPC: dimyristoylphosphatidylcholine; AFM: atomic force microscopy; ATRA: all trans retinoic acid; AMB: amphotericin B.
Competing interests
The author is a Founder of Lypro Biosciences Inc. and co-author of US Patent application No. 10/778,640 "Lipophilic drug delivery vehicle and methods of use thereof".
Acknowledgements and Funding
Work from the author's lab was funded by NIH grants HL64159 and AI 061354. The author thanks Ms. J. A. Beckstead for assistance with figure preparation.
References
- Fielding CJ. High Density Lipoproteins. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/MOL.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P, Ray KK, Cannon CP. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010;55:1283–1299. doi: 10.1016/j.jacc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Dudley-Brown S. A shot of good cholesterol: synthetic HDL, a new intervention for atherosclerosis. J Cardiovasc Nurs. 2004;19:421–424. doi: 10.1097/00005082-200411000-00015. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. full_text. [DOI] [PubMed] [Google Scholar]
- Weers PM, Narayanaswami V, Ryan RO. Modulation of the lipid binding properties of the N-terminal domain of human apolipoprotein E3. Eur J Biochem. 2001;268:3728–3735. doi: 10.1046/j.1432-1327.2001.02282.x. [DOI] [PubMed] [Google Scholar]
- Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J Lipid Res. 2001;42:1096–1104. [PubMed] [Google Scholar]
- Nath A, Atkins WM, Sligar SG. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry. 2007;46:2059–2069. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- Borch J, Hamann T. The nanodisc: a novel tool for membrane protein studies. Biol Chem. 2009;390:805–814. doi: 10.1515/BC.2009.091. [DOI] [PubMed] [Google Scholar]
- Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. full_text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayburt TH, Sligar SG. Membrane protein assembly into Nanodiscs. FEBS Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Unwin PN. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975;257:28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Bayburt TH, Grinkova YV, Sligar SG. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch Biochem Biophys. 2006;450:215–222. doi: 10.1016/j.abb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Blanchette CD, Cappuccio JA, Kuhn EA, Segelke BW, Benner WH, Chromy BA, Coleman MA, Bench G, Hoeprich PD, Sulchek TA. Atomic force microscopy differentiates discrete size distributions between membrane protein containing and empty nanolipoprotein particles. Biochim Biophys Acta. 2008;1788:724–731. doi: 10.1016/j.bbamem.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Baas BJ, Denisov IG, Sligar SG. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch Biochem Biophys. 2004;430:218–228. doi: 10.1016/j.abb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Das A, Sligar SG. Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer. Biochemistry. 2009;48:12104–12112. doi: 10.1021/bi9011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz AJ, Bayburt TH, Barnakov AN, Springer BA, Sligar SG. Functional reconstitution of Beta2-adrenergic receptors utilizing self-assembling Nanodisc technology. Biotechniques. 2006;40:601–602. doi: 10.2144/000112169. [DOI] [PubMed] [Google Scholar]
- Baker SE, Hopkins RC, Blanchette CD, Walsworth VL, Sumbad R, Fischer NO, Kuhn EA, Coleman M, Chromy BA, Létant SE, Hoeprich PD, Adams MW, Henderson PT. Hydrogen production by a hyperthermophilic membrane-bound hydrogenase in water-soluble nanolipoprotein particles. J Am Chem Soc. 2009;131:7508–7509. doi: 10.1021/ja809251f. [DOI] [PubMed] [Google Scholar]
- Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–67. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- Alami M, Dalal K, Lelj-Garolla B, Sligar SG, Duong F. Nanodiscs unravel the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J. 2007;26:1995–2004. doi: 10.1038/sj.emboj.7601661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Wang J, Tama F, Chollet L, Gogol EP, Collier RJ, Fisher MT. Three-dimensional structure of the anthrax toxin pore inserted into lipid nanodiscs and lipid vesicles. Proc Natl Acad Sci USA. 2010;107:3453–3457. doi: 10.1073/pnas.1000100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc. 2009;131:17777–17779. doi: 10.1021/ja907918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Grimme S, Ganesh B, Gopisetty A, Sheng JR, Martinez O, Jayarama S, Artinger M, Meriggioli M, Prabhakar BS. Nanodisc-incorporated hemagglutinin provides protective immunity against influenza virus infection. J Virol. 2010;84:361–371. doi: 10.1128/JVI.01355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RO. Nanodisks: hydrophobic drug delivery vehicle. Expert Opin Drug Deliv. 2008;5:343–351. doi: 10.1517/17425247.5.3.343. [DOI] [PubMed] [Google Scholar]
- Iovannisci DM, Beckstead JA, Ryan RO. Targeting nanodisks via an apolipoprotein - single chain variable antibody chimera. Biochem Biophys Res Commun. 2009;379:466–469. doi: 10.1016/j.bbrc.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsel S, Bolard J. Amphotericin B: new life for an old drug. Trends Pharmacol Sci. 1996;17:445–449. doi: 10.1016/S0165-6147(96)01012-7. [DOI] [PubMed] [Google Scholar]
- Oda MN, Hargreaves P, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high-density lipoprotein enriched with the polyene antibiotic, amphotericin B. J Lipid Res. 2006;47:260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- Nguyen T-S, Weers PMM, Raussens V, Wang Z, Ren G, Sulchek T, Hoeprich PD, Ryan RO. Amphotericin B induces interdigitation of apolipoprotein stabilized nanodisk bilayers. Biochim Biophys Acta. 2008;1778:303–312. doi: 10.1016/j.bbamem.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KG, Bishop J, Ryan RO, Titus R. Nanodisk-associated amphotericin B clears Leishmania major cutaneous infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2006;50:1238–1244. doi: 10.1128/AAC.50.4.1238-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell M, Choi BS, Ryan RO, Hancock M, Titus RG, Abebe T, Hailu A, Müller I, Rogers ME, Bangham CR, Munder M, Kropf P. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3:e480. doi: 10.1371/journal.pntd.0000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Adamson PC. All-Trans-Retinoic Acid Pharmacology and Its Impact on the Treatment of Acute Promyelocytic Leukemia. Oncologist. 1996;1:305–314. [PubMed] [Google Scholar]
- Guidoboni M, Zancai P, Cariati R, Rizzo S, Dal Col J, Pavan A, Gloghini A, Spina M, Cuneo A, Pomponi F, Bononi A, Doglioni C, Maestro R, Carbone A, Boiocchi M, Dolcetti R. Retinoic acid inhibits the proliferative response induced by CD40 activation and interleukin-4 in mantle cell lymphoma. Cancer Res. 2005;65:587–95. [PubMed] [Google Scholar]
- Kitareewan S, Blumen S, Sekula D, Bissonnette RP, Lamph WW, Cui Q, Gallagher R, Dmitrovsky E. G0S2 is an all-trans-retinoic acid target gene. Int J Oncol. 2008;33:397–404. [PMC free article] [PubMed] [Google Scholar]
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- Takitani K, Hino N, Terada Y, Kurosawa Y, Koh M, Inoue A, Kawakami C, Kuno T, Tamai H. Plasma all-trans retinoic acid level in neonates of mothers with acute promyelocytic leukemia. Acta Haematol. 2005;114:167–169. doi: 10.1159/000087893. [DOI] [PubMed] [Google Scholar]
- Redmond KA, Nguyen T-S, Ryan RO. All-trans retinoic acid nanodisks. Int J Pharm. 2007;339:246–250. doi: 10.1016/j.ijpharm.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AT, Evens AM, Anderson RJ, Beckstead JA, Sankar N, Sassano A, Bhalla S, Yang S, Platanias LC, Forte TM, Ryan RO, Gordon LI. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br J Haematol. 2010;150:158–169. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni F, Ponzoni M. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol. 2007;39:1747–1753. doi: 10.1016/j.biocel.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Epstein J, Sanderson IR, Macdonald TTX. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 1994;26:1–13. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative Medicine Review. 2009;14:141–153. [PubMed] [Google Scholar]
- Ghosh M, Singh ATK, Xu W, Sulchek T, Gordon LI, Ryan RO. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2010. in press . [DOI] [PMC free article] [PubMed]
- De Leon-Rodriguez LM, Lubag AJ, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Responsive MRI agents for sensing metabolism in vivo. Acc Chem Res. 2009;42:948–957. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skajaa T, Cormode DP, Falk E, Mulder WJ, Fisher EA, Fayad ZA. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6:2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- Cormode DP, Briley-Saebo KC, Mulder WJ, Aguinaldo JG, Barazza A, Ma Y, Fisher EA, Fayad ZA. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4:1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, Fisher EA, Fayad ZA, Mulder WJ. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Vucic E, Leupold E, Mulder WJ, Cormode DP, Briley-Saebo KC, Barazza A, Fisher EA, Dathe M, Fayad ZA. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging. 2008;3:233–242. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- Chen W, Jarzyna PA, van Tilborg GA, Nguyen VA, Cormode DP, Klink A, Griffioen AW, Randolph GJ, Fisher EA, Mulder WJ, Fayad ZA. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010;24:1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer NO, Blanchette CD, Chromy BA, Kuhn EA, Segelke BW, Corzett M, Bench G, Mason PW, Hoeprich PD. Immobilization of his-tagged proteins on nickel-chelating nanolipoprotein particles. Bioconjug Chem. 2009;20:460–465. doi: 10.1021/bc8003155. [DOI] [PubMed] [Google Scholar]
- Fischer NO, Infante E, Ishikawa T, Blanchette CD, Bourne N, Hoeprich PD, Mason PW. Conjugation to nickel-chelating nanolipoprotein particles increases the potency and efficacy of subunit vaccines to prevent West Nile encephalitis. Bioconjug Chem. 2010;21:1018–1022. doi: 10.1021/bc100083d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J, Torta F, Sligar SG, Roepstorff P. Nanodiscs for immobilization of lipid bilayers and membrane receptors: kinetic analysis of cholera toxin binding to a glycolipid receptor. Anal Chem. 2008;80:6245–6252. doi: 10.1021/ac8000644. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Miura S, Kawamura A, Uehara Y, Rye KA, Saku K. Newly developed reconstituted high-density lipoprotein containing sphingosine-1-phosphate induces endothelial tube formation. Atherosclerosis. 2007;194:159–168. doi: 10.1016/j.atherosclerosis.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc. 2009;131:1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Libeu CA, Newhouse Y, Hall SC, Witkowska HE, Weisgraber KH. Apolipoprotein E-dipalmitoylphosphatidylcholine particles are ellipsoidal in solution. J Lipid Res. 2007;48:1035–1044. doi: 10.1194/jlr.M600545-JLR200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Gogonea V, Lee X, Wagner MA, Li XM, Huang Y, Undurti A, May RP, Haertlein M, Moulin M, Gutsche I, Zaccai G, Didonato JA, Hazen SL. Double superhelix model of high density lipoprotein. J Biol Chem. 2009;284:36605–36619. doi: 10.1074/jbc.M109.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skar-Gislinge N, Simonsen JB, Mortensen K, Feidenhans'l R, Sligar SG, Lindberg B Møller, Bjørnholm T, Arleth L. Elliptical structure of phospholipid bilayer nanodiscs encapsulated by scaffold proteins: casting the roles of the lipids and the protein. J Am Chem Soc. 2010;132:13713–13722. doi: 10.1021/ja1030613. [DOI] [PMC free article] [PubMed] [Google Scholar]