Abstract
Recent studies have shown that oxidative stress contributes to the pathogenesis of muscle damage in dystrophic (mdx) mice. In this study we have investigated the role of NADPH oxidase as a source of the oxidative stress in these mice. The NADPH oxidase subunits gp91phox, p67phox and rac 1 were increased 2–3 fold in tibilais anterior muscles from mdx mice compared to wild type. Importantly, this increase occurred in 19 day old mice, before the onset of muscle necrosis and inflammation, suggesting that NADPH oxidase is an important source of oxidative stress in mdx muscle. In muscles from 9 week old mdx mice, gp91phox and p67phox were increased 3–4 fold and NADPH oxidase superoxide production was 2 times greater than wild type. In single fibers from mdx muscle NADPH oxidase subunits were all located on or near the sarcolemma, except for p67phox,which was expressed in the cytosol. Pharmacological inhibition of NADPH oxidase significantly reduced the intracellular Ca2+ rise following stretched contractions in mdx single fibers, and also attenuated the loss of muscle force. These results suggest that NADPH oxidase is a major source of reactive oxygen species in dystrophic muscle and its enhanced activity has a stimulatory effect on stretch-induced Ca2+ entry, a key mechanism for muscle damage and functional impairment.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked, degenerative muscle disease, which affects approximately 1 in 3500 males globally. DMD is caused by the absence of dystrophin, a large (427 kDa) protein connecting the cytoskeleton to a complex of sarcolemmal proteins, which bind to the extracellular matrix. Dystrophin was originally thought to have an important structural and stabilizing role during muscle contractions, which protected muscles from contraction-induced, mechanical damage to the membrane [1]. However, recent evidence from dystrophin-deficient muscles has revealed a more complicated picture, with the abnormal regulation of many ion channels and cell signaling pathways contributing to the disease pathophysiology [2]. In young DMD patients, muscle damage is followed by regeneration but as the disease manifests, regeneration is impeded and muscle fibers are progressively replaced with connective tissue and fatty deposits. Profound muscle weakness results in loss of mobility by about the age of 10–12, and eventually death around age 20–30, due to respiratory and/or cardiac failure.
Reactive oxygen species (ROS) have been implicated in a wide-range of human diseases. Over two decades ago, ROS were postulated to contribute to the pathogenesis of DMD, which led to a number of clinical trials using antioxidants [3]. Overall, these trials were disappointing in terms of clinical benefits. Retrospectively though, these clinical studies were often carried out on patients with advanced muscle degeneration and the antioxidants used were not membrane permeable. This point is highlighted by recent studies on the mdx mouse, an animal model of DMD, in which membrane permeable antioxidants administered to young mice ameliorate the progression of damage in both skeletal [4], [5], [6] and cardiac [7] muscle. Therefore, the timing and targeting of the antioxidant are both key factors to consider in designing these drugs as therapeutic strategies for DMD. In recent studies, we have also shown that ROS play an important role in mdx muscle damage produced by stretched (eccentric) contractions [6], [8]. Dystrophic muscles are extremely vulnerable to damage from stretched contractions, which are performed during everyday activities, such as walking downhill, when the quadriceps muscle acts as a brake to control the degree of knee flexion against the force of gravity.
An important follow up question to previous studies on oxidative stress is to elucidate the source(s) of excessive ROS production in dystrophic muscles. Given the inflammatory nature of dystrophy, an obvious potential source are ROS-producing cells such as macrophages and neutrophils. However, there is evidence that mdx muscles are oxidatively stressed before the onset of observable histological muscle damage and inflammation, which begins at around 3 to 4 weeks of age. For instance, increased expression of endogenous antioxidants, SOD and catalase, as well as lipid peroxidation in pre-necrotic mdx mice (up to 20 days of age) have been observed [9]. Similarly, increased levels of oxidized GSH, a measure of cellular ROS reactivity, in muscles of mdx mice of the same age, has been shown [10]. These findings imply that the loss of dystrophin initially triggers increased ROS production by a skeletal muscle-specific source, rather than by invading inflammatory cells, which are likely to contribute to oxidative stress during the damage/regeneration phase of the disease.
NADPH oxidase is a multi-protein, enzyme complex, which uses NADPH as a substrate to convert molecular oxygen to reactive oxygen species (ROS), usually superoxide or hydrogen peroxide (H2O2). It is highly expressed in inflammatory cells, such as neutrophils and macrophages, and is activated during phagocytosis to kill invading pathogens. The phagocyte NADPH oxidase consists of the membrane-bound proteins gp91phox, also called NOX2, and p22phox. In addition, there are several cytosolic subunits; the organizer subunit, p47phox, the activator subunit p67phox, the transport subunit p40phox, and the small GTP-ases rac1 or rac2, which are required for full activation of the enzyme [11]. This phagocytic NADPH oxidase complex is also present in a many other cell types including all muscle types; smooth, cardiac and skeletal [12]. The only phagocytic NADPH oxidase subunit not detected in skeletal muscle is p40phox [13]. Gene array data has shown significantly increased mRNA for the NADPH oxidase subunits gp91phox [14] and p67phox [15] in mdx hindlimb muscles. Moreover, NADPH oxidase activity is increased in mdx cardiac muscle [7], [16] and in mdx skeletal muscle fibers at rest and subjected due to hypo-osmotic swelling [17]. These results suggest that NADPH oxidase is an important source of ROS in mdx muscle, after the initial onset of muscle damage and regeneration. However, to test if NADPH oxidase is a major source of ROS in mdx muscle, studies are also needed on pre-necrotic mdx mice, younger than 3 weeks of age, to avoid the confounding effects of ROS stimulated by muscle degradation pathways and infiltrating inflammatory cells.
In the current study, we tested the hypothesis that NADPH oxidase is a major source of ROS production in mdx muscle, by measuring NADPH oxidase protein levels in pre-necrotic mdx mice. We also measured NADPH oxidase enzyme activity and protein expression in young adult mdx muscles. Finally, we investigated if NADPH oxidase contributed to stretch-induced muscle damage in mdx single fibers. In previous papers we have shown that both the stretch-induced force loss and the rise in intracellular Ca2+ are significantly attenuated when stretch-activated channel (SAC) blockers are used [18]. We have also shown that ROS stimulate Ca2+ entry through stretch-activated channels in mdx fibers, both at rest and following stretched contractions [8]. The data from this previous study also suggested that stretch-activated channels from mdx but not wild type muscle are hypersensitive to oxidative modification, either directly or via other ROS-activated signaling proteins such as src kinase. Therefore, in the present study, we investigated whether NADPH oxidase is a source of the stretch-induced ROS, which triggers increased intracellular Ca2+ in mdx muscle fibers.
Methods
Ethics Statement
Wild type (WT) and dystrophin-deficient mdx mice, both bred on the C57BL/10ScSnJ background, were purchased from Animal Resources Centre, Perth, Australia and from The Jackson Laboratory, USA. All experimental procedures were approved by the Animal Ethics Committee of the University of Sydney (protocol number: K22/6-2007/2/4625) and the Institutional Animal Care and Use Committee at the University of Washington (protocol number: 3298-02). Mice were euthanized either by I.P injection of 163 mg/kg pentobarbitone sodium (University of Sydney) or by CO2 inhalation followed by cervical dislocation (University of Washington).
Western Blotting
Tibialis anterior (TA) muscles of 18–19 day and 9 week old mice were homogenized (Polytron PT 1200, Kinematica, Littau/Lucerne, Switzerland) in a lysis buffer containing; 50 mM Tris (pH 7.5), 150 mM NaCl, 25 mM EGTA, 25 mM EDTA, 1% Triton X-100, protease inhibitor cocktail, calpain 1 inhibitor and phosphatase inhibitor (Sigma, USA). After 30 min incubation on ice, samples were centrifuged at 15,800 g at 4°C and the supernatant was collected. Total protein was measured by the Bradford assay technique (Bio-Rad, Hercules, CA, USA).
TA muscle lysates were immunoblotted as described previously [6]. Membranes were probed using mouse antibodies against rac1 (1∶500) (Cytoskeleton, CO), gp91phox (1∶1000) and p67phox (1∶500) (BD Biosciences, San Jose, CA), and rabbit antibodies against p22phox (1∶500) and p47phox (1∶500) (Santa Cruz, CA). The protein bands were detected with an ECL Plus kit (Amersham Pharmacia Biotech, UK) and visualized with an Alpha Innotech FluoChem SP Imaging System (San Leandro, CA, USA). Densitometry was performed using ImageJ software. Following imaging, membranes were incubated in Ponceau S solution (Sigma, USA), to conform equal loading of protein in each well.
NADPH-dependent superoxide assay
NADPH oxidase superoxide production was measured with the lucigenin chemiluminescent assay, using similar methods to those previously described [7]. Briefly, TA muscles from WT and mdx mice were homogenized in a detergent-free lysis buffer (pH 7.4) containing (mM); 150 NaCl, 50 Tris, 25 EGTA, 25 EDTA, as well as a protease inhibitor cocktail and phosphatase inhibitors. Protein concentrations of each sample were determined by Bradford assay. A total of 2 mg/ml of the protein homogenate, diluted in the same Tris buffer, was used for each experiment. Immediately before the experiment, NADPH (200 µM) and lucigenin (10 µM) were added from stock solutions, so that the final volume was 600 µl. Each sample was incubated at 37°C and the lucigenin-dependent light emission was detected by a photomultiplier-based luminometer. Various drugs were added to block potential sources of ROS; the non-specific NADPH oxidase inhibitor diphenyleneiodonium (DPI, 10 µM); the NOS inhibitor, N-nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 µM); the mitochondrial site I electron transport inhibitor, rotenone (20 µM); and the xanthine oxidase inhibitor, oxypurinol (100 µM).
NADPH oxidase immunostaining in single fibers
Flexor digitorum brevis (FDB) single fibers from 3 to 8 week old WT and mdx mice were isolated based on previous methods [19]. Briefly, FDB muscles were dissected in a solution containing; 138 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.06 mM MgCl2, 12.5 mM HEPES and 5.6 mM glucose. Muscles were then placed in the same solution containing 0.2% collagenase type I and 10% FCS and incubated with 5% CO2 in air at 37°C for 90 min. Muscles were placed in culture medium (DMEM and 10% FCS) for 30 min and then single fibers were dissociated by gently triturating with a plastic Pasteur pipette. Isolated fibers were plated in 24 well culture dishes on laminin-coated coverlsips and incubated at 37°C for at least 4 hours to allow for attachment. Single fibers were rinsed with PBS and fixed for 10 min (2% paraformaldehyde). Fibers were the permeabilized and blocked in PBS containing 0.25% saponin, 10% FCS and 1% BSA for 30 min. Primary antibodies, diluted in PBS and 0.1% saponin, were incubated overnight at 4°C. Secondary antibodies (AlexaFluor 488 or 555; Molecular Probes, Invitrogen), diluted 1∶1000 were incubated with 0.1% saponin and DAPI in PBS for 2 hrs at room temperature. Fluorescent images were taken by scanning confocal microscopy (Zeiss LSM 510 Meta), with 40 or 100 x oil immersion objectives, and viewed with Image J software.
Stretched contractions on mdx single fibers
Single muscle fibers from mdx mice were loaded with a Ca2+ indicator and subjected to stretched contractions, as described previously [18]. Briefly, single FDB muscle fibres from mdx mice (8–10 weeks of age) were dissected and attached, via aluminium tendon clips, to a force transducer and length controller. Fibers were perfused with a solution containing (mM); NaCl 121, KCl 5, CaCl2 1.8, MgCl2 0.5, NaH2P04 0.4, NaHCO3 24 and glucose 5.5. The solution was maintained at room temperature and constantly bubbled with 95% O2–5% CO2 (pH 7.4). Fibers were loaded with 10 µM Fluo4-AM (Molecular Probes) to measure the resting intracellular Ca2+ concentration ([Ca2+]i). Experiments were carried out on non-treated fibers (control) and fibers treated for 15 min with the NADPH oxidase inhibitor DPI (1 µM). Confocal microscopy was used to measure changes in [Ca2+]i before and after 10 stretched contractions, using our previous protocol [18]. Isometric force was also measured before and 10 min after the stretched contractions.
Statistics
All results are presented as the mean ± SEM. Statistical differences between groups (WT or mdx, control or treated) were determined using the Student's unpaired t-test. The level of statistical significance was set at P<0.05.
Results
NADPH oxidase proteins are increased in 9 week old mdx muscle
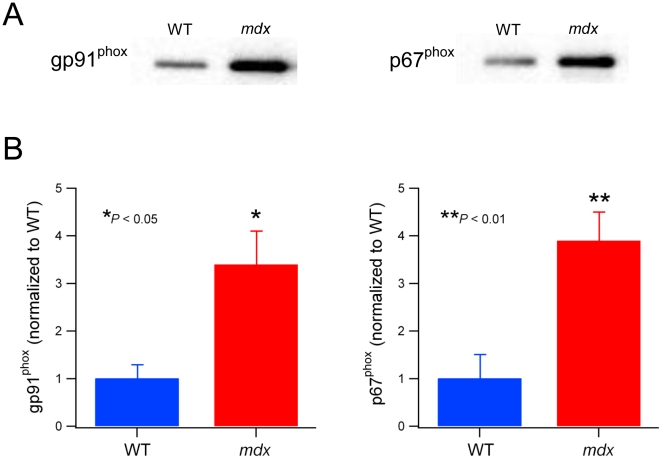
The NADPH oxidase catalytic subunit gp91phox is increased in 4–8 week old mdx skeletal muscles [17]. In the current study, we found a similar magnitude increase (3–4 fold) in TA muscle homogenates from 9 week mdx mice, compared to WT (Figure 1). In addition, at this age, we also measured the expression levels of the key activator subunit, p67phox. Interestingly, we found that this protein also increased by 3–4 fold in mdx muscle (see Figure 1). Both of these proteins were significantly greater for mdx compared to WT (gp91phox, P<0.05; p67phox, P<0.01).
Figure 1. NADPH oxidase subunits are increased in 9 week old mdx mice.
A. Representative western blots of gp91phox and p67phox from 9 week old WT and mdx TA muscles. B. Pooled results of densitometry analysis for gp91phox and p67phox from WT (n = 4) and mdx (n = 8) muscles.
NADPH oxidase-dependent superoxide production is increased in mdx muscle
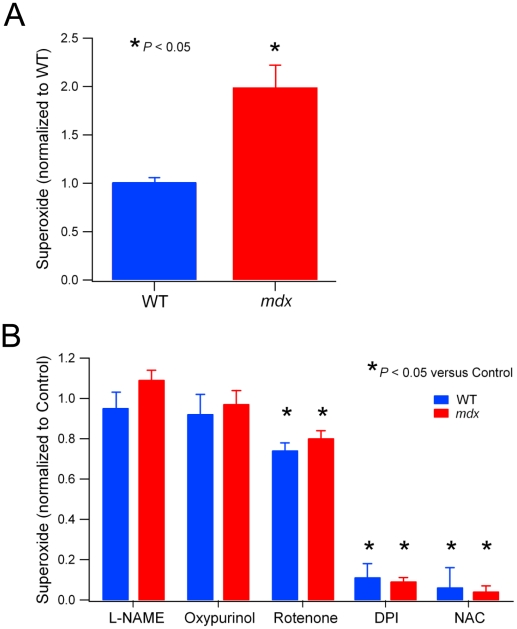
Given the increased protein expression of NADPH oxidase in mdx muscle, we then investigated whether this would result in enhanced NADPH oxidase activity. Previously, our lab showed that NADPH oxidase activity was increased in heart homogenates from mdx mice [7]. Therefore, we used the same procedure to investigate whether NADPH oxidase ROS production was increased in mdx skeletal muscle compared to WT. NADPH-dependent ROS (superoxide) production was measured in TA muscle homogenates from 9 week old WT and mdx mice. We used a well-established technique, the lucigenin chemiluminescent superoxide assay, using NADPH as the substrate (see Methods for details). Superoxide production was significantly increased (P<0.05) by 2 fold in mdx muscle compared to WT (Figure 2A). To establish that NADPH oxidase was the primary source of the increased superoxide production in mdx muscles, we used inhibitors of NADPH oxidase and the other main cellular ROS sources (Figure 2B). For both WT and mdx muscles, L-NAME and oxypurinol had no effect, while rotenone reduced superoxide by about 20% (P<0.05). However, the NADPH oxidase inhibitor DPI prevented more than 90% of the superoxide production (P<0.05), indicating that NADPH oxidase was the main source of the ROS in this assay. Like DPI, the antioxidant NAC, used as a positive control, almost completely prevented superoxide production (P<0.01).
Figure 2. NADPH oxidase superoxide production is increased in mdx muscle.
A. Lucigenin chemiluminescent assay was used to measure NADPH-dependent superoxide production from muscle homogenates of WT and mdx mice (see Methods for details). Values from each experiment were pooled for WT and mdx muscles (n = 5 in both groups). B. Inhibitors of various sources of ROS were added to the assay and superoxide production was compared to the control level (no inhibitor). Pooled values for WT and mdx mice.
NADPH oxidase subunits are differentially increased in 18–19 day old (pre-necrotic) mdx mice
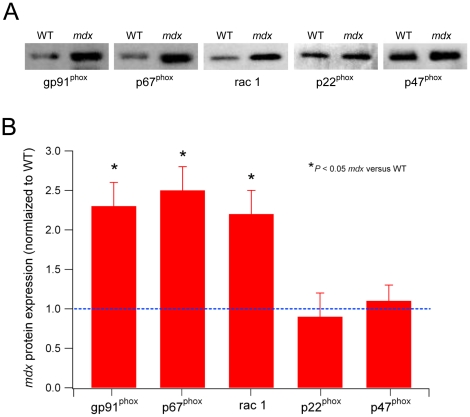
Between the ages of about 4 and 8 weeks, mdx muscles undergo extensive cycles of necrosis, inflammation and regeneration. Since inflammatory cells such as macrophages and neutrophils express NADPH oxidase, we wanted to test whether NADPH oxidase protein expression was increased in 18–19 day old, pre-necrotic, mdx muscles. This would determine whether the increased NADPH oxidase expression in mdx muscles was produced by skeletal muscle fibers, as a consequence of the lack of dystrophin, and not from inflammatory cells. In these experiments, we measured protein expression levels of all the NADPH oxidase subunits. As shown in Figure 3, three subunits, gp91phox, p67phox and rac1 were all significantly increased by 2–3 fold in mdx TA muscles compared to WT (P<0.05). The levels of the other two subunits, p22phox and p47phox were not significantly different between mdx and WT.
Figure 3. NADPH oxidase subunits are increased in 18–19 day old (pre-necrotic) mdx mice.
A. Representative western blots of all NADPH oxidase subunits from TA muscles of 18–19 day old WT and mdx mice. B. Pooled results from densitometry for the subunits showing mdx values normalized to WT (represented by the blue dotted line). The proteins gp91phox, p67phox and rac1 were all significantly increased in mdx muscle compared to WT, whereas p22phox and p47phox were not different (n = 4 samples for each group).
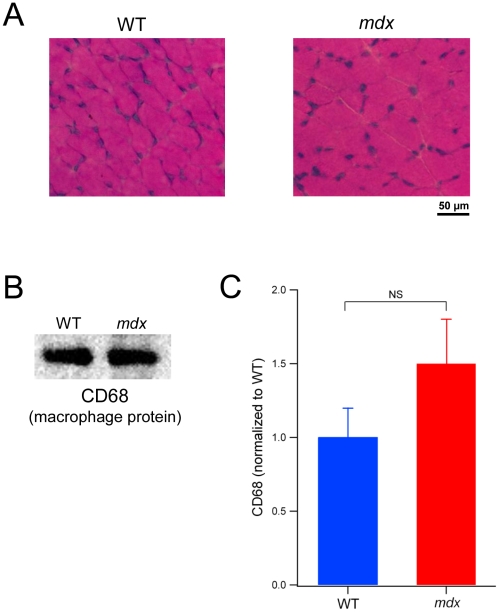
In order to confirm that the higher expression of NADPH oxidase was not derived from increased numbers of inflammatory cells, we firstly examined histological sections, by H&E staining. We found no evidence of inflammatory cell infiltration or myofiber necrosis in 18–19 day old mdx mice (Figure 4A). In addition, we also showed by immunoblotting that the macrophage protein CD68 was not significantly different between mdx and wild type mice at this age (Figure 4B and C).
Figure 4. Muscles of 18–19 day old mdx mice show no evidence of inflammatory cells.
A. Representative images of TA cross-sections from 19 day old WT and mdx mice stained with H&E. Note the normal histological appearance of the mdx muscle, which is devoid of necrotic fibers and infiltrating inflammatory cells. B. Sample western blot showing the macrophage protein, CD68, from TA homogenates of 18–19 day old WT and mdx mice. C. Pooled data of CD68 expression from western blots of 18–19 day old mice, showing no significant difference (NS) between WT and mdx muscles.
Localization of NADPH oxidase proteins in single muscle fibers
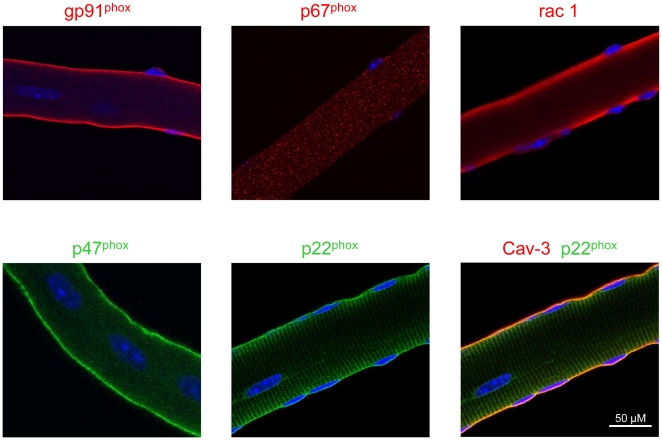
To determine the cellular localization of NADPH oxidase subunits we carried out immunocytochemistry on isolated single muscle fibers. Mice ranging in age from 3 to 9 weeks of aged were used and, in general, there was no observable difference in the localization of the various subunits as a function of age. Furthermore, the localization was similar between mdx and WT fibers. All of the subunits, with the exception of p67phox, were localized on or near the sarcolemma (Figure 5). A striated pattern of staining for gp91phox and particularly p22phox is probably due to t-tubule expression [20]. In addition to sarcolemmal localization, p47phox is diffusely found in the cytoplasm, while p67phox is only located in the cytoplasm in resting cells. In order to demonstrate sarcolemmal localization, p22phox (green) was co-labeled with caveolin-3 (red), the muscle-specific caveolin isoform, found in sarcolemmal caveolae. As shown in Figure 5, there was good co-localization between the two proteins, characterized in the merged image by the yellow staining along the sarcolemma.
Figure 5. NADPH oxidase immunostaining of single fibers from mdx mice.
Isolated mdx muscle fibers from the FDB muscle were immunostained using antibodies against the various NADPH oxidase subunits. Nuclei are stained by DAPI (blue). In the bottom right panel, p22phox (green) was co-immunostained with caveolin-3 (red) to demonstrate sarcolemmal localization (yellow).
Stretch-induced Ca2+ influx in mdx fibers is reduced by an NADPH oxidase inhibitor
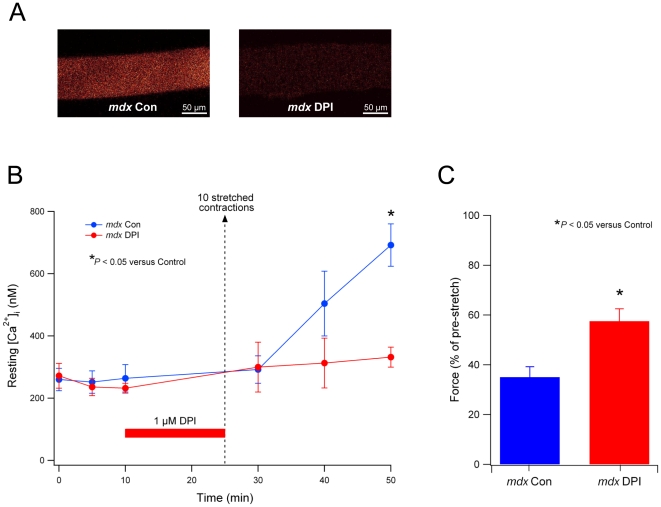
We have previously shown that the stretch-induced Ca2+ influx in mdx fibers occurs through stretch-activated channels [18]. Recently, we showed that stretch-induced ROS mediate this effect, since the Ca2+ influx could be prevented with an antioxidant [8]. In the current study, we extended these findings by determining whether NADPH oxidase was the source of the stretch-induced ROS, which triggers the opening of the SACs and the Ca2+ influx. In these experiments, we used only mdx fibers, since WT fibers show only a very small rise in resting intracellular Ca2+ following our stretched contraction protocol [18]. Single mdx fibers were incubated with or without the NADPH oxidase inhibitor DPI (1 µM) for 15 min before a series of 10 stretched contractions. The intracellular Ca2+ indicator Fluo-4 was used to measure changes in [Ca2+]i before and after the stretched contractions, using confocal microscopy. As shown previously [8], Ca2+ levels increased progressively for 25 min after the stretched contractions in mdx control fibers (Figure 6A and B). DPI greatly reduced the rise in Ca2+ after the stretched contractions, significant at 25 min compared to control fibers (P<0.05). As shown in Figure 6C, the reduction in muscle force after the stretched contractions was also significantly inhibited by DPI (P<0.05) by about 20%, which is comparable to the effect provided by SAC blockers [18] and the antioxidant Tiron [8].
Figure 6. NADPH oxidase inhibition reduces stretch-induced Ca2+ influx and force loss in mdx fibers.
A. Fluorescent confocal images showing intracellular Ca2+ (Fluo-4) for mdx single fibers taken 25 min after a series of 10 stretched contractions. Fibers were either untreated controls (mdx Con) or treated with the NADH oxidase inhibitor DPI (mdx DPI). B. Pooled data of the resting intracellular Ca2+ concentration ([Ca2+]i) as measured before and after 10 stretched contractions (dotted line with arrow). Values are shown for control fibers (n = 9; blue) and fibers treated with 1 µM DPI (n = 5; red) for 15 min before the stretched contractions. C. Pooled values showing the significantly greater muscle force after the stretched contractions for DPI- treated fibers (n = 5; red) compared to control fibers (n = 9; blue). Note that pre-stretch force values are represented by 100%.
Discussion
Oxidative stress is a hallmark feature of many pathological human conditions, including DMD [21]. In terms of understanding disease mechanisms and designing therapeutic strategies for muscular dystrophy, it is important to discern if this overproduction of ROS is a primary cause or secondary effect of the disease progression. Therefore, identifying the source(s) of the excessive ROS is necessary. In recent years, NADPH oxidase has emerged as an important source of ROS for normal physiological functions. However, enhanced activity is associated with pathological conditions affecting a wide range of tissues and organs, including the heart, kidney, lung and vasculature [12]. In skeletal muscle, relatively little is known about the role and regulation of NADPH oxidase. Recent work from us and others, using various antioxidants, has shown that ROS is a cause of both the chronic muscle damage in mdx mice and the acute damage from stretched contractions [4], [6], [8], [22]. In the current study, we hypothesized that NADPH oxidase was a major source of the enhanced ROS production in mdx skeletal muscle. Recent studies showed that protein expression of gp91phox was increased 3 fold in muscles from 4–8 week old mdx mice compared to wild type [17]. We confirmed this result in 9 week old mdx mice and additionally showed that the activator subunit, p67phox was increased by a similar amount. In addition, we found a two-fold increase in NADPH-dependent superoxide production from mdx muscle homogenates, which was almost completely prevented by the non-specific NADPH oxidase inhibitor DPI but not by blockers of NOS or xanthine oxidase, other possible sources of ROS. The mitochondrial complex I inhibitor, rotenone, provided a small but significant attenuation of superoxide, however this difference was the same for WT and mdx muscles. Therefore, this data suggests that NADPH oxidase ROS generation is greatly enhanced in mdx skeletal muscle, consistent with findings in mdx cardiac muscle [7].
Dystrophic muscles are characterized by cycles of myofiber damage, necrosis, inflammation and finally regeneration or fibrosis. In mdx mouse hind limb muscles, such as TA, EDL and soleus, this process is most prominent between about 4–9 weeks of age, during which time more than 50% of the muscle undergoes damage and regeneration, as evidenced by the number of fibers with central nuclei. During this period, infiltrating inflammatory cells are a key feature of the disease and can both exacerbate muscle damage as well as promote regeneration [23]. Upon activation, macrophages and neutrophils utilize NADPH oxidase to produce superoxide, which reacts to form more cytotoxic ROS that can damage neighboring cells. In skeletal muscle, ROS production by neutrophil NADPH oxidase has been shown to cause significant muscle damage following hindlimb unloading, which was attenuated in gp91phox null mice [24]. Thus, in order to show that skeletal muscle, and not phagocyte, NADPH oxidase is a major cause of oxidative stress in dystrophic muscle, we measured protein expression levels in young (18–19 day old) mdx mice before the onset of muscle damage and inflammatory cell infiltration and activation. Importantly, in young mdx muscle, we found a significant, 2–3 fold, increase in three NADPH oxidase subunits; gp91phox, p67phox and rac1. Expression of the other two subunits, p47phox and p22phox was not different between mdx and WT. In mdx muscles we found no histological evidence of muscle necrosis, or inflammatory cell infiltration, and expression of the macrophage protein, CD68, was not different between mdx and WT muscles. Normal muscles contain resident, non-activated, macrophages [25], which probably explains the detection of CD68 in WT and mdx muscles. Therefore, given the immunofluorescent detection of all NADPH oxidase subunits in single myofibers (see Figure 5), we postulate that the increased NADPH oxidase subunit levels in mdx muscles is most likely due to enhanced expression by skeletal muscle fibers themselves. As mentioned previously, there is evidence of oxidative stress in pre-necrotic mdx muscles [9], [10]. While other sources of ROS, such as mitochondria and xanthine oxidase might also contribute to the enhanced ROS production, our data suggests that NADPH oxidase is a major source of oxidative stress in dystrophin-deficient muscle, a conclusion also reached by Shkryl et al.,(2009) [17] (see below).
An interesting finding was the differential changes in the expression of the various NADPH oxidase subunits in mdx muscle, with gp91phox, p67phox and rac1 increased, and p22phox and p47phox the same as WT. This could be explained by different transcriptional regulation of the genes encoding these proteins. In support of our findings, both gp91phox [14] and p67phox [15] mRNA levels are greatly increased in mdx skeletal muscles. Transcriptional regulation of these subunits in mdx muscle is possibly mediated by the transcription factor NF-κB regulates, which is known to regulate NADPH oxidase gene transcription in phagocytes [26]. The DNA binding activity of NF-κB is greatly increased in the diaphragm of pre-necrotic 15 and 18 day old mdx mice [27], which supports the interpretation that NF-κB activity is involved in the upregulation of these subunits. Moreover, the same study showed that the ROS scavenger NAC reduced NF-κB activity in mdx muscle. Thus, a positive feedback loop whereby NF-κB increases NADPH oxidase expression and the resulting ROS production further stimulates NF-κB nuclear translocation and transcriptional activity may occur. Consistent with this idea, we have recently shown that chronic treatment of mdx mice with NAC, from 3 weeks of age, attenuates muscle damage and significantly reduces nuclear localization of NF-κB [6]. Currently, it is not clear why the other NADPH oxidase proteins, p22phox and p47phox did not also increase in mdx muscle. In normal mouse skeletal muscle, p22phox is the most abundant and p67phox the least abundant subunit, at least at the mRNA level [28]. Thus, assuming a stoichiometry of 1∶1 for the NADPH oxidase subunits [29], it is necessary for the least expressed subunits to be upregulated in order to form a greater number of active NADPH oxidase complexes. The three subunits that increased in the current experiments are all critical for full activation of the enzyme: p67phox and rac1 bind to each other and gp91phox to stimulate catalytic activity of the enzyme [12]. On the other hand, while p47phox is considered essential for activation in phagocytes and other cell types following agonist stimulation, it is not required for basal activity in smooth muscle cells [30]. Future experiments are required to determine the regulation and role of each NADPH oxidase subunit in both normal and dystrophic skeletal muscle.
We have previously shown in mdx fibers that the stretch-induced rise in cytosolic Ca2+ is mediated by SAC [18]. Moreover, blocking SAC in mdx muscles also significantly attenuated the force drop following stretched contractions and reduced membrane permeability [18], [31]. More recently, we found that these effects of SAC blockers could be replicated by antioxidants [6], [8], and in the current study, by the NADPH oxidase inhibitor DPI (see Figure 6). Taken together, these results imply that stretch of mdx muscle produces ROS, by activation of NADPH oxidase, which subsequently triggers Ca2+ entry through SAC leading to muscle damage and contractile dysfunction. Our interpretation is consistent with recent findings [17]. Using a different model of stretch, hypo-osmotic swelling, Shkryl et al., (2009) reported that NADPH oxidase triggered Ca2+ sparks in mdx fibers. They postulated that the Ca2+, triggered by NADPH oxidase-derived ROS, was later sequestered by mitochondria, which then generated additional ROS. Interestingly, basal ROS production from NADPH oxidase, but not mitochondria, was greater in mdx fibers compared to WT. Again, these findings suggest that stretch-induced NADPH oxidase ROS production precedes, and subsequently stimulates mitochondrial ROS production via Ca2+ uptake. Consistent with this idea, there is recent evidence that in 5 week old mdx mice, during the extensive muscle damage phase, mitochondria also contribute to ROS production [32].
Currently, the mechanism by which NADPH oxidase-dependent ROS activates SAC remains unclear. One possibility is that ROS activates SAC directly, via redox-sensitive sulfhydryl groups, or indirectly, by ROS-mediated signaling pathways. We have recently shown that TRPC1, a candidate for the overactive SAC in mdx muscle [33], was activated via ROS sensitive src kinase [8]. Furthermore, TRPC1 and src kinase were increased in mdx muscle, and src kinase inhibition significantly improved muscle force following stretched contractions in mdx EDL muscle [8]. Src kinase phosphorylates p47phox to initiate its translocation and organization of the other NADPH oxidase cytosolic subunits to the membrane-bound complex [34]. Thus, increased expression and activation of src kinase might be involved in the enhanced stretch-induced ROS production of NADPH oxidase and the associated Ca2+ influx. TRPV2 is another potential candidate for the SAC in mdx muscle [35]. A final point of consideration is that both TRPC1 [8] and src kinase [36] bind to Caveolin-3, which is upregulated in mdx muscles [37]. Here, we have shown that p22phox co-localized with caveolin-3 in single fibers (see Figure 5). We also have preliminary evidence that NADPH oxidase subunits are found in both caveolae and non-caveolae fractions in mdx and WT muscles (unpublished observations). Depending on the cell type and species, caveolae or lipid rafts can regulate activation [38] or inhibition [39] of NADPH oxidase. Therefore, in future experiments it will be important to elucidate whether caveolin-3 and caveolae play a role in the regulation of NADPH oxidase activity in mdx muscles.
In summary, this study has identified NADPH oxidase as a major source of ROS production in mdx muscle, which is upregulated during the period of oxidative stress that precedes muscle damage and inflammation. The other key finding is that NADPH oxidase is involved in the activation of a ROS-sensitive SAC in dystrophic muscle, triggered by stretched contractions. Inhibition of NADPH oxidase prevented the influx of Ca2+ into the muscle and provided protection against the force deficit. Therefore, investigating the regulation of NADPH oxidase expression and activity will be important to gain new insight into the mechanisms of damage in dystrophic muscle and to potentially develop new therapeutic approaches for DMD.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was funded by grants from the National Health and Medical Research Council, Australia, and National Institutes of Health (NIH). NPW is the recipient of a Fellowship from Parent Project Muscular Dystrophy (PPMD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW. Dysregulated intracellular signaling and inflammatory gene expression during initial disease onset in Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2009;88:502–522. doi: 10.1097/PHM.0b013e3181a5a24f. [DOI] [PubMed] [Google Scholar]
- 3.Rando TA. Oxidative stress and the pathogenesis of muscular dystrophies. Am J Phys Med Rehabil. 2002;81:S175–186. doi: 10.1097/00002060-200211001-00018. [DOI] [PubMed] [Google Scholar]
- 4.Dorchies OM, Wagner S, Vuadens O, Waldhauser K, Buetler TM, et al. Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–625. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 5.Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, et al. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am J Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitehead NP, Pham C, Gervasio OL, Allen DG. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 8.Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J Cell Sci. 2008;121:2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- 9.Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, et al. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. doi: 10.1016/s0022-510x(98)00258-5. [DOI] [PubMed] [Google Scholar]
- 10.Dudley RW, Khairallah M, Mohammed S, Lands L, Des Rosiers C, et al. Dynamic responses of the glutathione system to acute oxidative stress in dystrophic mouse (mdx) muscles. Am J Physiol Regul Integr Comp Physiol. 2006;291:R704–710. doi: 10.1152/ajpregu.00031.2006. [DOI] [PubMed] [Google Scholar]
- 11.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 13.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 14.Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, et al. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord. 2008;18:371–381. doi: 10.1016/j.nmd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng BS, Zhao P, Pattison JS, Gordon SE, Granchelli JA, et al. Regenerated mdx mouse skeletal muscle shows differential mRNA expression. J Appl Physiol. 2002;93:537–545. doi: 10.1152/japplphysiol.00202.2002. [DOI] [PubMed] [Google Scholar]
- 16.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 17.Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, et al. Reciprocal amplification of ROS and Ca(2+) signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Arch. 2009;458:915–928. doi: 10.1007/s00424-009-0670-2. [DOI] [PubMed] [Google Scholar]
- 18.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, et al. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravenscroft G, Nowak KJ, Jackaman C, Clement S, Lyons MA, et al. Dissociated flexor digitorum brevis myofiber culture system–a more mature muscle culture system. Cell Motil Cytoskeleton. 2007;64:727–738. doi: 10.1002/cm.20223. [DOI] [PubMed] [Google Scholar]
- 20.Hidalgo C, Sanchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 21.Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 22.Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, et al. Lipid peroxidation inhibition blunts nuclear factor-kappaB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen HX, Tidball JG. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol. 2003;553:833–841. doi: 10.1113/jphysiol.2003.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimorady-Esfahani A, Grounds MD, McMenamin PG. Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve. 1997;20:158–166. doi: 10.1002/(sici)1097-4598(199702)20:2<158::aid-mus4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, et al. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- 28.Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, et al. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal. 2008;10:559–574. doi: 10.1089/ars.2007.1792. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Hitt ND, Kleinberg ME. Stoichiometry of p22-phox and gp91-phox in phagocyte cytochrome b558. Biochemistry. 1995;34:16753–16757. doi: 10.1021/bi00051a024. [DOI] [PubMed] [Google Scholar]
- 30.Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circulation. 2004;109:1307–1313. doi: 10.1161/01.CIR.0000118463.23388.B9. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Menazza S, Blaauw B, Tiepolo T, Toniolo L, Braghetta P, et al. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum Mol Genet. 2010;19:4207–4215. doi: 10.1093/hmg/ddq339. [DOI] [PubMed] [Google Scholar]
- 33.Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 35.Zanou N, Iwata Y, Schakman O, Lebacq J, Wakabayashi S, et al. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett. 2009;583:3600–3604. doi: 10.1016/j.febslet.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 37.Vaghy PL, Fang J, Wu W, Vaghy LP. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 1998;431:125–127. doi: 10.1016/s0014-5793(98)00738-8. [DOI] [PubMed] [Google Scholar]
- 38.Vilhardt F, van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han W, Li H, Villar VA, Pascua AM, Dajani MI, et al. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]