Abstract
Background
The Salmonella genomic island 1 (SGI1) is a Salmonella enterica-derived integrative mobilizable element (IME) containing various complex multiple resistance integrons identified in several S. enterica serovars and in Proteus mirabilis. Previous studies have shown that SGI1 transfers horizontally by in trans mobilization in the presence of the IncA/C conjugative helper plasmid pR55.
Methodology/Principal Findings
Here, we report the ability of different prevalent multidrug resistance (MDR) plasmids including extended-spectrum β-lactamase (ESBL) gene-carrying plasmids to mobilize the multidrug resistance genomic island SGI1. Through conjugation experiments, none of the 24 conjugative plasmids tested of the IncFI, FII, HI2, I1, L/M, N, P incompatibility groups were able to mobilize SGI1 at a detectable level (transfer frequency <10−9). In our collection, ESBL gene-carrying plasmids were mainly from the IncHI2 and I1 groups and thus were unable to mobilize SGI1. However, the horizontal transfer of SGI1 was shown to be specifically mediated by conjugative helper plasmids of the broad-host-range IncA/C incompatibility group. Several conjugative IncA/C MDR plasmids as well as the sequenced IncA/C reference plasmid pRA1 of 143,963 bp were shown to mobilize in trans SGI1 from a S. enterica donor to the Escherichia coli recipient strain. Depending on the IncA/C plasmid used, the conjugative transfer of SGI1 occurred at frequencies ranging from 10−3 to 10−6 transconjugants per donor. Of particular concern, some large IncA/C MDR plasmids carrying the extended-spectrum cephalosporinase bla CMY-2 gene were shown to mobilize in trans SGI1.
Conclusions/Significance
The ability of the IncA/C MDR plasmid family to mobilize SGI1 could contribute to its spread by horizontal transfer among enteric pathogens. Moreover, the increasing prevalence of IncA/C plasmids in MDR S. enterica isolates worldwide has potential implications for the epidemic success of the antibiotic resistance genomic island SGI1 and its close derivatives.
Introduction
Acquisition of foreign DNA by horizontal gene transfer (HGT) is a crucial mechanism that allows bacteria to acquire new traits, and it represents a key driving force in bacterial evolution. HGT is widely recognized as the mechanism responsible for the widespread distribution of antibiotic resistance genes. Among HGT mechanisms, those specified by conjugative plasmids seem to be the most sophisticated and implicated in the diffusion of antibiotic resistance genes [1]. Bacterial conjugation appears to be a conserved molecular process by which transfer of DNA from a donor to a recipient cell occurs during a close cell-cell contact. In Gram-negative bacteria, conjugation requires the elaboration of a type IV secretion system, called the transferosome and the formation of a nucleoprotein complex involved in processing the DNA and delivering it to the transferosome, called the relaxosome [2]. Although conjugation is a conserved mechanism, conjugative systems (transferosome and relaxosome) have shown some differences depending on the incompatibility (Inc) group of plasmids [2]. Incompatibility is a manifestation of the relatedness of plasmids that share common replication controls. Incompatibility was defined as the inability of two related plasmids to be propagated stably in the same bacterial strain [3], [4].
Localization of antimicrobial resistance genes on transferable genetic elements such as broad-host range plasmids, integrative conjugative elements facilitates the HGT of these genes among bacteria and provides a rapid mean of spread [5]. Certain replicon types (correlated with Inc groups) are associated with multidrug resistance (MDR) from bacteria implicated in disease outbreaks or found in food-producing animals [6]. In this context, IncA/C MDR plasmids are widely distributed among foodborne pathogens such as Salmonella [7]. IncA/C replicon types are associated with plasmids carrying and disseminating extended-spectrum β-lactamase (ESBL) genes in animals and humans [6]. During the past decade, specific attention has been focused on IncA/C MDR plasmids that encode the AmpC β-lactamase CMY-2 (bla CMY-2 gene) especially among Salmonella enterica and Escherichia coli [5], [6], [8], [9].
On the other hand, the 43-kb Salmonella genomic island 1 (SGI1) is the first genomic island conferring a MDR phenotype identified in S. enterica [10]. SGI1 is an integrative mobilizable element (IME) which contains a complex class 1 integron, named In104, located at the 3′ end of the island [11]. The In104 complex integron confers the common penta-resistance profile to ampicillin (Amp), chloramphenicol (Chl), streptomycin (Str), sulphonamides (Sul) and tetracycline (Tet) of the epidemic MDR S. enterica serovar Typhimurium defined phage type DT104 (S. Typhimurium DT104) strains [10], [11]. Since the identification of SGI1 in S. Typhimurium DT104, variant SGI1 MDR complex integrons have been described in a wide variety of S. enterica serovars and more recently also in Proteus mirabilis [11]–[14]. The identification of SGI1 in P. mirabilis clinical isolates is of great concern as the spread of the SGI1 (or variants) MDR phenotype could have significant implications in other pathogenic bacteria.
In all cases and until now, in field or clinical S. enterica and P. mirabilis strains, SGI1 is found integrated into the bacterial chromosome within the last 18 bp of the trmE gene (also named thdF) [10], [11]. In 2005, we reported that SGI1 could be conjugally transferred from S. enterica donor strains to non-SGI1 S. enterica and E. coli recipient strains where it integrated into the recipient chromosome in a site-specific manner [15]. Briefly, after excision of SGI1 from the Salmonella chromosome, the conjugative mobilization in trans by the conjugative helper IncA/C plasmid pR55 occurs between donor and recipient strains. In the recipient cell, the circular form of SGI1 integrates in a site-specific manner at the 3′ end of the chromosomal trmE gene [15]. SGI1 appeared to be a non-self-transmissible but mobilizable element and was thus classified within the group of site-specific integrative mobilizable elements (IMEs) that are related to integrative conjugative elements (ICEs) [15].
In the present study, we report the conjugative in trans mobilization assays of SGI1 by several plasmids of different incompatibility groups. We have tested epidemic successful plasmids carrying Extended Spectrum Cephalosporins (ESC) resistance genes encoding CTX-Ms, TEM-52 ESBLs, and CMY-2 AmpC β-lactamase [16]. According to the results of our plasmid collection tested, we found that only the broad-host-range conjugative IncA/C plasmids were able to mobilize in trans SGI1. Conjugative IncHI2 and IncI1 plasmids carrying ESBL genes (bla CTX-M or bla TEM) were unable to mobilize SGI1. Although certain IncA/C MDR bla CMY-2 plasmids seemed to have lost their self-transferable capacity, some conjugative IncA/C MDR bla CMY-2 plasmids permitted the conjugative mobilization of SGI1 [5], [7], [8]. Our findings suggest that a close relationship, probably at a molecular conjugative process level, may exist between the IncA/C MDR plasmid family and the MDR genomic island SGI1.
Results and Discussion
The identification of SGI1 in the chromosome of several S. enterica serovars, i.e. Agona, Cerro, Derby, Dusseldorf, Emek, Haifa, Infantis, Kedougou, Kentucky, Kiambu, Kingston, Meleagridis, Newport, Paratyphi B, Tallahassee, Typhimurium, Virchow, and also in P. mirabilis suggested that SGI1 might be horizontally mobilized by conjugative elements concomitantly borne by field strains [11]–[15]. Mobilization experiments were undertaken to determine whether SGI1 was mobilizable in trans by different conjugative plasmids previously described as spreading among MDR Salmonella strains and/or other pathogenic Enterobacteriaceae [16]. We firstly introduced by conjugation a set of 17 conjugative ESBL plasmids (CTX-M-1, -2, -9 and TEM-52) of IncHI2 and IncI1 groups in the SGI1-carrying S. Agona strain 959SA97 previously described to conjugally transfer SGI1 in the presence of the conjugative IncA/C helper plasmid pR55 (Table 1 and data not shown) [15]. These ESBL IncHI2 and IncI1 plasmids are representative of the ESBL plasmid families that spread among several S. enterica serovars in animal and human isolates representing a major concern [17], [18]. In all mobilization assays between ESBL plasmids/SGI1-carrying S. Agona strain 959SA97 and the recipient E. coli K-12 strain BM14, no SGI1 transconjugants could be obtained in 3 repeated attempts (Table S1 in the supplemental material). In all mating experiments, control of conjugative transfer of the introduced helper plasmid was positive (data not shown). Two TEM-52 IncI1 conjugative plasmids p777SA01 and p04-3486 were initially isolated from S. Agona and S. Typhimurium field strains that also harboured SGI1 or SGI1-A variant, respectively (Table 1) [17]. Mobilization of SGI1 was also tested from these field strains and resulted also negatively (Table S1). These results indicated that SGI1 is not mobilized by our set of ESBL plasmids of IncHI2 and IncI1 groups from S. enterica to E. coli. These data suggested that IncHI2 and IncI1 plasmids are not able to mobilize SGI1. Moreover, these results indicated that the presence of any conjugative plasmid, which would provide all conjugal transfer functions for successful conjugation, is not the only sufficient prerequisite for mobilization of SGI1. However, our ESBL plasmid collection is mainly from S. enterica sources and does not contain plasmids of other Inc groups than HI2 and I1 that are also described to harbour various ESBL genes (bla TEM, bla SHV, bla CTX-M) in bacterial isolates from other sources such as E. coli from humans [19].
Table 1. Bacterial strains, and plasmids used in this study.
| Strains and plasmid | Relevant genotype, resistance profilea, original host or characteristics | Reference or source |
| S. enterica SGI1 donors | ||
| Agona 959SA97 | SGI1+; AmpChlStrSulTet | [10] |
| Agona 959SA97ΔS009::kan | SGI1ΔS009::kan +; AmpChlKanStrSulTet | [10] |
| Agona 47SA97 | SGI1-C+; StrSul | [30] |
| Agona 47SA97Δxis::kan | SGI1-CΔxis::kan +; KanStrSul | [30] |
| Agona 777SA01 | SGI1-A+; IncI1+ (TEM-52); AmpChlStrSulTetTmp-3GC | [17] |
| Albany 7205.00 | SGI1-F+; AmpChlSulTetTmp | [31] |
| Typhimurium 04-3486 | SGI1+; IncI1+ (TEM-52); AmpChlStrSulTet-3GC | [17] |
| E. coli recipient | ||
| BM14 | K-12 J53 derivative; F- pro met azi; AzR | Institut Pasteur, France |
| Plasmids | ||
| IncA/C pRA1 | Tra+; SulTet; A. hydrophila | [32] |
| IncA/C pIP40A | Tra+; AmpKanSul; P. aeruginosa | [33] |
| IncA/C pR55 | Tra+; AmpChlGenKanSul; K. pneumoniae | [24] |
| IncA/C pR16a | Tra+; AmpKanSul; P. stuartii | [21] |
| IncA/C p13688 | Tra+; AmpChlGenStrSulTetTmp-3GC; CMY-2; E. coli | [9] |
| IncA/C p13956 | Tra+; AmpChlStrSulTetTmp-3GC; CMY-2; E. coli | [9] |
| IncA/C pAM04528 | Tra−; AmpChlStrSulTet-3GC; CMY-2; S. Newport | [8] |
| IncA/C pN418 | Tra−; AmpChlGenKanStrSulTet-3GC; CMY-2; S. Heidelberg | [7] |
| IncFI pOX38 | Tra+; Kan; F factor derivative; E. coli | Institut Pasteur, France |
| IncFII R1-16 | Tra+; Kan; R1 derivative; E. coli | [20] |
| IncHI2 pCEB6542 | Tra+; SulTet-3GC; CTX-M-1; S. Llandoff | [34] |
| IncHI2 p1639-SA-00 | Tra+; AmpSulTetTmp-3GC; CTX-M-2; S. Virchow | [18] |
| IncHI2 p142-SA-01 | Tra+; AmpSulTetTmp-3GC; CTX-M-2; S. Virchow | [fernandez2007] |
| IncHI2 p3464b | Tra+; AmpStrSulTetTmp-3GC; CTX-M-9; S. Virchow | [35] |
| IncI1 p777-SA-01 | Tra+; Amp-3CG; TEM-52; S. Agona | [17] |
| IncI1 p04-3486 | Tra+; Amp-3CG; TEM-52; S. Typhimurium | [17] |
| IncI1 R112 | Tra+; Kan; S. Panama | [21] |
| IncL/M R69 | Tra+; AmpKanTet; S. Paratyphi B | [21] |
| IncN RPC3 | Tra+; KanStr; E. coli | [21] |
| IncP RP4 | Tra+; AmpKanTet; P. aeruginosa | [33] |
| IncW RSa | Tra+; ChlKanStrSul; Shigella flexneri | [22] |
abbreviations: Amp, ampicillin; Az, sodium azide; Chl, chloramphenicol; Gen, gentamicin; Kan, kanamycin; Str, streptomycin; Sul, sulphonamides; Tet, tetracyclines; Tmp, trimethoprim; 3GC, third generation cephalosporin.
In spite of these negative results, we decided to test reference conjugative plasmids of main different incompatibility groups previously described for plasmid typing [20]–[22]. Conjugative plasmids pRA1 (IncA/C), pOX38 (IncFI), R1-16 (IncFII), R112 (IncI1), R69 (IncL/M), RPC3 (IncN), RP4 (IncP), and Rsa (IncW) were introduced by conjugation into SGI1-carrying S. Agona strain 959SA97 or SGI1-C-carrying S. Agona strain 47SA97 depending on antibiotic selection used to introduce the plasmid and thereafter for the transfer of SGI1 (Table 1). The presence of each reference plasmid in S. Agona SGI1 donor strains was confirmed by PCR based-replicon typing [3]. Among all the reference plasmids tested, only the IncA/C reference plasmid pRA1 initially isolated from Aeromonas hydrophila in 1971 was able to mobilize variant SGI1-C from S. Agona donor strain 47SA97 in mating experiments with the E. coli recipient strain BM14 (Table 2). E. coli SGI1-C transconjugants showed the antibiotic resistance profile conferred by SGI1-C (StrSul) and the additional resistance to sodium azide. The presence of SGI1 or variants of it was confirmed by several PCRs indicating that the entire SGI1 was presented in E. coli transconjugants and integrated into the chromosome (data not shown). The kanamycin resistance conferred by plasmid pRA1 was not transferred to the E. coli recipient. The absence of plasmid pRA1 in E. coli transconjugants was also confirmed by the specific IncA/C replicon PCR (data not shown) [3]. These results indicated that the IncA/C reference plasmid pRA1 was not transferred into the transconjugants tested and confirmed that SGI1 was mobilized in trans by pRA1. Using the IncA/C reference plasmid pRA1 as helper plasmid, E. coli SGI1-C transconjugants were found at a frequency of around 10−3 (Table 2). All other conjugative reference plasmids of IncFI, IncFII, IncI1, IncL/M, IncN, IncP, and IncW were unable to mobilize SGI1 or variant SGI1-C in repeated mating experiments (Table S1). In all mating experiments, conjugative transfer control of the different conjugative helper plasmids was positive (data not shown). These results suggested that the in trans mobilization of SGI1 may be correlated with the presence of a helper conjugative plasmid of the IncA/C family in the Salmonella SGI1 donor strain.
Table 2. SGI1 mobilization by IncA/C plasmids.
| S. enterica donor strain | SGI1 variant | Conjugative plasmid | SGI1 transfer frequencya | ||
| Field strain | |||||
| Agona 959SA97 | SGI1 | − | <10−9 | ||
| Agona 47SA97 | SGI1-C | − | <10−9 | ||
| Albany 7205.00 | SGI1-F | − | <10−9 | ||
| Derivative of field strain | |||||
| Agona 959SA97ΔS009::kan | SGI1ΔS009::kan | – | <10−9 | ||
| Agona 47SA97Δxis::kan | SGI1-CΔxis::kan | – | <10−9 | ||
| Transconjugant strain | |||||
| Agona 47SA97 | SGI1-C | IncA/C pRA1 | 9.8 10−3 | ||
| Agona 47SA97 | SGI1-C | IncA/C pIP40a | 1.9 10−2 | ||
| Agona 959SA97 | SGI1 | IncA/C pR55 | 1.9 10−3 | ||
| Agona 47SA97 | SGI1-C | IncA/C pR55 | 3.4 10−3 | ||
| Albany 7205.00 | SGI1-F | IncA/C pR55 | 7.2 10−5 | ||
| Agona 959SA97 | SGI1 | IncA/C pR16a | 4.4 10−3 | ||
| Agona 47SA97 | SGI1-C | IncA/C pR16a | 8.9 10−3 | ||
| Agona 47SA97Δxis::kan | SGI1-CΔxis::kan | IncA/C pR55 | 3.1 10−7 | ||
| Agona 959SA97ΔS009::kan | SGI1ΔS009::kan | IncA/C p13688 (CMY-2) | 6.7 10−5 | ||
| Agona 959SA97ΔS009::kan | SGI1ΔS009::kan | IncA/C p13956 (CMY-2) | 2.2 10−5 | ||
| Agona 47SA97Δxis::kan | SGI1-CΔxis::kan | IncA/C p13688 (CMY-2) | 5.2 10−6 | ||
| Agona 47SA97Δxis::kan | SGI1-CΔxis::kan | IncA/C p13956 (CMY-2) | 2.2 10−6 | ||
| Albany 7205.00 | SGI1-F | IncA/C pAM04528 (CMY-2) | <10−9 | ||
| Albany 7205.00 | SGI1-F | IncA/C pN418 (CMY-2) | <10−9 | ||
the frequency of transfer was calculated by dividing the number of SGI1 transconjugants by the number of SGI1 donor cells. Transfer frequencies correspond to the result of one experiment which has been repeated two times and showing the same results.
To assess whether the IncA/C plasmid family is specifically implicated in the mobilization of SGI1, and whether different IncA/C plasmids may mobilize in trans SGI1, we introduced by conjugation different MDR IncA/C plasmids into SGI1-carrying S. Agona strain 959SA97 or SGI1-C-carrying S. Agona strain 47SA97 or SGI1-F-carrying S. Albany strain 7205.00 (Table 1). A first set of historical MDR IncA/C plasmids isolated at the end of the 1960′s like the reference IncA/C plasmid pRA1, i.e. pIP40a (from Pseudomonas aeruginosa in 1969), pR16a (from Providencia Stuartii in 1966), and pR55 (from Klebsiella pneumoniae in 1969) that was firstly used to experimentally mobilize SGI1, was tested in SGI1 mobilization assays (Table 1) [15], [21], [23], [24]. E. coli SGI1 transconjugants were obtained in all mobilization assays (Table 2). Transconjugants showed the antibiotic resistance profile of the SGI1 variant horizontally transferred and susceptibility to specific antibiotic markers of the different helper IncA/C plasmids used in the donor strains. All these results were confirmed by PCRs on E. coli transconjugants (data not shown). Depending on the helper conjugative IncA/C plasmid used, and also on the S. enterica SGI1 donor strain, the SGI1 transfer frequencies were found to range from 10−3 to 10−5. These results indicated that several conjugative IncA/C plasmids were able to mobilize in trans SGI1 or variants of it. Thus, these results strengthen the hypothesis that there is a specific relation between the IncA/C plasmid family and the ability to mobilize SGI1.
To further confirm this hypothesis, other current MDR IncA/C plasmids encoding the extended spectrum cephalsporinase CMY-2 were also tested in SGI1 mobilization assays (Table 1). Since 2000, the emergence of extended-spectrum cephalosporin-resistant S. enterica carrying IncA/C plasmid-mediated CMY-2 AmpC β-lactamase has been reported in numerous countries around the world. Such CMY-2 IncA/C plasmids have been also identified in E. coli isolates [8], [9]. Two CMY-2 IncA/C plasmids p13688 and p13956 isolated from E. coli animal strains were introduced by conjugation from an E. coli transconjugant to S. Agona derivative strain 959SA97ΔS009::kan or S. Agona derivative strain 47SA97Δxis::kan [9]. These mutant strains contain a kanamycin marker in SGI1 that was necessary for the selection of SGI1 in mating experiments according to the large antibiotic resistance profile of these plasmids (Table 1). The ORF S009 initially annotated in the SGI1 sequence revealed to be non-functional, thus the insertion of the kanamycin marker gene would not affect the transfer of SGI1 (B. Doublet, unpublished results). Strain 47SA97Δxis::kan has been previously described and the deletion of the xis gene in SGI1-C did not abolish the SGI1 transfer but slightly affected its transfer frequency [15]. Like with the previously tested IncA/C plasmids, these 2 conjugative CMY-2 IncA/C plasmids allowed the mobilization of SGI1 at frequencies ranging from 10−5 to 10−6 (Table 2). Two other current CMY-2 IncA/C plasmids, pAM04528 and pN418, isolated from representative strains of clonal epidemic S. Heidelberg and S. Newport in the U.S. were also studied to mobilize SGI1 [7], [8]. Conjugation experiments from the original S. enterica host strains to E. coli recipient strain failed for the CMY-2 IncA/C plasmid pAM04528 and yielded many E. coli transconjugants for the CMY-2 IncA/C plasmid pN418. Then, the CMY-2 IncA/C plasmid pN418 was introduced by conjugation into SGI1-F-carrying S. Albany strain 7205.00 and plasmid pAM04528 was transferred by electroporation into the same SGI1 donor strain. For both plasmids no E. coli SGI1 transconjugants were obtained in 3 independent assays of SGI1 mobilization (Table 2). Control of conjugative transfer of helper plasmid was positive for pN418 and as suspected, negative for pAM04528. As expected, these results indicated that a non-self-transferable IncA/C plasmid, i.e. pAM04528 from S. Newport, was not able to mobilize in trans SGI1. However surprisingly, the CMY-2 MDR IncA/C plasmid pN418 from S. Heidelberg, which is self-transmissible, was also not able to mobilize SGI1. These two plasmids have been previously described in different studies and seemed to be very similar [7], [8]. Thus, these data suggested that there might exist genetic differences between these two CMY-2 MDR IncA/C plasmids and also between the 2 others CMY-2 MDR IncA/C plasmids isolated from E. coli strains of this study that could explain the efficiency or not to mobilize SGI1. Moreover, the difference in SGI1 transfer rates ranging from 10−3 to 10−6 could be another result of transfer function differences between IncA/C plasmids. These results are in accordance with other studies which showed that transfer rates for different IncA/C plasmids can vary by as much as 104 fold [5], [7], [25].
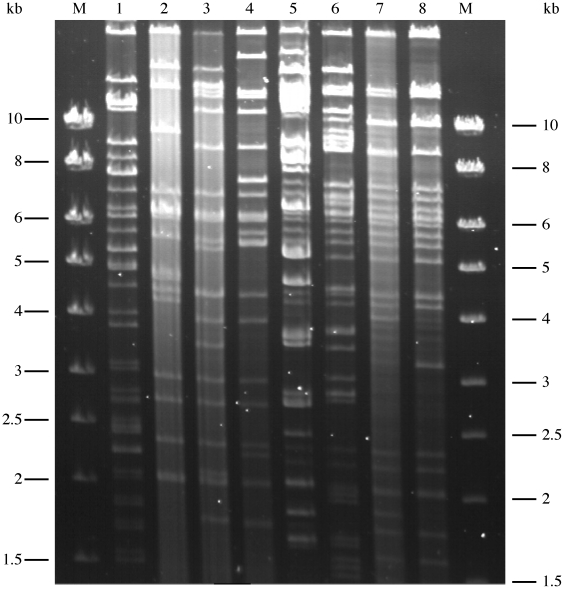
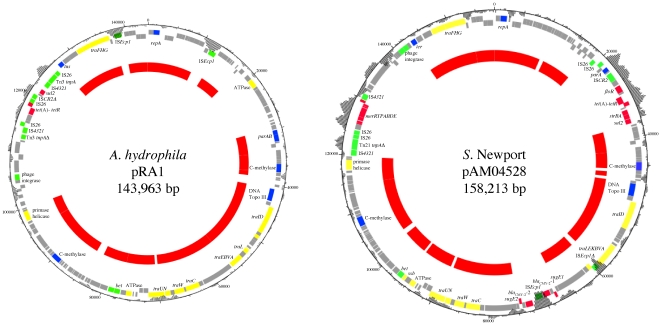
A comparative genetic analysis of the large IncA/C MDR plasmids used in this study was set up based on restriction profile and comparison of two sequenced plasmids pRA1 and pAM04528 to assess their genetic relationship and to determine if their transfer efficiency could be explained by particular traits. According to the full-length sequences of plasmids pRA1 and pAM04528, enzymatic digestion of the historical and current IncA/C MDR plasmids was performed using restriction enzyme DraI. Among the IncA/C MDR plasmids studied, the historical plasmids pR55, pR16, and pIP40a showed related DraI patterns being only different by few fragments of different sizes (Figure 1). The same result was also observed for the 4 current epidemic CMY-2 IncA/C MDR plasmids, i.e. p13956, p13688, pN418, and pAM04528 (Figure 1). However, this second set of plasmids presented really distinct profiles compared to the historical ones. Interestingly, the IncA/C reference plasmid pRA1 revealed a distinct DraI profile from all other IncA/C plasmids (Figure 1). Sequence comparison of the two sequenced IncA/C plasmids pRA1 and pAM04528 used in this study allowed to define a common IncA/C plasmid backbone between these 2 plasmids which represents around 100 kb in size indicated as an inner red circle in Figure 2. This common IncA/C plasmid backbone showed an overall genomic synteny and a nucleotide identity ranging from 88 to 94%. Moreover, the functional predictions inferred from the plasmid annotations showed that this IncA/C backbone corresponds to essential functions of a plasmid lifestyle such as replication, maintenance, and conjugative transfer (Figure 2). The main regions of difference between these plasmids correspond to different antibiotic resistance gene clusters and insertion sequences as shown by the deviating nucleotide composition (Figure 2), which seem to be acquired by insertion/transposition events. It is worth to note that although a large common IncA/C backbone is shared, the DraI restriction profiles of these plasmids are very different from each other (Figure 1).
Figure 1. Restriction profile analysis of IncA/C plasmids digested by the restriction enzyme DraI.
Lane 1, pRA1; lane 2, pR55; lane 3, pR16a; lane 4, pIP40a; lane 5, p13956; lane 6, p13688; lane 7, pN418; lane 8, pAM04528; M, kbp molecular marker (Smartladder, Eurogentec, Seraing, Belgium).
Figure 2. Circular representations of previously sequenced IncA/C plasmids pRA1 and pAM04528 and their common IncA/C backbone (red inner circle).
Nucleotide composition (GC plot) is represented on each plasmid and genes were color coded, depending on functional annotations, as follows: plasmid replication/maintenance, blue; transposition/recombination, green; conjugative plasmid transfer, yellow; antimicrobial resistance, red; other functions/hypothetical proteins, gray. Distance scales in base pair are given around each map. Sequences and annotations of pRA1 and pAM04528 are accessible in GenBank database under accession numbers FJ705807 and FJ621587, respectively.
A recent study from Lindsey et al. showed that the combination of Salmonella, IncA/C plasmids, and MDR is very ancient. In their work, they demonstrated an interesting relation between the presence/absence of IncA/C MDR plasmids, the presence/absence of SGI1 and epidemic successful serovars of S. enterica like Typhimurium DT104 and Newport [6]. MDR plasmids belonging to the IncA/C family are widely distributed among Salmonella and other Enterobacteriaceae from animal sources and have caused considerable concern in public health community [6], [7]. Moreover, a stronger association of MDR with IncA/C replicon was observed in Salmonella than with other replicon types [6]. This association is probably related to a highly conserved IncA/C plasmid backbone into which horizontally acquired antibiotic resistance fragments were integrated in few sites [25].
An important trait for the epidemic spread of IncA/C plasmids lies in their conjugative self-transmissibility. Welch et al. demonstrated that a large majority of IncA/C plasmids found in Salmonella strains were able to transfer horizontally [25]. However, several studies showed that the conjugal transfer could not be demonstrated for all CMY-2 MDR IncA/C plasmids of epidemic S. Newport isolates in the U.S. [25]. Poole et al. also observed that CMY-2 MDR IncA/C plasmids from Salmonella strains rarely transferred when it was the only replicon detected in the donor strain [5]. Regarding the majority of large MDR IncA/C plasmids sequenced and their self-transferability when it is known, there are few differences, which could explain the conjugative efficiency or not. The only difference identified involving tra genes was a partial duplication of traC genes associated with the duplication of the bla CMY-2 locus in non-self-transferable plasmid identified in S. Newport isolates [7], . Only one larger fragment upstream of the parAB cluster is absent from pAM04528 but present on the IncA/C reference plasmid pRA1 [7]. However, this region contains several ORFs of unknown function and only one putative ATPase which could be implicated in transfer function. When transferability of sequenced plasmids were known, no correlation was observed between the presence of this region and the self-transferability [5], [7], [8]. The loss of transfer efficiency of current epidemic CMY-2 IncA/C plasmids of S. Newport isolates may be due to numerous insertion sequences present on these plasmids compared to historical ones. However, several questions remain to be answered: (i) Why the self-transferable IncA/C plasmid pN418 is unable to mobilize SGI1; and (ii) What and/or where is the specific link between IncA/C transferability and SGI1 mobilization. To answer these questions, further studies need to be undertaken on specific transfer elements of SGI1 to connect them to IncA/C transfer functions.
In summary, we have shown that the MDR genomic island SGI1 is specifically mobilized in trans by the conjugative IncA/C plasmid family. Depending on the IncA/C plasmid used, the conjugative transfer of SGI1 occurred at frequencies ranging from 10−3 to 10−6 transconjugants per donor. Of particular concern, some large IncA/C MDR plasmids carrying the extended-spectrum cephalosporinase bla CMY-2 gene were shown to mobilize in trans SGI1. To the best of our knowledge, this study represents the first description of a specific relation between an incompatibility plasmid group and the specific mobilization of another mobile genetic element. Related IncA/C MDR plasmids have been detected in several fish pathogens such as Aeromonas, Yersinia, Photobacterium, Edwardsiella indicating that IncA/C plasmids mediates environmental MDR dissemination between bacteria from mammalian enteric flora and from aquatic ecosystem [7], [8], [25]–[27]. Thus, aquatic environment may be a favourable ecologic niche where horizontal transfer takes place between different bacterial genera. More than just their own dissemination, conjugative IncA/C plasmids may also contribute to the spread of the antibiotic resistance genomic island SGI1 and its close-derivatives among enteric pathogens and potentially more widely.
Materials and Methods
Bacterial strains, media, plasmids and antibiotic susceptibility testing
The Salmonella strains harbouring SGI1 or variants of it used in conjugation experiments are described in Table 1. S. enterica strains 959SA97 (serovar Agona harbouring SGI1), 47SA97 (serovar Agona harbouring the SGI1-C variant), 7205.00 (serovar Albany harbouring the SGI1-F variant) were used as donor strains [15]. E. coli K-12 strain BM14 was used as recipient strain. All strains were grown at 37°C in brain heart infusion broth or agar plates. The Salmonella-Shigella (SS) medium with addition of appropriate antibiotics was used for selection of S. enterica donors and E. coli SGI1 transconjugants in mobilization experiments. Conjugative helper plasmids of different host origins used in mobilization experiments are listed in Table 1. These plasmids (except pAMO4528 and pN418) have been previously described to be self-transmissible (Tra+) [see references in Table 1]. Donor, recipient, and transconjugant strains were screened for antibiotic resistance by the disk diffusion method on Mueller-Hinton agar plates [28]. Susceptibility was tested using disks containing the following antibiotics: Amx (10 µg), Chl (30 µg), Kan (30 IU), Gen (15 µg), Str (10 IU), Sul (200 µg), Tet (30 IU), Tmp (5 µg) and for third generation cephalosporins, ceftriaxone (30 µg), cefepime (30 µg), and ceftiofur (30 µg). All antibiotic disks were purchased from BioRad (Marnes la Coquette, France).
Bacterial conjugations
Bacterial conjugation was performed to introduce the different helper plasmids into the SGI1-containing S. enterica strains. Briefly, end-log exponential phase liquid cultures of an E. coli donor containing helper plasmid and a recipient SGI1-carrying S. enterica strain were mixed together in a approximately 1∶4 ratio. After overnight incubation without shaking at 37°C, the mating was streaked on appropriate selective SS agar plates. Antibiotics for which resistances were conferred by SGI1, were used to select Salmonella SGI1 recipient strains and antibiotic resistances displayed by helper plasmids were chosen to select transconjugants.
SGI1 mobilization assays were performed by mixing S. enterica SGI1 donor strain harbouring different helper plasmid and the sodium azide-resistant E. coli recipient strain BM14 together with a donor-to-recipient ratio of 4∶1. This broth was incubated overnight at 37°C without shaking. The next day, the cells were streaked on appropriate selective SS agar plates. Sodium azide (500 µg/ml) was used to select against Salmonella donor cells, and Str (50 µg/ml) and/or Tet (10 µg/ml) and/or Tmp (40 µg/ml) to select against unmated recipient cells. Salmonella donors in the mating were numbered on SS agar plates supplemented with antibiotics selecting for SGI1 (Str, Tet, Tmp) and the helper plasmid (antibiotic resistance only conferred by the plasmid and not by SGI1). Appropriate control plates were performed for the conjugative transfer of the helper plasmid in each mating experiment. The SGI1 frequency of transfer was determined by dividing the number of E. coli SGI1 transconjugants by the number of Salmonella donor cells.
SGI1 PCR detection, PCR-based replicon typing, IncA/C plasmid restriction profile, and sequence analysis
Detection of SGI1 and its location in Salmonella strains and in E. coli transconjugants were performed using primers corresponding to the left and right junctions in the chromosome as described previously [15], [23]. Primers U7-L12 (located in the trmE gene) and 104D (located in the yidY gene) corresponding to the Salmonella chromosome and primers EcU7-L12 and Ec104D (located in the tnaL gene) corresponding to the E. coli chromosome were used to assess SGI1 junctions in its specific attachment site in Salmonella and E. coli, respectively (Table S1).
Conjugative helper plasmids in E. coli donor strains were typed by the PCR-based replicon typing as previously described [3]. The same method was applied to confirm the presence of the different helper plasmids in the Salmonella SGI1 donor strains. Additional PCRs were performed to amplify known resistance genes carried by the helper plasmids especially for ESC resistance genes bla TEM, bla CTX-M, bla CMY, and for phenicol resistance genes, cat and floR (Table S1).
To assess the genetic relationship between IncA/C helper plasmids used to mobilize SGI1, plasmid DNA was extracted and purified from E. coli host strains with the QIAGEN plasmid midi kit (Courtaboeuf, France) used according to the manufacturer's recommendations. According to the sequence of the IncA/C reference plasmid pRA1, plasmid DNA was digested with the restriction enzyme DraI (Promega, Charbonnières-les-Bains, France). Fragments of DNA were separated by electrophoresis in 0.6% ultra pure DNA grade Agarose gel (BioRad, Marnes la Coquette, France).
Sequence comparisons at the nucleotide level were carried out with the BLAST algorithm using the Artemis Comparison Tool [29]. Plasmid maps of pRA1 and pAM04528 were designed using DNA plotter according the annotations in GenBank database under accession numbers FJ705807 and FJ621587, respectively. Their common IncA/C backbone was assigned for region of high nucleotide identity and of genomic synteny.
Supporting Information
SGI1 mobilization assays by different incompatibility group plasmids.
(DOC)
Acknowledgments
We thank I. Monchaux for expert technical assistance. We also thank A. Carattoli (Istituto Superiore di Sanita, Rome), P. Courvalin (Institut Pasteur, Paris), and the Health Protection Agency Culture Collections for providing reference plasmids of different incompatibility groups.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by public funds from the French National Institute of Agronomic Research. GD was supported by a doctoral fellowship from the Ministère de l'Education Nationale et de la Recherche, France. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Amábile-Cuevas CF, Chicurel ME. Bacterial Plasmids and gene flux. Cell. 1992;70:189–199. doi: 10.1016/0092-8674(92)90095-t. [DOI] [PubMed] [Google Scholar]
- 2.De la Cruz F, Frost LS, Meyer RJ, Zechner EL. Conjugative DNA metabolism in Gram-negative bacteria. FEMS Microbiol Rev. 2010;34:18–40. doi: 10.1111/j.1574-6976.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- 3.Carattoli A, Bertini A, Vila L, Falbo V, Hopkins KL, et al. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Carattoli A. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother. 2009;53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole TL, Edrington TS, Brichta-Harhay DM, Carattoli A, Anderson RC, et al. Conjugative transferability of the A/C plasmids from Salmonella enterica isolates that posses or lack bla CMY in the A/C plasmid backbone. Foodborne Path Dis. 2009;6:1185–1194. doi: 10.1089/fpd.2009.0316. [DOI] [PubMed] [Google Scholar]
- 6.Lindsey RL, Fedorka-Cray PJ, Frye JG, Meinersmann RJ. IncA/C plasmids are prevalent in multidrug-resistant Salmonella enterica isolates. Appl Environ Microbiol. 2009;75:1908–1915. doi: 10.1128/AEM.02228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, et al. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol. 2009;191:4750–4757. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Call DR, Singer RS, Meng D, Broschat SL, Orfe LH, et al. bla CMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob Agents Chemother. 2010;54:590–596. doi: 10.1128/AAC.00055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meunier D, Jouy E, Lazizzera C, Doublet B, Kobisch M, et al. Plasmid-borne florfenicol and ceftiofur resistance encoded by the floR and bla CMY-2 genes in Escherichia coli isolates from diseased cattle in France. J Med Microbiol. 2010;59:467–471. doi: 10.1099/jmm.0.016162-0. [DOI] [PubMed] [Google Scholar]
- 10.Boyd D, Peters GA, Cloeckaert A, Sidi Boumedine K, Chaslus-Dancla E, et al. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J Bacteriol. 2001;183:5725–5732. doi: 10.1128/JB.183.19.5725-5732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey MR, Boyd DA, Olson AB, Doublet B, Cloeckaert A. The genetics of Salmonella genomic island 1. Microbes Infect. 2006;8:1915–1922. doi: 10.1016/j.micinf.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed AM, Hussein AIA, Shimamoto T. Proteus mirabilis clinical isolate harbouring a new variant of Salmonella genomic island 1 containing the multiple antibiotic resistance region. J Antimicrob Chemother. 2007;59:184–190. doi: 10.1093/jac/dkl471. [DOI] [PubMed] [Google Scholar]
- 13.Boyd DA, Shi X, Hu QH, Ng LK, Doublet B, et al. Salmonella Genomic Island 1 (SGI1), Variant SGI1-I, and a New Variant SGI1-O in Proteus mirabilis Clinical and Food Isolates from China. Antimicrob Agents Chemother. 2008;52:340–344. doi: 10.1128/AAC.00902-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doublet B, Poirel L, Praud K, Nordmann P, Cloeckaert A. European clinical isolate of Proteus mirabilis harbouring the Salmonella genomic island 1 variant SGI1-O. J Antimicrob Chemother. 2010;65:2260–2262. doi: 10.1093/jac/dkq283. [DOI] [PubMed] [Google Scholar]
- 15.Doublet B, Boyd D, Mulvey MR, Cloeckaert A. The Salmonella genomic island 1 is an integrative mobilizable element. Mol Microbiol. 2005;55:1911–1924. doi: 10.1111/j.1365-2958.2005.04520.x. [DOI] [PubMed] [Google Scholar]
- 16.Su LH, Chu C, Cloeckaert A, Chiu CH. An epidemic of plasmids? Dissemination of extended-spectrum cephalosporinases among Salmonella and other Enterobacteriaceae. FEMS Immunol Med Microbiol. 2008;52:155–168. doi: 10.1111/j.1574-695X.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 17.Cloeckaert A, Praud K, Doublet B, Bertini A, Carattoli A, et al. Dissemination of an extended-spectrum-β-lactamase bla TEM-52 gene-carrying IncI1 plasmid in various Salmonella enterica serovars isolated from poultry and humans in Belgium and France between 2001 and 2005. Antimicrob Agents Chemother. 2007;51:1872–1875. doi: 10.1128/AAC.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García Fernández A, Cloeckaert A, Bertini A, Praud K, Doublet B, et al. Comparative analysis of IncHI2 plasmids carrying bla CTX-M-2 or bla CTX-M-9 from Escherichia coli and Salmonella enterica strains isolated from poultry and humans. Antimicrob Agents Chemother. 2007;51:4177–4180. doi: 10.1128/AAC.00603-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcades G, Deschamps C, Boyd A, Gautier V, Picard B, et al. Replicon typing of plasmids in Escherichia coli producing extended-spectrum beta-lactamases. J Antimicrob Chemother. 2009;63:67–71. doi: 10.1093/jac/dkn428. [DOI] [PubMed] [Google Scholar]
- 20.Couturier M, Bex F, Bergquist PL, Maas WK. Identification and classification of bacterial plasmids. Microbiol Reviews. 1988;52:375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabbert YA, Scavizzi MR, Witchitz JL, Gerbaud GR, Bouanchaud DH. Incompatibility groups and the classification of fi− resistance factors. J Bacteriol. 1972;112:666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussel A, Chabbert YA. Taxonomy and epidemiology of Gram-negative bacterial plasmids studied by DNA-DNA filter hybridization in formamide. J Gen Microbiol. 1978;104:269–276. doi: 10.1099/00221287-104-2-269. [DOI] [PubMed] [Google Scholar]
- 23.Doublet B, Golding GR, Mulvey MR, Cloeckaert A. Secondary chromosomal attachment site and tandem integration of the mobilizable Salmonella genomic island 1. PLoS One. 2008;3:e2060. doi: 10.1371/journal.pone.0002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witchitz JL, Chabbert YA. Résistance transferable à la gentamicine. Ann Inst Pasteur. 1971;121:733–742. [PubMed] [Google Scholar]
- 25.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, et al. Multiple antimicrobial resistance in plague: an emerging pluc health risk. PLoS One. 2007;2:e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh D, Cunningham M, Ji B, Fekete FA, Parry EM, et al. Transferable, multiple antibiotic and mercury resistance in atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J Antmicrob Chemother. 2008;61:1221–1228. doi: 10.1093/jac/dkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch TJ, Evenhuis J, White DG, McDermott PF, Harbottle H, et al. IncA/C plasmid-mediated florfenicol resistance in the catfish pathogen Edwarsiella ictaluri. Antimicrob Agents Chemother. 2009;53:845–846. doi: 10.1128/AAC.01312-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Members of the SFM antibiogramm committee. comité de l'antibiogramme de la société française de microbiologie report 2003. Int J Antimicrob Agents. 2003;21:364–391. doi: 10.1016/s0924-8579(03)00021-9. [DOI] [PubMed] [Google Scholar]
- 29.Carver T, Berriman M, Tivey A, Patel C, Böhme U, et al. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics. 2008;24:2672–2676. doi: 10.1093/bioinformatics/btn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd D, Cloeckaert A, Chaslus-Dancla E, Mulvey MR. Characterization of variant Salmonella genomic island 1 multidrug resistance regions from serovars Typhimurium DT104 and Agona. Antimicrob Agents Chemother. 2002;46:1714–1722. doi: 10.1128/AAC.46.6.1714-1722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doublet B, Lailler R, Meunier D, Brisabois A, Boyd D, et al. Variant Salmonella genomic island 1 antibiotic resistance gene cluster in Salmonella enterica serovar Albany. Emerg Infect Dis. 2003;9:585–591. doi: 10.3201/eid0905.020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoki T, Egusa S, Ogata Y, Watanabe T. Detection and resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol. 1971;65:343–349. doi: 10.1099/00221287-65-3-343. [DOI] [PubMed] [Google Scholar]
- 33.Datta N, Hedges RW, Shaw EJ, Sykes RB, Richmond MH. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971;108:1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cloeckaert A, Praud K, Lefevre M, Doublet B, Pardos M, et al. Extended-spectrum-β-lactamase bla CTX-M-1 gene-carrying IncI1 plasmid in Salmonella enterica isolates from poultry and Humans in France, 2003-2008. Antimicrob Agents Chemother. 2010;54:4484–4486. doi: 10.1128/AAC.00460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weill FX, Lailler R, Praud K, Kérouanton A, Fabre L, et al. Emergence of extended-spectrum-β-lactamase (CTX-M-9)-producing multiresistant strains of Salmonella enterica Serotype Virchow in Poultry and Humans in France. J Clin Microbiol. 2004;42:5767–5773. doi: 10.1128/JCM.42.12.5767-5773.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SGI1 mobilization assays by different incompatibility group plasmids.
(DOC)